Abstract
Sex is considered an understudied variable in health research. Schizophrenia is a brain disorder with known sex differences in epidemiology and clinical presentation. We systematically reviewed the literature for sex-based differences of diffusion properties of white matter tracts in schizophrenia. We then conducted a meta-analysis examining sex-based differences in the genu and splenium of the corpus callosum in schizophrenia. Medline and Embase were searched to identify relevant papers. Studies fulfilling the following criteria were included: (1) included individuals with a diagnosis of schizophrenia, (2) included a control group of healthy individuals, (3) included both sexes in the patient and the control groups, (4) used diffusion tensor imaging, and (5) involved analyzing metrics of white matter microstructural integrity. Fractional anisotropy (FA) was used as the measure of interest in the meta-analysis. Of 730 studies reviewed, 75 met the inclusion criteria. Most showed no effect of sex, however, those that did found either that females have lower FA than males, or that the effect of disease in females is larger than that in males. The findings of the meta-analysis in the corpus callosum supported this result. There is a recognized need for studies on schizophrenia with a sufficient sample of female patients. Lack of power undermines the ability to detect sex-based differences. Understanding the sex-specific impact of illness on neural circuits may help inform development of new treatments, and improvement of existing interventions.
Keywords: white matter tracts, fractional anisotropy, genu, splenium, corpus callosum, gender, sex
Introduction
As sex is known to affect human brain development,1,2 disorders that are likely to have a genetic or neurodevelopmental origin may affect the male brain differently than the female brain. Sex may be an especially relevant factor for schizophrenia, where incidence,3,4 age-at-onset,5 clinical manifestation,6 disease prognosis,6,7 and treatment response7 all differ between male and female patients.
Schizophrenia has been characterized as a disorder of disconnectivity.8 Considerable evidence for changes in the white matter tracts connecting brain regions has been demonstrated in people with schizophrenia compared to healthy controls.9–11 An assessment of sex-related differences in white matter among schizophrenia patients themselves may provide insight into sex-specific etiology, clinical differences, or treatment targets that may be useful for addressing specific patient needs.
Diffusion tensor imaging (DTI) allows us to indirectly measure white matter microstructure by recording diffusion of water molecules. Several review articles have summarized findings from DTI studies on schizophrenia patients.9–14 In addition, 2 meta-analyses have been conducted on articles where DTI properties of the corpus callosum in schizophrenia were examined.15,16 However, to our knowledge, no systematic review or meta-analysis has been conducted to date on the effect of sex on diffusion measures of white matter in schizophrenia patients.
Sex differences in brain morphology of schizophrenia patients are often seen in regions that are normally different between the sexes on average,17 such as the corpus callosum. Sex differences in the macrostructure of the corpus callosum have been observed in healthy subjects18 and schizophrenia patients.19 Sex differences have also been observed in the fractional anisotropy (FA) of the corpus callosum in healthy subjects.19–23 Two meta-analyses have shown structural differences in the corpus callosum between schizophrenia patients and controls.15,16 In addition, several studies have shown functional abnormalities in the corpus callosum linked to disruption of interhemispheric communication in schizophrenia.24,25 Findings of abnormal interhemispheric transfer in schizophrenia was also shown using event-related activity.26 An early review on studies of the corpus callosum found that a thicker corpus callosum in schizophrenia patients was associated with both more negative symptoms and earlier age of onset.27 As both of these are more common in males, it may be that corpus callosum abnormalities in schizophrenia are sex-dependent. Indeed, an exploratory meta-regression in a study showed that sex may explain differences in diffusion properties of the corpus callosum in schizophrenia.15 Sex differences in other white matter tracts in healthy individuals have been shown.28,29 However, given the on-average sex differences of the corpus callosum in healthy individuals, findings supporting corpus callosum abnormalities related to clinical phenomena more commonly associated with males, and the large number of studies published on this structure, we also chose to conduct a meta-analysis using the corpus callosum.
In this systematic review, findings of sex differences in DTI studies on schizophrenia are examined. We included both cross-sectional and prospective DTI studies of patients with schizophrenia, which also had a comparison group of healthy subjects. The outcome measure of interest was any white matter microstructural property obtained from DTI scans, with a particular focus on the most commonly reported measures of FA and mean diffusivity (MD). This article has 3 components: a systematic review of sex-related findings in studies on all white matter tracts; a more detailed review on only those articles that study the genu and splenium of the corpus callosum; and a meta-analysis on FA of the genu and splenium in males and females.
Methods
Search Strategy
Medline (1946 to April 8, 2016) and Embase (1974 to April 8, 2016) databases were searched for all studies that used DTI to compare microstructural properties between patients with schizophrenia and healthy controls. The search strategy was developed in consultation with a medical librarian at the University of Toronto and is included in the supplementary material document (supplementary figure S1). The sensitivity of the search strategy was tested and revised using 24 relevant articles that were retrieved using a Google Scholar search and identified in a literature review on DTI findings in schizophrenia.30 Additional studies were identified by doing backward and forward citation searching on all studies that met inclusion criteria. Authors were contacted via email and ResearchGate to request full-text articles that were inaccessible through other means. The literature search strategy was updated on August 11, 2016 to include new studies published after the initial search. There is no review protocol for this systematic review. This systematic review was written in accordance with PRISMA guidelines.31
Inclusion and Exclusion Criteria
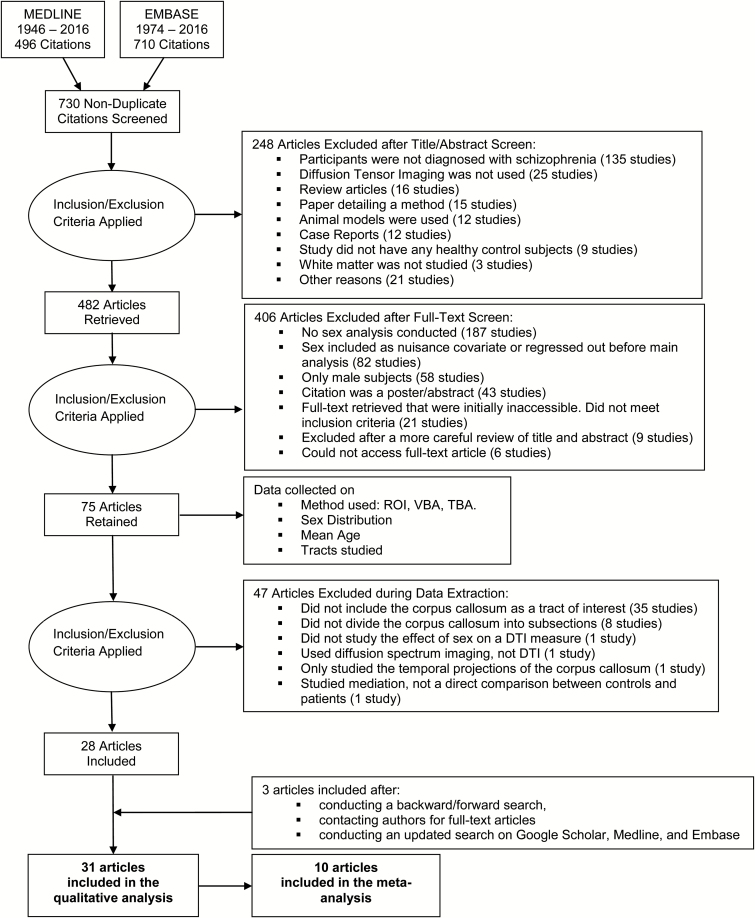
Study titles and abstracts were screened using the following inclusion criteria: (1) the sample included people with a diagnosis of schizophrenia, (2) the study had a control group of healthy subjects, (3) both males and females were included in the patient group and the control group, (4) the study used DTI, and (5) image processing involved computing and analyzing DTI-acquired metrics of white matter microstructural integrity. Only articles published in or translated to English were included. Posters and abstract for conference proceedings were excluded as they are not peer-reviewed and do not contain most of the information required for data synthesis. However, non-peer-reviewed articles and theses were retained to avoid publication bias. The articles that passed the initial screening were filtered again to only include articles that conducted and reported results of sex-based analyses. This screening was conducted by scanning the full text for the terms: sex, gender, men, women, male, female, boy, and girl, and only keeping studies that used these terms in the context of a sex analysis. To maintain the sensitivity of the search, the term “sex analysis” is used broadly. Any mention of the terms above qualified the study to be included, with the exception of those studies that only mentioned these terms to report the sex distribution in their study or to indicate that their samples were sex-matched. The retrieved studies were filtered a third time to isolate those that studied the genu and splenium of the corpus callosum. The same technique as the second filter was used for the third filter with the following terms: corpus, callosum, genu, and splenium. Data were screened by author S.S. The data screening process has been represented as a flowchart in figure 1.
