Abstract
Stressor exposure induces neuronal remodeling in specific brain regions. Given the persistence of stress-related illnesses, key next steps in determining the contributions of neural structure to mental health are to identify cell types that fail to recover from stressor exposure and to identify “trigger points” and molecular underpinnings of stress-related neural degeneration. We evaluated dendrite arbor structure on hippocampal CA1 pyramidal neurons before, during, and following prolonged exposure to one key mediator of the stress response – corticosterone (cortisol in humans). Basal dendrite arbors progressively simplified during a 3-week exposure period, and failed to recover when corticosterone was withdrawn. Corticosterone exposure decreased levels of the dendrite stabilization factor Abl2/Arg nonreceptor tyrosine kinase and phosphorylation of its substrates p190RhoGAP and cortactin within 11 days, suggesting that disruption of Arg-mediated signaling may trigger dendrite arbor atrophy and, potentially, behavioral abnormalities resulting from corticosterone exposure. To test this, we administered the novel, bioactive Arg kinase activator, 5-(1,3-diaryl-1H-pyrazol-4-yl)hydantoin, 5-[3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl]-2,4-imidazolidinedione (DPH), in conjunction with corticosterone. We found that repeated treatment corrected CA1 arbor structure, otherwise simplified by corticosterone. DPH also corrected corticosterone-induced errors in a hippocampal-dependent reversal learning task and anhedoniclike behavior. Thus, pharmacological compounds that target cytoskeletal regulators, rather than classical neurotransmitter systems, may interfere with stress-associated cognitive decline and mental health concerns.
1. Introduction
Chronic stress can lead to alterations in neuron structure and function in several brain regions, including the hippocampus where stressor exposure induces dendrite atrophy in rodent models (McEwen et al., 2016). In humans, morphological, functional, and structural changes in the brain correlate with stress load, depressive episodes, and sensitivity to antidepressant treatment (Sheline, 2000; Sheline et al., 2003; Koolschijn et al., 2009; Lorenzetti et al., 2009). Further, stress-induced structural changes can be cumulative (Seo et al., 2014), and in rodents, neural remodeling – both atrophy and hypertrophy, depending on brain region – correlates with impairments in attentional function (Liston et al., 2006), hippocampal-dependent learning and memory (Sousa et al., 2000), and depression-like behavior (Gourley et al., 2013). The complexity of the stress response, however, has made elucidating the mechanisms by which stressors remodel neurons difficult. Further, discrete cell types have different sensors and response mechanisms for stress-associated signals, highlighting the need to investigate cell type-specific effects.
Assessing the impact of discrete components of the stress response may have utility in identifying stress-mediated mechanisms that modify neural structure. In rodents, exposure to elevated levels of the primary glucocorticoid, corticosterone (CORT, cortisol in humans), is sufficient to disrupt hippocampal dendrite structure at the level of dendritic spines, as well as the entire dendrite arbor (McEwen et al., 2016; Conrad et al., 2017). Mechanisms by which CORT disrupts dendrite arbors are not well understood, despite the central role dendrite shape, size, and branching pattern play in circuit formation and function. For instance, dendrites provide surface area to house dendritic spines, and they also inform the spatial specificity of synaptic connections and determine computational integration and summation of postsynaptic responses (Koleske, 2013). Understanding how dendrite structure is impacted by CORT may be essential to understanding how stress impacts brain function.
Landmark studies indicated that both stress and CORT cause atrophy of hippocampal CA3 apical dendrite arbors, and arbor structure rebounds when CORT levels normalize (reviewed in Conrad et al., 2017). By contrast, in hippocampal CA1, CORT-induced basal dendrite atrophy does not recover following a 7-day CORT washout period (Gourley et al., 2013). Hippocampal CA1 neurons integrate inputs from CA3 and the entorhinal cortex to coordinate hippocampal-dependent decision making (Kelemen and Fenton, 2016). Identifying intracellular mechanisms that contribute to stressor-related vulnerabilities may thus shed light onto the mechanisms by which stress and CORT lead to behavioral consequences that also persist beyond the period of exposure.
Previous work from our group has identified an integrin-Abl2/Arg kinase-p190RhoGAP cascade that attenuates RhoA-ROCK signaling to stabilize hippocampal CA1 dendrite arbors (Moresco et al., 2005; Sfakianos et al., 2007; Warren et al., 2012; Kerrisk et al., 2013; Lin et al., 2013; Koleske, 2013). The current study provides evidence that CORT exposure disrupts this dendrite stabilization pathway. More specifically, we find that elevated CORT reduces levels of Arg, as well as phosphorylation of Arg substrates p190RhoGAP and cortactin, in the hippocampus. Consistent with this pattern, phosphorylation of the RhoA-ROCK signaling target cofilin is also increased. These changes coincide with reductions in dendrite arbor complexity in hippocampal CA1 and occur at a point when the levels and activities of other cytoskeletal regulatory elements are intact. Finally, we show that the novel small molecule Arg activator, DPH, blocks CORT-induced CA1 dendrite atrophy and rescues depression-like behavior and deficiencies in hippocampal-dependent decision making. Together, these findings suggest that pharmacotherapies that target dendrite-stabilizing cytoskeletal regulatory mechanisms may impede stress-associated cognitive decline and mental health concerns.
2. Materials and methods
2.1. Subjects
Subjects were wild type C57BL/6 mice bred in-house from Jackson Labs stock. Experiments were initiated between postnatal day (P) 31–49, a timeline consistent with our previous investigation of hippocampal CA1 dendritic arbors (Gourley et al., 2013). Female and male mice were used for immunoblotting, immunoprecipitation, and behavioral studies. Anatomical studies were conducted using males. Studies were approved by the Emory and Yale IACUCs, as appropriate.
2.2. CORT exposure
4-Pregnen-11β-21-DIOL-3-20-DIONE-21-hemisuccinate (CORT; Steraloids) was dissolved in water (25 µg/ml free-base, ~4.9 mg/kg/day) (Gourley et al., 2013; Gourley et al., 2008). Mice were euthanized following 4, 10–11, or 20–22 days of exposure (the latter time points referred to as 11-day and 21-day, respectively). Additional groups were exposed to CORT for 21 days, then euthanized following a 7- or 20–22-day washout period (referred to as +7 day washout and +21 day washout, respectively) Timelines are provided in the figures.
Mice in behavioral experiments were exposed to CORT for 21 days, then regular water replaced the CORT solution, and behavioral testing commenced.
