Abstract
Background
Direct acting antiviral hepatitis C virus (HCV) therapies are highly effective but costly. Wider adoption of an 8-week ledipasvir/sofosbuvir treatment regimen could result in significant savings, but may be less efficacious compared with a 12-week regimen. We evaluated outcomes under a constrained budget and cost-effectiveness of 8 vs 12 weeks of therapy in treatment-naïve, noncirrhotic, genotype 1 HCV-infected black and nonblack individuals and considered scenarios of IL28B and NS5A resistance testing to determine treatment duration in sensitivity analyses.
Methods
We developed a decision tree to use in conjunction with Monte Carlo simulation to investigate the cost-effectiveness of recommended treatment durations and the population health effect of these strategies given a constrained budget. Outcomes included the total number of individuals treated and attaining sustained virologic response (SVR) given a constrained budget and incremental cost-effectiveness ratios.
Results
We found that treating eligible (treatment-naïve, noncirrhotic, HCV-RNA <6 million copies) individuals with 8 weeks rather than 12 weeks of therapy was cost-effective and allowed for 50% more individuals to attain SVR given a constrained budget among both black and nonblack individuals, and our results suggested that NS5A resistance testing is cost-effective.
Conclusions
Eight-week therapy provides good value, and wider adoption of shorter treatment could allow more individuals to attain SVR on the population level given a constrained budget. This analysis provides an evidence base to justify movement of the 8-week regimen to the preferred regimen list for appropriate patients in the HCV treatment guidelines and suggests expanding that recommendation to black patients in settings where cost and relapse trade-offs are considered.
Keywords: budget impact, cost-effectiveness, NS5A, IL28B
At least 3 million individuals are infected with hepatitis C virus (HCV) in the United States [1, 2]. New therapies to treat HCV are very effective, with cure rates >95%, but they are costly. Because of the high prevalence of HCV and the high cost of treatment, the budgetary impact of treating HCV is high [3]. As a result, many payers in the United States restrict access to HCV treatment to patients with more advanced liver fibrosis and to those without recent substance use [4]. As medication prices are coming down, some payers have loosened their HCV treatment coverage restrictions, but many, especially Medicaid programs, continue to limit access to HCV therapy [4].
A means of controlling medication cost is to shorten treatment duration. One of the most common profiles of HCV-infected patients in the United States is HCV genotype 1 infected individuals who are treatment naïve, have a serum HCV RNA <6 million copies/mL, without cirrhosi [5–8]. In such patients, a common treatment regimen is co-formulated ledipasvir/sofosbuvir (LDV/SOF). Per US Food and Drug Administration (FDA)–approved labeled indication, shortening the LDV/SOF treatment course in such patients from 12 to 8 weeks represents a savings of 33% [9]. Though real-world data suggest excellent cure rates with the 8-week regimen in appropriate patients [10, 11], there is concern that in some patients, especially those with co-factors such as black race [12], HIV co-infection, NS5A resistance-associated substitutions (RAS), and/or hepatosteatosis, the 8-week treatment course may be inadequate. And although the recent approval of glecaprevir/pibrentasvir brings another 8-week regimen to the clinics for treatment-naïve patients with HCV infection, some payers will prefer LDV/SOF based on negotiated prices. Further, while treatment options for “salvage” HCV regimens have been recently approved, willingness of insurers to pay for them is uncertain. Mandated shortening of treatment to 8 weeks may therefore increase the pool of patients who are difficult to treat for HCV.
Understanding the trade-offs between cost and efficacy for 8- and 12-week treatment courses is critical. We therefore employed an existing microsimulation model of HCV infection, the Hepatitis C Cost-Effectiveness Model (HEP-CE), to examine the economic value associated with 8- and 12-week treatment regimens. We considered treatment for black patients and nonblack patients and considered strategies for identifying patients best treated with 12 weeks of LDV/SOF, including testing for host-related factors (interleukin-28B [IL28B] genotype) or virus-related factors (NS5A resistance). We identified threshold treatment efficacies and cost that changed conclusions, and we considered the decision assuming both an open treatment budget and a fixed capacity system.
METHODS
Model Structure
We first built a decision tree to describe the effectiveness of the 8- and 12-week strategies in black and nonblack patients (Figure 1). The model begins with treatment eligible patients presenting for treatment. The efficacy of either the 8- or 12-week course of LDV/SOF determines the sustained virologic response (SVR) rate after the first round of therapy. Those who fail firstline therapy are either retained in care with salvage therapy of sofosbuvir/velpatasvir/voxilaprevir for 12 weeks or are lost to follow-up [13]. Those retained either attain SVR or fail therapy and never attain SVR. The decision tree estimates per-person therapy costs of first- and second-line therapy and estimates the proportion of the population achieving SVR.
