Abstract
Background:
The present study aims to comparatively evaluate the isolation and quantification of stem cells derived from dental pulp and periodontal ligament of a permanent tooth and to assess their viability and proliferation on a platelet-rich fibrin (PRF) scaffold.
Materials and Methods:
A total of 15 systemically healthy individuals between the age group of 15–25 years requiring third molar or orthodontic premolar extractions. Teeth were extracted atraumatically and transported to the laboratory. Stem cells were isolated from dental pulp and periodontal ligament. After attaining more than 90% confluency by the 7th day, these cells were tested for their viability and characterization. Stem cells were also incubated with PRF and viability was assessed on the 7th day.
Results:
The mean number of cell for dental pulp stem cells (DPSCs) and periodontal ligament stem cell (PDLSC) was statistically insignificant (P > 0.05). The mean live cell viability was compared between DPSC (98.07%) and PDLSC (98%). Both DPSC and PDLSC showed a high percentage of expression of CD73 markers, 30.40% and 29.80%, respectively. However, DPSCs and PDLSCs lacked expression of CD34 expressing only 3.47% and 3.53%, respectively. PRF membrane as a scaffold exhibited no cytotoxic effects on DPCS's or PDLSC's. The cell viability of cells cultured with PRF was statistically insignificant (P > 0.05) when compared to the cells cultured with culture media.
Conclusion:
The study thus indicates that dental pulp and periodontal ligament are both rich sources of mesenchymal stem cells and can be successfully used for obtaining stem cells. PRF exhibits no cytotoxic effects on the cells and can be used in conjunction with dental stem cells.
Key words: Dental pulp, mesenchymal stem cells, periodontal ligament, platelet-rich fibrin
INTRODUCTION
Periodontitis is an inflammatory disease in which specific microorganism cause destruction of the periodontium, apical migration of the junctional epithelium, formation of pocket, and ultimately resulting into tooth loss.[1] Initial treatment focuses on the elimination of the etiological factors.[2] Periodontal therapy aims at regeneration of tissues that are lost due to disease activity.[3] Various biomaterials including autogenous and allogenic bone grafts in conjunction with guided tissue regeneration or alone have been employed for the regeneration of these tissues.[4]
Stem cells are primitive cells that possess the potential to undergo replications (self-renewal) and multilineage differentiation.[5] Advances in stem cell research have unveiled that stem cells have a promising role in regenerative medicine.[6] Mesenchymal Stem cells were first isolated from the bone marrow by Friedenstein et al. in 1966. The author described them as a stem cell population capable of differentiating into at least one mesenchymal lineage.[7]
Gronthos in 2000 was the first to successfully isolate stem cells from the human dental pulp stem cells (DPSCs) and characterized them as multipotent stem cell population that can differentiate into adipocytes and neural-like cells.[8] Melcher in 1985 put forth the concept that stem cells may be present in the periodontal tissue periodontal ligament stem cells (PDLSCs) based on a hypothesis that the multilineage cells of the periodontium originated from a single ancestral cell population.[9] In 1987, McCulloch et al. identified a trifling population of undifferentiated cells from the periodontal ligament.[10]
In 2001, Choukroun et al. introduced the concept of platelet-rich fibrin (PRF). A second generation platelet concentrate obtained without using any anticoagulants or gelifying agents. It has strong fibrin architecture with entrapped platelets and leukocyte cytokines. It consists of various growth factors and glycoproteins which are slowly released over several days. As a result, PRF enhances soft- and hard-tissue healing while providing mechanical protection to both the surgical and grafted sites.[11]
The present study aimed to comparatively evaluate the isolation and quantification of stem cells derived from dental pulp and periodontal ligament of a permanent tooth and to assess their viability and proliferation on a PRF scaffold.
MATERIALS AND METHODS
This in vitro study was reviewed and approved by the Institutional Research Review Board and Ethical Committee, Jaipur Dental College, Jaipur. A total of 15 systemically healthy individuals between the age group of 15–25 years requiring third molar or orthodontic premolar extractions were included in this study. Cases wherein teeth were noncarious, and atraumatic extractions were possible were included in the study. The subjects were selected based on the inclusion and exclusion criteria. The selected subjects were then explained the nature of the study and a signed consent form was attained.