2.3. Gland harvesting
Adrenal and thymus glands were extracted post-mortem following 11 days of CORT by midline dissection and weighed in pairs.
2.4. Biocytin injection of hippocampal neurons
Mice were deeply anaesthetized with pentobarbital. As previously described (Sfakianos et al., 2007), hippocampal slices (400 µm) were prepared and maintained in a standard interface chamber at 31 °C. Individual CA1 pyramidal neurons were injected with 4% biocytin solution in 2 M potassium acetate solution, pH 7.5. Neurons were injected with 200 ms current injections of 4 nA at 2 Hz for 20 min. Only neurons that maintained a membrane potential and fired action potentials during this entire period were analyzed. Sections containing injected neurons were fixed in 4% paraformaldehyde overnight, cryoprotected in 30% sucrose, then re-sectioned at 40 µm, and visualized using standard avidin-horseradish peroxidase (HRP) staining (Vectastain Elite ABC; Vector Laboratories). Approximate ranges of injection were 3.7–4.0 mm lateral, −2.5 to −4.5 mm ventral, and −3.3 to −3.5 mm caudal of bregma.
2.5. Morphometric analysis of dendrites
Serial sections containing dye-filled neurons were traced sequentially starting at the cell body and moving in the + and − directions under 100 × magnification using a light microscope outfitted with a Z drive. Cells were then reconstructed using Neurolucida software (MicroBrightField). As is standard practice, sections were apposed using landmarks and were aligned at high magnification by joining interrupted primary and secondary branches based on position, orientation, and dendrite thickness as well as other local tissue markers. Z-stack series of individual biocytin-labeled neurons were considered complete only when clean dendrite-free sections were detectable on the far + Z and − Z margins. Sholl analysis, total dendrite length, and branch point number were determined using NeuroExplorer (MicroBrightField). Neurons were traced by an experimenter blind to group. A maximum of 5 neurons were sampled from each animal. Outliers were identified as values > 1.5 times the interquartile distance either below the first or above the third quartile and excluded.
2.6. Immunoblotting
Mice were euthanized by rapid decapitation at the time points indicated, and brains were extracted and frozen at −80 °C. Brains were sectioned into 1 mm coronal sections using a chilled brain matrix. Tissue punches (1 mm diameter) were aimed at the dorsal-intermediate hippocampus, where CA1-rich samples could easily be collected, and ventral hippocampus, where CA3-rich samples could readily be collected. Tissues were homogenized by sonication in lysis buffer [200 µl: 137 mM NaCl, 20 mM tris-Hcl (pH = 8), 1% NP-40, 10% glycerol, 1:100 Phosphatase Inhibitor Cocktails 2 and 3, 1:1000 Protease Inhibitor Cocktail (Sigma)], and stored at −80 °C. Protein concentrations were determined using a Bradford colorimetric assay (Pierce).
Equal amounts of protein (15 µg) were separated by SDS-PAGE on 7.5% gradient Tris-glycine gels (Bio-Rad). Following PVDF membrane transfer, blots were blocked with 5% nonfat milk for 1 h. Membranes were incubated with primary antibodies at 4 °C overnight and then incubated in horseradish peroxidase secondary antibodies for 1 h. Immunoreactivity was assessed using a chemiluminescence substrate (Pierce) and measured using a ChemiDoc MP Imaging System (Bio-Rad). Densitometry values were individually normalized to the corresponding loading control, which did not change as a function of CORT exposure, and then normalized to the control sample mean from the same membrane in order to control for fluorescence variance between gels. Phospho-protein levels were normalized to the corresponding total protein levels.
Primary antibodies are listed in Supplementary Table 1.
2.7. Immunoprecipitation
Tissue homogenization and immunoprecipitation were performed in ice-cold lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 2 mM EDTA, and 2.5% CHAPS with protease and phosphatase inhibitors) as described previously (Hernandez et al., 2004). Briefly, hippocampi were dissected in ice-cold PBS, homogenized and sonicated, and spun to remove debris, followed by removal of detergent using BioBeads (Bio-Rad) overnight at 4 °C with gentle mixing. Protein extract was standardized to 1 mg/ml and precleared at 4 °C for 20 min with protein A/G agarose resin (Pierce). Supernatants were then rotated with anti-p190RhoGAP (clone D2D6; Millipore) at 4 °C overnight, and immune complexes were bound to protein A/G agarose resin at 4 °C for 1 h with gentle rotation. After centrifugation, supernatant was kept for depletion analysis. Resin was washed three times with 1 ml of lysis buffer and resuspended in 30 µl of 1 × LSB. Samples were boiled for 10 min and separated via SDS-PAGE before transfer to nitrocellulose for immune-detection. Equal volumes of input and supernatant were loaded and verified by total protein staining.
2.8. DPH treatment in vivo
DPH (Sigma and MedChemExpress) was suspended in 17% DMSO and PBS and administered at 30 mg/kg, i.p., 1 ml/100 g. In the case of acute DPH treatment, mice were given a single administration of DPH and euthanized 4 h later for immunoblotting experiments. In the case of repeated administration, DPH was delivered daily starting on day 11 of the CORT exposure period, corresponding to the time point when dendrite atrophy first became detectable in our anatomical studies. DPH treatment then continued throughout the remainder of the CORT exposure period. During this time, DPH was prepared fresh every 3 days. Then, behavioral testing commenced, or mice were euthanized for cellular morphology studies, with euthanasia occurring 16–20 h following the final injection.
2.9. Instrumental reversal task
Mice were trained to nose poke for 20 mg grain-based food reinforcers (Bio-Serv) using standard illuminated Med-Associates conditioning chambers equipped with 2 nose poke recesses located on opposite sides of one wall. Mice were reinforced for responding on one nose poke recess according to a variable ratio 2 schedule of reinforcement. Responding on the remaining recess was non-reinforced. Mice were trained once/day for 7–9 days (i.e., 7–9 training sessions for each mouse) until they clearly distinguished between the reinforced and non-reinforced responses. Sessions were 25 min long. Response rates generated during the final 7 sessions for all mice were compared between groups and are shown.
Next, a 25 min hippocampal-dependent reversal test was conducted (Gourley et al., 2010). Mice were reinforced for responding on the aperture located on the opposite side of the chamber, whereas the previously-reinforced response no longer generated food. Persisting in generating the non-reinforced response is considered erroneous, and erroneous response rates were compared between groups. The percentage of total responses that were correct was also compared between groups.
