Abstract
Background
There is limited information about the hazards of cigarettes, smokeless tobacco, and waterpipe in the Middle East. The aim of this study was to determine the association between different types of tobacco use and earlier death in the Golestan Cohort Study.
Methods
The Study includes 50,045 adults (aged 40–75) from northeastern Iran. The baseline questionnaire (2004–2008) assessed information about use of cigarettes, chewing tobacco (nass), and waterpipe. To assess the use of each type of tobacco compared with never tobacco users, we used Cox regression models adjusted for age, socioeconomic status, area of residence, education, and other tobacco used, and stratified by sex, ethnicity, and opium use.
Results
17% of participants reported a history of cigarette smoking, 7.5% chewing tobacco (nass), and 1.1% smoking waterpipe, and these figures declined in the later birth cohorts. During a median follow-up of 8 years, 4,524 deaths occurred (mean age 64.8±9.9 years). Current (HR=1.44; 95%CI: 1.28–1.61) and former (HR=1.35; 95%CI: 1.16–1.56) cigarette smokers had higher overall mortality relative to never tobacco users. The highest cigarette-associated risk was for cancer death among current heavy smokers (HR=2.32; 95%CI: 1.66–3.24). Current nass chewing was associated with overall mortality (HR=1.16; 95%CI: 1.01–1.34), and there was a 61% higher risk of cancer death in people chewing nass more than 5 times a day. We observed an association between the cumulative lifetime waterpipe use (waterpipe-years>=28) and both overall (HR=1.66; 95%CI: 1.11–2.47), and cancer mortality (HR=2.82; 95%CI: 1.30–6.11).
Conclusions
Regular use of cigarettes, smokeless tobacco and waterpipe were associated with the risk of earlier death (particularly from cancer) in our cohort.
Introduction
Most high-income countries have experienced an epidemic of smoking-related diseases during the twentieth century, first in men, and then in women.[1] While this epidemic seems to have plateaued in many of these countries,[2] tobacco use, including cigarette smoking and alternative tobacco products, are gradually reaching similar epidemic proportions in many low and middle income countries, where 85% of the world’s 1.3 billion smokers live.[3] Many of these countries do not have effective tobacco control policies and strategies in place.[4] A pooled analysis of cohort studies in Asia showed that in adults above the age of 45, smoking accounted for 15.8% of deaths among men and 3.3% of deaths in women in 2004.[5] This study also showed diversity in smoking habits and its health effects across different countries in Asia, but there were no countries from the Middle East in this analysis.
Alternative tobacco products, such as smokeless tobacco and the waterpipe are gaining popularity in many parts of the world, particularly among the youth.[3 6] Promotional materials targeted at smokers often suggest that smokeless tobacco (chewed or snuff) may be a safer alternative to smoking.[7] Waterpipe use has also regained popularity since 1990 in many parts of the world, particularly the Middle East and Africa, and this trend is extending to the US and other Western countries. [8] This is thought to be mainly driven by its renewed popularity among women and the youth, caused by the introduction of flavored tobacco, the café culture associated with waterpipe smoking, easier cultural exchange, [8] and the lack of specific regulatory policies.[9] The World Health Organization (WHO) has identified an urgent need to study the health effects of waterpipe smoking.[10] Against claims regarding the “relative safety” of such alternative products compared to cigarettes, studies have shown many potential hazards[11–13], but their long-term impact on earlier death is largely unknown.[14] Regular use of many such products in the Middle East provides a good opportunity to study this aspect of tobacco toxicity.
We conducted this study to compare the overall and cause-specific mortality rates among users of cigarettes, chewing tobacco, and waterpipe, with never tobacco users in the Golestan Cohort Study, a prospective cohort of 50,045 adults in Iran, during which detailed and validated information have been collected on the lifetime exposure to all of these risk factors.[15]
Methods
Study population
After a feasibility pilot study in 2003,[16] a total of 50,045 adult participants, aged 40 to 75 years, were enrolled prospectively in the Golestan Cohort Study (GCS) from January 2004 through June 2008.[15] The cohort participants were enrolled from those who lived in Gonbad City (20%) and 326 villages (80%) in Golestan Province, northeastern Iran.
Measurements
The GCS general questionnaire included detailed information on the participants’ life-style including the use of different types of tobacco products, opium use and alcohol drinking, as well as demographic characteristics, residential history, occupation, socioeconomic status, and past medical history of chronic diseases. Data were obtained on the types of tobacco used including cigarettes, chewed tobacco (nass), waterpipe, and pipe, and the ages of starting and stopping, daily consumption amount, and frequency of use. Nass, also known as Naswar, a chewable smokeless tobacco, is a mixture of tobacco, ash, and lime that is widely used in the Central Asian Republics, Afghanistan, Iran, Pakistan, and in South Africa.[12 17] Waterpipe, also known as hookah, shisha, hubbly bubbly, narghile, or qualyan, is a device used to smoke tobacco which passes the smoke through water before it is inhaled, and it is estimated to be used by 100 million people around the world.[18]
Definition of exposures
Tobacco users were defined as those who consumed any type of tobacco product at least once a week for 6 months. Cigarette and nass users were further classified as former (those who quit more than 1 year before enrollment) or current users at baseline, and categorized by starting age and average lifetime intensity of use. Waterpipe use could not be classified in a similar way both because of the small number of users and the intermittent nature of its use.[19] Cumulative waterpipe use (waterpipe-years) was calculated by multiplying duration of use by average number of times per day during each period of use, and was summed over the periods. Waterpipe-years were then categorized into tertiles (less than 5, 5–28, and more than 28). If the participant used multiple types of tobacco, or used them intermittently, data were recorded separately for each type and period of use. Pipe use was very uncommon, and very few used it exclusively, so it was not assigned a separate category for analysis. To assess the accuracy of the baseline tobacco questionnaire, we compared the answers with a second re-assessment after an average of 5 years among 11,418 randomly selected individuals, and only 3.6% of smoking reports were inconsistent with the baseline.
Cause of death ascertainment
All of the GCS participants are annually followed up through active telephone surveys and home visits. The follow-up success rate through March 2015 was over 99% (402 lost to follow-up). In addition to annual active follow-up, the GCS uses other sources, such as local health workers’ reports and monthly provincial death registration reports, to reduce the time interval between death and ascertainment of the cause. The details of the GCS methods to evaluate the cause of death are discussed elsewhere.[20]. We used the 10th revision of International Classification of Diseases (ICD-10) codes to classify the cause of death; the most prevalent causes of death in this population were ischemic heart disease (ICD-10 codes I20–I25), cerebrovascular accidents (I60–I69), cancer (C00–C97), respiratory disease (J00–J99), and external causes (S00–T88).
Statistical analysis
Follow-up continued until loss to follow-up, death, or March 31, 2015, whichever came first. We fitted Cox proportional hazards models to estimate unadjusted and adjusted total and cause-specific mortality hazard ratios (HR) and 95% confidence intervals (95% CI) in relation to the exposures of interest, including type of tobacco used, age at start, and per day average amount of use. The potential confounders in the models were sex, age at baseline, residential place (urban vs. rural), ethnicity (Turkmen vs. others), highest attained educational level (none/less than high school/high school/college or higher), opium use (yes vs. no), quartiles of a composite wealth score[21], and the use of other tobacco types (never, former, and current). Further inclusion of alcohol consumption and body mass index (BMI) did not materially change the hazard ratios (data not shown), so they were not included in the final models. In all the models, “never tobacco users” (people who reported they had never used any tobacco product regularly) were the universal reference category, and we used age as the time-scale variable.