Figure 1.
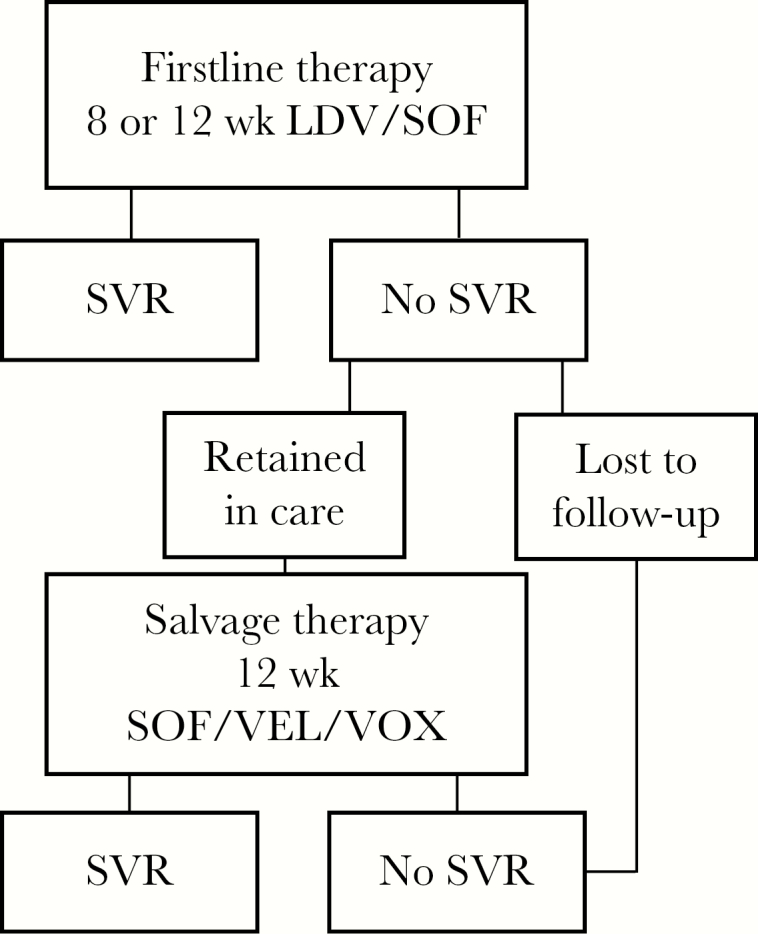
Decision tree evaluating 8-week vs 12-week therapy for HCV. Figure 1 is a schematic of our model. All individuals begin the analysis ready for treatment. Following firstline therapy, individuals’ chance of attaining SVR is based on the efficacy of the firstline regimen. Among those failing to achieve SVR, they are either retained in care or lost to follow-up. Those lost to follow-up never achieve SVR. Those retained are treated with salvage therapy and attain SVR with a probability equal to the efficacy of the salvage therapy. At the end of each branch (SVR or no SVR), lifetime cost and QALY outcomes are calculated via the HEP-CE model. Abbreviations: HCV, hepatitis C virus; HEP-CE, Hepatitis C Cost-Effectiveness Model; QALY, quality-adjusted life-year; SVR, sustained virologic response.
Next, we used the HEP-CE model to estimate the lifetime medical costs and quality-adjusted life-years (QALYs) of each strategy, discounted 3% annually [14]. The HEP-CE Model is a Monte Carlo lifetime simulation of HCV infection, screening, and treatment, summarized in greater detail in the published literature [15–17]. The model inputs are summarized in Table 1. Where possible, inputs were informed by the relevant clinical trials.
Table 1.