Sample and cell culture
Following atraumatic extractions, the teeth were transported to the laboratory immediately in a micro-centrifuge tube (Appendauf tube) containing the transport media (Dulbecco Modified Eagle's Medium [DMEM] with penicillin/streptomycin). At the laboratory, the tooth was first rinsed thoroughly with Dulbecco's phosphate buffered saline comprising of added antibiotic and antimycotic agents. The periodontal ligament tissue present on the root surface was scraped and processed further. Dental pulp was extracted from the same tooth. A round carbide bur was used to section the tooth at the cemento-enamel junction. The pulp tissue was then gently removed from the chamber. The collected tissues were cut using Castroviejo scissors into small fragments referred to as “explants.” These explants were seeded on previously collagen-coated culture plates, and culture media (DMEM + strep/penic + epidermal growth factor + basic fibroblast growth factor + insulin + fetal bovine serum) was added. The culture plates were incubated at 37°C with 95% humidified air and 5% CO2 The media was changed on alternate days.[12] By 7th day, the cell culture dishes showed >80% confluency for DPSC [Figure 1] and PDLSC [Figure 2]. These cell layers were then disengaged with 0.25% trypsin to form cell suspension which is used for further investigations including cell viability, immunocytochemistry, and PRF seeding.
Figure 1.
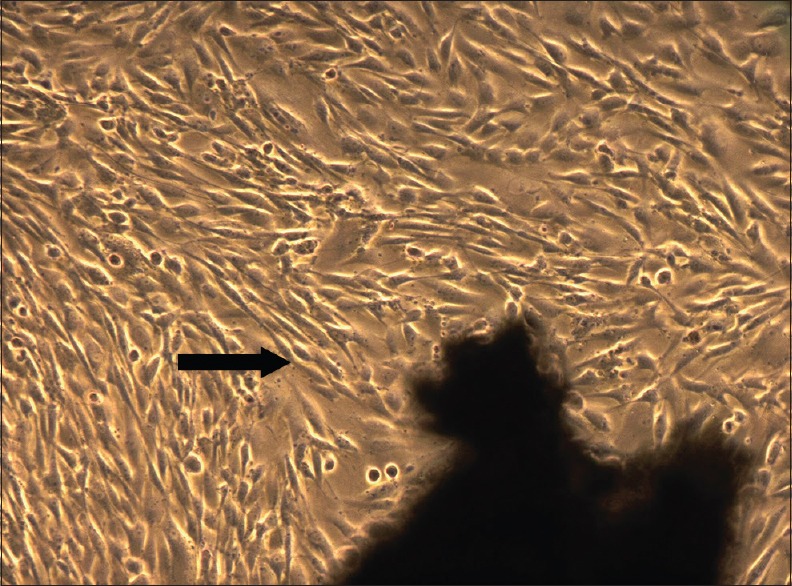
Dental pulp stem cells observed under phase contrast microscope, 7th day (5X magnification)–confluent cell layer observed
Figure 2.
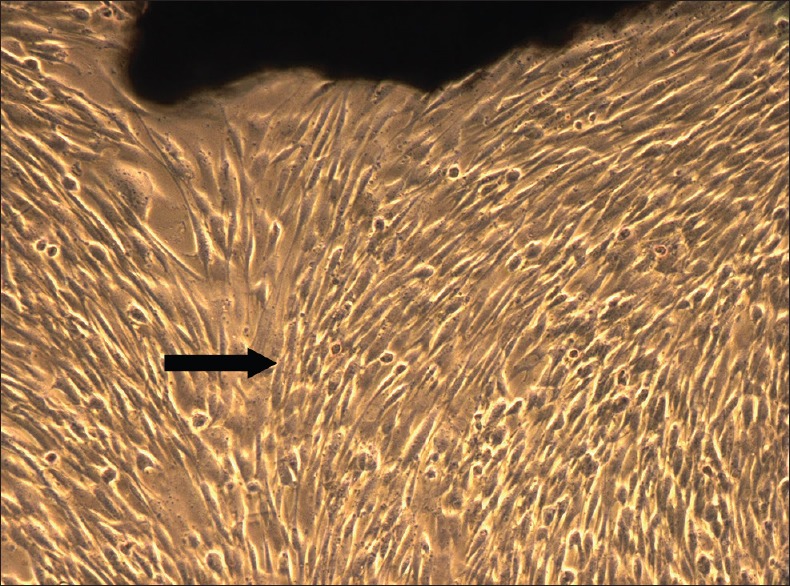
Periodontal ligament stem cell observed under phase contrast microscope, 7th day (5X magnification)–confluent cell layer observed
Preparation of platelet-rich fibrin
Blood samples of the respective subjects were withdrawn in 10 ml glass-coated plastic tubes (vacutainers) without any anticoagulant. Samples were centrifuged immediately at 3000 rpm for 10 min. The resultant layers formed comprised of a base of red blood cells, acellular plasma on the top, and a PRF clot in the centre.[11] The fibrin clot was separated from the underlying red blood cells. The PRF clot was placed between sterile dry gauze, and gentle pressure was applied to make a membrane. The PRF membrane was cut into 1 cm × 1 cm dimensions and placed onto a culture dish. Each PRF membrane was covered with 5 ml suspension of cells in the culture dishes and incubated for 7 days. Culture medium was changed on day 1, 3, and 5 and observed under the phase contrast microscope. By the 7th day, the cells reached >80% confluency [Figure 3]. These cells were then detached using trypsin and checked for cell viability.