All survival analyses were stratified by sex. Two variables did not meet the proportionality assumption using Schoenfeld residuals (ethnicity and opium use), so all multivariable Cox models were also stratified on these variables.[22] Individuals with a prior diagnosis of ischemic heart disease (IHD), cerebrovascular accident (CVA), chronic obstructive pulmonary disease (COPD), and cancer at baseline were excluded from survival analyses. Three types of sensitivity analysis were also performed: 1. excluding the first 24 months of follow-up, 2. restricting all analyses to men as about 95% of cigarette smokers were male, and 3. classifying the individuals who quit smoking within 5 years of enrollment as current smokers.
In a random subgroup of the original cohort (n=11,418), a second round of risk factor assessment was done about 5 years after the initial enrollment. These results were used to compare smoking trends over time. Standardized mortality rates (SMR) per 100,000 person-years of follow up were calculated using the WHO 2000 standard world population.
All statistical analyses were conducted using STATA statistical software, version13 (StataCorp Inc., College Station, TX).
Results
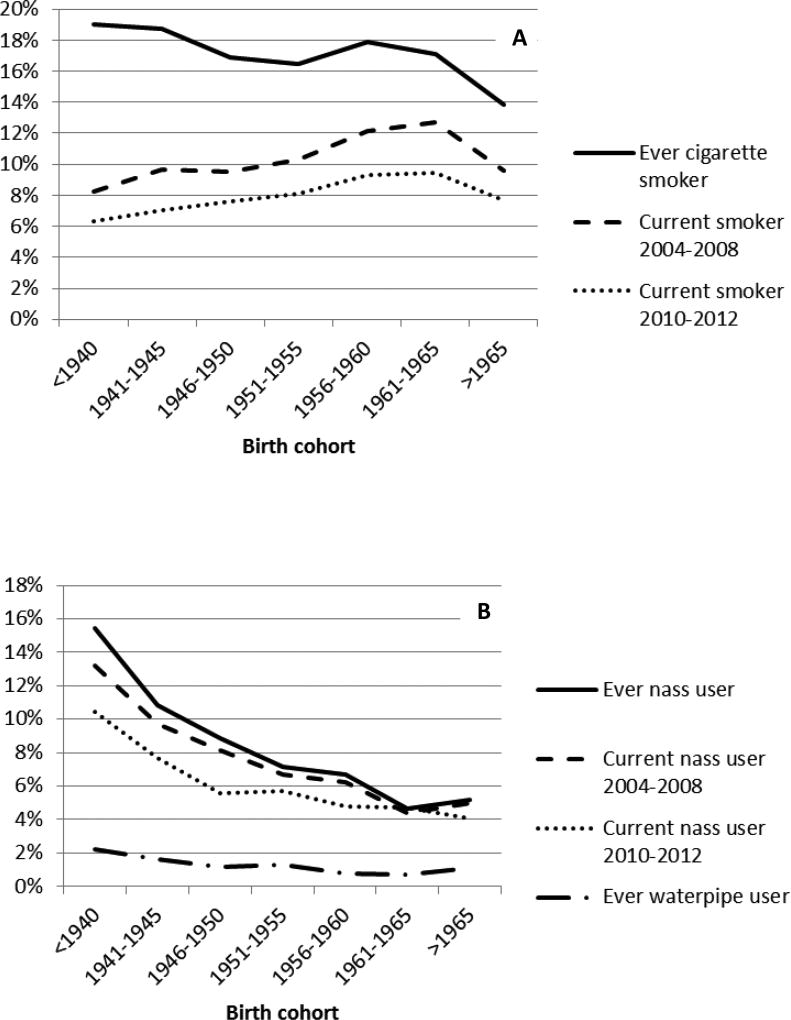
A history of cigarette smoking was reported by 8662 participants (17%), 94.9% of whom were male (Table 1). They smoked, on average, 14.6 (standard deviation:10.7) cigarettes per day, and began smoking at an average age of 25.9 (standard deviation:10.5). Table 1 shows other characteristics of the cohort participants by their smoking status. Cigarette smokers were more likely to be Turkmen, rural, and have a body mass index (BMI) below 25. There were significantly more opium users among both former and current smokers (50.6% and 52.6%, respectively) than among never smokers (9.7%). Also, on average, former smokers smoked more cigarettes per day, and had started smoking at an earlier age than current smokers. Nass and waterpipe use were significantly more common among former cigarette smokers compared with both current smokers and never smokers (Table 1). Figure 1 shows the trends of tobacco use at baseline and the re-assessment after an average of 5 years, by the birth year of the participants. As the figure shows, except for an increase among individuals born during 1955–1965, there are fewer ever cigarette smokers among younger cohorts, and about 32% of current smokers quit as they grew older, while only 1% of non-smokers picked up smoking. Nass use shows a constant drop by age and birth cohort.
Table 1.
Baseline characteristics of Golestan Cohort Study participants according to cigarette smoking status
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Never smoker (n= 13,016) |
Former smoker (n= 3,087) |
Current smoker (n=5,131) |
Never smoker (n=28,367) |
Former smoker (n=126) |
Current smoker (n=318) |
||
| SMR per 105 person-years (95% CI) | 1,106 (1,043 to 1,169) | 1,560 (1,412 to 1,708) | 1,682 (1,521 to 1,844) | 1,028 (726 to 1,331) | 1,474 (870 to 2,079) | 2,234 (1,428 to 3,041) | |
|
| |||||||
| Age a | 53.5 (9.6) | 55.5 (9.5) | 51.2 (8.5) | 51.3 (8.6) | 57.5 (9.1) | 53.1 (8.4) | |
|
| |||||||
| Ethnicity | Turkmen | 9,698 (74.5) | 2,257 (73.1) | 4,097 (79.8) | 20,983 (74.0) | 39 (31.0) | 179 (56.3) |
| non-Turkmen | 3,318 (25.5) | 830 (26.9) | 1,034 (20.2) | 7,384 (26.0) | 87 (69.0) | 139 (43.7) | |
|
| |||||||
| residence | urban | 2,176 (16.7) | 661 (21.4) | 1,095 (21.3) | 5,988 (21.1) | 34 (27.0) | 78 (24.5) |
| rural | 10,840 (83.3) | 2,426 (78.6) | 4,036 (78.7) | 22,379 (78.9) | 92 (73.0) | 240 (75.5) | |
|
| |||||||
| education | none | 6,583 (50.6) | 1,624 (52.6) | 2,210 (43.1) | 24,308 (85.7) | 109 (86.5) | 284 (89.3) |
| Up to 8 years | 4,321 (33.2) | 1,057 (34.2) | 1,960 (38.2) | 3,328 (11.7) | 12 (9.5) | 30 (9.4) | |
| High school | 1,490 (11.4) | 292 (9.5) | 764 (14.9) | 604 (2.1) | 2 (1.6) | 3 (0.9) | |
| University | 622 (4.8) | 114 (3.7) | 197 (3.8) | 127 (0.4) | 3 (2.4) | 1 (0.3) | |
|
| |||||||
| BMI | <25 | 5,892 (45.2) | 1,688 (54.7) | 3,289 (64.1) | 9,205 (32.5) | 66 (52.4) | 199 (62.6) |
| 25–29 | 4,970 (38.2) | 960 (31.1) | 1,341 (26.1) | 9600 (33.8) | 34 (27.0) | 67 (21.1) | |
| >=30 | 2,154 (16.5) | 439 (14.2) | 501 (9.8) | 9562 (33.7) | 26 (20.6) | 52 (16.4) | |
|
| |||||||
| Opium ever use | 1,942 (14.9) | 1,546 (50.1) | 2,658 (51.8) | 2072 (7.3) | 72 (57.1) | 210 (66.0) | |
|
| |||||||
| Alcohol ever use | 396 (3.0) | 439 (14.2) | 873 (17) | 17 (0.1) | 1 (0.8) | 3 (0.9) | |
|
| |||||||