Fig. 1.
PRISMA flow diagram.
Data Extraction
To understand how sex affects white matter findings in schizophrenia across all tracts, data on sex-related findings, average age, and DTI image processing method were extracted by author LS for studies on all white matter tracts. Given the additional analyses for the corpus callosum, measures including quality assessment and details of DTI processing were collected only for those articles that studied the genu and splenium. A list of all measures collected in the data extraction form is included in the supplementary material document. Data were extracted by L.S., and any ambiguities were flagged and later discussed between S.S. and L.S. until a consensus was reached.
Additionally, statistical summary measures were collected for the meta-analysis. FA is a commonly used DTI measure that is sensitive to changes in white matter microstructural integrity. FA means and SDs were extracted from studies on the genu and splenium, separately for males and females. Authors were contacted if sex-separated FA values were not reported in the article.
Quality Assessment
A modified version of the Newcastle-Ottawa Scale (NOS)32 was used to assess the quality of all studies that included the genu and splenium as regions of interest. The questions on representativeness of cases and selection of controls were removed as all cases were consecutive and all healthy subjects were community controls. The scale was modified to check comparability for age and sex as both are important factors. An extra point was given to studies that matched cases and controls on one additional factor. As sex-related analyses were not the primary objective of most studies in this review, 3 questions were added to assess the emphasis on sex analyses in the studies. The quality assessment criteria are detailed in table 1.
Table 1.
Modified Newcastle-Ottawa Scale for Quality Assessment
| Legend | Description |
|---|---|
| Adequacy of the case definition | |
| + | Independent validation (1+ person or process) to ensure diagnostic accuracy |
| − | No description of independent validation process |
| Adequacy of control definition | |
| + | Explicitly states that controls have no history of mental illness |
| − | No mention of procedure to verify history of mental illness |
| Comparability of cases and controls on the basis of age | |
| + | Cases and controls are matched for age and/or analyses are adjusted for age |
| − | No description of comparability based on age |
| Comparability of cases and controls on the basis of sex | |
| + | Cases and controls are matched for sex and/or analyses are adjusted for sex |
| − | No description of comparability based on sex |
| Comparability of cases and controls on the basis of another variable (not age/sex) | |
| + | Cases and controls are matched for stated variable and/or analyses are adjusted for stated variable |
| − | No description of comparability based on stated variable |
| Medication summary | |
| + | Clear description of medication status for case group |
| − | No description of medication status for case group |
| Correction for multiple comparisons | |
| + | Clear description of process to correct for multiple comparisons in analyses |
| − | No description of process to correct for multiple comparisons in analyses |
| Sex and/or gender in title of journal article | |
| + | Sex and/or gender is mentioned in the title of the journal article |
| − | No mention of sex and/or gender in the title of the journal article |
| Sex analysis in abstract of journal article | |
| + | Sex analysis is described in the journal abstract |
| − | No description of sex analysis in abstract |
| Significance of sex finding mentioned in discussion section | |
| + | Significance of sex finding is discussed in above mentioned section |
| − | No discussion of sex finding in above mentioned section |
Meta-analysis
Statistical summary measures were categorized into 4 groups: male-genu, female-genu, male-splenium, and female-splenium. Heterogeneity of the data was tested for all 4 groups. As the 2 splenium groups had moderately high I2 values, a random-effects pair-wise meta-analysis was conducted using the DerSimonian-Laird method.33 Hedges’ g was used as the effect size measure in the meta-analysis. Forest plots were generated to examine the effect of sex on the difference in FA between patients and controls. To assess the contribution of each study to the overall effect, a leave-one-out meta-analysis was conducted on all 4 groups. In addition, a test for skewness was conducted34 to determine if any study included in the meta-analysis contained data that had a non-normal distribution. Funnel plots were also generated to assess publication bias and systematic heterogeneity. As standard error is known to be superior to other measures of study size,35 standard error was plotted against Hedges’ g in the funnel plots. Contours were drawn at the 90% and 95% CI. Any group that had more than 1 study outside the 90% CI was tested for systematic heterogeneity by conducting exploratory meta-regressions.
Meta-regression
Age is known to affect white matter FA in both patients and controls.36,37 Thus, age was regressed against effect size to assess potential impact on difference in FA between patients and controls in sex-stratified samples. Age was calculated by averaging the reported average age for patients and controls, weighted by the number of people in each group. Exploratory meta-regressions were conducted in which the following variables were regressed against effect size: quality assessment score, year of publication, number of diffusion-weighted gradient directions, slice thickness, image processing method, scanner company, magnetic field strength, and country.
Subgroup Analyses
The meta-regression did not explain the heterogeneity in the meta-analysis so a subgroup analysis was conducted. Studies were categorized into voxel-based, region-of-interest-based, or tractography-based analyses.
Results
Systematic Review—Studies on all White Matter Tracts
Study Characteristics.
Out of 730 unduplicated studies, 75 studies were retained. Data on sex findings, sex distribution, mean age, tracts studied, and the method used for DTI analysis were extracted (table 2). The sample size in the included studies ranged from 16 to 252 participants (median across studies = 65), and the mean age of participants ranged from 14 to 65 years (median across studies = 32.8). Twenty-five studies used a region-of-interest-based method, 24 studies used a voxel-based approach, and 24 studies used a tract-based method for DTI analysis. In 45 out of 75 studies, at least 60% of patients were male. The corpus callosum was the most frequently analyzed tract. It was studied in 52% of the included studies (table 3). Seventy-two percent of the studies on the corpus analyzed the genu and splenium separately.
Table 2.