2.10. Sucrose consumption and locomotor activity
Next, female mice were given ad libitum access to food and water and housed individually with bedding and nesting materials for 24 h. Water was then removed 19 h prior to test, when mice were given access to a water bottle containing 1% w/v sucrose (Gourley et al., 2008). Bottles were weighed before and after the 1-hour test, and the difference in weight was normalized to each animal's body weight. Upon analysis, 3 values > 2 standard deviations above the mean were identified – likely due to fluid spillage – and excluded.
During this time, cages were positioned in customized locomotor monitoring frames (Med-Associates) equipped with 16 photobeams. Total beam breaks were collected over the 24-hour period and compared between groups. Ambulatory counts (> 2 sequential photo-beams) and stereotypy-like counts (repetitive breaking of the same beam, as during grooming) were also extracted.
Estrous status was assessed on the sucrose consumption test day in accordance with Byers et al., 2012.
2.11. Statistics
Neuron morphometric measures, densitometry values, nose poke response metrics, sucrose consumption values, and locomotor scores were compared by analysis of variance (ANOVA) with repeated measures when appropriate. Sucrose consumption was also analyzed by ANOVA as a function of animals' estrous phases. In case of interactions or main effects between > 2 groups, Tukey's post-hoc comparisons were applied.
3. Results
3.1. CORT simplifies hippocampal CA1 dendrites
We first aimed to identify the time point when CORT-induced atrophy of hippocampal CA1 dendrite arbors first became detectable. To this end, mice were exposed to CORT and euthanized following 11 or 21 days of exposure. To test for persistence of anatomical deficits, if any, additional groups were exposed to CORT for 21 days, followed by a 7- or 21-day washout period prior to euthanasia (timeline in Fig. 1a).
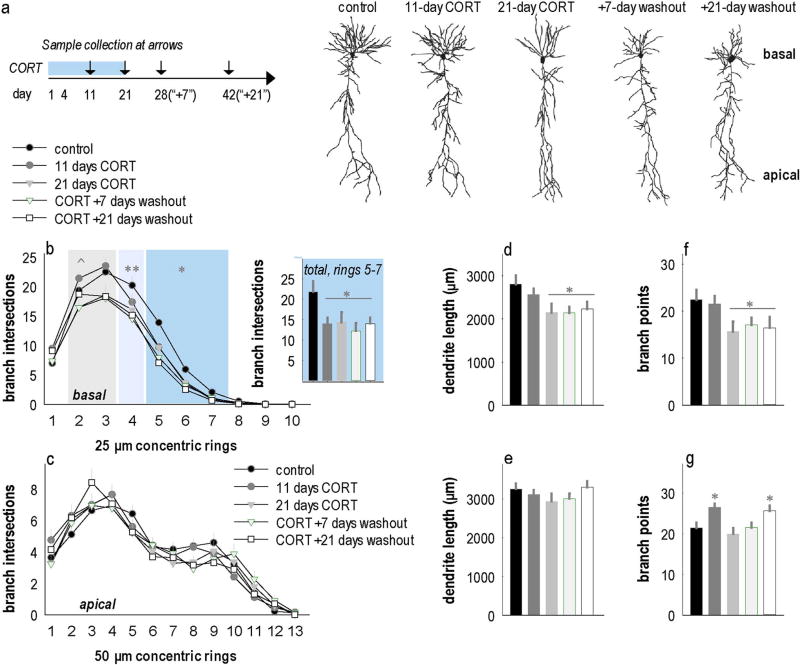
Fig. 1.
Basal hippocampal CA1 dendrites progressively regress with repeated CORT exposure. (a) Experimental timeline indicating CORT exposure and sample collection time points. (b) Camera lucida renderings of representative neurons. (c) Prolonged CORT exposure induced durable dendrite arbor regression, with atrophy on distal branches emerging as early as 11 days of exposure, then progressing to more proximal branches with more prolonged exposure. Inset: 11 days of exposure reduced branch intersections on distal dendrites (rings 5–7) in a manner that was indistinguishable from atrophy caused by longer exposure periods [main effect F(4,105) = 4.6, p = 0.002]. (d) Prolonged CORT exposure did not impact the complexity of apical trees of hippocampal CA1 neurons. (e) Overall basal dendrite lengths were reduced with 21 days of CORT exposure, and this did not recover. (f) Overall apical dendrite length did not change. (g) Basal dendrite branch points were also reduced with 21 days of CORT exposure, and counts did not recover. (h) Apical branch points were elevated after 11 days of CORT exposure and also with 21 days of recovery following a 21-day exposure period. Means + SEMs, *p < 0.05 vs. control; **p < 0.05 control vs. CORT + washout groups, ^p < 0.1 control vs. CORT + washout groups. n = 17–23 neurons/group; 8–12 mice/group.
Analysis of complete dendrite reconstructions (Fig. 1b) revealed that CORT progressively induced degeneration of basal dendrite arbors. Specifically, we detected significant losses in branch intersections 125–175 µm from the soma following 11 days of CORT exposure [interaction F(4,105) = 2.8, p = 0.03] (Fig. 1c). We additionally confirmed that adrenal and thymus glands were atrophied, as would be expected with exogenous CORT exposure (Suppl. Fig. 1).
With 21 days of exposure, we found similar reductions in dendrite branching within 125–175 µm from the soma, and that these defects had extended proximally, to 100 µm from the soma, with trends for reductions also detected at 50–75 µm from the soma (Fig. 1c). By contrast, CORT did not affect apical dendrite intersections [interaction F(4,95) = 1.1, p = 0.35] (Fig. 1d). Thus, 11 days of CORT exposure is sufficient to trigger basal dendrite retraction in hippocampal CA1. This basal dendrite atrophy worsens with longer CORT exposure, and it fails to recover when CORT is removed (Fig. 1c).
Paralleling these findings, total basal, but not apical, dendrite length was reduced by 21 days of CORT exposure, and failed to recover [basal F(4,106) = 3.1, p = 0.02; apical F(4,99) = 1.5, p = 0.2] (Fig. 1e–f). Basal dendrite branch points also decreased following 21 days of CORT, and did not recover [F(4,106) = 2.5, p < 0.05] (Fig. 1g). By contrast, apical branch points increased following 11 days of CORT exposure and at the latest washout period tested [F(4,99) = 3.2, p = 0.02] (Fig. 1h). Thus, 11 days of CORT exposure triggers modifications in CA1 basal dendrite arbor structure. Dendrite simplification worsens with further exposure such that deficiencies in overall length and branching become detectable, and they fail to recover within 21 days.