| Nass use | Never | 11,516 (88.5) | 1,648 (53.4) | 4,502 (87.7) | 28,092 (99.0) | 101 (80.2) | 308 (96.9) |
| Former | 153 (1.2) | 147 (4.8) | 37 (0.7) | 23 (0.1) | 4 (3.2) | 2 (0.6) | |
| Current | 1,347 (10.3) | 1,292 (41.9) | 592 (11.5) | 252 (0.9) | 21 (16.7) | 8 (2.5) | |
|
| |||||||
| Waterpipe use | Never | 12,913 (99.2) | 3,013 (97.6) | 5,105 (99.5) | 28,024 (98.8) | 112 (88.9) | 306 (96.2) |
| Former | 50 (0.4) | 47 (1.5) | 7 (0.1) | 106 (0.4) | 10 (7.9) | 3 (0.9) | |
| Current | 53 (0.4) | 27 (0.9) | 19 (0.4) | 237 (0.8) | 4 (3.2) | 9 (2.8) | |
|
| |||||||
| Age when smoking started | <20 | 1,081 (35.0) | 1,150 (22.4) | 9 (7.1) | 19 (6.0) | ||
| 20–24 | 956 (31.0) | 1,391 (27.1) | 25 (19.8) | 18 (5.7) | |||
| 25–29 | 425 (13.8) | 705 (13.7) | 18 (14.3) | 20 (6.3) | |||
| >=30 | 625 (20.2) | 1,885 (36.7) | 74 (58.7) | 261 (82.1) | |||
|
| |||||||
| Years of cigarette smoking | <10 | 1,299 (42.1) | 921 (17.9) | 83 (65.9) | 178 (56.0) | ||
| 10–19 | 789 (25.6) | 1,063 (20.7) | 22 (17.5) | 68 (21.4) | |||
| 20–29 | 510 (16.5) | 1,528 (29.8) | 11 (8.7) | 30 (9.4) | |||
| >=30 | 489 (15.8) | 1,619 (31.6) | 10 (7.9) | 42 (13.2) | |||
|
| |||||||
| Average lifetime cigarettes per dayb | <10 | 889 (28.8) | 1,744 (34.0) | 72 (57.1) | 200 (62.9) | ||
| 10–19 | 518 (16.8) | 1,385 (27.0) | 8 (6.3) | 53 (16.7) | |||
| >=20 | 1,662 (53.8) | 1,957 (38.1) | 45 (35.7) | 55 (17.3) | |||
BMI: body mass index, SMR: standardized mortality rate, CI: confidence interval
Numbers show frequencies (percentage) except for age which is mean (SD), and SMR which is rate per 100,000 (95%CI)
Mean number of cigarettes smoked per day during the time the person smoked. The numbers do not add up since in 71 men and 11 women, average cigarettes per day could not be calculated.
Figure 1.
Proportion of A. cigarette smoking, and B. alternative tobacco use, by birth cohorts in the Golestan Cohort Study
During 391,208 person-years of follow-up (median duration of 8 years), until 31 March 2015, 4,524 deaths occurred among 50,045 cohort participants. The underlying cause of death was confirmed in 3,796 individuals. Among these, the major causes of death were ischemic heart disease (1,294 deaths: 34%), cancer (897 deaths: 24%), cerebrovascular accidents (624 deaths: 16%), external causes (217 deaths: 6%), and respiratory diseases (187: 5%). The most common causes of cancer death were cancers of esophagus (20.0%), stomach (19.7%), and lung (6.2%). Individuals who had reported a prior history of any of these diseases at baseline were excluded from survival analyses (ischemic heart disease (n=3,051), cerebrovascular accidents (n=429), chronic respiratory disease (n=3,035), and cancer (n=159), since the diagnosis may have altered their smoking habits.
Both current (HR=1.44; 95%CI: 1.28 to 1.61) and former (HR=1.35; 95%CI: 1.16 to 1.516) cigarette smokers had higher total mortality compared with never tobacco users (Table 2).
Table 2.
Overall and cause-specific mortality by cigarette use in the Golestan Cohort Study
| Overall mortality | IHD mortality | CVA mortality | Cancer mortality |
Respiratory disease mortality |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Number1 | Deaths1 | crude HR (95% CI) |
adjusted HR2 (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
||
| Never tobacco users | 34,544 | 2,141 | 1 | 1 | 1 | 1 | 1 | 1 | |
|
| |||||||||
| Former smokers3 | 2,551 | 353 | 1.55 (1.37 to 1.75)** | 1.35 (1.16 to 1.56)** | 1.17 (0.87 to 1.58) | 1.10 (0.72 to 1.69) | 1.36 (0.97 to 1.88) | 1.58 (0.74 to 3.37) | |
| Current smokers | 4,889 | 566 | 1.79 (1.61 to 1.99)** | 1.44 (1.28 to 1.61)** | 1.34 (1.07 to 1.68)** | 1.06 (0.75 to 1.50) | 1.69 (1.33 to 2.16)** | 1.76 (1.00 to 3.19)* | |
|
| |||||||||
| Smoking start age3 | |||||||||
| Former | <20 | 887 | 109 | 1.62 (1.33 to 1.98)** | 1.37 (1.09 to 1.73)** | 1.46 (0.93 to 2.30) | 1.44 (0.76 to 2.72) | 1.31 (0.79 to 2.19) | 2.00 (0.63 to 6.42) |
| 20–24 | 773 | 107 | 1.59 (1.30 to 1.94)** | 1.38 (1.09 to 1.75)** | 0.86 (0.50 to 1.50) | 1.36 (0.71 to 2.60) | 1.33 (0.79 to 2.23) | 1.32 (0.37 to 4.70) | |
| 25–29 | 345 | 45 | 1.40 (1.04 to 1.89)** | 1.20 (0.87 to 1.66) | 1.35 (0.73 to 2.51) | 0.79 (0.27 to 2.27) | 1.13 (0.56 to 2.28) | 1.25 (0.25 to 6.28) | |
| >=30 | 546 | 92 | 1.41 (1.14 to 1.75)** | 1.17 (0.92 to 1.49) | 1.26 (0.79 to 1.99) | 0.77 (0.37 to 1.62) | 0.90 (0.50 to 1.63) | 0.69 (0.17 to 2.84) | |
| <20 | 1,031 | 123 | 2.03 (1.68 to 2.46)** | 1.71 (1.40 to 2.09)** | 1.21 (0.79 to 1.86) | 1.19 (0.63 to 2.27) | 2.30 (1.54 to 3.44)** | 3.25 (1.28 to 8.23)** | |