Demographic Information for all Studies in the Systematic Review and Meta-analysis
| Author | Year | N—SCZ | % Males | N—HC | % Males | Mean Age (SD)—SCZ | Mean Age (SD)—HC | Dx Criteria | Image Processing | Main Finding Related to Sex |
|---|---|---|---|---|---|---|---|---|---|---|
| Savadjiev P, Whitford T, Hough M, et al. | 2014 | 39 | 56% | 33 | 48% | M: 16.5 (0.97); F: 16.0 (1.0) | M: 15.7 (1.5) F: 15.2 (1.1) | K-SADS-PL | TBA | Diagnosis by sex interaction in left anterior CC for shape normalized dispersion |
| Domen P, Michielse S, Gronenschild E, et al. | 2013 | 85 | 68% | 80 | 36% | 28.3 (7) | 30.9 (10.8) | DSM-IV (CASH) | VBA | No significant group by sex interactions for FA for whole sample. Stratified analyses (equal groups) revealed patient-control differences in females and sibling-patient differences in males |
| Caprihan A, Abbott C, Yamamoto J, et al. | 2011 | 65 | 68% | 125 | 66% | 33.65 (11.38) | 31.21 (9.90) | DSM-IV | SBM | Sex differences only significant for superior longitudinal fasciculus (group by sex interaction P = .056) and not the CC. |
| Rametti G, Junqué C, Falcón C, et al | 2009 | 25 | 48% | 24 | 46% | 31.8 (7) | 32.2 (6.8) | DSM-IV | VBA | Females with SCZ had lower FA in the GCC than males; no significant differences in control group or in ADC values |
| Schneiderman J, Buchsbaum M Mehmet Haznedar M, et al. | 2009 | 58 | 69% | 48 | 58% | Y: 17.3 (2.1); O: 43.1 (10.8) | Y: 17.1 (2.1) O: 42.2 (11.5) | DSM-IV | ROI | In the right hemisphere, males with SCZ had lower FA than controls in GCC and SCC but higher FA in BCC; females with SCZ show lower FA in BCC and posterior portion of splenium and higher FA in genu and anterior portion of splenium. In the left hemisphere, males with SCZ had lower FA than controls except in anterior genu; females showed similar trend. |
| Rotarska-Jagiela A, Schönmeyer R, Oertel V, et al. | 2008 | 24 | 50% | 24 | 50% | 39 (9.35) | 39.21 (8.95) | DSM-IV | ROI | Women had decreased FA values in inferior genu but no significant gender by diagnosis interaction for CC |
| Price G, Cercignani M, Parker G, et al. | 2006 | 18 | 44% | 21 | 29% | 23.6 (6.3) | 29.4 (7.1) | DIP/DSM-IV | TBA | FA was lower in females (both in patients and controls) in the CC (esp. genu)—but no gender by group interaction |
| Price G, Bagary M, Cercignani M, et al. | 2005 | 20 | 70% | 29 | 38% | 24.95 | 28.06 | DSM-IV | ROI | For women (irrespective of group) FA was lower; trend for MD to be higher in men |
| Kumra S, Ashtari M, Mcmeniman M, et al. | 2004 | 12 | 75% | 9 | 67% | 16.5 (1.8) | 15.5 (1.7) | K-SADS-PL/ DSM-IV | ROI | Exploratory sex analyses conducted but results not reported |
| Agartz I, Jesper C, Andersson L, et al. | 2001 | 20 | 55% | 24 | 63% | 38.4 (7.9) | 42.2 (6.7) | DSM-IV | VBA | Sex analyses built in design but regressed out |
| Ellison-Wright I, Nathan P, Bullmore E, et al. | 2014 | 21 | 81% | 21 | 67% | 34.2 (10.9) | 31.5 (9.1) | DSM-IV | VBA | Median FA did not differ significantly by gender in the genu of the CC, yet the average FA was 2% greater in males than females. Authors suggest the gender imbalance of their sample may have reduced power to detect a change. |
| Wright S, Hong L. Winkler A, et al. | 2015 | 46 | 65% | 31 | 16% | 37.5 (13.4) | 38.8 (14.3) | DSM-IV | VBA | Sex as covariate in mediation model—No sex effect |
| Arnedo J, Mamah D, Baranger D, et al. | 2015 | 47 | 66% | 36 | 47% | 37.3 (8.5) | 36.9 (9.1) | DSM-IV | VBA | No effect of sex based on one factor ANOVA using univariate GLM when exploring the relationship between voxels and proportion of gender. |
| Zhang F, Qiu L, Yuan L, et al. | 2014 | 28 | 54% | 26 | 50% | 25.93 (8.06) | 26.43 (7.46) | DSM-IV | VBA | No change in FA value regarding sex of patients |
| Collinson S, Chyi Gan S, San Woon P, et al. | 2014 | 113 | 74% | 73 | 64% | Y: 29.31 (7.03); O: 37.0 (9.01) | 32.24 (10.17) | DSM-IV | ROI | No gender effects in either 2 patient group (FEP vs chronic) or controls re: CC midsagittal area, FA or volume comparisons. |
| Kochunov P, Glahn D, Rowland L, et al. | 2013 | 58 | 88% | 60 | 80% | 37.7 (12.0) | 37.5 (11.9) | DSM-IV | TBA | No effect of sex with the beta coefficient for sex being nonsignificant for all tracts |
| Foong J, Maier M, Clark C, et al. | 2000 | 20 | 75% | 25 | 64% | 37.65 | 33.84 | DSM-IV | ROI | No significant sex differences for either the SCZ or HC group in the CC |
| Carpenter D, Tang C, Friedman J, et al. | 2008 | 76 | 68% | 77 | 68% | 36 (15) | 37 (17) | DSM-IV/CASH | TBA | Sex was not a significant factor for any of the tracts |
| Cheung A Cheung V Cheung C, et al. | 2008 | 26 | 50% | 25 | 44% | 28.5 (9.4) | 28.2 (9.2) | DSM-IV-TR | VBA | No significant intergroup difference in sex |
| Mitelman S, Nikiforova Y, Canfield E, et al. | 2009 | 49 | 86% | 16 | 56% | 42.69 (12.29) | 41.63 (12.23) | NR | ROI | Sex was added as independent factor in an ANCOVA—no separate interaction with sex was reported due to lack of significance |
| Voineskos A, Lobaugh N, Bouix S, et al. | 2010 | 50 | 58% | 50 | 58% | Y: 40 (12); O: 65 (6) | Y: 38 (10); O: 66 (7) | DSM-IV | TBA | No significant main effect of gender, gender by group interaction, or group by gender by tract interaction |
| Gasparotti R, Valsecchi P, Carletti F, et al. | 2009 | 21 | 52% | 21 | 62% | 28.52 (8.79) | 27.42 (7.31) | DSM-IV-TR | ROI | No significant contributions of gender in ANCOVA— trend for males to have larger difference in FA in splenium between patients and controls |
| Knöchel C, Oertel-Knöchel V, Schönmeyer R, et al. | 2012 | 16 | 56% | NR | NR | 37.57 (7.84) | 39.31 (10.98) | DSM-IV-TR | ROI | No main effect of gender on anatomical and DTI scores |
| Kitiş O, Eker M, Zengin B, et al. | 2011 | 25 | 56% | 17 | 53% | 38.1 (12.0) | 33.4 (10.4) | DSM-IV | ROI | Sex was thought not to have any relation with FA values—was not considered a confounding factor |
| Boos H, Mandl R, van Haren N, et al. | 2013 | 126 | 80% | 109 | 50% | 26.64 (5.58) | 27.30 (8.15) | DSM-IV | TBA | Males had higher FA compared to females for the right ILF only; no significant differences between groups in mean FA for all WM tracts for males only (SCZ) and females only analyses (siblings/HC) |
| Skudlarski P, Schretlen D, Thaker G, et al. | 2013 | 109 | 60% | 104 | 41% | 33.8 (1.0) | 38.9 (1.3) | DSM-IV | VBA | Whole brain mean FA— men had higher values; no sex by FA by clinical diagnosis; sex showed interaction in 5 regions in comparison of bipolar probands and HC |
| Leroux E, Delcroix N, & Dollfus S | 2015 | 17 | 76% | 17 | 76% | 34.8 (8.5) | 36.5 (9.5) | DSM-IV | TBA | Differences in FA in men controls vs men with schizophrenia (decreased FA—associated with increase in RD) |
| Schwehm A, Robinson D, Gallego J, et al. | 2016 | 113 | 68% | 111 | 62% | M (Y): 21.1 (3.3); F (Y): 19.9 (4.0); M (O): 45 (8.7); F (O): 46.2 (9.7) | M (Y): 20.1 (4.1); F (Y): 20.7 (2.5); M (O):43.6 (8.8); F (O): 45.7 (9.1) | DSM-IV | TBA | Males had higher FA compared to females; females had a higher RD than males; group × sex interaction for FA/MD/ RD where female patients had lower FA across all WM tracts compared with healthy females; Females had lower FA in SLF compared with male patients in younger group |
| Chyi GS | 2011 | 120 | 71% | 75 | 65% | M: 32.2 (8.29); F: 34.53 (10.17) | M: 30.88 (8.30); F: 34.28 (12.64) | DSM-IV | ROI | Main effect for sex was not significant in any CC region nor was the interaction between sex and diagnosis significant |