Model Inputs to Evaluate 8 vs 12 Weeks of Treatment of HCV Genotype 1
| Input | Value | Sensitivity Range Evaluated | Source |
|---|---|---|---|
| Cohort characteristics | |||
| Mean age | 53 | 30–65 | [12] |
| Proportion male, % | 60 | 0–100 | [12] |
| Average age at HCV infection | 26 | 16–36 | [27] |
| HCV disease progression | |||
| Median y to cirrhosis from infection | 25 | 15–35 | [28], [29] |
| Median y to first liver- related event after cirrhosis | 11 | 6–17 | [30] |
| Liver-related mortality with compensated cirrhosis, deaths/100 PY | 1.4 | 0.7–2.8 | [30] |
| Liver-related mortality with decompensated cirrhosis, deaths/100 PY | 12 | 6–24 | [30] |
| Reduction in liver mortality after SVR, % | 94 | 81–98 | [31] |
| HCV therapy efficacy, % | |||
| SVR of 8-wk regimen LDV/ SOF in black patients | 96.3 | 72–100 | [12] |
| SVR of 8-wk regimen LDV/ SOF in nonblack patients | 96.9 | 72–100 | [12] |
| SVR of 12-wk regimen LDV/ SOF in black patients | 98.9 | 72–100 | [12] |
| SVR of 12-wk regimen LDV/ SOF in nonblack patients | 97.1 | 72–100 | [12] |
| SVR of 12-wk regimen sofosbuvir/velpatasvir/ voxilaprevir | 97.3 | 0–100 | [32] |
| Retention to salvage treatment, % | 24 | 0–100 | [13] |
| Costs | |||
| Non-HCV-related medical costs, $ per mo | |||
| Background medical costs (without HCV) | 110–1100 | 55–1650 | [33] |
| Laboratory testing costs, $ | |||
| IL28B genotype test | 68.52 | 0–200 | [34] |
| NS5A test | 231.23 | 0–400 | [34] |
| HCV related medical costs, $ per mo | |||
| No cirrhosis | 245 | 185–305 | [35] |
| Mild to moderate cirrhosis | 440 | 315–550 | [35] |
| Decompensated cirrhosis | 830 | 620–1050 | [35] |
| HCV therapy, $ per 4 wk | |||
| LDV/SOF | 18900 | 9000–28 000 | [23] |
| Sofosbuvir/velpatasvir/ voxilaprevir | 18654 | 11 250–33 750 | [23] |
| Quality of life | |||
| After achieving SVR | 0.74–0.92 | 0.60–1 | [36], [37] |
| No to moderate fibrosis | 0.89 | 0.75–1 | 38–40] |
| Cirrhosis | 0.62 | 0.55–0.75 | [38], [39] |
| Decompensated cirrhosis | 0.48 | 0.40–0.60 | [38], [39] |
Cost-effectiveness Analysis
In the cost-effectiveness analysis, we simulated a cohort without constrained treatment capacity, evaluating the effect of treating all individuals. The model estimates of the lifetime cost and QALY per person. We modeled the effect of no treatment, treatment with an 8-week LDV/SOF regimen, and treatment with a 12-week LDV/SOF regimen. We sorted regimens in order of increasing lifetime cost and then calculated the incremental cost and QALYs associated with increasingly expensive strategies compared with the next least costly strategy. We calculated incremental cost-effectiveness ratios (ICERs) by dividing the incremental cost by the incremental QALYs. We used the commonly cited US willingness-to-pay threshold of $100 000 per QALY gained to interpret ICERs [14].
Budget Constrained Analysis
We evaluated the effect of each treatment strategy by assuming a fixed budget constraint. This analysis assumed the budgetary perspective of a public payer, department of health, or department of corrections with a fixed pharmacy budget and therefore considered only the costs of first- and second-line therapy. As an example, we chose a $10 000 000 fixed budget. Using the decision tree from the cost-effectiveness analysis, we found the maximum number of individuals who could be treated while keeping the budget at or below the constraint.
Scenario Analyses
We also explored 2 potential testing strategies that could evaluate who would have success using an 8-week regimen and who may benefit from longer therapy: detecting interleukin-28B (IL28B) polymorphisms and testing for the prevalence of NS5A RAS [18]. The difference in efficacy observed between black and nonblack patients has been in part attributed to differences in IL28B polymorphisms [19]. While the role of IL28B in pegylated interferon-alpha treatment was well established, the effect of IL28B polymorphisms on treatment with direct acting antivirals (DAAs) such as LDV/SOF has been attenuated; however, differences in SVR rates among IL28B genotypes persist [19, 20]. Some studies suggest that certain NS5A RAS can affect SVR achievement, although NS5A resistance testing is rare [18]. We evaluated the effect of IL28B testing and treating individuals with either an 8-week (CC genotypes) or 12-week (non-CC genotypes) course of therapy. Next, we considered a scenario in which all patients received HCV viral genotyping for NS5A ledipasvir-specific RAS, including substitutions at the following positions: K24G/N/R, M28A/G/T, Q30E/G/H/L/K/R/T, L31I/F/M/V, P32L, S38F, H58D, A92K/T, and Y93C/F/H/N/S in genotype 1a patients and L31F/I/M/V, P32L, P58D, A92K, and Y93C/H/N/S in genotype 1b patients [18]. Our approach to parameterizing these analyses is covered in the Supplementary Appendix.
To further evaluate the robustness of our results, we conducted several sensitivity analyses. We varied the efficacy and cost of therapy to determine the effect of price reductions and evaluated the effects of retention, the age of the cohort, and the availability of salvage treatment.