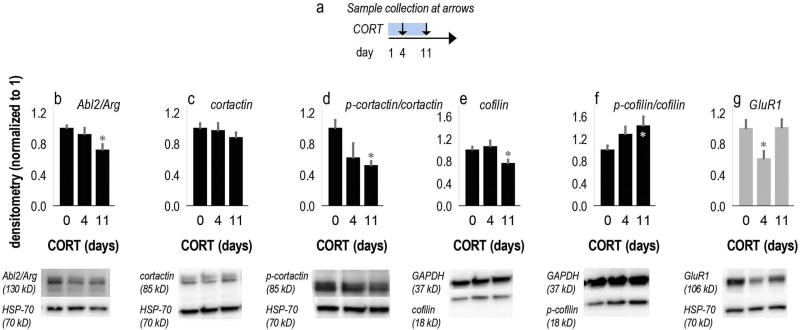
Based on these findings, we measured the levels and activities of a panel of cytoskeletal regulatory factors following 11 days of CORT. An additional group was euthanized 1 week earlier, following 4 days of CORT (Fig. 2a). In samples that would be expected to primarily contain hippocampal CA1 tissue, 11 days of CORT exposure reduced Arg levels by 28% [F(2,21) = 6.27, p = 0.007] (Fig. 2b) (Suppl. Table 2 also summarizes all effects.). Consistent with this finding, phosphorylation of the Arg binding partner and substrate cortactin was also reduced [F(2,16) = 3.55, p = 0.05] (Fig. 2c,d), whereas total cortactin levels were unchanged [F < 1] (Fig. 2c). Cofilin phosphorylation was increased [F(2,21) = 3.86, p = 0.04] (Fig. 2e,f), and total coflin levels were reduced [F(2,21) = 4.93, p = 0.02] (Fig. 2e). As phosphorylation inactivates cofilin, these effects would be associated with greatly diminished cofilin activity. We also measured AMPA GluR1, as CORT has previously been shown to regulate hippocampal GluR1 levels (Kvarta et al., 2015). GluR1 was diminished with 4 days of CORT exposure as expected [F(2,19) = 4, p = 0.04] (Fig. 2g) and consistent with previous findings indicating that GluR1 levels also decrease with repeated stressor exposure (Schmidt et al., 2010). Unlike the cytoskeletal regulators tested, however, GluR1 levels normalized with 11 days of exposure.
Fig. 2.
CORT exposure regulates cytoskeletal regulatory elements in the hippocampus. (a) Experimental timeline outlining sample collection points. (b) In CA1-rich samples, levels of the Abl2/Arg nonreceptor tyrosine kinase and (c–d) phosphorylation of its substrate cortactin were reduced by 11 days of CORT exposure. (e) Levels of cofilin decreased, and (f) phosphorylation increased. (g) GluR1 was also diminished by 4 days of CORT exposure, but recovered by 11 days of exposure. Means + SEMs, *p < 0.05 vs. 0-day control. Representative blots are below with the corresponding loading controls, HSP-70 (70 kDa) and GAPDH (37 kDa), which did not change as a function of CORT exposure. Protein levels in control mice were consistent with prior investigations (e.g., Shapiro et al., 2017). Further detailed results and comparisons to CA3-rich samples are presented in Suppl. Table 2. n = 6–8/group.
Our finding that the structure of hippocampal CA1 basal dendrites fails to recover despite removal of CORT differs from reports on dentate granule and CA3 dendrite arbors, which recover following CORT or stressor exposure (Sousa et al., 2000; Vyas et al., 2004; Maiti et al., 2008; Hoffman et al., 2011). Accordingly, we find that tissue extracts enriched for hippocampal CA3 were largely spared changes in cytoskeletal regulators (Suppl. Table 2). Levels of the key cytoskeletal factor LIM kinase 2 were, however, rapidly elevated by CORT (within 4 days; Suppl. Table 2); this may contribute to the exquisite reactivity of hippocampal CA3 neurons to stressor exposure (Tata and Anderson, 2010).
3.2. An Arg kinase activator induces p190RhoGAP phosphorylation
p190RhoGAP-A (p190RhoGAP) is a major substrate of Arg in the postnatal mouse brain (Hernandez et al., 2004) and is the predominant phosphotyrosine-containing protein of 190 kDa detected by the 4G10 antibody in mouse brain extract (Brouns et al., 2001; Hernandez et al., 2004). We first verified that the tyrosine-phosphorylated protein of 190 kDa in mouse hippocampal lysate was immunoprecipitated with anti-p190RhoGAP antibodies (Fig. 3a), as in whole-brain homogenates. We then used anti-phosphotyrosine immunoblotting with 4G10 to measure phospho-p190RhoGAP levels following CORT exposure.
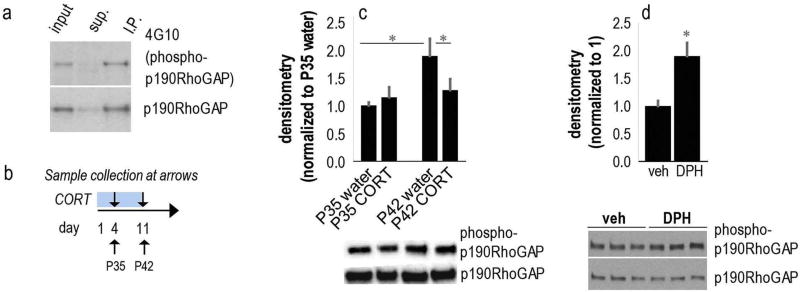
Fig. 3.
Bi-directional regulation of hippocampal p190RhoGAP phosphorylation by CORT and the Arg activator DPH. (a) First we confirmed that the 190 kDa band recognized by anti-phosphotyrosine immunoblotting is phospho-p190RhoGAP. Lanes, from left to right represent input of hippocampal homogenate, supernatant after immunoprecipitation with anti-p190RhoGAP antibodies, and the immunoprecipitate. The upper blot was probed with the 4G10 anti-phosphotyrosine antibody, while the lower blot was probed with anti-p190RhoGAP. (b) Experimental timeline: Mice were exposed to CORT starting at P31 and euthanized at P35 or P42 (arrows). (c) p190RhoGAP phosphorylation increased as a function of age in typical (control) mice, but this was mitigated with CORT exposure. Representative blots are below. n = 6–8/group. (d) In a separate experiment, mice were injected with DPH and euthanized 4 h later. DPH increased hippocampal p190RhoGAP phosphorylation. Representative blots below. n = 5/group. Means + SEMs, *p < 0.05 vs. control or as otherwise noted.