| Voineskos A, Foussias G, Lerch J, et al. | 2013 | 77 | 68% | 79 | 61% | Deficit: 32.1 (8.29); Non- deficit: 43 (14) | 43 (14) | DSM-IV | TBA | No sex analyses conducted. |
| Liu X, Lai Y, Wang X, et al. | 2013 | 17 | 41% | 17 | 35% | 40.7 (11.1) | 34.12 (8.13) | DSM-IV | VBA | Significant effect of sex in gender separated analysis but when combining 2 genders no significant correlation between FA and GM (trend between FA and CT of lateral orbitofrontal cortex) |
| Goghari V, Billiet T, Sunaert S, et al. | 2014 | 25 | 52% | 27 | 48% | 41.3 (10.8) | 40.7 (11.1) | DSM-IV | TBA | Gender as covariate in analysis—no sex findings |
| Miyata J, Sasamoto A, Koelkebeck K, et al. | 2012 | 26 | 62% | 32 | 50% | 34.5 (8.9) | 39.0 (11) | DSM-IV | VBA | Gender as covariate in analysis; in exploratory analysis no main effect of gender or gender by diagnosis interaction |
| Jane Tseng C, Chien Y, Liu C, et al. | 2015 | 32 | 53% | 32 | 53% | 34.2 (6.3) | 32 (6.5) | DSM-IV | TBA | Gender matched and controlled for gender in linear regression—no specific sex findings |
| Fujiwara H, Namiki C, Hirao K, et al. | 2007 | 42 | 50% | 24 | 50% | 36.6 (6.0) | 35.2 (5.9) | DSM-IV | ROI | Lateralized findings of anterior CB abnormality not gender specific in schizophrenia |
| Knöchel C, O ‘dwyer L, Alves G, et al. | 2011 | 28 | 46% | 22 | 55% | 40.82 (12.04) | 41.94 (10.51) | DSM-IV | VBA | No impact of gender on fiber integrity results |
| Wigand M, Kubicki M, Clemm Von Hohenberg C, et al. | 2015 | 33 | 73% | 33 | 73% | W/ halluc.: 43.5 (8.19) W/out halluc.: 39.67 (3.39) | 44.97 (11.39) | DSM-IV | VBA | Sex Included as a covariate—none of the covariates were related to diffusion measures |
| Schneiderman J, Hazlett E, Chu K, et al. | 2011 | 96 | 72% | 93 | 59% | 25.9 (4.6) | 35.77 (18.12) | DSM-IV | ROI | Group x sex interaction— area 41 on the left: female patients had lower FA than female controls and men have no difference between groups; sex by subgroup (acute vs chronic interaction) —acute men have higher FA than women in dorsolateral, frontal, orbitofrontal and superior temporal regions |
| Fitzsimmons J, Schneiderman J, Whitford T, et al. | 2014 | 38 | 87% | 40 | 85% | Y: 21.56 (4.25) O: 41.90 (9.29) | Y: 23.47 (4.86) O: 43.90 (6.34) | DSM-IV | TBA | ANOVA repeated with male subjects only (too few females)—study overall observed increases in trace, axial and radial diffusivity in patients with FEP relative to controls and patients with chronic SCZ in the cingulum |
| Szeszko P, Robinson D, Ashtari M, et al. | 2008 | 33 | 64% | 30 | 60% | 25.1 (4.1) | 25.9 (4.6) | DSM-IV | VBA | Neither main effect of sex or group × sex interaction were significant |
| Garver D, Holcomb J, Christensen J. | 2008 | 13 | 69% | 14 | 50% | Drug responder: 35 (11); Poor responder 32 (12) | 29 (7) | DSM-IV | VBA | No sex related effects |
| Nugent K, Chiappelli J, Sampath H, et al. | 2015 | 45 | 73% | 53 | 52% | 38.9 (13.1) | 39.4 (12.3) | DSM-IV | TBA | Sex was not significantly associated with tract- averaged FA in either group |
| Segal D, Mehmet Haznedar M, Hazlett E, et al. | 2010 | 47 | 74% | 38 | 61% | Y: 24.8; O: 42.1 | 39.1 | DSM-IV | ROI | No significant interactions between gender and diagnosis except in the left hemisphere PCG gray matter volume in recent- onset SCZ group (only 2 women)—gender was then omitted as covariate |
| Takei K, Yamasue H, Abe O, et al. | 2008 | 31 | 39% | 65 | 37% | 33.8(9.0) | 34.7 (9.7) | DSM-IV | VBA | No significant interaction between gender and diagnosis (separate analyses for each gender were also not significant) |
| Zhang X, Fan F, Chen D, et al. | 2016 | 39 | 41% | 30 | 43% | 28.87 (10.22) | 24.47 (7.89) | DSM-IV | VBA | Sex as covariate—after adjusting for gender, partial correlation showed significant: (1) negative correlation between FA and PANSS + scores in L cerebellum, (2) positive correlation between FA values and PANSS general psychopathology in CC, and (3) positive correlation between FA values and PANSS total score in CC. |
| Phillips O, Nuechterlein K, Clark K, et al. | 2009 | 23 | 74% | 22 | 77% | 34.65 (9.17) | 30.87 (9.52) | DSM-IV | TBA | No sex or asymmetry effects or interactions between diagnosis and sex or hemisphere |
| Bertisch H, Li D Hoptman M, et al. | 2010 | 27 | NR | 48 | NR | NR | NR | DSM-IV | VBA | Significant effect of sex for Pegboard right hand raw score only (re: heritability)—no significant DTI sex effects |
| Collin G, Kahn R, de Reus M, et al. | 2014 | 40 | 90% | 51 | 43% | 30.6 (6.1) | 29.4 (8.6) | DSM-IV | TBA | No significant effect of sex even after analyzing male subjects separately. |
| Urči-Blake B, Van Der Meer L, Pijnenborg G, et al. | 2015 | 45 | 76% | 19 | 53% | 35 (11) | 34 (11) | DSM-IV | VBA | No significant associations of gender with FA or effective connectivity strengths |
| Miyata J, Hirao K, Namiki C, et al. | 2007 | 40 | 50% | 36 | 50% | 37.40 (9.56) | 36.42 (7.86) | DSM-IV | ROI | Neither a significant main effect of gender nor a significant gender × diagnosis interaction was found |
| Agarwal N, Rambaldelli G, Perlini C, et al. | 2008 | 71 | 65% | 75 | 52% | 40.48 (11.89) | 39.73 (10.94) | DSM-IV | ROI | Sex as covariate; when 2 groups stratified by sex, male patients had significantly greater ADC values in comparison with men in control group (except in left anterior ADC values) and female patients with SCZ had greater ADC values for right but not left side compared with women in control group |
| Okugawa G, Nobuhara K, Minami T, et al. | 2006 | 21 | 52% | 21 | 52% | 31.1 (6.7) | 30.4 (4.9) | DSM-IV | ROI | No sex effect of FA in right or left sides or of the ADC in right or left sides |
| Kalus P, Slotboom J, Gallinat J, et al. | 2004 | 15 | 60% | 15 | 60% | 32.27 (10.67) | 30.27 (6.69) | ICD-10 | ROI | No significant main effects of sex on TIV or significant between-subjects effects of sex |
| Anderson D, Ardekani B, Burdick K, et al. | 2013 | 35 | 66% | 56 | 55% | 30.6 (10.9) | 31.9 (9.4) | DSM-IV | ROI | Main effect of sex for FA with males having higher FA compared to females across all brain regions; No significant main effect of sex for MD and no region- by-sex and region-by-group- by-sex interactions for FA or MD |
| Kalus P, Slotboom J, Gallinat J, et al. | 2004 | 14 | 64% | 15 | 60% | 27.93 (6.35) | 31.37 (5.97) | ICD-10 | ROI | Gender matched + as covariate. No significant main effect of sex on TIV |
| Kunimatsu N, Aoki S, Kunimatsu A, et al. | 2012 | 39 | 49% | 40 | 50% | M: 29.1 (6.7); F: 29.8 (7.0) | M: 30.0 (5.2); F: 29.9 (8.0) | DSM-IV | TBA | (1) Gender-combined and gender-separated analyses: lower FA in SCZ vs controls bilaterally for anterior cingulum, UF and Fx; (2)For ADC for gender combined analysis CC significantly higher ADC than controls (but not in gender-separated); (3) Bilateral anterior cingulum & body of cingulum, left fornix and bilateral UF also significantly higher ADC for patients in gender combined; (4) Gender- separated analysis females have higher ADC in right anterior cingulum and left fornix while males have higher ADC in left anterior cingulum, right body of cingulum and bilateral UF |
| White T, Schmidt M, Karatekin C | 2009 | 29 | 62% | 41 | 61% | 14.2 (3.4) | 14.8 (2.8) | K-SADS-PL | TBSS/VBA | No significant differences in number of potholes between males and females |