Figure 3.
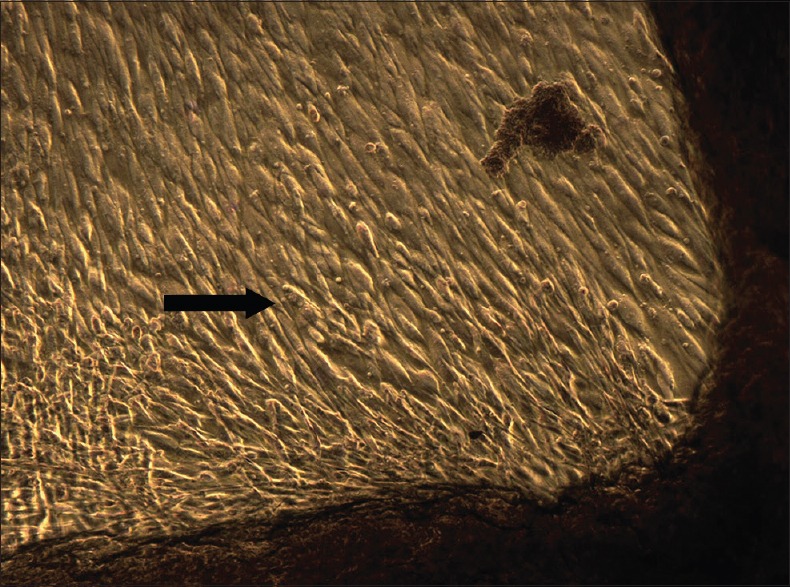
Cells seeded with platelet-rich fibrin, 7th day (5X magnification)–confluent cell layer observed
Cell viability
A cell suspension of an approximate concentration of 1 × 105 to 2 × 105 cells/ml was prepared by diluting cells in a complete media without serum. 0.5 ml of this cell suspension was placed in a screw cap test tube. 0.1 ml of 0.4% Trypan Blue Stain was added to the cell suspension. The screw cap test tube was shaken and then left undisturbed for 5 min at room temperature (15°C–30°C). A hemocytometer was filled with the suspension and observed under a microscope. Nonviable cells were stained, however, the viable cells did not take up the stain [Figure 4].[13]
Figure 4.
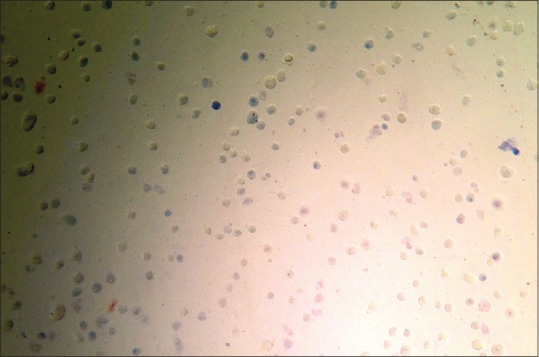
Hemocytometer as observed under light microscope-nonviable cells are stained and viable cells excluded the stain
Immunocytochemistry
The procedure for immunochemistry utilized 4% paraformaldehyde for fixing the cells for duration of 15 min. Following fixing, 0.25% Triton X-100 was used permeabilizing the cells. The next step included blocking and incubating the cells with (Mouse monoclonal CD73 and CD34) primary antibodies for 1 h. A rabbit secondary antibody was utilized for incubating for 45 min. Incubation procedure was then carried out using 0.1–1 μg/ml Hoechst or 4',6-diamidino-2-phenylindole (DNA stain) for a period for 1 min.[14]
Statistical analysis
SPSS (statistical package for social sciences) version 21.0 (IBM Corp: Armonk, NY, USA)and Epi-info version 3.0 were selected for analyzing the results statistically. The analysis was done using unpaired or independent t-test for comparison of mean value between 2 groups and paired or dependent t-test was used for comparison of 2 mean values obtained from a same group or a pair of values obtained from the same sample. The P value was taken significant when < 0.05 (P < 0.05) and Confidence interval of 95% was taken.