| Current | 20–24 | 1,271 | 132 | 1.78 (1.48 to 2.14)** | 1.50 (1.23 to 1.82)** | 1.78 (1.27 to 2.50)** | 0.86 (0.43 to 1.70) | 1.54 (1.00 to 2.38)* | 1.83 (0.65 to 5.19) |
| 25–29 | 646 | 70 | 1.92 (1.51 to 2.46)** | 1.61 (1.25 to 2.07)** | 0.92 (0.51 to 1.68) | 0.89 (0.35 to 2.23) | 2.06 (1.24 to 3.40)** | 2.86 (0.93 to 8.83) | |
| >=30 | 1,941 | 241 | 1.69 (1.47 to 1.95)** | 1.36 (1.17 to 1.58)** | 1.18 (0.88 to 1.60) | 1.16 (0.75 to 1.78) | 1.63 (1.19 to 2.24)* | 1.46 (0.68 to 3.14) | |
|
| |||||||||
| Cigarettes per day3,4 | |||||||||
| <10 | 784 | 113 | 1.43 (1.17 to 1.74)** | 1.32 (1.06 to 1.64)** | 1.51 (1.01 to 2.28)* | 0.73 (0.35 to 1.53) | 1.09 (0.65 to 1.83) | 1.46 (0.46 to 4.60) | |
| Former | 10–19 | 424 | 49 | 1.34 (1.00 to 1.79)** | 1.20 (0.89 to 1.64) | 0.72 (0.33 to 1.56) | 0.72 (0.25 to 2.02) | 1.49 (0.82 to 2.70) | 1.12 (0.22 to 5.74) |
| >=20 | 1,328 | 188 | 1.64 (1.40 to 1.92)** | 1.31 (1.06 to 1.62)** | 1.26 (0.82 to 1.93) | 1.47 (0.84 to 2.55) | 1.03 (0.63 to 1.69) | 1.09 (0.35 to 3.42) | |
| <10 | 1,783 | 187 | 1.68 (1.43 to 1.96)** | 1.40 (1.19 to 1.66)** | 1.28 (0.92 to 1.78) | 1.16 (0.71 to 1.89) | 1.45 (1.01 to 2.10)* | 1.34 (0.55 to 3.29) | |
| Current | 10–19 | 1,283 | 135 | 1.58 (1.31 to 1.89)** | 1.34 (1.10 to 1.63)** | 1.14 (0.77 to 1.69) | 0.59 (0.28 to 1.24) | 1.75 (1.18 to 2.59)** | 2.10 (0.87 to 5.09) |
| >=20 | 1,772 | 240 | 2.20 (1.90 to 2.54)** | 1.76 (1.50 to 2.07)** | 1.63 (1.19 to 2.24)** | 1.28 (0.78 to 2.09) | 2.32 (1.66 to 3.24)** | 2.01 (0.87 to 4.62) | |
HR: hazard ratio; CI: confidence interval; IHD: ischemic heart disease; CVA: cerebrovascular accidents;
p<0.05,
p<0.001
Excluding individuals with a baseline disease.
Cox regression models stratified by sex, ethnicity and opium, and adjusted for age, socioeconomic status, residence, education, nass and waterpipe use.
The reference category for all models are the same “never tobacco users”.
In 59 smokers (7 dead), average cigarettes per day could not be calculated.
Current cigarette smoking had a stronger association with mortality due to IHD (HR=1.34; 95%CI: 1.07 to 1.68), cancer (HR=1.69; 95%CI: 1.33 to 2.16) and respiratory diseases (HR=1.76; 95%CI: 1.00 to 3.19). Earlier age of smoking initiation was also associated with higher overall and cause-specific mortality (Table 2). We also observed a dose-dependent increase in the risk of all-cause mortality among current smokers with the average number of cigarettes smoked per day (Table 2). Among cause-specific deaths, in particular, cancer mortality more than doubled in the current heavy smokers (>20 cigarettes per day) compared to never tobacco users.
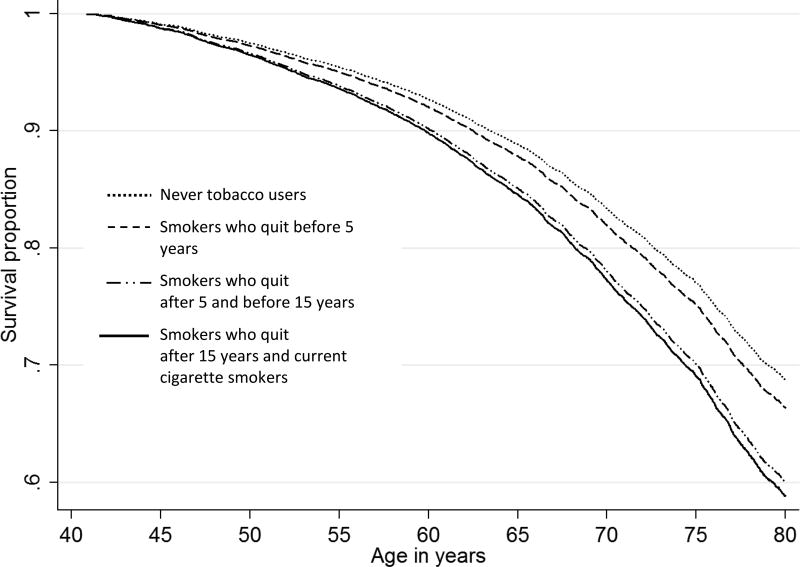
In order to evaluate the effects of cessation on earlier death in former cigarette smokers, we analyzed the duration of smoking before they quit (Figure 2). Former smokers who quit after 15 years of cigarette smoking had identical survival curves to current smokers, and had worse survival than former smokers with a shorter smoking history or never tobacco users. Those who smoked for less than 5 years had similar survival curves as never tobacco users.
Figure 2.
Survival among never tobacco users and cigarette smokers, by the duration of smoking, in the Golestan Cohort Study. The curves are based on Cox regression models stratified by sex, ethnicity and opium, and adjusted for age, socioeconomic status, residence, education, nass and waterpipe use.
Current nass chewing was associated with overall mortality (HR=1.16; 95%CI: 1.01 to 1.34). The highest mortality risk associated with nass chewing was a 61% higher risk of cancer death in people chewing nass, on average, more than 5 times a day (Table 3). The association between nass initiation age and mortality was more complex, and seemed to peak among people starting around the ages of 25–30. However, it is important to consider nass use in the context of cigarette smoking, as about 41% of former cigarette smokers currently used nass (Table 1), and out of 1464 former smokers who used nass, 1211 (82.7%) started nass after quitting cigarettes. Therefore, we also analyzed mortality in association with different combinations of cigarette and nass use (Table 4). Chewing nass had a particularly strong association with cancer mortality: while being a former cigarette smoker alone was not associated with a higher risk, nass use substantially increased the risk of death from cancer among former smokers.
Table 3.