| Epstein K, Cullen K, Mueller B, et al. | 2014 | 55 | 56% | 55 | 49% | 16.9 (1.7) | 16.5 (2.6) | K-SADS-PL & DSM-IV | TBA | Sex as covariate—no main sex analysis |
| Chan W, Yang G, Chia M, et al. | 2010 | 39 | 77% | 64 | 59% | 28.8 (6.8) | 32.3 (10.2) | DSM-IV | VBA | Sex as covariate—no main sex analysis |
| Prasad K, Upton C, Schirda C, et al. | 2015 | 39 | 56% | 29 | 31% | 26.83 (8.53) | 27.14 (6.75) | DSM-IV | TBA | Sex as covariate—no main sex analysis |
| Epstein K, Kumra S | 2015 | 34 | 65% | 29 | 45% | 16.4 (1.9) | 16.5 (2.2) | DSM-IV | TBA | Sex as covariate—no main sex analysis |
| Lee D, Smith G, Su W | 2012 | 14 | 86% | 29 | 21% | 20.9 | 23.6 | DSM-IV | VBA | Gender was not related to tract volume or FA |
| Price G Cercignani M Parker G, et al. | 2007 | 19 | 58% | 23 | 48% | 23.8 (6.18) | 29.6 (7.17) | DSM-IIIR/ DSM-IV | TBA | Greater tract coherence in males on the left UF irrespective of group |
| Andreone N, Tansella M, Cerini R, et al. | 2007 | 68 | 57% | 64 | 53% | 41.39 (11.68) | 40.70 (11.6) | DSM-IV | ROI | ADC measures greater in men and women with schizophrenia compared to controls |
| Steel R, Bastin M, Mcconnell S, et al. | 2001 | 10 | 50% | 10 | 40% | 34 (14) | 35 (7) | DSM-IV | ROI | In males with SCZ FA was slightly reduced in all 4 ROIs but not statistically significant; In females with schizophrenia there was an increase (P < .01) in FA in right occipital region |
| White T, Magnotta V, Bockholt H, et al. | 2009 | 114 | 74% | 138 | 59% | Y: 25.2 (6.7); O: 36.4 (11.0) | Y: 25.2 (6.6); O: 34.0 (11.3) | DSM-IV or CASH | ROI | Sex as covariate—no main sex analysis |
| Stephen J, Coffman B, Jung R, et al. | 2013 | 29 | 86% | 29 | 72% | 37.1 (2.9) | 38.1 (2.2) | DSM-IV-TR | jICA | Match on sex and as covariate—gender not predictive of RT |
| Seitz J, Zuo J, Lyall A, et al. | 2016 | 30 | 67% | 30 | 60% | 21.76 (4.73) | 21.88 (3.38) | DSM-IV-TR | TBA | Matched on sex and controlled for gender and age—no main sex analysis |
| Sun Y, Chen Y, Lee R, et al. | 2016 | 31 | 45% | 28 | 46% | 31.9 (9.7) | 31.8 (9.3) | NR | TBA | Gender-matched and sex as covariate- no main sex analysis |
| James A, Joyce E, Lunn D, et al. | 2016 | 37 | 62% | 24 | 50% | 16.3 (1.1) | 15.9 (1.4) | K-SADS-PL & DSM5 | TBA | Females had superior processing speed (no gender-specific DTI finding) |
| Mallas E, Carletti F, Chaddock C, et al. | 2016 | 63 | 79% | 124 | 54% | 33.78 (10.70) | 35.79 (13.40) | DSM-IV | VBA | Sex was not a significant predictor of FA |
| Gawłowska-Sawosz M, Rabe-Jabłońska J, Gębski P, et al. | 2015 | 30 | 50% | 30 | 50% | 20.2 (2.48) | 21.53(2.66) | DSM-IV | ROI | Within the women subgroups there were significant differences of FA in right anterior limb of internal capsule but no differences for men |
Note: ADC, apparent diffusion coefficient; CC, corpus callosum; FA, fractional anisotropy; ROI, region of interest analysis; SBM, source based morphometry; TBA, tract-based Analysis; VBA, voxel-based analysis.
Table 3.
Number of Studies out of 75 With Data on White Matter Tract Reported
| Tract | Frequency |
|---|---|
| Corpus callosum | 39 |
| Cingulum | 21 |
| Superior longitudinal fasciculus | 18 |
| Uncinate fasciculus | 15 |
| Inferior fronto-occipital fasciculus (IFOF) | 13 |
| Internal capsule | 12 |
| Thalamic radiations | 12 |
| Inferior longitudinal fasciculus (ILF) | 10 |
| Corona radiata | 9 |
| Fornix | 7 |
| Corticospinal tract | 8 |
| External capsule | 6 |
| Arcuate fasciculus | 6 |
| Sagittal stratum (ILF + IFOF) | 5 |
| Major and minor forceps | 4 |
| Superior fronto-occipital fasciculus (SFOF) | 3 |
| Tapetum | 2 |
| Optic radiations | 1 |
Sex-Related Findings.
Out of 75 studies, 20 tested the interaction of sex with diagnostic group (controls/patients) and 12 studies conducted sex-stratified analyses. Twelve studies only included sex as a nuisance covariate in their statistical models and 6 studies conducted sex-related analyses but did not report the findings of those analyses. Fifty-one studies found no effect of sex and/or no sex-by-diagnosis interaction. Only 24 studies had a statistically significant sex-related finding.
Commissural Fibers.
Thirty-nine out of 75 articles studied the corpus callosum. Nine of these found a significant sex-related effect. One study showed that female patients had lower FA compared to female controls in the genu, body, and forceps minor in sex-separated analyses; however, no difference was observed between the male groups.38 Several studies reported lower FA in females compared to males. One study found that females had lower FA in the inferior genu,39 but no sex by diagnosis interaction. Two more studies, with shared datasets, found that females had decreased FA values in the genu, irrespective of diagnostic group.40,41 Another study found that female patients had lower FA than male patients; however, there were no sex differences between male and female controls.42 One study calculated shape-normalized dispersion and found that in the left anterior corpus callosum, dispersion was highest in male patients, followed by female controls, followed by male controls and female patients.43 In another study, male patients had lower relative anisotropy (RA) than controls in the genu and splenium, but higher RA in the body of the corpus callosum in the right hemisphere; female patients had lower RA in the body of the corpus and posterior portion of splenium and higher RA in the genu and anterior portion of splenium.44 In the left hemisphere, male patients had lower RA than controls except in the anterior genu; females showed a similar trend. One study showed that females had a trend of higher MD than males,40 and another study found that apparent diffusivity coefficient was significantly higher in patients compared to controls at the corpus callosum in a sex-combined analysis but not in a sex-separated analysis.45 Finally, a study showed that differences in radial diffusivity were not significant after females were removed from the analysis.46 Many studies observed lower FA in females compared to males in the corpus callosum. Significant differences between males and females were observed using several DTI measures.
Projection Fibers.
Two out of 15 studies on the internal capsule reported significant sex specific findings. One study reported lower FA in the right anterior limb of the internal capsule for female patients relative to female controls, but observed no differences between the 2 male groups.47 Another study using RA analyzed adolescents and adult subjects focusing on the internal capsule.44 In adolescents, male controls had lower RA in the right hemisphere and higher RA in the left hemisphere compared to male patients. The opposite finding was reported in females where female controls had higher RA in the right hemisphere and lower RA in the left hemisphere compared to female patients. Surprisingly, the finding for adult males was similar to the finding for female adolescents. For adult females, no difference was observed in the left hemisphere and controls had lower RA than patients in the right hemisphere. However, the other 13 (out of 15) studies showed no effect of sex on the internal capsule and the significant findings of the 2 remaining studies cannot be compared as both used a different DTI measure. The external capsule, corticospinal tract, and corona radiata were tracts of interest for 6, 8, and 9 studies respectively. None reported significant sex-specific findings.
Association Fibers.
