Abstract
We compared change in HIV reservoir DNA following continued antiretroviral therapy (ART) vs short treatment interruption (TI) in early ART-treated Kenyan infants. While HIV DNA in the reservoir decayed with continued ART, HIV DNA levels were similar to pre-TI HIV DNA reservoir levels in most children after short TI.
Keywords: antiretroviral therapy, HIV DNA, infants, reservoir, treatment interruption
During acute HIV infection, a reservoir of long-lived infected cells is established that persists during antiretroviral treatment (ART) and causes viral rebound upon ART cessation [1, 2]. Experimental nonhuman primate models show that this reservoir is generated within days of infection [3]. Initiating ART early in HIV infection may limit reservoir size, increase time to viral rebound upon treatment cessation, and increase likelihood of post-treatment viral control—but does not ensure remission [2, 4–6]. In case reports, HIV-infected infants with early ART had long periods of remission [7, 8], encouraging efforts to identify interventions that augment early ART to promote post-treatment control in pediatric populations. Evaluating these approaches requires analytical treatment interruption (TI). Thus, it is important to understand whether short TI leads to sustained increases in latently infected cells in the HIV reservoir.
Few studies have measured the impact of TI on HIV reservoir. In adults, TI has been associated with initially increased HIV DNA levels, which return to pre-TI levels after >6 months of ART resumption, suggesting that TI may not cause a lasting increase in HIV-infected cell reservoirs [6, 9]. In contrast, a preliminary report from the Children with HIV Early Antiretroviral Therapy trial (CHER) showed sustained increased HIV DNA levels in 17 infants 2–3 years following a median 11-month TI [10], and a smaller study showed similar results [11]. To understand the impact of shorter TI in infants, we quantified blood HIV DNA reservoir levels of Kenyan infants who were randomized to continued ART vs TI in the Optimizing Pediatric HIV-1 Treatment study (OPH).
METHODS
Study Population
OPH (NCT00428116) was a randomized controlled trial in which, following 24 months of continuous ART (median age at initiation, 5 months), children were randomized to continued ART or TI [12]. Blood was collected at 3-month intervals for CD4 measurements in real time and stored for HIV viral load (performed retrospectively). ART restart criteria were CD4% <20–25%, or >one-third decrease from peak CD4, more advanced World Health Organization stage, or weight-for-age decrease, as previously described [12]. After ART restart, there was no further interruption. Children were excluded from this substudy if HIV RNA was ≥1000 copies/mL during the 6 months prior to the HIV DNA measurements at 24 months post–ART initiation (time of randomization) and 42 months post–ART initiation (18 months following randomization; n = 27), or if samples were not available (n = 1). Seven of these children had virally suppressed samples available for HIV DNA measurement at 75 months post–ART initiation (51 months following randomization).
Laboratory Methods
Plasma HIV RNA was quantified using the Gen-Probe HIV-1 RNA assay (Gen Probe, San Diego, CA) with a limit of detection (LOD) of 2.18 log10 copies/mL. DNA was extracted from peripheral blood mononuclear cells (PBMCs) using QIAamp DNA (Qiagen, Valencia, CA). Cellular DNA was quantified using RPP30 ddPCR assay (Bio-Rad, Hercules, CA). HIV DNA was quantified in duplicate by in-house cross-subtype pol polymerase chain reaction (PCR) [13] modified for ddPCR, with LOD of 5 copies/106 cells determined using previously validated DNA from ACH2 cells that have a single HIV provirus per cell and HIV-negative genomic DNA controls (Supplementary Figure 1). If results were below the LOD or were >2-fold discordant between duplicates, additional replicates were performed until >2.5e5 cells were tested. HIV DNA was normalized to RPP30 and HIV DNA copies/106 PBMCs reported.
Statistical Analysis
Analysis was performed using R (version 3.4). HIV DNA fold change was compared between continued and TI arms using the Wilcoxon rank-sum test. Correlation between postrandomization peak HIV RNA and HIV DNA fold change was determined using Spearman’s rank-order correlation.