RESULTS
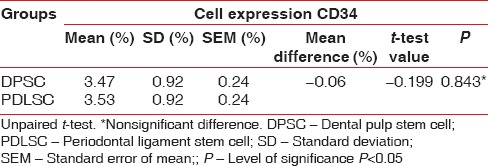
In the present study, the mean number of cell for DPSC was 4.49 × 105 and for the PDLSC was 4.51 × 105 [Table 1]. There was no statistically significant difference in the number of cells obtained from the DPSC and PDLSC. The mean live cell viability was compared between DPSC (98.07%) and PDLSC (98%) [Table 2]. Statistically, no significant difference was observed between the DPSC and PDLSC. Both DPSC and PDLSC showed a high percentage of expression of CD73 markers [Figure 5], 30.40% and 29.80%, respectively [Table 3]. The difference between DPSC and PDLSC was not statistically significant. However, DPSCs and PDLSCs lacked expression of hematopoietic marker, CD34 [Figure 6] expressing only 3.47% and 3.53%, respectively [Table 4].
Table 1.
Comparison of number of cells between dental pulp stem cell and periodontal ligament stem cell
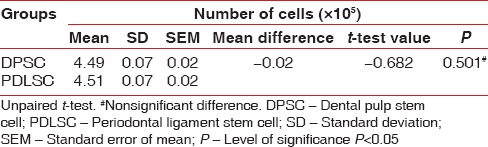
Table 2.
Comparison of live cell viability between dental pulp stem cell and periodontal ligament stem cell
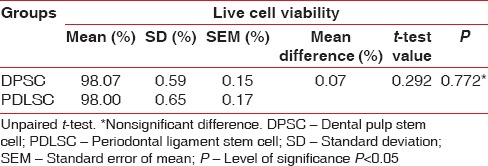
Figure 5.
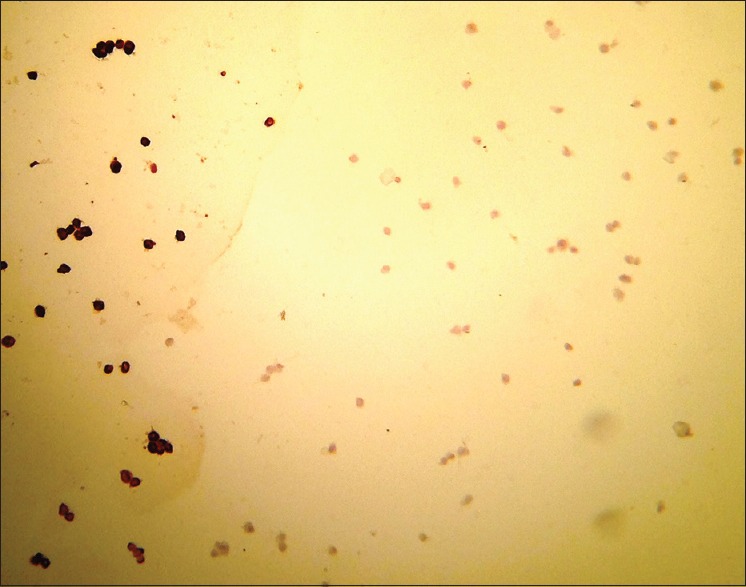
CD73 observed under light microscope-positive cells are dark stained cells
Table 3.
Comparison of cell expression of CD73 between dental pulp stem cell and periodontal ligament stem cell
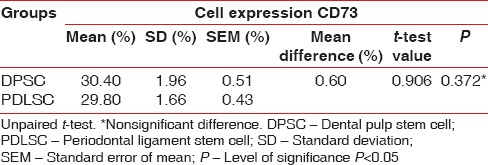
Figure 6.
CD34 observed under light microscope-positive dark stained cells are not seen
Table 4.