Findings from studies on association fibers are briefly summarized here. A more detailed description of the findings can be found in the supplementary material. Ninety percent of the studies on the cingulum, 83% of studies on the SLF, and 87% of studies on the UF found no significant sex-related effect. Two studies on the ILF reported significant findings. The results of both studies were congruent reporting higher mean FA in males compared to females.48,49 Only one study on the thalamic radiations showed a significant effect of sex. This study found that male patients had lower RA than male controls in the right anterior thalamic radiation, however, female patients had higher RA than female controls.44 Only one out of 8 studies on the fornix reported significant results. This study reported that patients with schizophrenia had decreased FA on both sides of the fornix irrespective of sex-group separation.45 Sex-separated analyses also revealed increased apparent diffusion coefficient (ADC) values in the left fornix for female patients. The superior fronto-occipital fasciculus (SFOF), inferior fronto-occipital fasciculus (IFOF), optic radiations, arcuate fasciculus (AF), and the sagittal stratum (IFOF + ILF) were tracts of interest in 3, 13, 1, 6, and 5 studies respectively with none reporting significant sex-specific findings.
Meta-analysis on the Genu and Splenium of the Corpus Callosum
Study Characteristics.
The 31 included studies were published between 2000 and 2016, with 8 studies published in the first 8 years and 23 studies published in the last 8 years of the 16-year period. These 31 studies were conducted across 13 different countries, with most studies conducted in the United States. Sixteen studies exclusively studied the corpus callosum. Eighteen studies reported duration of illness for schizophrenia patients, which ranged from a mean of 2 years to 33 years (median across studies = 13). Thirteen out of the 31 studies reported age at illness onset which ranged from 13 to 30 years (median across studies = 24). All studies analyzed FA. Twelve studies analyzed an additional measure with MD (7 studies) being the most common measure after FA.
Quality Assessment.
Quality assessment ratings for the 31 studies on the genu and splenium are summarized in supplementary table S1. The studies ranged from a score of 3 to 10 where 0 is the minimum achievable score and 10 the maximum. Four studies scored < 5 in the quality assessment. Only one out of these 4 studies was included in the meta-analysis. Leave-one-out meta-analysis showed that exclusion of this study did not change results.
None of the samples from the 10 studies included in the meta-analysis had a non-normal distribution when tested for skewness. The funnel plots showed that one study in the female-genu group, 2 studies in the 2 male groups, and 3 studies in the female-splenium groups were outside the 90% CI (supplementary figure S4). Thus, exploratory meta-regressions were run on the male-genu, male-splenium, and female-splenium groups. Results on the exploratory meta-regressions are reported in the supplementary material document.
Meta-analysis.
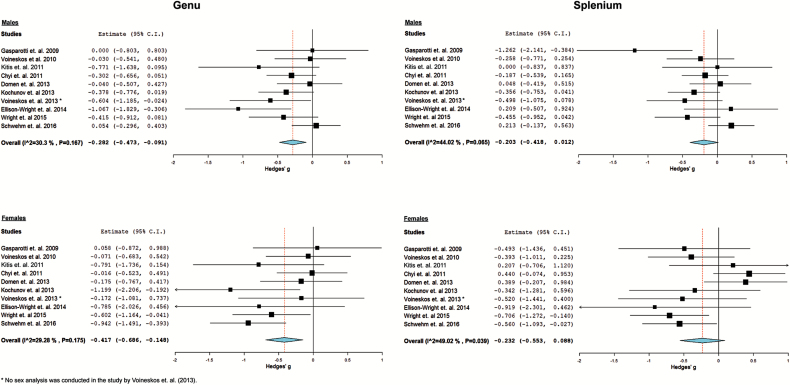
FA was significantly different between patients and controls in the genu of the corpus callosum for both males (Hedges’ g = −0.282, 95% CI [−0.473, −0.091], P = .004) and females (Hedges’ g = −0.417, 95% CI [−0.686, −0.148], P = .002; figure 2). FA in the splenium was not significantly different in patients compared to controls for both sexes. Even though females had a larger effect size than males in both regions, the CIs overlapped; thus, the difference between the female and male groups was not statistically significant. Results of the leave-one-out meta-analysis, diagnostic group stratified meta-analysis, meta-regression, a post hoc power analysis, and subgroup analysis are reported in the supplementary material.
Fig. 2.
Forest plots: standardized mean difference in fractional anisotropy between patients and controls.
Discussion
This is the first systematic review and meta-analysis to our knowledge that summarizes findings of the effect of sex on DTI-based white matter metrics in schizophrenia patients and controls. We conducted a systematic review on all white matter tracts, followed by a more focused review and meta-analysis on the genu and splenium of the corpus callosum. While the majority of studies showed no effect of sex on white matter tracts, 7 showed that females had a lower FA than males regardless of diagnostic group, 3 showed that only female patients have a lower FA than female controls with no difference observed in males, and 2 showed that female patients had a lower FA than male patients. The meta-analysis showed a significant difference in FA between patients and controls at the genu, but not the splenium, for both sexes. Effect sizes of group differences were larger for females in both regions, but were not significantly different from the effect observed in males. In studies that used tractography, a significant difference for females but not males was present in the splenium, and a significant difference was present for both sexes in the genu. In this subgroup, the effect size for females was almost twice as large as the effect size in males at both the genu and the splenium, however, this difference was not statistically significant.
Even though only 15% of studies in this review indicated a statistically significant sex-related finding, all findings were in the same direction showing either that females have a lower anisotropy than males, or that disease-related differences were larger in females than in males. Our meta-analytic findings in the genu and the splenium of the corpus callosum support this result. An earlier meta-analysis of DTI studies did not examine effects of sex; however, a closer examination showed that those studies with fewer men had a larger effect of disease at the splenium.15 When taken together, these results may seem counterintuitive, given that men have more severe cognitive impairment, negative symptoms, earlier age of onset, and a higher likelihood of treatment resistance. However, another interpretation consistent with these results is that the female brain is “protected” against schizophrenia. Other disorders that occur more frequently in males, such as autism spectrum disorder, have also found that females can require a larger biological “hit” to develop the condition.50,51 Females with autism have an excess of large deleterious copy number variants and single-nucleotide variants. This suggests that females require greater alteration to normal brain functioning to develop autism.50 Further support for this interpretation comes from animal studies where developmental and genetic models of schizophrenia show that male mice are more vulnerable to develop schizophrenia than female mice.52 From a neuroanatomical perspective, female brains are more lateralized anteriorly, with greater hemispheric differentiation, and fewer interhemispheric connections.53 Some have argued that schizophrenia is caused by abnormal transfer of information or reverse transmission of information.53 As males have more fibers in the anterior portion of the corpus callosum, there is more myelination and a greater likelihood of reverse transmission.53 As females have fewer connections and have a more differentiated brain anteriorly, they may require a bigger change in microstructural integrity of fibers to manifest the symptoms of schizophrenia.
Nevertheless, a large proportion of studies showed no effect of sex on diffusion properties of white matter tracts in schizophrenia and it is important to consider possible factors that may have influenced this null finding. Our interpretation is that most studies were underpowered, not just in overall sample size, but in number of females included. Recruiting female schizophrenia patients is challenging as fewer females are diagnosed with schizophrenia compared to males.54 Forty-two percent of schizophrenia patients are females, yet, only 34% of a non-epidemiological study’s sample is comprised of female patients.55 Thus, some studies have a higher proportion of males in the patient group compared to the control group. As females and patients have both been shown to have lower FA than their counterparts in this review, this disproportionate ratio of sexes would result in an underestimation of the difference in FA between patients and controls. Aggregating values across the 2 sexes may result in higher variability in the measure of interest, making it difficult to detect differences between patients and controls.
Only 4% of studies showed a significant interaction of sex with diagnostic group. The meta-analysis shows that for the genu and splenium, the effect in females is larger than the effect in males but is not statistically significant. Thus, a sex-by-diagnosis interaction may exist, however, it may require a large sample size to be detected. Studies included in this review may be underpowered to detect that effect. Additionally, interaction effects and sex-separated analyses cut the power of these studies, which are already likely underpowered. The subgroup analysis showed that in the female groups, overall effect calculated based on studies using a tract-based approach was significant, however, the effect calculated using the region-of-interest approach was not. The lack of a positive result for the region of interest (ROI) studies could be explained by the large CI around the effect size. The small number of ROI studies and the fact that 2 out of the 3 studies were not peer-reviewed could have contributed toward this variability.