RESULTS
Cohort Characteristics During Initial 24 Months of ART
During the OPH study, children were treated with 24 months of ART (range, 23–28 months), after which 42 were randomized to TI (n = 21) or continued ART (n = 21). Fourteen children from OPH met criteria for this laboratory substudy (see “Methods”), 7 in each arm. At ART initiation, the median age of the 14 infants was 4.8 months (interquartile range [IQR], 4.4–7 months), median CD4% was 22.5% (IQR, 15%–25%), and the median viral load was 6.5 log10 copies/mL (IQR, 5.7–6.9 log10 copies/mL) (Supplementary Table 1). Initial ART was NNRTI-based for 10 infants and PI-based for 4 infants. Twenty-four months following ART initiation (the time of randomization), 2 children were still on NNRTI-based ART, and 12 were taking PI-based ART; the median CD4% had risen to 35% (IQR, 32%–40%), while the median viral load dropped below detection (Supplementary Table 1).
Treatment Interruption
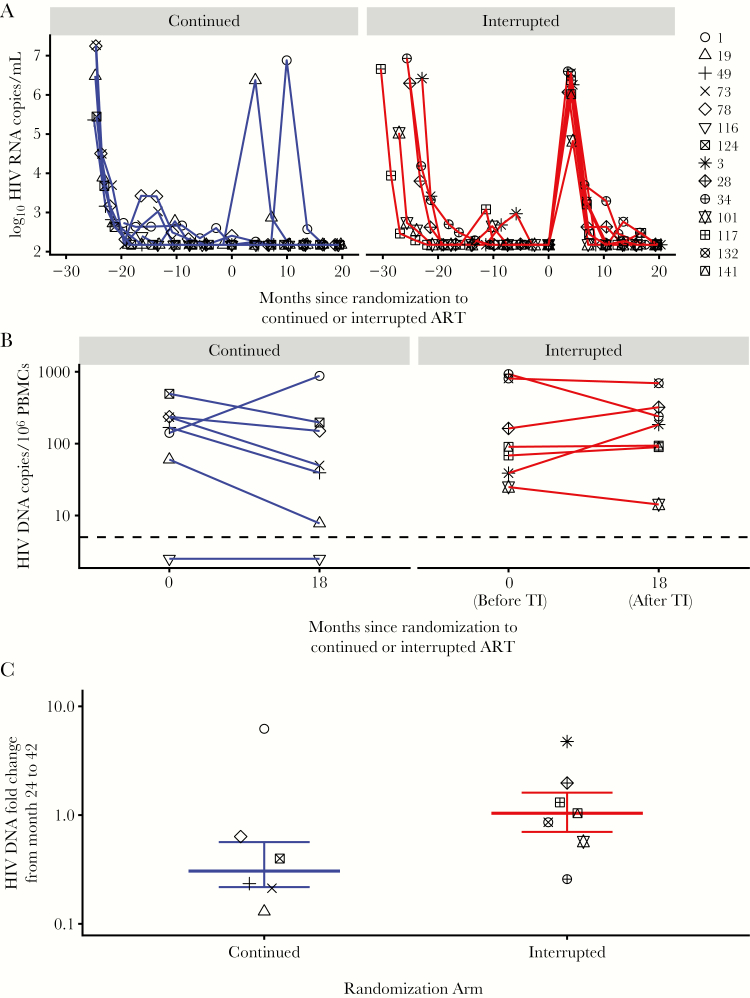
At the first postrandomization visit (~3 months after TI), all 7 children in the TI arm exhibited viral rebound (Figure 1A) and met CD4-based criteria to restart ART, resulting in a median of 106 days (IQR, 104–119 days) off ART. Of the 7 children in the continued arm, 5 had continuous viral suppression while 2 had high HIV RNA levels at a single time point: infant #19 at 3 months and #1 at 9 months postrandomization (Figure 1A). Three months after randomization, the median viral load was 6.3 log10 copies/mL and was below detection (<2.18 log10 copies/mL), and the median CD4% were 21% and 42% in the TI and continued arms, respectively. Eighteen months after randomization, all 14 children were on PI-based ART with viral loads below detection. The median CD4% were 34% (IQR, 30.5%–35.5%) in the TI group and 40% (IQR, 32.5%–42%) in the continued group.
Figure 1.