RESULTS
Cost-effectiveness Analysis
The cost-effectiveness was similar among black and nonblack patients as regimen costs were the same and efficacy rates were similar (Table 2). Among all patients, an 8-week course of LDV/SOF resulted in a discounted lifetime medical cost of $226 000 and a quality-adjusted lifetime expectancy of 15.2 QALYs, yielding ICERs of less than $11 000/QALY (Table 2). When employing the 8-week regimen, 97% of black patients ultimately attained SVR compared with 98% of nonblack patients. Four percent of black patients and 3% of nonblack patients needed a second course of therapy because they failed the 8-week regimen, 0.03% of black patients and 0.02% of nonblack patients were left without treatment options after failing both HCV treatment regimens, and 2.8% of black patients and 2.4% of nonblack patients were lost to follow-up between the first and second lines of therapy. A 12-week regimen resulted in fewer patients requiring retreatment (1.1% vs 3.7% for black patients and 2.9% vs 3.1% for nonblack patients), fewer patients being left without treatment options (0.01% vs 0.03% for black patients and 0.020% vs 0.022% for nonblack patients), and fewer patients lost to follow-up (0.8% vs 2.8% for black patients and 2.2% vs 2.4% for nonblack patients). However, the 12-week regimen increased costs by $18000 per black patient and $19 000 per nonblack patient, with a commensurate increase in QALYs of less than 0.1 per black patient and less than 0.01 per nonblack patient, yielding an ICER compared with the 8-week regimen of $212000/QALY for black patients and $2 850 000 for nonblack patients (Table 2).
Table 2.
Cost-effectiveness and Fixed Budget Analysis of Treating Noncirrhotic, Treatment-Naïve, Genotype 1 HCV-Infected Individuals
| Cost, $ | Incr. Cost, $ | QALY | Incr. QALY | ICER, $ | % SVR | No. Treated w/$10 000 000 | |
|---|---|---|---|---|---|---|---|
| Black patients | |||||||
| Not treated | 182 000 | - | 10.98 | - | - | 0 | 0 |
| 8-wk | 225 000 | 43 700 | 15.16 | 4.18 | 10 400 | 97.2 | 261 |
| 12-wk | 244 000 | 18 700 | 15.24 | 0.09 | 218 000 | 99.2 | 175 |
| Nonblack patients | |||||||
| Not treated | 182 000 | - | 10.98 | - | - | 0 | 0 |
| 8-wk | 225 000 | 43 600 | 15.18 | 4.20 | 10 400 | 97.6 | 261 |
| 12-wk | 244 000 | 18 900 | 15.19 | 0.01 | 2 860 000 | 97.8 | 175 |
Budget Constrained Analysis
In the presence of a fixed pharmacy budget of $10 000 000, 261 patients could be treated with an 8-week regimen, with 254 black and 255 nonblack patients attaining SVR, while 175 could be treated under the 12-week regimen, with 174 black and 171 nonblack patients attaining SVR. While the 12-week strategy yielded a higher probability of SVR among those who were treated, using an 8-week regimen allowed for almost 50% more individuals to be cured (Table 2).
IL28B Testing Scenario Analyses
In black patients, 8-week therapy had a lifetime cost of $227 000 and 15.0 QALYs for an ICER of $11 000 compared with no treatment, and 93.9% of patients reached SVR (Table 3). Treating based on the IL28B polymorphism increased costs by $16 000 and quality of life by less than 0.1 QALYs, resulting in an ICER of $190 000 compared with an 8-week regimen for all patients without IL28B testing (Table 3). Treating all patients with 12-week therapy was slightly more expensive, increasing costs by $2000, and quality of life by just under 0.01 QALYs, producing an ICER of $267 000 compared with the IL28B testing strategy (Table 3). Among nonblack patients, where the prevalence of IL28B non-CC polymorphisms is low relative to black patients, IL28B testing was a dominated strategy. In this cohort, treating all patients with an 8-week course had an ICER of $11 000 compared with no treatment, while the 12-week regimen had an ICER of $212000 compared with the 8-week regimen (Table 3). In both black and nonblack patients, an 8-week treatment course was preferred to treating patients based on the results of an IL28B test.
Table 3.