For measurements of protein levels following CORT, we exposed mice to CORT starting at P31, then euthanized mice after 4 or 11 days, at P35 or P42 (Fig. 3b). In contrast to all other proteins, whose levels or activity were not altered by age, we found that phospho-p190RhoGAP increased as a function of age from P35 to P42 in hippocampal tissue (Fig. 3c). Importantly, CORT blocked this age-dependent elevation, decreasing levels by 11 days of exposure [interaction F(1,23) = 4.2, p = 0.05] (Fig. 3c), consistent with reduced Arg levels at the same time point (Fig. 2).
We next measured phospho-p190RhoGAP to verify the efficacy of the novel small molecule activator of Arg, DPH. Systemic injection of 30 mg/kg increased p190RhoGAP phosphorylation 4 h following injection (t8 = 3.4, p = 0.008) (Fig. 3d).
3.3. DPH recovers dendrite arbor structure
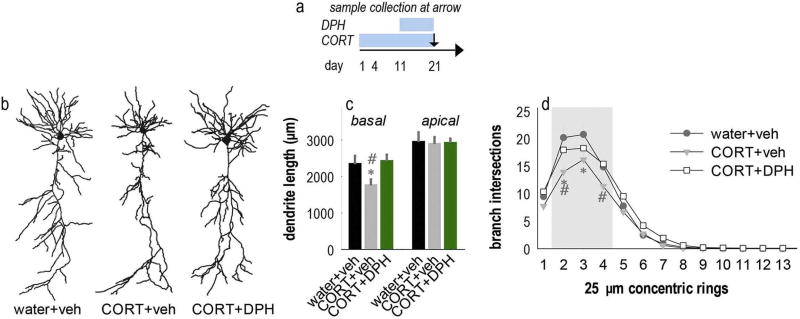
Given that CORT exposure simplifies basal dendrites and reduces Arg and phospho-p190RhoGAP levels, we tested whether stimulating Arg with DPH could prevent CORT-induced dendrite arbor atrophy. Mice were exposed to CORT and treated with DPH beginning at day 11 of CORT exposure when dendrite atrophy was first detectable (again, Figs. 1c, 4a). Then, dendrites were imaged and reconstructed at 21 days of CORT exposure (Fig. 4b). CORT decreased basal arbor length as expected, and DPH fully blocked this effect [F(2,47) = 6, p = 0.005] (Fig. 4c). Apical arbor length did not differ between groups [F < 1] (Fig. 4c). Sholl analyses of the basal arbors revealed interactions between distance and group [F(26,658) = 1.97, p = 0.003]. Post-hoc comparisons indicated that CORT simplified basal dendrite arbors, while DPH corrected these deficiencies (Fig. 4d).
Fig. 4.
DPH corrects CORT-induced deficiencies in dendrite arborization. (a) Experimental timeline indicating CORT and DPH administration periods and sample collection time point. (b) Representative neurons. (c) Mice were exposed to 21 days of CORT, with either DPH or its vehicle introduced at day 11. CORT decreased arbor length as expected, but DPH blocked this loss. As in our initial studies, apical arbors were unaffected. (d) Sholl analyses revealed that chronic CORT again decreased basal branch intersections, but DPH intervention corrected this deficiency. Bars = means + SEMs, *p < 0.05 vs. control; #p < 0.05 vs. CORT +DPH. n = 16–22 neurons/group; 6–9 mice/group.
3.4. DPH confers behavioral resilience to CORT
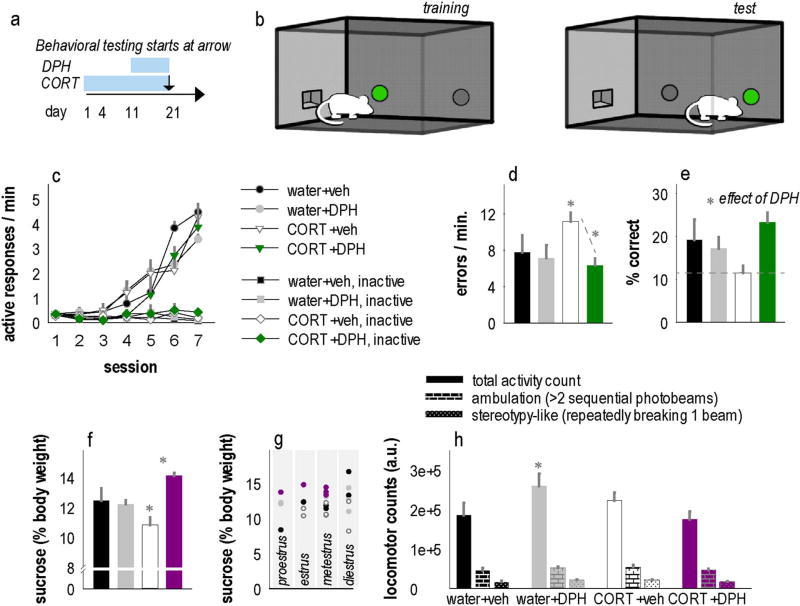
We next tested whether DPH could block behavioral and structural deficits induced by CORT. While exposing mice to CORT, we began treating mice with DPH starting on day 11, corresponding to the onset of basal dendrite simplification (again, Figs. 1c, 5a). At 21 days, CORT exposure and DPH treatment were discontinued, and mice were tested in a hippocampal-dependent instrumental reversal task. First, mice were trained to nose poke for food reinforcers in operant conditioning chambers, with one of two nose poke responses reinforced. Then, mice were required to “reverse” their response to the opposite side of the chamber to continue to receive reinforcement (Fig. 5b). The ability to reverse the initially trained response was measured.
Fig. 5.
The Arg activator DPH prevents CORT-induced behavioral abnormalities. (a) Experimental timeline indicating CORT and DPH administration periods and timing of behavioral testing. (b) We utilized a spatial reversal learning task in which mice are initially trained to nose poke on one aperture, then the location of the active operand is reversed to a nose poke recess in a different location in the chamber. (c) Male mice acquired the food-reinforced response and responded minimally on the inactive aperture, however (d) CORT increased errors in the reversal phase, and DPH corrected this deficiency. (e) DPH also increased the percentage of responses that were reinforced. (f) In female mice, CORT induced anhedonic-like sucrose neglect, a depression-like behavior, and this was fully corrected by DPH, an antidepressant-like response. (g) Sucrose consumption patterns could not be accounted for by estrous phase at the time of testing. (h) Further, CORT and CORT + DPH groups locomoted a similar amount (although the DPH-alone group was more active). Bars and symbols = means + SEMs. *p < 0.05 vs. control or as indicated. n = 6–8/group.