Comparison of cell expression of CD34 between dental pulp stem cell and periodontal ligament stem cell
In this study, the cell viability of DPSC and PDLSC cultured with PRF was compared with the viability of DPSC and PDLSC cultured in vitro, respectively. Statistically no significant differences were seen between the two groups during the 7-day culture period [Table 5]. PRF membrane as a scaffold exhibited no cytotoxic effects on DPCS's or PDLSC's. The cells were found attached to the edges of the PRF membrane under observation by phase-contrast microscopy, exhibiting a spindle-shaped morphology.
Table 5.
Comparison of live cell viability between dental pulp stem cell and periodontal ligament stem cell cultured with platelet-rich fibrin

DISCUSSION
In the present study, functional and quantifiable differences between DPSCs and PDLSC were evaluated. 15 systemically healthy subjects requiring third molar or orthodontic premolar extractions were included in the study. The dental pulp and the periodontal ligament tissues were procured from the extracted teeth followed by the preparation of explants. Growth media was added to the culture dishes containing the explants and placed in the incubator. The growth media was periodically changed and the culture dishes were checked for stem cells on the 7th day under a phase contrast microscope and assessed for their viability. The cells were then subcultured and further investigated for their viability and proliferation on a PRF membrane.
Adult stem cells were successfully isolated from human dental tissues in a study conducted by Young Jo et al., 2007. The authors isolated cells from periodontal ligament (PDLSC), dental pulp (DPSC), mandibular bone marrow (MBMSC) and the periapical follicle (PAFSC) and studied their properties. Stem cells from derived from these sources had the potential to differentiate into mineral nodule forming cells and adipocytes.[15]
Shi et al. in 2005, in an ex vivo study expanded stem cells from the dental pulp, periodontal ligament, and human exfoliated deciduous teeth. These cells extracted from different sources presented various markers in relation to mesenchymal stem cells, bone, endothelium, dentin, neural tissue, and smooth muscle.[16]
On comparing DPSC and PDLSC, difference in the number of cells was statistically insignificant (DPSC was 4.49 × 105 and for the PDLSC was 4.51 × 105) and cell viability (DPSC - 98.07% and PDLSC - 98%) was observed. Both DPSC and PDLSC showed a high percentage of expression of CD73 markers, 30.40% and 29.80%, respectively. However, DPSC and PDLSC lacked expression of hematopoietic marker, CD34 expressing only 3.47% and 3.53%, respectively.
The findings of the current study are in accordance to a study done by Hakki et al., 2015. The authors successfully isolated and compared the stemness and proliferation of the stem cells derived from the pulp and periodontal ligament. Both cells showed high expression of CD73 whereas the expression of CD34 was < 2%.[17] These results coincide with a study conducted by Ponnaiyan et al. 2012. The authors analyzed the immune-phenotypical differences between DPSC and PDLSC. They concluded that DPSC and PDLSC showed strong expression of CD73 and minimal expression of CD34.[18]
In this study, the cell viability of DPSC and PDLSC cultured with PRF was compared with the viability of DPSC and PDLSC cultured in vitro, respectively. Statistically, no significant differences were seen between the two groups during the 7-day culture period. Li et al. in 2013 stated that PRF is a functionally complex regenerative scaffold favoring tissue-specific alveolar bone augmentation and soft-tissue regeneration through progenitor-specific mechanisms.[19]
In the present study, PRF membrane as a scaffold, exhibited no cytotoxic effects on DPCS's or PDLSC's. The cells were found attached to the edges of the PRF membrane under observation by phase-contrast microscopy, exhibiting a spindle-shaped morphology. These results are in agreement to a study done by Huang et al. in 2010.[12] Similar results were shown in studies done by Wu et al., 2009,[20] Dohan et al., 2009,[21] and Chang et al., 2010.[22] These studies concluded that PRF demonstrated no cytotoxicity toward cells including preadipocytes, dermal prekeratinocytes, osteoblasts, oral epithelial cells, gingival fibroblasts, and periodontal ligament cells. Consistently osteoblasts, gingival fibroblast, and periodontal ligament cells were found to be attached at the edge of PRF membrane.
The study thus indicates that dental pulp and periodontal ligament are both rich sources of mesenchymal stem cells and can be successfully used for obtaining stem cells. Dental pulp and periodontal ligament-derived stem cells isolated from the same subject show similar cell expression of CD73 and CD34. However, further studies are required for better understanding of cell differentiation mechanisms. This would enable the use of optimal culture conditions to attain ideal cell populations for the treatment of diseases.