Study quality may also be a factor that influenced the null findings. As data on quality were only collected for studies on the genu and splenium of the corpus callosum, only those studies will be discussed here. In one study, a t test was conducted to see if FA on average differed between males and females.56 By averaging across patients and controls in the male and female groups, the specific effect of diagnosis was not considered. Based on the null result of the t test, sex was not included as a covariate in further analyses in this study. Moreover, the number of male and female patients and controls was not mentioned in the text or in any figures or tables. The poor method of sex analysis and the reporting bias may have resulted in the null effect of sex on the corpus callosum FA. Moreover, often-important sex-related findings may be missed because many studies are simply using sex as a nuisance covariate instead of conducting a sex-based analysis. Averaged FA for the whole brain was computed in one of the included studies.57 Averaging FA across large areas of the brain may cancel out any effects of sex on individual regions of the brain. In another study, the corpus callosum was not separated into its subparts.58 The genu and splenium of the corpus callosum originate from different neurodevelopmental structures and develop during different neurodevelopmental stages19 and are thus influenced by different environmental factors. Moreover, those 2 regions connect very different parts of the brain,59 and the splenium has been shown to have a higher FA than the genu in several studies.20,59,60 In one study, the genu is grouped together with the forceps minor, and splenium grouped with the forceps major, thus the exclusive effect of sex on the genu and splenium cannot be ascertained from this study’s findings.61
Limitations and Strengths
Our greatest challenge was that summary statistics from only 33% of the studies on the genu and splenium were available for the meta-analysis. As most studies did not report sex-separated values for FA, authors were contacted to obtain those values. Data from 21 out of the 31 studies could not be obtained as authors either did not respond or did not have the data available. As a result, most of the 10 studies were published recently and the meta-analysis does not reflect findings from studies published before 2009. Despite our attempts to control for many variables, it remains the case that several confounds were present, among them DTI acquisition quality, analytic method, sex distribution, duration of illness, effect of medication, and treatment history. Although the linear effect of age on FA was tested in the meta-regression, the nonlinear effect of age62,63 on FA could not be examined. This review also had several strengths. A comprehensive search strategy was used to retrieve relevant studies. The strategy was developed in consultation with a medical librarian and was also tested for comprehensiveness by ensuring that all relevant studies from a previous review on DTI findings in schizophrenia and all key studies identified through a Google Scholar search were included in the results of the search. This review followed PRISMA guidelines and thus all components of the review have been reported in a clear and complete manner. Studies in this review were conducted across 13 different countries and were not geographically restricted. Unpublished work or work that did not undergo review was not ignored. The meta-analysis included a thesis and an article published in a non-peer reviewed journal. Publication bias and heterogeneity among studies were rigorously tested.
Implications and Future Research
Studies on white matter tracts, but more generally on brain structure or function, should conduct either a sex-by-diagnosis interaction, or a sex-separated analysis, or both. As such, they should be adequately powered to do so. Including sex as a nuisance covariate allows the effect of the main predictor or variable to be isolated from the effect of sex; however, it does not help understand how sex could explain variability in the main variable of interest. Thus, simply including sex as a covariate is not sufficient. Federal funding agencies such as the National Institute of Mental Health in the United States, and the Canadian Institutes of Health Research in Canada have begun to prioritize the importance of such approaches.64,65 Most researchers recognize that patients and controls are separate groups and report DTI measures separately for the 2 groups; however, very few studies consider the 2 sexes as different enough to report the summary statistics separately for the 2 sexes. As several studies in this review reported a main effect of sex, DTI studies on schizophrenia should also report summary statistics for their variable of interest separately for males and females.
There is a recognized need for studies on schizophrenia with a large enough sample of female patients. Being underpowered increases the chance of type II error and severely undermines the ability to detect differences between male and female patients that may help explain the epidemiological and clinical differences observed between male and female schizophrenia patients. More importantly, effects of existing and novel treatments may be different for males and females. Understanding the sex-specific impact of illness on neural circuits may help inform development of new treatments, and improvement of existing ones.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
The authors did not receive any specific funding for the present study. S.S. is supported by an Ontario Graduate Scholarship. A.N.V. receives funding from the Canadian Institutes of Health Research, Ontario Mental Health Foundation, Canada Foundation for Innovation, Brain and Behavior Research Foundation, CAMH Foundation, and the National Institute of Mental Health (R01MH099167 and R01MH102324).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Raznahan A, Lee Y, Stidd R, et al. Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proc Natl Acad Sci U S A. 2010;107:16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Erus G, Battapady H, Satterthwaite TD, et al. Imaging patterns of brain development and their relationship to cognition. Cereb Cortex. 2015;25:1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kraepelin E. Dementia Praecox and Paraphrenia. Malabar, FL: Krieger Publ Co; 1971. https://scholar.google.ca/scholar?hl=en&as_sdt=0,5&q=1.%09Kraepelin+E+Dementia+Praecox+and+Paraphrenia.+Barclay+RMtrans.+New+York,+NY+Krieger+1971%3B. Accessed October 17, 2016. [Google Scholar]
- 4. Castle DJ, Murray RM. The neurodevelopmental basis of sex differences in schizophrenia. Psychol Med. 2016;21:565–575. [DOI] [PubMed] [Google Scholar]
- 5. Loranger AW. Sex difference in age at onset of schizophrenia. Arch Gen Psychiatry. 1984;41:157. [DOI] [PubMed] [Google Scholar]
- 6. Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand. 2000;101:3–38. [DOI] [PubMed] [Google Scholar]
- 7. Szymanski S, Lieberman JA, Alvir JM. Gender differences in onset of illness, treatment response, course, and biologic indexes in first-episode schizophrenic patients. Am J Psychiatry. 1995;152:698–703. [DOI] [PubMed] [Google Scholar]
- 8. Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl. 1999;395:68–79. [DOI] [PubMed] [Google Scholar]
- 9. Kubicki M, McCarley R, Westin C-F, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters BD, Blaas J, de Haan L. Diffusion tensor imaging in the early phase of schizophrenia: what have we learned?J Psychiatr Res. 2010;44:993–1004. [DOI] [PubMed] [Google Scholar]
- 11. Kuswanto CN, Teh I, Lee TS, Sim K. Diffusion tensor imaging findings of white matter changes in first episode schizophrenia: a systematic review. Clin Psychopharmacol Neurosci. 2012;10:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. [DOI] [PubMed] [Google Scholar]
- 13. Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur Psychiatry. 2008;23:255–273. [DOI] [PubMed] [Google Scholar]
- 14. Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. [DOI] [PubMed] [Google Scholar]
- 15. Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2011;129:149–155. [DOI] [PubMed] [Google Scholar]
- 16. Zhuo C, Liu M, Wang L, Tian H, Tang J. Diffusion tensor MR imaging evaluation of callosal abnormalities in schizophrenia: a meta-analysis. PLoS One. 2016;11:e0161406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abel KM, Drake R, Goldstein JM. Sex differences in schizophrenia. Int Rev Psychiatry. 2010;22:417–428. [DOI] [PubMed] [Google Scholar]
- 18. Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. http://www.ncbi.nlm.nih.gov/pubmed/2731030 Accessed September 16, 2016. [DOI] [PubMed] [Google Scholar]
- 19. Liu F, Vidarsson L, Winter JD, Tran H, Kassner A. Sex differences in the human corpus callosum microstructure: a combined T2 myelin-water and diffusion tensor magnetic resonance imaging study. Brain Res. 2010;1343:37–45. [DOI] [PubMed] [Google Scholar]
- 20. Westerhausen R, Kreuder F, Dos Santos Sequeira S, et al. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res Cogn Brain Res. 2004;21:418–426. [DOI] [PubMed] [Google Scholar]
- 21. Menzler K, Belke M, Wehrmann E, et al. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage. 2011;54:2557–2562. [DOI] [PubMed] [Google Scholar]
- 22. Westerhausen R, Walter C, Kreuder F, Wittling RA, Schweiger E, Wittling W. The influence of handedness and gender on the microstructure of the human corpus callosum: a diffusion-tensor magnetic resonance imaging study. Neurosci Lett. 2003;351:99–102. http://www.ncbi.nlm.nih.gov/pubmed/14583391 Accessed September 16, 2016. [DOI] [PubMed] [Google Scholar]
- 23. Shin Y-W, Kim DJ, Ha TH, et al. Sex differences in the human corpus callosum: diffusion tensor imaging study. Neuroreport. 2005;16:795–798. http://www.ncbi.nlm.nih.gov/pubmed/15891572 Accessed September 16, 2016. [DOI] [PubMed] [Google Scholar]
- 24. Mohr B, Pulvermüller F, Cohen R, Rockstroh B. Interhemispheric cooperation during word processing: evidence for callosal transfer dysfunction in schizophrenic patients. Schizophr Res. 2000;46:231–239. [DOI] [PubMed] [Google Scholar]
- 25. Endrass T, Mohr B, Rockstroh B. Reduced interhemispheric transmission in schizophrenia patients: evidence from event-related potentials. Neurosci Lett. 2002;320:57–60. [DOI] [PubMed] [Google Scholar]
- 26. Frodl T, Meisenzahl EM, Müller D, et al. Corpus callosum and P300 in schizophrenia. Schizophr Res. 2001;49:107–119. [DOI] [PubMed] [Google Scholar]
- 27. Coger RW, Serafetinides EA. Schizophrenia, corpus callosum, and interhemispheric communication: a review. Psychiatry Res. 1990;34:163–184. [DOI] [PubMed] [Google Scholar]
- 28. Lei X, Han Z, Chen C, Bai L, Xue G, Dong Q. Sex differences in fiber connection between the striatum and subcortical and cortical regions. Front Comput Neurosci. 2016;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kumar R, Chavez AS, Macey PM, Woo MA, Harper RM. Brain axial and radial diffusivity changes with age and gender in healthy adults. Brain Res. 2013;1512:22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wheeler AL, Voineskos AN. A review of structural neuroimaging in schizophrenia: from connectivity to connectomics. Front Hum Neurosci. 2014;8:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 32. Wells G, Shea B, O‘Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxfordasp. Accessed October 17, 2016.