In initial training, CORT- and DPH-exposed mice clearly differentiated between the active and inactive response apertures, with increased response rates on the active aperture [effect of response F(1,22) = 147, p < 0.001], and no effects of CORT or DPH [interactions p > 0.13] (Fig. 5c). Upon reversal, however, CORT-exposed mice generated more errors and had difficulty performing the spatial reversal (Fig. 5d). DPH reduced these errors [interaction F(1,21) = 5, p = 0.04] (Fig. 5d) and increased response accuracy overall (% responses that were reinforced) [main effect F(1,20) = 5.6, p = 0.03] (Fig. 5e). Thus, DPH corrects CORT-induced deficits in hippocampal-dependent learning and memory.
Prolonged exposure to CORT can cause depression-like behaviors (e.g., Gourley et al., 2008, 2013), so we tested female cohorts further using a sucrose consumption test, a classical assay of anhedonic-like behavior in rodents. Prior low-dose CORT decreased sucrose consumption in females, an anhedonic-like consequence (see also Mekiri et al., 2017). Meanwhile, DPH increased consumption, an anti-depressant-like effect [interaction F(1,25) = 10.6, p = 0.003] (Fig. 5f). There were no differences in general liquid intake (not shown). Also, sucrose consumption patterns could not be attributable to estrous phase at test [phase F(3,25) = 0.39, p = 0.76] (Fig. 5g), in agreement with a recent report using other estrous assessment approaches (Mekiri et al., 2017).
In the 24 h preceding sucrose consumption testing, locomotor activity counts were collected and found to be elevated in the DPH-alone group [interaction F(1,28) = 5.3, p = 0.03], but activity in DPH + CORT and CORT-alone mice did not differ from control, suggesting that the DPH-mediated recovery of sucrose consumption in CORT-exposed mice could not be attributable to locomotor activity changes (Fig. 5h). We also analyzed locomotor counts that could be specifically attributed to repetitive stereotypy-like behavior vs. ambulatory behavior. While an interaction effect was detected for stereotypy-like counts, post-hoc comparisons were non-significant [F(1,28) = 6, p = 0.02] (Fig. 5h). Ambulatory counts did not differ [F(1,28) = 2.2, p = 0.15] (Fig. 5h).
4. Discussion
Stress hormones regulate dendrite morphology in distinct brain regions, including the hippocampus. Landmark investigations identified dendrite atrophy following chronic stressor or CORT exposure (Sousa et al., 2000; Woolley et al., 1990), but the key signaling targets of stressor exposure were not identified. Here, we aimed to identify changes in the levels or activities of cytoskeletal regulatory factors at, or preceding, a “trigger point” when CORT-related arbor change became detectable. With 11 days of CORT exposure, we found that the basal arbors of hippocampal CA1 neurons had begun to simplify, and this effect was worsened with additional exposure, such that gross changes in arbor length and branch points became detectable. We thus focused on identifying biochemical events occurring at, and preceding, the 11-day time point. Our investigation revealed down-regulation of the Arg nonreceptor tyrosine kinase, a key regulator of cytoskeletal structure. Stimulating Arg kinase with the activator DPH protected hippocampal CA1 arbor structure in CORT-exposed mice. DPH also corrected CORT-induced deficits in a hippocampal-dependent learning and memory test and also anhedonic-like behavior, protecting against the durable effects of chronic CORT. These findings provide the first evidence, to our knowledge, that this compound impacts behavior.
4.1. Stress-related structural reorganization of hippocampal neurons
Hippocampal CA3 neurons are highly sensitive to stressor exposure (Tata and Anderson, 2010). CA1 neurons are poorly characterized by comparison, regarded as more resilient than their CA3 counterparts (Conrad et al., 2017). This perspective is based at least in part on investigations using large bolus CORT doses (33–40 mg/kg) that occlude normal circadian CORT cycling (Sousa et al., 2000; Woolley et al., 1990; Morales-Medina et al., 2009). By contrast, the oral CORT exposure procedure utilized here mimics CORT secretion during restraint stress and leaves diurnal cycling intact, with CORT levels during the inactive, daytime cycle that do not differ from control, and levels that are elevated ≥ 4-fold during the active night cycle (Gourley et al., 2008). As in prior investigations, we find that chronic (3 weeks) oral CORT exposure results in dendrite simplification on basal CA1 arbors but not apical CA1 arbors (Morales-Medina et al., 2009; Gourley et al., 2013; Fig. 1). Interestingly, 3 weeks of CORT exposure also reduces dendritic spine density on basal CA1 arbors, but dendritic spine density normalizes after a 7-day washout period while basal arbors do not recover (Gourley et al., 2013). It is possible that dendritic spine loss following CORT – although recoverable – triggers persistent basal arbor simplification. Glucocorticoid receptors aggregate on dendritic spines in CA1 (Jafari et al., 2012). Further, chronic stressor exposure potentiates amygdalar output and increases dendritic spine density and length in the basal nuclei (Correll et al., 2005; Padival et al., 2013), relevant because amygdala-CA1 coupling increases, while CA3-CA1 coupling decreases, with stressor exposure, all effects that persists beyond the stressor exposure period (Ghosh et al., 2013). Additional experiments are needed to uncover mechanisms driving persistent dendrite simplification.
Here, we capitalized on our finding that hippocampal dendrites began to atrophy with 11 days of CORT exposure to identify possible dendrite stability regulators that were impacted by CORT. Immunoblotting revealed that Arg levels decreased during this time frame, and CORT also reduced phosphorylation of its substrate p190RhoGAP. Arg mediates the stability of dendrites and dendritic spines (Moresco et al., 2005; Sfakianos et al., 2007). In particular, it phosphorylates and activates p190RhoGAP in the postnatal brain to keep RhoA GTPase activity low (Hernandez et al., 2004). A failure to activate p190RhoGAP-mediated RhoA attenuation, for example in Argdeficient (arg−/−) mice, reduces neuronal dendrites (Sfakianos et al., 2007; Lin et al., 2013). Supporting the perspective that CORT-induced reduction of Arg and phospho-p190RhoGAP triggers structural change, we find that pharmacological stimulation of Arg and p190RhoGAP (with the novel Arg activator DPH) blocks dendrite atrophy, suggesting that enriching Arg-p190RhoGAP-mediated RhoA silencing combats stress-related dendrite degeneration. These findings complement new evidence that corticosterone and corticotropin-releasing factor synergistically act on RhoA within dendritic spines to destabilize their structure (Chen et al., 2016).