Overall and cause-specific mortality by use of alternative tobacco products (smokeless tobacco (nass) and waterpipe) in the Golestan Cohort Study
| Overall mortality | IHD mortality | CVA mortality | Cancer mortality | Respiratory disease mortality |
|||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Number1 | Deaths1 | crude HR (95% CI) |
adjusted HR2 (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
||
| Never tobacco users | 34,544 | 2,141 | 1 | 1 | 1 | 1 | 1 | ||
|
| |||||||||
| Former nass users3 | 275 | 45 | 1.26 (0.93 to 1.70) | 0.98 (0.73,1.30) | 1.04 (0.55,1.96) | 0.53 (0.19,1.51) | 1.31 (0.70,2.45) | 1.14 (0.31,4.16) | |
| Current nass users | 2,999 | 480 | 1.73 (1.56 to 1.93)** | 1.16 (1.01,1.34)* | 1.16 (0.87,1.55) | 0.98 (0.65,1.47) | 1.42 (1.04,1.94)** | 0.7 (0.29,1.70) | |
|
| |||||||||
| Nass chewing start age3 | |||||||||
| <20 | 200 | 40 | 1.57 (1.15 to 2.16)** | 1.11 (0.79 to 1.54) | 1.20 (0.63 to 2.30) | 0.55 (0.17 to 1.76) | 1.67 (0.89 to 3.15) | ND | |
| 20–24 | 256 | 36 | 1.34 (0.96 to 1.87) | 1.13 (0.83 to 1.54) | 1.05 (0.53 to 2.11) | 0.54 (0.16 to 1.75) | 1.28 (0.63 to 2.58) | 1.38 (0.35 to 5.46) | |
| 25–29 | 203 | 40 | 2.36 (1.72 to 3.24)** | 1.61 (1.17 to 2.21)** | 0.83 (0.34 to 2.06) | 0.84 (0.26 to 2.72) | 2.04 (1.04 to 3.97)* | 0.69 (0.08 to 5.60) | |
| 30–34 | 419 | 76 | 2.06 (1.63 to 2.61)** | 1.26 (0.98 to 1.63) | 1.40 (0.83 to 2.36) | 1.43 (0.73 to 2.80) | 1.54 (0.89 to 2.67) | 1.25 (0.37 to 4.27) | |
| >=35 | 2,196 | 333 | 1.61 (1.43 to 1.82)** | 1.08 (0.93 to 1.26) | 1.13 (0.82 to 1.56) | 0.96 (0.62 to 1.50) | 1.30 (0.92 to 1.83) | 0.7 (0.27 to 1.80) | |
|
| |||||||||
| Nass chewing times per day3,4 | |||||||||
| <=3 | 798 | 117 | 1.48 (1.22 to 1.79)** | 1.07 (0.87 to 1.32) | 1.45 (1.00 to 2.11) | 0.78 (0.42 to 1.44) | 1.01 (0.62 to 1.66) | 0.16 (0.02 to 1.32) | |
| 3–5 | 872 | 147 | 1.95 (1.64 to 2.32)** | 1.34 (1.09 to 1.63)* | 1.42 (0.96 to 2.11) | 0.87 (0.47 to 1.61) | 1.55 (1.02 to 2.38)* | 0.89 (0.30 to 2.66) | |
| >5 | 1,496 | 249 | 1.65 (1.44 to 1.90)** | 1.13 (0.95 to 1.35) | 0.81 (0.55 to 1.20) | 1.06 (0.66 to 1.70) | 1.61 (1.12 to 2.30)** | 0.99 (0.37 to 2.60) | |
|
| |||||||||
| Ever Waterpipe users3 | 411 | 54 | 1.41 (1.08 to 1.85)* | 1.30 (0.98 to 1.73) | 0.85 (0.46 to 1.63) | 1.10 (0.50 to 2.42) | 1.75 (0.95 to 3.21) | 0.40 (0.05 to 3.15) | |
| Cumulative waterpipe-years (Tertiles)3 | |||||||||
| <4.5 | 156 | 10 | 0.98 (0.53 to 1.82) | 0.87 (0.46 to 1.64) | 0.59 (0.15 to 2.42) | 0.58 (0.08 to 4.26) | 2.08 (0.76 to 5.70) | ND | |
| 4.5–28 | 156 | 17 | 1.39 (0.86 to 2.24) | 1.30 (0.80 to 2.12) | 0.88 (0.28 to 2.76) | 2.00 (0.72 to 5.57) | 0.45 (0.06 to 3.21) | ND | |
| >28 | 140 | 26 | 1.77 (1.20 to 2.60)** | 1.66 (1.11 to 2.47)* | 1.08 (0.44 to 2.67) | 0.82 (0.20 to 3.36) | 2.82 (1.30 to 6.11)** | ND | |
HR: hazard ratio; CI: confidence interval; IHD: ischemic heart disease; CVA: cerebrovascular accidents;
p<0.05,
p<0.001
Excluding individuals with a baseline disease.
Cox regression models stratified by sex, ethnicity and opium, and adjusted for age, socioeconomic status, residence, education, and cigarette smoking.
The reference category for all models are the same “never tobacco users”.
In 96 nass users (12 dead), average nass use per day could not be calculated.
Table 4.
Overall and cause-specific mortality by combinations of cigarette and smokeless tobacco (nass) in the Golestan Cohort Study
| Number1 | Deaths1 | Overall mortality | IHD mortality | CVA mortality | Cancer mortality | Respiratory disease mortality |
||
|---|---|---|---|---|---|---|---|---|
| crude HR (95% CI) |
adjusted HR2 (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
adjusted HR (95% CI) |
|||
| Never tobacco users3 | 34,544 | 2,141 | 1 | 1 | 1 | 1 | 1 | 1 |
| Former cigarette only | 1,320 | 162 | 1.37 (1.16 to 1.62)** | 1.30 (1.10 to 1.54)** | 1.23 (0.89 to 1.70) | 1.06 (0.65 to 1.73) | 1.15 (0.77 to 1.73) | 1.17 (0.44 to 3.09) |
| Current cigarette only | 4,299 | 482 | 1.79 (1.60 to 1.99)** | 1.45 (1.29 to 1.64)** | 1.32 (1.05 to 1.67)* | 1.06 (0.74 to 1.53) | 1.75 (1.37 to 2.25)** | 1.73 (0.94 to 3.19) |
| Current nass only4 | 1,393 | 236 | 1.60 (1.39 to 1.85)** | 1.17 (1.00 to 1.36)* | 1.15 (0.85 to 1.55)* | 0.64 (0.16 to 2.61) | 1.40 (1.01 to 1.95)* | 0.56 (0.20 to 1.52) |
| Former cigarette + current Nass4 | 1,056 | 163 | 1.87 (1.58 to 2.21)** | 1.33 (1.11 to 1.59)** | 0.92 (0.62 to 1.37) | 1.15 (0.69 to 1.91) | 1.65 (1.13 to 2.39)** | 1.80 (0.80 to 4.04) |
| Current cigarette + current Nass4 | 523 | 75 | 1.94 (1.53 to 2.46)** | 1.28 (1.00 to 1.64)* | 1.17 (0.71 to 1.92) | 1.07 (0.53 to 2.18) | 1.67 (1.02 to 2.75)* | 1.09 (0.31 to 3.82) |
HR: hazard ratio; CI: confidence interval; IHD: ischemic heart disease; CVA: cerebrovascular accidents;
p<0.05,
p<0.001
Excluding individuals with a baseline disease.
Cox regression models stratified by sex, ethnicity and opium, and adjusted for age, socioeconomic status, residence, education, and waterpipe use.
The reference category for all models are the same “never tobacco users”.
There were only 97 exclusive former nass users, so the models for this category and its combinations could not be constructed.
As Table 3 shows, any waterpipe use showed a borderline association with higher overall and cancer mortality, and there was a significant association between the highest level of cumulative lifetime waterpipe use (waterpipe-years>=28) and both overall (HR=1.66; 95%CI: 1.11 to 2.47), and cancer mortality (HR=2.82; 95%CI: 1.30 to 6.11). The number of regular waterpipe smokers was relatively small, and the models for death from respiratory disease did not converge.
Since opium use affected the risk estimates in our models more than other confounders, we stratified our main results by opium use in Supplementary Tables 1 and 2. In general, the associations with cigarette smoking were stronger among opium users, while waterpipe use was only associated with overall and cancer mortality among never opium users.