(A) Plasma HIV RNA copies/mL over time in infants randomized to continued antiretroviral therapy (ART; left) or interrupted ART (right). Limit of detection was 2.18 log10 copies HIV RNA/mL. (B) Comparison of total HIV DNA copies/106 peripheral blood mononuclear cells (PBMCs) before (24 month after initial ART) and after randomization to treatment interruption (18 months later) in children in the continued arm (left) or interrupted arm (right). Limit of detection was 5 copies of HIV DNA/106 PBMCs. (C) HIV DNA fold change following randomization to treatment interruption (comparing HIV DNA at randomization and 18 months later) in infants randomized to continued (left) or interrupted ART (right).
HIV DNA Reservoir Dynamics
After 24 months of initial ART, children randomized to continued ART had a median of 168 HIV DNA copies/106 PBMCs (IQR, 101–235 HIV DNA copies/106 PBMCs) in the reservoir, and children in the TI arm had a median of 90 HIV DNA copies/106 PBMCs (IQR, 54–485 DNA copies/106 PBMCs; P = .9). Eighteen months later, after children in the TI arm had resumed ART for >15 months, HIV DNA levels were median 50 HIV DNA copies/106 PBMCs (IQR, 24–174 HIV DNA copies/106 PBMCs) and 185 HIV DNA copies/106 PBMCs (IQR, 92–280 HIV DNA copies/106 PBMCs) in the continued and TI arms, respectively (P = .53). During the 18 months after randomization, 1 of 7 children in the continued arm had increased HIV DNA, 5 of 7 had decreased HIV DNA (range, 0.13–0.63), and 1 of 7 children had HIV DNA levels below detection at both time points (Figure 1B, Supplementary Table 1). In the TI arm, 3 of 7 children had increases in HIV DNA (range, 1.3–4.7), while 4 of 7 had decreased or unchanged HIV DNA levels (range, 0.26–1.04). The median HIV DNA declines during the 18 months after randomization were –5.69 HIV DNA copies/1e6 PBMCs/month in the continued arm and 0.18 in the interrupted arm. The median HIV DNA fold changes were 0.32 (IQR, 0.22–0.58) in children randomized to continued ART and 1.04 (IQR, 0.72–1.64) in children randomized to TI (P = .14) (Figure 1C). Similar results were observed when we excluded the 2 children in the continued arm with postrandomization viremia: median HIV DNA fold changes were 0.32 (IQR, 0.23–0.46) vs 1.04 (IQR, 0.71–1.64) in the continued and TI arms, respectively (P = .04). In children with viremia during the 18 months after randomization, change in HIV DNA did not correlate with peak viremia (Spearman’s rho, 0.12; P = .77).
In a limited number of children with longer follow-up (2 in the TI arm and 5 in the continued arm), a virally suppressed sample was available from 51 months after randomization (75 months after initial ART). The median HIV DNA declines from 0 to 51 months following randomization were –1.96 HIV DNA copies/1e6 PBMCs/month in the continued arm and –0.37 in the interrupted arm. The median HIV DNA fold changes were 0.44 (IQR, 0.44–0.75) and 0.92 (IQR, 0.84–1.00) in children randomized to continued ART and TI, respectively (Supplementary Figure 2).
DISCUSSION
We measured HIV DNA in the reservoir in children that started ART during the first year of life and were randomized 2 years later to continue or interrupt ART. We compared HIV DNA levels at randomization and again 18 months later, after all children had resumed ART for >15 months and achieved viral suppression. HIV DNA decayed in children with continued viral suppression, while the median HIV DNA fold change after TI was 1.04, suggesting that TI lessens the decay that occurs on continued ART. Indeed, for 2 children in the TI arm with ~4 years of follow-up, HIV DNA reservoir size remained relatively unchanged over time (Supplementary Figure 2). The fact that most children in the TI arm had similar HIV DNA levels before and after TI suggests that the reservoir reseeding that occurred during TI was followed by decay after ART resumption. Mechanisms of reservoir seeding include new infections of cells that become quiescent and clonal proliferation of cells containing provirus [14, 15]. Our study could not distinguish between these mechanisms as we did not characterize the reservoir composition or replication competence due to limited sample volume and cell viability. In addition, our cohort did not include infants treated with very early ART (within hours of birth), which may have different viral reservoir decay dynamics.