Cost-effectiveness Scenario Treating Patients Based on an IL28B or NS5A Test
| Cost, $ | Incr. Cost, $ | QALY | Incr. QALY | ICER, $ | % SVR | |
|---|---|---|---|---|---|---|
| IL-28B genotype test | ||||||
| Black patients | ||||||
| Not treated | 182 000 | - | 10.98 | - | - | 0 |
| 8-wk | 226 000 | 44 100 | 15.02 | 4.04 | 10 900 | 93.9 |
| IL28B tested | 243 000 | 16 800 | 15.11 | 0.09 | 196 000 | 95.9 |
| 12-wk | 244 000 | 1800 | 15.11 | 0.01 | 273 000 | 96.1 |
| Nonblack patients | ||||||
| Not treated | 182 000 | - | 10.98 | - | - | 0 |
| 8-wk | 226 000 | 44 000 | 15.03 | 4.06 | 10 900 | 94.3 |
| IL28B tested | 243 000 | 16 900 | 15.11 | 0.07 | Dominated | 95.9 |
| 12-wk | 244 000 | 18 700 | 15.12 | 0.09 | 218 000 | 96.2 |
| NS5A test | ||||||
| Not treated | 182 000 | - | 10.98 | - | - | 0 |
| 8-wk | 226 000 | 43 800 | 15.10 | 4.13 | 10 600 | 95.8 |
| NS5A tested | 229 000 | 3570 | 15.16 | 0.06 | 62 300 | 97.2 |
| 12-wk | 244 000 | 14 900 | 15.25 | 0.09 | 170 000 | 99.2 |
NS5A Testing Scenario
We found that 8-week therapy cost $227 000 with 15.1 QALYs, yielding an ICER of $10 900 compared with no treatment (Table 3). Treating patients based on NS5A RAS increased costs by $3230 and quality of life by 0.06 QALYs, resulting in an ICER of $56 500. Treating all patients with a 12-week regimen increased costs by $14 400 over the NS5A testing strategy and increased QALYs by 0.09, producing an ICER of $164 000. With an ICER of less than $100 000 per QALY, administering an NS5A test and treating based on RASs was preferred to treating all patients with either an 8- or 12-week treatment course regardless of RAS. We found that NS5A testing was cost-effective as long as the SVR rate for 8 weeks of therapy in patients with RAS conferring more than 100-fold resistance to ledipasvir was 88% or less.
Sensitivity Analyses
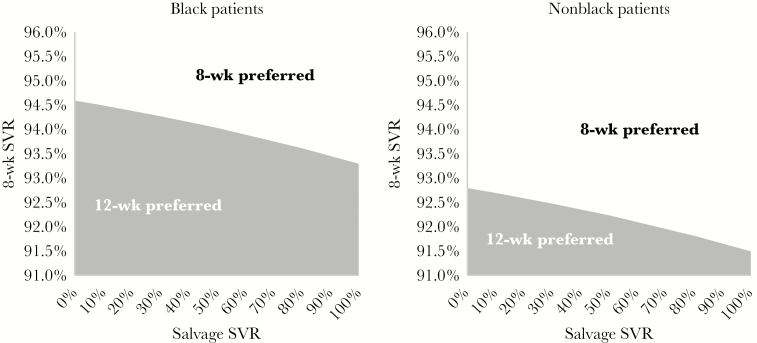
Two-way sensitivity analyses identified thresholds of 8-week treatment efficacy and salvage therapy efficacy where 12-week LDV/SOF therapy is preferred (Figure 2). Assuming that salvage therapy cures 97.3% of those who fail an 8-week course of LDV/SOF, the 8-week treatment regimen was preferred from a cost-effectiveness perspective unless 8-week treatment efficacy was <93.4% for black patients or <91.6% for nonblack patients. In the extreme case of a completely ineffective salvage therapy (SVR = 0%), we found that 8-week therapy remained preferred from a cost-effectiveness perspective, as long as the 8-week regimen resulted in an SVR greater than 94.5% for black patients and 92.7% for nonblack patients. With a constrained budget, 8-week treatment resulted in more individuals cured unless the efficacy of 8-week therapy was <65.9% for black patients and <64.7% for nonblack patients.
Figure 2.
Sensitivity analysis of SVR of 8-week therapy and salvage therapy. Figure 2 depicts the results of our sensitivity analysis of the effect of 8-week regimen SVR and salvage therapy SVR. The x-axis displays the SVR range of the salvage regimen, and the y-axis depicts the SVR of the 8-week regimen. Holding constant the efficacy of the 12-week regimen, we vary the salvage SVR from 0% to 100% and find the corresponding 8-week regimen efficacy threshold that results in 12-week therapy to be preferred. In the figure, the downward sloping line is that threshold, with the shaded region underneath representing where the 12-week regimen is preferred. Areas above each threshold shaded region indicate where the 8-week regimen is preferred. The threshold for black patients is higher compared with nonblack patients because in our primary data source [18] the 12-week efficacy of LDV/SOF was higher among black patients (98.9%) than it was among nonblack (97.1%) patients, which makes 12 weeks of therapy more attractive in general. Abbreviations: LDV, ledipasvir; SOF, sofosbuvir; SVR, sustained virologic response.
Next, we found that when the monthly cost of LDV/SOF was $8883 (47% of the current Federal Supply Schedule costs = $18 900) 12-week therapy was the preferred strategy for black patients. Because the 8-week and 12-week efficacies were similar for nonblack patients, the 12-week regimen was not preferred unless the monthly price of LDV/SOF fell to less than 4% ($750) of the Federal Supply Schedule cost.