In the course of these experiments, we discovered that phosphop190RhoGAP levels increased nearly 2-fold in the hippocampus in healthy mice between P35–P42, a period when Arg knockout mice develop significant hippocampal CA1 dendrite arbor loss (Sfakianos et al., 2007). It is possible that failures in this developmentally-appropriate surge in p190RhoGAP phosphorylation could contribute to the timing of dendrite loss in Arg-deficient mutant mice.
Reductions in phospho-p190RhoGAP, as following CORT exposure, are associated with increased RhoA activity (Hernandez et al., 2004; Sfakianos et al., 2007; Warren et al., 2012; Kerrisk et al., 2013). Accordingly, we found that phosphorylation of cofilin, a major down-stream target of RhoA-ROCK signaling, is also increased following CORT. Because cofilin phosphorylation inactivates the protein, this profile is associated with less cofilin activity. Interestingly, disruption of cofilin function by phosphorylation is associated with increased dendritic spine size or stability (Shi et al., 2009), while CORT instead eliminates dendritic spines in hippocampal CA1 (Gourley et al., 2013; Morales-Medina et al., 2009). It could be that the observed inhibition of cofilin activity is insufficient to protect against CORT-induced dendritic spine loss. An additional consideration, however, is that our tissue dissections contain multiple cell types and both apical and basal dendritic trees. Future investigations could aim for greater specificity in understanding cell type- or cell subregion-specific alterations in cofilin activity.
We also identified reduced phosphorylation of the Arg substrate cortactin, an actin-binding protein that stimulates the Arp2/3 complex to nucleate actin filament branches (e.g., Boyle et al., 2007; Courtemache et al., 2015). Arg also promotes cortactin binding to actin, and the two proteins cooperate to stabilize actin filament branches (Courtemache et al., 2015; MacGrath and Koleske, 2012). Disruption of Arg function reduces cortactin localization to dendritic spines in hippocampal neurons, leading to reduced spine actin and net spine destabilization (Lin et al., 2013). Taken together, it may be that CORT-induced reductions in cortactin activity contribute to the reduction of dendritic spines in hippocampal CA1 upon CORT exposure (Gourley et al., 2013; Morales-Medina et al., 2009).
CORT and stressor exposure decrease levels of the AMPA receptor subunit GluR1 in the hippocampus, and this effect can be blocked by metyropone, a CORT synthesis inhibitor (Kvarta et al., 2015; Schmidt et al., 2010). We replicated this decline in GluR1 in hippocampal extracts, showing that CORT decreased levels within 4 days of exposure. Interestingly, levels recovered by 11 days of CORT exposure, when dendrite structure deficiencies first emerged. While CORT-induced degradation of GluR1-mediated neuroplasticity could conceivably be part of a neuronal sequela that ultimately triggers dendrite regression, additional studies specifically testing this hypothesis would be necessary, given this temporal discord.
4.2. The Arg stimulator DPH yields behavioral resistance to CORT
Given our finding that CORT exposure decreases Arg kinase levels in the hippocampus, coinciding with dendrite atrophy, we hypothesized that stimulating Arg could prevent CORT-related behavioral impairments. Using systemic administration of the Arg activator DPH at a dose that stimulated phosphorylation of the Arg substrate p190RhoGAP in the hippocampus, we found that DPH indeed blocked CORT-induced decision-making deficits otherwise characteristic of hippocampal damage (Gourley et al., 2010) in male mice. Thus, DPH blocked CORT-induced hippocampal-dependent learning and memory impairments. We also tested sucrose consumption in CORT-exposed female mice. As in CORT-exposed males (Gourley et al., 2013; Gourley et al., 2008), CORT exposed females consumed less sucrose, which is thought to model anhedonia in humans (see also Mekiri et al., 2017). A key next step in these experiments is to determine whether DPH corrects anhedonic-like behavior or decision-making abnormalities following stressor exposure.
Together, our findings suggest that effective therapeutic strategies for stress-related illnesses could include agents that directly target regulators of actin polymerization or turnover, or those that act indirectly — for example, ketamine, an NMDA receptor antagonist, has rapid antidepressant-like properties that are associated with dendritic spine proliferation in deep-layer prefrontal cortex (Li et al., 2010). Recent investigations indicate that behavioral benefits are likely attributable to actions on apical, but not basal, branches (Liu et al., 2015). Meanwhile, the degeneration of basal, but not apical, hippocampal CA1 arbors coincides with depression-like behavior (Gourley et al., 2013; Fig. 5). Further elucidation of the specific cell types and cell subregions that contribute to stressor-related disease, recovery, and resilience may shed light onto the mechanisms by which certain treatment approaches are effective, while others fail, and may also illuminate new strategies to combat illness.
Supplementary Material
Acknowledgments
This work was supported by PHS grants NS089662 and NS089439 (AJK) and MH101477 (SLG); Children's Healthcare of Atlanta (SLG); and training grants F31NS090767, T32NS007224, F31MH109208, and T32GM008602. We thank A. Allen for technical assistance and for the illustrations in Fig. 5 and E. Barfield for assistance with gland extraction.
The components of this project performed at the Yerkes National Primate Research Center were also supported by the Office of Research Infrastructure Programs/OD P51OD011132.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mcn.2017.10.007.
The authors report no conflict of interest.