Our study did not quantify HIV DNA in tissue reservoirs and was limited by small sample size with few evaluated time points. However, our data add substantially to the 2 previous studies on changes in HIV DNA following TI in children [10, 11], and they support results from adult cohorts [6, 9]. Analysis of 15 adults in the SPARTAC trial with transient TI showed that HIV DNA returned to pre-TI levels after ≥6 months of ART [6]. Another TI study of 10 adults with very low HIV DNA levels in the reservoir prior to a median TI of 4 weeks observed similar results, with HIV DNA levels returning to pre-TI values after treatment resumption [9]. These studies suggest that increases in HIV DNA can be minimized or reversed by rapid treatment resumption; however, larger studies with longer follow-up are needed.
Our findings add a new perspective that complements prior TI studies in perinatally infected infants, which focused on longer TI. The largest pediatric TI reservoir study to date included 17 infants treated earlier (<12 weeks at ART initiation) and with longer TI (median, 11 months) than in our study, and observed increased HIV DNA in the reservoir 26 months after treatment resumption [10]. Another study included only 3 infants with TI and showed 52.4-, 1.8-, and 12.2-fold increases in HIV DNA following TI of 0.75, 6.8, and 71 months, respectively [11]. Here we show that a short interruption of ~3 months does not appear to have sustained impact on HIV DNA levels in the reservoir in children; however, it may lessen the rate of decay provided by early continued ART. These data suggest that reseeding of the reservoir in pediatric HIV may be minimized with frequent viral load monitoring during short analytical TI.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the OPH study participants and their caregivers, without whom this research would not be possible; and the OPH administrative, clinical, and data teams for their dedication and support.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (grant number R01 AI076105), the National Institute of Child Health and Development (grant numbers R01 HD023412, K24 HD054314 and R01 HD094718), and the University of Washington Center for AIDS Research grant from the National Institutes of Health([AI027757).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chun TW, Engel D, Berrey MM et al. . Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A 1998; 95:8869–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Persaud D, Palumbo PE, Ziemniak C et al. . Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS 2012; 26:1483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whitney JB, Hill AL, Sanisetty S et al. . Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 2014; 512:74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li JZ, Etemad B, Ahmed H et al. . The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sáez-Cirión A, Bacchus C, Hocqueloux L et al. ; ANRS VISCONTI Study Group. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williams JP, Hurst J, Stöhr W et al. ; SPARTACTrial Investigators. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife 2014; 3:e03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frange P, Faye A, Avettand-Fenoël V et al. ; ANRS EPF-CO10 Pediatric Cohort and the ANRS EP47 VISCONTI study group. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 3:e49–54. [DOI] [PubMed] [Google Scholar]
- 8. Persaud D, Gay H, Ziemniak C et al. . Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calin R, Hamimi C, Lambert-Niclot S et al. ; ULTRASTOP Study Group. Treatment interruption in chronically HIV-infected patients with an ultralow HIV reservoir. AIDS 2016; 30:761–9. [DOI] [PubMed] [Google Scholar]
- 10. Payne H, Watters S, Hsaio M et al. . Early ART and sustained virologic suppression limits HIV proviral DNA reservoir: CHER evidence. In special issue: absracts from the 2015 Conference on Retroviruses and Opportunistic Infections. Top Antiviral Med 2015; 23:16. [Google Scholar]
- 11. Martínez-Bonet M, Puertas MC, Fortuny C et al. . Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis 2015; 61:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wamalwa D, Benki-Nugent S, Langat A et al. . Treatment interruption after 2-year antiretroviral treatment initiated during acute/early HIV in infancy. AIDS 2016; 30:2303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benki S, McClelland RS, Emery S et al. . Quantification of genital human immunodeficiency virus type 1 (HIV-1) DNA in specimens from women with low plasma HIV-1 RNA levels typical of HIV-1 nontransmitters. J Clin Microbiol 2006; 44:4357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maldarelli F, Wu X, Su L et al. . HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 2014; 345:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner TA, McLaughlin S, Garg K et al. . HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014; 345:570–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.