When we varied retention in care after failing an 8-week regimen, we found that at any level of follow-up for salvage therapy (0%–100%), the 8-week regimen remained preferred, likely because firstline therapy is so efficacious.
The findings were robust in all other deterministic sensitivity analyses, including changing the efficacy and cost of therapy, retention, the age of the cohort, and the availability of salvage treatment (Supplementary Appendix).
DISCUSSION
This cost-effectiveness analysis, both with and without a fixed budget constraint, demonstrates that among treatment-naïve, genotype 1 HCV-infected individuals without cirrhosis, an 8-week treatment regimen provides good value for the money and is preferred to a 12-week regimen in both black and nonblack patients. While 8-week treatment results in more treatment failures, resources invested in extending therapy to 12 weeks would likely be more productively invested in other HCV-related health care interventions, such as expanding HCV screening or improving HCV linkage to care. We found that 8 weeks of therapy was preferred even though our rate of retreatment was low (24%) [13]; additional investments in linkage to care would likely increase the attractiveness if 8 weeks of treatment. Furthermore, when presented with a fixed budget constraint, the 8-week regimen results in nearly 50% more individuals attaining SVR than the 12-week regimen, yielding better population outcomes. This finding is particularly relevant for health systems faced with a fixed budget such as correctional systems or Medicaid, and this type of analysis could be useful to settings outside of the United States grappling with similar cost/efficacy trade-offs. In scenario analyses, however, we demonstrate that NS5A testing might be a good strategy for both controlling cost and minimizing poor outcomes. Guidelines should consider the value of NS5A testing, and future research should evaluate the real-world performance of such an individualized approach.
The American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) HCV treatment guidance recently added the 8-week LDV/SOF regimen to the recommended regimen list for treatment-naïve, genotype 1 HCV-infected individuals without cirrhosis who have a baseline HCV RNA <6 million IU/mL. However, there are caveats regarding which individuals are best suited for this shortened course of therapy; thus many clinicians remain concerned about mandating shortened treatment courses that can increase the risk of relapse for their individual patients. However, early trials showing decreased efficacy did not limit 8-week treatment to patients with HCV RNA <6 million copies, as would later be recommended. When we considered efficacy stratified by RNA, the relative efficacy rates of 8- and 12-week therapy in both black and nonblack patients were similar. Furthermore, we provide additional strategies here that may improve provider comfort with patient-tailored approaches.
While differences in treatment outcomes by race persist in the era of DAA treatment, these differences are less dependent on the IL28B polymorphism [21]. In a scenario considering the usefulness of IL28B testing to prioritize black patients for 8 vs 12 weeks of LDV/SOF, we found that treating based on IL28B polymorphism is not preferred from a cost-effectiveness standpoint, likely because the test does not provide adequate information to risk stratify. It is possible that the linked polymorphism IFNL4-ΔG/TT (rs368234815) may provide more resolution, especially in black patients [22]; however, commercial testing is not yet available.
In contrast, our results suggest that baseline testing for NS5A RAS that convey >100-fold ledipasvir phenotypic resistance is part of a potentially attractive treatment strategy. Work from Sarrazin et al. demonstrated that treating patients who are infected with a virus with baseline NS5A RAS with a 12-week regimen increases SVR by nearly 13 percentage points (from 82.8% with 8 weeks to 95.7% with 12 weeks) [18]. Our model results suggest that this large gain in SVR at a modest test cost provides good economic value and might be the ideal strategy to reduce cost and avoid higher relapse.
While in the current environment we demonstrate that overall 8-week treatment is preferred, there are important caveats. It is possible that future price negotiations and market competition result in the price of LDV/SOF falling to the point that an additional month of therapy for all patients provides good value and is cost-effective. In our analysis, we find that that occurs at around $8900 for a month of therapy in black patients, approximately 50% of the Federal Supply Schedule price and 25% of the average wholesale price of LDV/SOF [1, 23]. It is possible that some insurers or health systems have already crossed this threshold, or may do so with the downward pressure on prices due to competition with the release of the new 8-week regimen of glecaprevir/pibrentasvir. If so, those systems would secure the best possible outcomes by treating all black patients for 12 weeks. Among nonblack patients, the threshold price that results in 12-week therapy being cost-effective is very low and likely not realistic in the near future ($750 per month of therapy).
These data support the decision by the AASLD/IDSA Guidance Panel to recommend the 8-week regimen, regardless of price points, for nonblack patients. While this analysis also supports 8 weeks in black patients, it acknowledges a higher relapse rate with the 8-week regimen and a need for salvage with a second course of approved therapies. Furthermore, in the setting where the cost of LDV/SOF is less than $8900 per month (or $17 800 per treatment), the trade-off of higher relapse and cost is not needed. The guidance panel makes recommendations based on safety and efficacy and does not consider cost per se [24]. Thus, these data are more likely to support population-, health system–, and health insurer–level decisions, where fixed budgets imply that using an 8-week regimen could allow more black patients to be treated.
