Abstract
Aim:
The aim of this study is to determine the effectiveness of stevia as a daily mouthrinse among 12–15 year-old schoolchildren in Nellore District, Andhra Pradesh.
Materials and Methods:
A randomized, controlled triple blind trial was carried out among 108 children in B. V. S Municipallity Girls High school in Nellore. Children were randomly allocated into four groups comprising 27 in each group. Four mouthrinses composed of 0.2% chlorhexidine gluconate; 0.05% sodium fluoride, 10.6% stevioside and placebo were provided to the study participants. Plaque index, gingival index, and International Caries Detection and Assessment System (ICDAS) II, respectively, were used for assessing oral condition. The mouthrinsing was carried out for 6 months.
Results:
Group C showed maximum reduction of 8% and 10% on plaque and gingival scores, respectively, followed by Group A and Group B. However, Group D participants showed 1.5% and 1.8% increase in plaque and gingival scores, respectively. Analysis of ICDAS scores at 6 months indicated that values recorded were same as baseline for all the three groups except that for Group D, there was an increase in the prevalence of cavitated lesion D2–6from 5.6% to 5.8%.
Conclusion:
Stevia demonstrated very potent antiplaque and antigingivitis properties as compared to other mouthrinses at the end of 6 months trial.
Key words: Antigingivitis, antiplaque, mouthrinse
INTRODUCTION
Oral diseases are certainly pandemic and are of such morbidity that the case for prevention and indeed treatment is harder to sustain. Nevertheless, for the most common dental diseases, caries, and periodontitis there is a widespread demand for the preventive strategies aimed at the individual or the population.
At the core of this preventive foundation is oral hygiene and plaque control. The area of oral hygiene has undergone recent developments that have turned a mundane participant into a field of surprising growth and research. Oral care regimens have included the cleansing of the teeth and gingiva with various implements and ingredients.[1]
Mechanical methods of plaque control are the most widely accepted techniques for plaque removal. Chemotherapeutics is a broad term encompassing agents that may affect microorganisms, hard and soft tissues in the oral cavity. Mouthrinsing is one of the routes of administration of antiplaque and antimicrobial agents. Beginning in the 1960's, the preventive and therapeutic studies of oral antimicrobials began to shift toward fluorides and chlorhexidine digluconate compounds for prevention and control of dental diseases.[1]
A natural, noncaloric sweetener stevia has recently received much interest for use in oral hygiene products as it is proved to be potent antimicrobial without local side effects. However, there is less literature regarding the usage of Stevia. The probable reason may be lack of economical interest in finding a healthy substitute for sugar. In this perspective, the present study aimed to determine the effectiveness of Stevia as a daily mouthrinse among 12–15-year-old schoolchildren in Nellore District, Andhra Pradesh.
MATERIAL AND METHODS
Study design
A randomized, controlled, triple blind, parallel repeated measure study was carried out between January 2014 and August 2014, among female children aged 12–15 years selected from secondary school of Nellore city, Andhra Pradesh.
Source of data
The source of data was primary in nature, and it was obtained through clinical examination.
Study setting
The study was carried out in the government school which was selected from the nearby locality of the Nellore city. Since this study was a clinical trial spread over a period of 6 months (January 2014 to August 2014) maintaining the interest of proximity and feasibility B. V. S Municipality Girls High School was selected.
Study participants
Female children of 12–15 years age from VII, VIII, and 1X standards who satisfied the inclusion criteria and had informed consent were recruited in the study till the estimated sample size was met. The female students were randomly numbered and allocated into four different groups by table of random numbers.
Ethical clearance and informed consent
The ethical clearance was obtained from institutional review board of Narayana Dental College and Hospital. Permission was obtained from the headmistress of the school to conduct the study in school premises. Purpose of the study was explained to the headmistress and parents/guardians of the children. The informed consent was obtained from their parents/guardians and the headmistress of the respective school.
Training and calibration exercises
Before starting the trial, multiple sessions of training for the assessment of clinical parameters were performed with a calibrated professional in the Department of Public Health Dentistry, Narayana Dental College and Hospital. After the examiner was trained according to the calibrated professional's criteria, the intraexaminer reproducibility was assessed using kappa statistics for plaque index (PlI), GI, International Caries Detection and Assessment System (ICDAS). The kappa value for PlI, GI, and ICDAS was 0.78 which was stated as acceptable.
Pilot study
A pilot study was conducted before the main study among 27 participants to standardize the methodology with diagnostic instruments and criteria, data recording, mouthrinsing procedure.
Sampling procedure
The sample size was estimated based on the previous reported literature of stevia and chlorhexidine conducted by Zanela et al.[2] and Slavutzky [3] which revealed the percentage plaque score reduction between the stevia and chlorhexidine as 82% and 41.2%, respectively. The formula for sample size calculation was as follows:
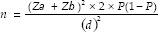
According to the percentage reduction of plaque scores of chlorhexidine and stevia as reported by literature P was calculated as follows:
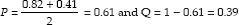
When the values are substituted in the for,
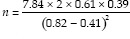
n = 22.