We conducted three types of sensitivity analysis. First, we dropped the first two years of the follow-up (861 of the deaths occurred in this period). The results were essentially the same as those including all of the follow-up period, and thus they are not shown. Since cigarette smokers and nass users were mainly men, we conducted a male-only analysis as well, which did not make any changes in the results (data not shown). Finally, we grouped individuals who quit smoking within 5 years from enrollment (n=647) with current smokers. This resulted in some attenuation of the association between both current smoking and overall mortality from 1.44 to 1.36 (95% CI: 1.23 to 1.51) and former cigarette smoking and overall mortality from 1.35 to 1.23 (95% CI: 1.07 to 1.41).
Discussion
In this population, current and former cigarette smokers and current nass users were at increased risk of earlier mortality, particularly from cancer. Former cigarette smokers who smoked for less than 5 years had similar survival curves to never tobacco users, but using smokeless tobacco after quitting increased the chance of dying from cancer compared to those who did not use it. Waterpipe use had similar but weaker effects on mortality, which were most pronounced with high lifetime cumulative use.
Current cigarette smokers in our study had a 44% increased risk of earlier death compared to never tobacco users. The magnitudes of the associations between cigarette smoking and earlier death in our study were smaller than those seen in most high-income countries,[23 24] but similar to those from East Asia[14 25] and other low- and middle-income countries still in the early stages of the tobacco epidemic.[5 26] The risk ratios in these populations are also very similar to those seen in the US at the beginning of the tobacco epidemic: current smokers in the CPS-I cohort (1959–1965) had relative risks of 1.76 (male) and 1.35 (female) for mortality from all causes, which increased in CPS-II (1982–1988), and reached 2.8 (both sexes) in five contemporary US cohorts (2000–2010)[1]. As one possible explanation, standardized mortality rates among non-smokers in the US have dropped from 4,142 per 105 in men above 55 and 2,884 per 105 in women above 55 in CPS-I cohort, to 1918 and 1248, respectively, in the contemporary cohorts. In our population, never smoking men and women above 55 had standardized mortality rates of 2,435 and 2,351, respectively (data not shown), which are also higher than contemporary rates in the US.
Another possible reason for the differences in risk may be due to smoking patterns: smokers in our population generally smoked fewer cigarettes, and started smoking later compared to many Western countries. Many previous studies,[27] as well as our findings, have showed a strong association between early age at smoking initiation and earlier death. Although the type of cigarettes used may be considered as another potential source of differences, cigarettes used in Iran are almost exclusively manufactured, and more than 60% are foreign cigarettes.[28] These cigarettes are imported, or smuggled mainly from other Middle East countries.[29] Many of the domestically-produced cigarettes are also under international brands, and a large volume of the tobacco used in them is imported. A study comparing the nicotine levels of foreign and domestic brands showed no difference between the two.[30]
The prevalence of smoking in Golestan province is lower than the national average in Iran; the prevalence of current smoking among above the age of 45 in the 2007 national survey of adults (the same time our cohort study started) was 29.5% in men and 3.3% in women.[31] Countries in the Middle East share many features of this smoking pattern; in most of them male smoking prevalences are 19.7–34.7%, and women smoke up to ten times less than men.[32] These rates, which are higher than those in sub-Saharan Africa but lower than the rates in Southeast Asia and Eastern Europe, are also lower than the smoking prevalence in the US at the beginning of the tobacco epidemic (54.1% in men and 38.1% in women).[33] However, a meta-regression of the WHO global smoking data showed that in the past decade, the Eastern Mediterranean region has experienced the fastest growth in smoking rates in the world among both among men and women, and about 73% of its population live in areas experiencing such a rapid rise.[34] Given our findings of higher mortality risk, particularly from cancer, this rapid growth heralds a rapid rise in tobacco-related mortality in this region. Moreover, the use of opium and alternative tobacco products may affect the health risks associated with smoking. For example, opium use increases the overall and cause-specific mortality rates[35], and modifies the association between tobacco use and mortality (Supplementary Table 1).
We observed an association between chewing tobacco and earlier mortality, particularly from cancer. Associations between chewing tobacco and cancers, especially those of the esophagus, lung, pancreas, and oral cavity have been previously reported.[11] Many people who use smokeless tobacco want to avoid the restriction of smoking in public places, many have had multiple quitting efforts in the past, and many become dual users.[36] Our results also showed that dual users, especially former smokers who chew tobacco, and those who pick up the habit in early adulthood are particularly at risk of dying from cancer. Studies from India, where chewing tobacco is a common form of smokeless tobacco particularly among women, have shown higher mortality compared with nonusers, from a number of specific causes, including respiratory diseases and cancer.[37 38] For example, the Bombay Cohort Study showed a relative risk of 2.60 (95% CI: 1.78–3.80) for deaths due to neoplasms among smokeless tobacco users. We did not find a significant association between using nass and cardiovascular death. The cardiovascular health risks of chewing smokeless tobacco have also been studied in several previous studies, and the results have been inconsistent.[11–13 39–41] In the INTERHEART study, which was a case-control study including 27,089 MI cases and controls from 52 countries, an OR of 2.23 was observed for non-fatal MI among tobacco chewers, which rose to 4.09 in people who also smoked cigarettes.[42] One difficulty in comparing the results of different studies is that the smokeless tobacco is used in many different forms and preparations.[43]
Previous epidemiologic studies have suggested increased risk of several health conditions associated with waterpipe use, but most of these studies have been cross sectional or retrospective[44]. We have also previously reported a correlation between cumulative waterpipe smoking and self-reported heart disease in a separate cross-sectional analysis in this population.[41] In 2010, Akl et al., systematically reviewed all previous evidence and reported that among 24 eligible studies, the quality of evidence for the different outcomes varied from very low to low, according to the GRADE approach for rating the quality of evidence.[45] The updated review in 2016, showed a promising number of 19 new studies added in the 6 years between the two studies, but still most of these were cross-sectional studies.[46] To the best of our knowledge, only one previous prospective cohort study has reported the association of waterpipe use and mortality[47]. In this study of 20,033 individuals in the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh, although waterpipe use was associated with earlier mortality, it was difficult to distinguish the effects from cigarette smoking, as more than 99% of waterpipe users also smoked cigarettes.
One problem that complicates studying waterpipe is the fact that its use is most often intermittent, and many measures of intensity and dependence devised for cigarettes (past month smoking, former vs. current use etc.) cannot capture its variation.[19 48] On the other hand, lifetime cumulative exposure to waterpipe has been collected in a few studies, including a report by Sibai et al. showing a threefold increased risk of severe coronary stenosis associated with 40 waterpipe-years or more use.[49] We did not observe any association between waterpipe use and cardiovascular mortality, but showed higher risks of total and cancer mortality in waterpipe users compared to never tobacco users among people with more than 28 waterpipe-years of cumulative exposure.
Our study had several limitations: within a subset of the cohort with a second data collection 5 years after enrollment, about 25% of current cigarette smokers quit smoking during the follow-up; this might have resulted in some bias towards null, as these people are still classified as current smokers in our analyses. We did not have any assessment of exposure to second-hand tobacco smoke, so some of our never tobacco users may have actually been exposed to tobacco products to some extent. As another limitation, waterpipe use in Golestan is lower than the national average, and our waterpipe analyses were underpowered, particularly when compared to those of cigarettes and nass. Our study has several strengths, including its prospective design, large sample size, minimal loss to follow-up, prior validation of self-reported opium use and outcome measures, and the availability of data on important potential confounders. We also collected detailed data on lifetime tobacco and opium use, allowing us to investigate the mortality hazards of all of the main types of tobacco use and their combinations in one single population.