Further studies are vital to highlight the exact mechanism of action of PRF for dental tissue regeneration both in vitro and in vivo. In vitro, experiments are very helpful to assay the biological effects of PRF, but they are limited in their ability to stimulate the clinical conditions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Proceedings of the World Workshop in Clinical Periodontics. Chicago: The American Academy of Periodontology; 1989. The American Academy of Periodontology; pp. I/23–4. [Google Scholar]
- 2.Garrett S, Bogle G. Periodontal regeneration: A review of flap management. Periodontol 2000. 1993;1:100–8. [PubMed] [Google Scholar]
- 3.Karring T, Nyman S, Gottlow J, Laurell L. Development of the biological concept of guided tissue regeneration – Animal and human studies. Periodontol 2000. 1993;1:26–35. [PubMed] [Google Scholar]
- 4.Kao RT, Conte G, Nishimine D, Dault S. Tissue engineering for periodontal regeneration. J Calif Dent Assoc. 2005;33:205–15. [PubMed] [Google Scholar]
- 5.Narang S, Sehgal N. Stem cells: A potential regenerative future in dentistry. Indian J Hum Genet. 2012;18:150–4. doi: 10.4103/0971-6866.100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rai S, Kaur M, Kaur S. Applications of stem cells in interdisciplinary dentistry and beyond: An overview. Ann Med Health Sci Res. 2013;3:245–54. doi: 10.4103/2141-9248.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 8.Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 9.Melcher AH. Cells of periodontium: Their role in the healing of wounds. Ann R Coll Surg Engl. 1985;67:130–1. [PMC free article] [PubMed] [Google Scholar]
- 10.McCulloch CA, Nemeth E, Lowenberg B, Melcher AH. Paravascular cells in endosteal spaces of alveolar bone contribute to periodontal ligament cell populations. Anat Rec. 1987;219:233–42. doi: 10.1002/ar.1092190304. [DOI] [PubMed] [Google Scholar]
- 11.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Huang FM, Yang SF, Zhao JH, Chang YC. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J Endod. 2010;36:1628–32. doi: 10.1016/j.joen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Freshney RI. Culture of Animal Cells-A Manual of Basic Technique. New York: Alan R. Liss, Inc.; 1987. p. 117. [Google Scholar]
- 14.Nethercott HE, Brick DJ, Schwartz PH. Immunocytochemical analysis of human pluripotent stem cells. Methods Mol Biol. 2011;767:201–20. doi: 10.1007/978-1-61779-201-4_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73. doi: 10.1089/ten.2006.0192. [DOI] [PubMed] [Google Scholar]
- 16.Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S, et al. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–9. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 17.Hakki SS, Kayis SA, Hakki EE, Bozkurt SB, Duruksu G, Unal ZS, et al. Comparison of mesenchymal stem cells isolated from pulp and periodontal ligament. J Periodontol. 2015;86:283–91. doi: 10.1902/jop.2014.140257. [DOI] [PubMed] [Google Scholar]
- 18.Ponnaiyan D, Bhat KM, Bhat GS. Comparison of immuno-phenotypes of stem cells from human dental pulp and periodontal ligament. Int J Immunopathol Pharmacol. 2012;25:127–34. doi: 10.1177/039463201202500115. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Pan S, Dangaria SJ, Gopinathan G, Kolokythas A, Chu S, et al. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. Biomed Res Int 2013. 2013:638043. doi: 10.1155/2013/638043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CL, Lee SS, Tsai CH, Lu KH, Zhao JH, Chang YC, et al. Platelet-rich fibrin increases cell attachment, proliferation and collagen-related protein expression of human osteoblasts. Aust Dent J. 2012;57:207–12. doi: 10.1111/j.1834-7819.2012.01686.x. [DOI] [PubMed] [Google Scholar]
- 21.Dohan Ehrenfest DM, Diss A, Odin G, Doglioli P, Hippolyte MP, Charrier JB, et al. In vitro effects of choukroun's PRF (platelet-rich fibrin) on human gingival fibroblasts, dermal prekeratinocytes, preadipocytes, and maxillofacial osteoblasts in primary cultures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:341–52. doi: 10.1016/j.tripleo.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Chang IC, Tsai CH, Chang YC. Platelet-rich fibrin modulates the expression of extracellular signal-regulated protein kinase and osteoprotegerin in human osteoblasts. J Biomed Mater Res A. 2010;95:327–32. doi: 10.1002/jbm.a.32839. [DOI] [PubMed] [Google Scholar]