This analysis has several limitations. First, the price of HCV treatment varies significantly among payers, and there is evidence of large price reductions following negotiations for exclusivity [25]. We attempted to capture this lower cost by using the Federal Supply Schedule, but it is possible this is not the appropriate metric. Next, due to data availability, we had to use heterogeneous data sources for the base case and 2 scenarios. Although the absolute efficacy values do not always match perfectly among the 2 scenarios and base case, the relative efficacies are internally consistent. While more research is needed to explore combinations of HCV viral load, IL28B genotype, RAS presence, and fibrosis in depth, we believe our results are a valuable first step in understanding the potential value in different testing and treatment strategies. Finally, while there are a number of treatments available, we focused on LDV/SOF alone as a firstline regimen. While the approval of glecaprevir/pibrentasvir provides another 8-week treatment option primarily in treatment-naïve patients, we believe that LDV/SOF will have continued relevance in the clinic. Price negotiations leading to steep discounts for LDV/SOF make prices difficult to compare, even given the lower published wholesale acquisition cost of glecaprevir/pibrentasvir ($13 200/4 weeks) compared with LDV/SOF ($31 500/4 weeks) [1, 25]. LDV/SOF has been available since 2014, and many providers have experience with that regimen. Given the similarity in efficacy between LDV/SOF and glecaprevir/pibrentasvir and the recommendation of LDV/SOF in the AASLD/IDSA treatment guidelines, the 2 regimens will likely continue as competitors. As such, our findings likely apply to glecaprevir/pibrentasvir as well. In particular, there are questions around the role of NS5A resistance in glecaprevir/pibrentasvir that clinical trials were unable to answer [26]. Our finding that NS5A resistance testing is likely cost-effective represents an important research space for maximizing the efficacy of glecaprevir/pibrentasvir in particular populations.
While highly efficacious therapies can cure HCV with few side effects in as little as 8 weeks, many individuals and payers are struggling with the cost. For LDV/SOF, our results indicate that 8-week therapy is cost-effective and can result in better population outcomes in both black and nonblack patients compared with 12-week therapy, even with lower rates of SVR. Future research demonstrating the real-world effectiveness of NS5A testing could improve outcomes still further, while controlling cost. This analysis provides an evidence base supporting the movement of the 8-week regimen to the preferred regimen list for appropriate patients in the HCV treatment guidelines. Wider use of the similarly effective, significantly less expensive 8-week regimen could result in the ability to treat more individuals and improve population health.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Financial support. This study was supported by the National Institute on Drug Abuse (grant numbers P30DA040500, R01DA031059). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies or the US government.
Potential conflicts of interest. Dr. Kim reports funding from Gilead Sciences. Dr. Naggie reports funding or support from Abbvie, Bristol Meyers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Merck, Tacere, Vertex Pharmaceuticals, Eviral Hep, IDSA, IAS-USA, Platform Q Health Inc., and Practice Point Communications. Drs. Morgan and Linas have no conflicts to report.
References
- 1. Micromedex Solutions. Drug Topics Red Book Online Available at: http://www.micromedexsolutions.com. Accessed 13 September 2017.
- 2. Edlin BR, Eckhardt BJ, Shu MA et al. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015; 62:1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med 2015; 162:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barua S, Greenwald R, Grebely J et al. Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015; 163:215–23. [DOI] [PubMed] [Google Scholar]
- 5. Messina JP, Humphreys I, Flaxman A et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015; 61:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller CL, Johnston C, Spittal PM et al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology 2002; 36:737–42. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Notes from the field: risk factors for hepatitis C virus infections among young adults – Massachusetts, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1457–8. [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention. Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002–2009. MMWR Morb Mortal Wkly Rep 2011; 60:537–41. [PubMed] [Google Scholar]
- 9. Gilead Sciences. Havoni Package Insert Available at: http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf. Accessed 11 March 2017.