Although former smokers in our study started smoking at an earlier age and smoked more cigarettes, they had lower risks of overall and cause-specific mortality compared with current smokers. The magnitude of this decrease in risk depended on the length of time they smoked before quitting; among former smokers, people who smoked less than five years before they quit had the most favorable outcome, and had mortality risks very similar to never tobacco users. Among all risk factors of non-communicable diseases, tobacco is the one that can be best reduced by appropriate policies.[50] The measures by the WHO Framework Convention on Tobacco Control (WHO MPOWER measures) target a 30% reduction in tobacco use in adults by 2025. This is while 70% of the population in the Eastern Mediterranean region live in countries where this goals seems unachievable.[34] To avert an already increasing tide of mortality due to non-communicable disease, especially cancer, reinforcement of local policies to implement MPOWER mandates, focusing on all types of tobacco use and local tobacco culture, is essential.
Supplementary Material
What is already known on this topic
-
-
Use of tobacco products is a worldwide health problem with substantial diversity in patterns of use and types of products, but it is understudied in many low and middle-income countries.
-
-
There is paucity of information regarding the long term effects of non-cigarette tobacco, and almost no evidence for the association between waterpipe use (as a rising habit in many parts of the world) and mortality.
What this study adds
-
-
All types of tobacco increased the risk of earlier death (particularly from cancer) in our study. We also showed increased risk of earlier death, compared to never tobacco users, among people who switched to smokeless tobacco after quitting cigarettes, and those who have regularly smoked the waterpipe for a long time.
-
-
These results emphasize the need for global tobacco control and show that the focus of anti-tobacco efforts must not be confined to cigarette smoking.
Acknowledgments
We thank the study participants for their cooperation over many years; the Behvarz (community health workers) in the study areas for their help; and Goharshad Goglani, Karim Aghcheli, Masoud Sotoudeh, Ramin Shakeri, Alireza Sadjadi, and Amir Sharifi from the Digestive Disease Research Institute of Tehran University of Medical Sciences. We also thank the general physicians, nurses, and nutritionists in the enrolment teams for their collaboration and assistance and Golestan University of Medical Sciences (Gorgan, Iran), and the chiefs of the Gonbad and Kalaleh health districts for their close collaboration and support.
Funding: The Golestan Cohort Study was supported by Tehran University of Medical Sciences (grant No: 81/15), Cancer Research UK (grant No: C20/A5860), the Intramural Research Program of the NCI, National Institutes of Health, and various collaborative research agreements with the IARC. The study has also received special support from the Social Security Organization of Iran Golestan Branch. The funders had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Abbreviations
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CPS
Cancer Prevention Studies
- CVA
cerebrovascular accident
- CVD
cardiovascular disease
- DDRI
Digestive Disease Research Institute
- GCS
Golestan Cohort Study
- HR
hazard ratio
- IARC
International Agency for Research on Cancer
- IHD
ischemic heart disease
- NCI
National Cancer Institute
- SMR
Standardized mortality rate
- VAQ
verbal autopsy questionnaire
- WHO
World Health Organization
Footnotes
Author contributions: Arash Etemadi, Hooman Khademi, Farin Kamangar: Writing the manuscript, data analysis, approving the final draft. Arash Etemadi, Farin Kamangar, Neal D. Freedman, Paul DP Pharoah, Christian Abnet, Paolo Boffetta, Sanford Dawsey, Paul Brennan, Reza Malekzadeh: Study design, editing and approving the final draft. Hossein Poustchi, Akram Pourshams, Shahin Merat, Elham Jafari, Farhad Islami, Masoud Khoshnia, and Shahryar Semnani: data collection, approving the final draft. Masoud Khoshnia, Samad Gharavi, Alireza Norouzi: Ascertainment of etiology of death, approving the final draft. The authors worked on the manuscript independently from the funders. They had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Reza Malekzadeh is the guarantor.
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work other than those described above; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical Approval: All cohort participants signed a written informed consent at enrolment, and the study methods were approved by appropriate ethics committees at Tehran University of Medical Sciences, the US National Cancer Institute (NCI), and the International Agency for Research on Cancer (IARC).
Transparency declaration: The manuscript's guarantor affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Data sharing: No additional data available.
References
- 1.Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–64. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer. 2009;9:655–64. doi: 10.1038/nrc2703. [DOI] [PubMed] [Google Scholar]
- 3.Giovino GA, Mirza SA, Samet JM, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–79. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 4.Saleheen D, Zhao W, Rasheed A. Epidemiology and public health policy of tobacco use and cardiovascular disorders in low- and middle-income countries. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1811–9. doi: 10.1161/ATVBAHA.114.303826. [DOI] [PubMed] [Google Scholar]
- 5.Zheng W, McLerran DF, Rolland BA, et al. Burden of total and cause-specific mortality related to tobacco smoking among adults aged >/= 45 years in Asia: a pooled analysis of 21 cohorts. PLoS medicine. 2014;11:e1001631. doi: 10.1371/journal.pmed.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayaraghavan M, Pierce JP, White M, Messer K. Differential use of other tobacco products among current and former cigarette smokers by income level. Addictive behaviors. 2014;39:1452–8. doi: 10.1016/j.addbeh.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter CM, Connolly GN, Ayo-Yusuf OA, Wayne GF. Developing smokeless tobacco products for smokers: an examination of tobacco industry documents. Tob Control. 2009;18:54–9. doi: 10.1136/tc.2008.026583. [DOI] [PubMed] [Google Scholar]
- 8.Maziak W, Ben Taleb Z, Bahelah R, et al. The global epidemiology of waterpipe smoking. Tobacco Control. 2015;24:3–12. doi: 10.1136/tobaccocontrol-2014-051903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jawad M, El Kadi L, Mugharbil S, Nakkash R. Waterpipe tobacco smoking legislation and policy enactment: a global analysis. Tobacco Control. 2015;24:60–65. doi: 10.1136/tobaccocontrol-2014-051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO Study Group on Tobacco Product Regulation (TobReg) 2. Geneva: World Health Organization; 2015. TobReg - Advisory Note Waterpipe Tobacco Smoking: Health Effects, Research Needs and Recommended Actions by Regulators. [Google Scholar]
- 11.Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. The lancet oncology. 2008;9:667–75. doi: 10.1016/S1470-2045(08)70173-6. [DOI] [PubMed] [Google Scholar]
- 12.Smokeless tobacco and some tobacco-specific N-nitrosamines. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization, International Agency for Research on Cancer. 2007;89:1–592. [PMC free article] [PubMed] [Google Scholar]