- 33. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. http://www.ncbi.nlm.nih.gov/pubmed/3802833 Accessed September 16, 2016. [DOI] [PubMed] [Google Scholar]
- 34. Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200 http://www.ncbi.nlm.nih.gov/pubmed/8916759 Accessed September 16, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. [DOI] [PubMed] [Google Scholar]
- 36. Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28:226–235. http://www.ncbi.nlm.nih.gov/pubmed/17296985 Accessed September 16, 2016. [PMC free article] [PubMed] [Google Scholar]
- 37. Jones DK, Catani M, Pierpaoli C, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Domen PA, Michielse S, Gronenschild E, et al. ; for G.R.O.U.P Microstructural white matter alterations in psychotic disorder: a family-based diffusion tensor imaging study. Schizophr Res. 2013;146:291–300. [DOI] [PubMed] [Google Scholar]
- 39. Rotarska-Jagiela A, Schönmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DEJ. The corpus callosum in schizophrenia-volume and connectivity changes affect specific regions. Neuroimage. 2008;39:1522–1532. [DOI] [PubMed] [Google Scholar]
- 40. Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA. The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry. 2005;76:585–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Price G, Cercignani M, Parker GJ, et al. Abnormal brain connectivity in first-episode psychosis: a diffusion MRI tractography study of the corpus callosum. Neuroimage. 2007;35:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rametti G, Junqué C, Falcón C, et al. A voxel-based diffusion tensor imaging study of temporal white matter in patients with schizophrenia. Psychiatry Res Neuroimaging. 2009;171:166–176. [DOI] [PubMed] [Google Scholar]
- 43. Savadjiev P, Whitford TJ, Hough ME, et al. Sexually dimorphic white matter geometry abnormalities in adolescent onset schizophrenia. Cereb Cortex. 2014;24:1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schneiderman JS, Buchsbaum MS, Haznedar MM, et al. Age and diffusion tensor anisotropy in adolescent and adult patients with schizophrenia. Neuroimage. 2009;45:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kunimatsu N, Aoki S, Kunimatsu A, et al. Tract-specific analysis of white matter integrity disruption in schizophrenia. Psychiatry Res Neuroimaging. 2012;201:136–143. [DOI] [PubMed] [Google Scholar]
- 46. Leroux E, Delcroix N, Dollfus S. Left-hemisphere lateralization for language and interhemispheric fiber tracking in patients with schizophrenia. Schizophr Res. 2015;165:30–37. [DOI] [PubMed] [Google Scholar]
- 47. Gawłowska-Sawosz M, Rabe-Jabłońska J, Gębski P, Chomczyński P. Internal capsule integrity and its sex-related structural differences in early-onset schizophrenia – diffusion tensor imaging study. Psychiatr Pol. 2015;49:349–361. [DOI] [PubMed] [Google Scholar]
- 48. Boos HB, Mandl RC, van Haren NE, et al. Tract-based diffusion tensor imaging in patients with schizophrenia and their non-psychotic siblings. Eur Neuropsychopharmacol. 2013;23:295–304. [DOI] [PubMed] [Google Scholar]
- 49. Schwehm A, Robinson DG, Gallego JA, et al. Age and sex effects on white matter tracts in psychosis from adolescence through middle adulthood. Neuropsychopharmacology. 2016;41:2473–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jacquemont S, Coe BP, Hersch M, et al. A higher mutational burden in females supports a “female protective model” in neurodevelopmental disorders. Am J Hum Genet. 2014;94:415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proc Natl Acad Sci U S A. 2013;110:5258–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hill RA. Sex differences in animal models of schizophrenia shed light on the underlying pathophysiology. Neurosci Biobehav Rev. 2016;67:41–56. [DOI] [PubMed] [Google Scholar]
- 53. Crow TJ, Paez P, Chance SA. Callosal misconnectivity and the sex difference in psychosis. Int Rev Psychiatry. 2007;19:449–457. [DOI] [PubMed] [Google Scholar]
- 54. McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67–76. [DOI] [PubMed] [Google Scholar]
- 55. Longenecker J, Genderson J, Dickinson D, et al. Where have all the women gone?: participant gender in epidemiological and non-epidemiological research of schizophrenia. Schizophr Res. 2010;119:240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kitiş O, Eker MC, Zengin B, et al. The disrupted connection between cerebral hemispheres in schizophrenia patients: a diffusion tensor imaging study. Türk Psikiyatr Derg. 2011;22:213–221. http://www.ncbi.nlm.nih.gov/pubmed/22143946 Accessed August 22, 2016. [PubMed] [Google Scholar]
- 57. Kochunov P, Glahn DC, Rowland LM, et al. Testing the hypothesis of accelerated cerebral white matter aging in schizophrenia and major depression. Biol Psychiatry. 2013;73:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ćurčić-Blake B, van der Meer L, Pijnenborg GH, David AS, Aleman A. Insight and psychosis: functional and anatomical brain connectivity and self-reflection in Schizophrenia. Hum Brain Mapp. 2015;36:4859–4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. J Magn Reson Imaging. 2005;21:735–743. [DOI] [PubMed] [Google Scholar]
- 60. Chepuri NB, Yen Y-F, Burdette JH, Li H, Moody DM, Maldjian JA. Diffusion anisotropy in the corpus callosum. AJNR Am J Neuroradiol. 2002;23:803–808. http://www.ncbi.nlm.nih.gov/pubmed/12006281 Accessed September 16, 2016. [PMC free article] [PubMed] [Google Scholar]
- 61. Caprihan A, Abbott C, Yamamoto J, et al. Source-based morphometry analysis of group differences in fractional anisotropy in schizophrenia. Brain Connect. 2011;1:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Westlye LT, Walhovd KB, Dale AM, et al. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20: 2055–2068. [DOI] [PubMed] [Google Scholar]
- 63. Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. [DOI] [PubMed] [Google Scholar]
- 64. Johnson JL, Greaves L, Repta R. Better science with sex and gender: facilitating the use of a sex and gender-based analysis in health research. Int J Equity Health. 2009;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Clow BN; Atlantic Centre of Excellence for Women’s Health Rising to the Challenge : Sex- and Gender-Based Analysis for Health Planning, Policy and Research in Canada. Halifax, NS, Canada: Atlantic Centre of Excellence for Women’s Health; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.