- 10. Kowdley KV, Sundaram V, Jeon CY et al. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology 2017; 65:1094–103. [DOI] [PubMed] [Google Scholar]
- 11. Lai JB, Witt MA, Pauly MP et al. Eight- or 12-week treatment of hepatitis C with ledipasvir/sofosbuvir: real-world experience in a large integrated health system. Drugs 2017; 77:313–8. [DOI] [PubMed] [Google Scholar]
- 12. Wilder JM, Jeffers LJ, Ravendhran N et al. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: a retrospective analysis of phase 3 data. Hepatology 2016; 63:437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maier MM, Ross DB, Chartier M et al. Cascade of care for hepatitis C virus infection within the US veterans health administration. Am J Public Health 2016; 106:353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neumann PJ, Russell LB, Sanders GD, Siegel JE, Ganiats TG.. Cost Effectiveness in Health and Medicine. 2nd ed Oxford: Oxford University Press; 2017. [Google Scholar]
- 15. Linas BP, Barter DM, Leff JA et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One 2014; 9:e97317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Linas BP, Barter DM, Morgan JR et al. The cost-effectiveness of sofosbuvir-based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med 2015; 162:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linas BP, Morgan JR, Pho MT et al. Cost-effectiveness and cost-containment in the era of interferon-free therapies to treat HCV genotype 1. Open Forum Infect Dis 2016; doi:10.1093/ofid/ofw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarrazin C, Dvory-Sobol H, Svarovskaia ES et al. Prevalence of resistance-associated substitutions in HCV NS5A, NS5B, or NS3 and outcomes of treatment with ledipasvir and sofosbuvir. Gastroenterology 2016; 151:501–12, e1. [DOI] [PubMed] [Google Scholar]
- 19. Backus LI, Belperio PS, Shahoumian TA et al. Real-world effectiveness of ledipasvir/sofosbuvir in 4,365 treatment-naive, genotype 1 hepatitis C-infected patients. Hepatology 2016; 64:405–14. [DOI] [PubMed] [Google Scholar]
- 20. Heim MH, Bochud PY, George J. Host - hepatitis C viral interactions: the role of genetics. J Hepatol 2016; 65:22–32. [DOI] [PubMed] [Google Scholar]
- 21. Su F, Green PK, Berry K, Ioannou GN. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology 2017; 65:426–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Brien TR, Kottilil S, Feld JJ et al. Race or genetic makeup for hepatitis C virus treatment decisions?Hepatology 2017; 65:2124–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. US Department of Veterans Affairs. Pharmaceutical Prices Available at: https://www.va.gov/oal/business/fss/pharmPrices.asp. Accessed 16 January 2017.
- 24. AASLD/IDSA/IAS–USA. Recommendations for testing, managing, and treating hepatitis C Available at: www.hcvguidelines.org. Accessed 13 November 2017.
- 25. Weisman R. Harvard Pilgrim negotiates discount on pricey hepatitis drug Available at: http://www.bostonglobe.com/business/2015/01/29/harvard-pilgrim-becomes-first-regional-health-insurer-negotiate-discount-hepatitis-drugs/cHGccEiJT8NDTnKqpTejfP/story.html. Accessed 11 March 2015.
- 26. Kwo PY, Poordad F, Asatryan A et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol 2017; 67:263–71. [DOI] [PubMed] [Google Scholar]
- 27. Freeman AJ, Dore GJ, Law MG et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001; 34:809–16. [DOI] [PubMed] [Google Scholar]
- 28. Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008; 48:418–31. [DOI] [PubMed] [Google Scholar]
- 29. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–32. [DOI] [PubMed] [Google Scholar]
- 30. Bruno S, Zuin M, Crosignani A et al. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol 2009; 104:1147–58. [DOI] [PubMed] [Google Scholar]
- 31. van der Meer AJ, Veldt BJ, Feld JJ et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012; 308:2584–93. [DOI] [PubMed] [Google Scholar]
- 32. Gane EJ, Shiffman ML, Etzkorn K et al. Sofosbuvir/velpatasvir in combination with ribavirin for 24 weeks is effective retreatment for patients who failed prior NS5A containing DAA regimens: results of the GS-US-342–1553 study. J Hepatol 2016; 64:S147–8. [Google Scholar]
- 33. Agency for Healthcare Research and Quality. Total health services-mean and median expenses per person with expense and distribution of expenses by source of payment: medical expenditure panel survey household component data Generated interactively. Available at: http://meps.ahrq.gov/mepsweb/. Accessed 15 July 2017.
- 34. Truven Health Analytics. MarketScan Commercial Claims and Encounters. 2017. Available at: https://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases. Accessed 15 September 2017. [Google Scholar]
- 35. Davis KL, Mitra D, Medjedovic J et al. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol 2011; 45:e17–24. [DOI] [PubMed] [Google Scholar]
- 36. Sherman KE, Muir A, Aggarwal J et al. Health-related quality of life among genotype 1 treatment experienced chronic hepatitis C patients: post-hoc anlyses of data from the Realize clinical trial. Amsterdam, the Netherlands: European Association for the Study of the Liver; 2013. [Google Scholar]
- 37. Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006; 26:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chong CA, Gulamhussein A, Heathcote EJ et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003; 98:630–8. [DOI] [PubMed] [Google Scholar]
- 39. Grieve R, Roberts J, Wright M et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 2006; 55:1332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stein K, Dalziel K, Walker A et al. Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess 2002; 6:1–122. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.