References
- Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr. Biol. 2007;17:445–451. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Settleman J. p190 RhoGAP is the principal Src substrate in brain and regulates axon growth, guidance and fasciculation. Nat. Cell Biol. 2001;3:361–371. doi: 10.1038/35070042. [DOI] [PubMed] [Google Scholar]
- Byers SL, Wiles MV, Dunn SL, Taft RA. Mouse estrous cycle identifcation tool and images. PLoS One. 2012;7:e35538. doi: 10.1371/journal.pone.0035538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Molet J, Lauterborn JC, Trieu BH, Bolton JL, Patterson KP, Gall CM, Lynch G, Baram TZ. Converging, synergistic actions of multiple stress hormones mediate enduring memory impairments after acute simultaneous stresses. J. Neurosci. 2016;36:11295–11307. doi: 10.1523/JNEUROSCI.2542-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Ortiz JB, Judd JM. Chronic stress and hippocampal dendritic complexity: methodological and functional considerations. Physiol. Behav. 2017;178:66–81. doi: 10.1016/j.physbeh.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol. Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Courtemache N, Gifford SM, Simpson MA, Pollard TD, Koleske AJ. Abl2/Arg-related gene stabilizes actin filaments, stimulates actin branching by actin-related protein 2/3 complex, and promotes actin filament severing by cofilin. J. Biol. Chem. 2015;290:4038–4046. doi: 10.1074/jbc.M114.608117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Laxmi TG, Chattarji S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J. Neurosci. 2013;33:1723–1744. doi: 10.1523/JNEUROSCI.0638-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal BDNF restores motivational and forced swim performance after corticosterone. Biol. Psychiatry. 2008;64:884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur. J. Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Swanson AM, Koleske AJ. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J. Neurosci. 2013;33:3107–3112. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez SE, Settleman J, Koleske AJ. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Able-related gene tyrosine kinase. Curr. Biol. 2004;14:691–696. doi: 10.1016/j.cub.2004.03.062. [DOI] [PubMed] [Google Scholar]
- Hoffman AN, Krigbaum A, Ortiz JB, Mika A, Hutchinson KM, Bimonte-Nelson HA, Conrad CD. Recovery after chronic stress within spatial reference and working memory domains: correspondence with hippocampal morphology. Eur. J. Neurosci. 2011;34:1023–1030. doi: 10.1111/j.1460-9568.2011.07820.x. [DOI] [PubMed] [Google Scholar]
- Jafari M, Seese RR, Babayan AH, Gall CM, Lauterborn JC. Glucocorticoid receptors are localized to dendritic spines and influence local actin signaling. Mol. Neurobiol. 2012;46:304–315. doi: 10.1007/s12035-012-8288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen E, Fenton AA. Coordinating different representations in the hippocampus. Neurobiol. Learn. Mem. 2016;129:50–59. doi: 10.1016/j.nlm.2015.12.011. [DOI] [PubMed] [Google Scholar]
- Kerrisk ME, Greer CA, Koleske AJ. Integrin α3 is required for late postnatal stability of dendrite arbors, dendritic spines and synapses, and mouse behavior. J. Neurosci. 2013;33:6742–6752. doi: 10.1523/JNEUROSCI.0528-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ. Molecular mechanisms of dendrite stability. Nat. Rev. Neurosci. 2013;14:536–550. doi: 10.1038/nrn3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvarta MD, Bradbrook KE, Dantrassy HM, Bailey AM, Thompson SM. Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. J. Neurophysiol. 2015;114:1713–1724. doi: 10.1152/jn.00359.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:954–959. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Yeckel MF, Koleske AJ. Abl2/Arg controls dendritic spine and dendrite arbor stability via distinct cytoskeletal control pathways. J. Neurosci. 2013;33:1846–1857. doi: 10.1523/JNEUROSCI.4284-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:2870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Ota KT, Dutheil S, Duman RS, Aghajanian GK. Ketamine strengthens CRF-activated amygdala inputs to basal dendrites in mPFC layer V pyramidal cells in the prelimbic but not infralimbic subregion, a key suppressor of stress responses. Neuropsychopharmacology. 2015;40:2066–2075. doi: 10.1038/npp.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Fornito A, Yucel M. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J. Affect. Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- MacGrath SM, Koleske AJ. Arg/Abl2 modulates the affinity and stoichiometry of binding of cortactin to F-actin. Biochemistry. 2012;51:6644–6653. doi: 10.1021/bi300722t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti P, Muthuraju S, Ilavazhagan G, Singh SB. Hypobaric hypoxia induces dendritic plasticity in cortical and hippocampal pyramidal neurons in rat brain. Behav. Brain Res. 2008;189:223–243. doi: 10.1016/j.bbr.2008.01.007. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekiri M, Gardier AM, David DJ, Guilloux JP. Chronic corticosterone administration effects on behavioral emotionality in female c57bl6 mice. Exp. Clin. Psychopharmacol. 2017;25:94–104. doi: 10.1037/pha0000112. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J. Chem. Neuroanat. 2009;38:266–272. doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Moresco EM, Donaldson S, Williamson A, Koleske AJ. Integrin-mediated dendrite branch maintenance requires Abelson (Abl) family kinases. J. Neurosci. 2005;25:6105–6118. doi: 10.1523/JNEUROSCI.1432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padival MA, Blume SR, Rosenkranz JA. Repeated restraint stress exerts different impact on structure of neurons in the lateral and basal nuclei of the amygdala. Neuroscience. 2013;246:230–242. doi: 10.1016/j.neuroscience.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, et al. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J. Neurosci. 2010;30:16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R. Cumulative adversity sensitizes neural response to acute stress: association with health symptoms. Neuropsychopharmacology. 2014;39:670–680. doi: 10.1038/npp.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakianos MK, et al. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability, and behavior. J. Neurosci. 2007;27:10982–10992. doi: 10.1523/JNEUROSCI.0793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LP, Parsons RG, Koleske AJ, Gourley SL. Differential expression of cytoskeletal regulatory factors in the adolescent prefrontal cortex: implications for cortical development. J. Neurosci. Res. 2017;95:1123–1143. doi: 10.1002/jnr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medial comorbidity. Biol. Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am. J. Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- Shi Y, Pontrello CG, DeFea KA, Reichardt LF, Ethell IM. Focal adhesion kinase acts downstream of EphB receptors to maintain mature dendritic spines by regulating cofilin activity. J. Neurosci. 2009;29:8129–8142. doi: 10.1523/JNEUROSCI.4681-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OFX, Paul-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Tata DA, Anderson BJ. The effects of chronic glucocorticoid exposure on dendritic length, synapse numbers and glial volume in animal models: implications for hippocampal volume reductions in depression. Physiol. Behav. 2010;99:186–193. doi: 10.1016/j.physbeh.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Warren MS, Bradley WD, Gourley SL, Lin YC, Simpson MA, Reichardt LF, Greer CA, Taylor JR, Koleske AJ. Integrin beta1 signals through Arg to regulate postnatal dendritic arborization, synapse density, and behavior. J. Neurosci. 2012;32:2824–2834. doi: 10.1523/JNEUROSCI.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.