- 13.Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes Control. 2005;16:347–58. doi: 10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- 14.Ramadas K, Sauvaget C, Thomas G, Fayette JM, Thara S, Sankaranarayanan R. Effect of tobacco chewing, tobacco smoking and alcohol on all-cause and cancer mortality: a cohort study from Trivandrum, India. Cancer Epidemiol. 2010;34:405–12. doi: 10.1016/j.canep.2010.04.006. doi: S1877-7821(10)00068-8 [pii] 10.1016/j.canep.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Pourshams A, Khademi H, Malekshah AF, et al. Cohort Profile: The Golestan Cohort Study--a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol. 2010;39:52–9. doi: 10.1093/ije/dyp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pourshams A, Saadatian-Elahi M, Nouraie M, et al. Golestan cohort study of oesophageal cancer: feasibility and first results. Br J Cancer. 2005;92:176–81. doi: 10.1038/sj.bjc.6602249. doi: 6602249 [pii] 10.1038/sj.bjc.6602249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zakiullah, Saeed M, Muhammad N, et al. Assessment of potential toxicity of a smokeless tobacco product (naswar) available on the Pakistani market. Tob Control. 2011 doi: 10.1136/tc.2010.042630. [DOI] [PubMed] [Google Scholar]
- 18.Ward KD, Hammal F, VanderWeg MW, et al. Are waterpipe users interested in quitting? Nicotine Tob Res. 2005;7:149–56. doi: 10.1080/14622200412331328402. doi: U03T810711538J26 [pii] 10.1080/14622200412331328402. [DOI] [PubMed] [Google Scholar]
- 19.Maziak W. Rise of waterpipe smoking. Bmj-Brit Med J. 2015;350 doi: 10.1136/bmj.h1991. doi: Artn H1991 10.1136/Bmj.H1991. [DOI] [PubMed] [Google Scholar]
- 20.Khademi H, Etemadi A, Kamangar F, et al. Verbal autopsy: reliability and validity estimates for causes of death in the Golestan Cohort Study in Iran. PLoS One. 2010;5:e11183. doi: 10.1371/journal.pone.0011183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islami F, Kamangar F, Nasrollahzadeh D, et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38:978–88. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein JP, Moeschberger ML. Survival analysis : techniques for censored and truncated data. 2. New York: Springer; 2003. [Google Scholar]
- 23.Pirie K, Peto R, Reeves GK, Green J, Beral V, Million Women Study C. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381:133–41. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 25.Gu D, Kelly TN, Wu X, et al. Mortality attributable to smoking in China. N Engl J Med. 2009;360:150–9. doi: 10.1056/NEJMsa0802902. doi: 360/2/150 [pii] 10.1056/NEJMsa0802902. [DOI] [PubMed] [Google Scholar]
- 26.Liu BQ, Peto R, Chen ZM, et al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411–22. doi: 10.1136/bmj.317.7170.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakata R, McGale P, Grant EJ, Ozasa K, Peto R, Darby SC. Impact of smoking on mortality and life expectancy in Japanese smokers: a prospective cohort study. BMJ. 2012;345:e7093. doi: 10.1136/bmj.e7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heydari G, Tafti SF, Telischi F, et al. Prevalence of smuggled and foreign cigarette use in Tehran, 2009. Tob Control. 2010;19:380–2. doi: 10.1136/tc.2009.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batmanghelidj E, Heydari G. Sanctions, Smuggling, and the Cigarette: The Granting of Iran Office of Foreign Asset Control's Licenses to Big Tobacco. Int J Prev Med. 2014;5:138–44. [PMC free article] [PubMed] [Google Scholar]
- 30.Taghavi S, Khashyarmanesh Z, Moalemzadeh-Haghighi H, et al. Nicotine content of domestic cigarettes, imported cigarettes and pipe tobacco in iran. Addict Health. 2012;4:28–35. [PMC free article] [PubMed] [Google Scholar]
- 31.Meysamie A, Ghaletaki R, Haghazali M, et al. Pattern of tobacco use among the Iranian adult population: results of the national Survey of Risk Factors of Non-Communicable Diseases (SuRFNCD-2007) Tob Control. 2010;19:125–8. doi: 10.1136/tc.2009.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng M, Freeman MK, Fleming TD, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–92. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease C, Prevention. Cigarette smoking among adults--United States, 2006. MMWR. Morbidity and mortality weekly report. 2007;56:1157–61. [PubMed] [Google Scholar]
- 34.Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015;385:966–76. doi: 10.1016/S0140-6736(15)60264-1. [DOI] [PubMed] [Google Scholar]
- 35.Khademi H, Malekzadeh R, Pourshams A, et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50,000 adults in Iran. BMJ. 2012;344:e2502. doi: 10.1136/bmj.e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClave-Regan AK, Berkowitz J. Smokers who are also using smokeless tobacco products in the US: a national assessment of characteristics, behaviours and beliefs of 'dual users'. Tob Control. 2011;20:239–42. doi: 10.1136/tc.2010.039115. [DOI] [PubMed] [Google Scholar]
- 37.Gupta PC, Pednekar MS, Parkin DM, Sankaranarayanan R. Tobacco associated mortality in Mumbai (Bombay) India. Results of the Bombay Cohort Study. Int J Epidemiol. 2005;34:1395–402. doi: 10.1093/ije/dyi196. [DOI] [PubMed] [Google Scholar]
- 38.Gajalakshmi V, Kanimozhi V. Tobacco chewing and adult mortality: a case-control analysis of 22,000 cases and 429,000 controls, never smoking tobacco and never drinking alcohol, in South India. Asian Pac J Cancer Prev. 2015;16:1201–6. doi: 10.7314/apjcp.2015.16.3.1201. [DOI] [PubMed] [Google Scholar]
- 39.Huhtasaari F, Lundberg V, Eliasson M, Janlert U, Asplund K. Smokeless tobacco as a possible risk factor for myocardial infarction: a population-based study in middle-aged men. Journal of the American College of Cardiology. 1999;34:1784–90. doi: 10.1016/s0735-1097(99)00409-x. [DOI] [PubMed] [Google Scholar]
- 40.Huhtasaari F, Asplund K, Lundberg V, Stegmayr B, Wester PO. Tobacco and myocardial infarction: is snuff less dangerous than cigarettes? BMJ. 1992;305:1252–6. doi: 10.1136/bmj.305.6864.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Islami F, Pourshams A, Vedanthan R, et al. Smoking water-pipe, chewing nass and prevalence of heart disease: a cross-sectional analysis of baseline data from the Golestan Cohort Study, Iran. Heart. 2013;99:272–8. doi: 10.1136/heartjnl-2012-302861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647–58. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 43.Gupta R, Gurm H, Bartholomew JR. Smokeless tobacco and cardiovascular risk. Archives of internal medicine. 2004;164:1845–9. doi: 10.1001/archinte.164.17.1845. [DOI] [PubMed] [Google Scholar]
- 44.El-Zaatari ZM, Chami HA, Zaatari GS. Health effects associated with waterpipe smoking. Tobacco Control. 2015;24:31–43. doi: 10.1136/tobaccocontrol-2014-051908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akl EA, Gaddam S, Gunukula SK, Honeine R, Jaoude PA, Irani J. The effects of waterpipe tobacco smoking on health outcomes: a systematic review. Int J Epidemiol. 2010;39:834–57. doi: 10.1093/ije/dyq002. [DOI] [PubMed] [Google Scholar]
- 46.Waziry R, Jawad M, Ballout RA, Al Akel M, Akl EA. The effects of waterpipe tobacco smoking on health outcomes: an updated systematic review and meta-analysis. Int J Epidemiol. 2016 doi: 10.1093/ije/dyw021. [DOI] [PubMed] [Google Scholar]
- 47.Wu F, Chen Y, Parvez F, et al. A prospective study of tobacco smoking and mortality in Bangladesh. PLoS One. 2013;8:e58516. doi: 10.1371/journal.pone.0058516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maziak W, Ben Taleb Z, Jawad M, et al. Consensus statement on assessment of waterpipe smoking in epidemiological studies. Tob Control. 2016 doi: 10.1136/tobaccocontrol-2016-052958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sibai AM, Tohme RA, Almedawar MM, et al. Lifetime cumulative exposure to waterpipe smoking is associated with coronary artery disease. Atherosclerosis. 2014;234:454–60. doi: 10.1016/j.atherosclerosis.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 50.Kontis V, Mathers CD, Bonita R, et al. Regional contributions of six preventable risk factors to achieving the 25 × 25 non-communicable disease mortality reduction target: a modelling study. Lancet Glob Health. 2015;3:e746–57. doi: 10.1016/S2214-109X(15)00179-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.