Sample size was 22 in each group. The estimated sample size was 22 in each group at an accepted minimum possible error of 0.05% and 90% power. To the sample of 22 participants, 25% of the sample was added to compensate for the loss of sample due to attrition over a period of 6 months, so the final sample consisted of 27 children.
Study procedure
The eligible students in the school were randomly allocated using table of random numbers into four groups that are Group A, Group B, Group C, and Group D. Narayana Pharmacy College was approached with a request to prepare daily the required quantity of mouthrinses containing 0.2% chlorhexidine, 0.05% sodium fluoride, and 10% stevia and placebo, in a precoded assignment, namely Group A, Group B, Group C, and Group D. The participants assigned with the particular group code were provided with a mouthrinse of same group.
The investigator did not know the composition of the mouthrinse received by participants of the respective group. The same was true for the participants and the biostatistician who analyzed the data; hence this, study is triple blinded. Once the study was completed the pharmacist of Narayana Pharmacy College did the decoding of four mouthrinse groups.
The students were instructed to immediately report any spells of sickness, change in taste perception, soreness of tongue, edema/redness, or burning sensation of the oral mucosa, visible staining during the study to the class teacher or the investigator. Such a finding was duly recorded in the pro forma.
Data collection instrument
At the baseline examination, a custom-made pro forma was used to record basic demography details. The students were examined on a straight chair under the natural light using a mouth mirror, probe, explorer, and chip blower. Clinical examinations were performed by the investigator herself for recording PlI as suggested by Silness and Loe [4] and gingival bleeding using the criteria suggested by Loe and Silness [5] and ICDAS II [6] criteria. The examinations were conducted at baseline, 3 months, and 6 months.
Monitoring of the daily mouthrinsing activity
The investigator herself supervised the daily mouthrinsing activity performed by the participants in the school. The class teacher and also the respective group leader were trained to carry out the activity in the absence of investigator.
Statistical analysis
Demographic details and oral hygiene practices were analyzed at baseline using descriptive statistics. For comparison of proportions of oral hygiene practices between groups, Chi-square test was used. One-way Analysis of Variance was used to compare the mean plaque and gingival score values between groups. Tukey honestly significant difference post hoc test was used for multiple comparisons of mean plaque and gingival score values between groups. Mean and percentage change in plaque, gingival scores, and dental caries prevalence within groups at different time periods were also calculated using descriptive statistics.
RESULTS
Demographic details of study participants
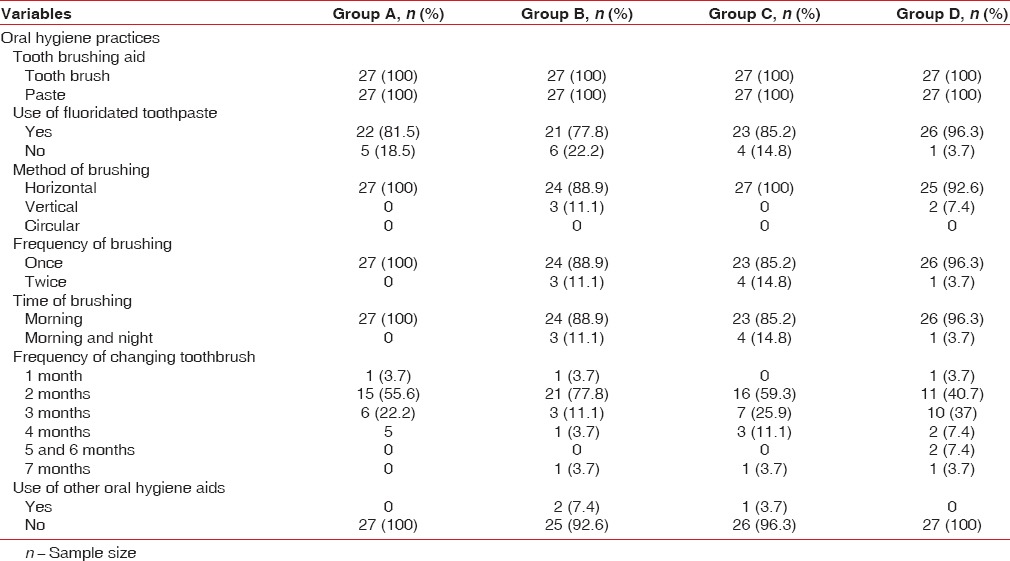
Analysis of data revealed that all the female participants from the four groups were using toothbrush and toothpaste for cleaning. Majority of the participants from four groups were using fluoridated toothpaste as material aid for cleaning and were practicing horizontal method for brushing with a habit of once daily in the morning. The frequency of change of toothbrush was once in 2 months by majority of the participants in four groups. However, no other oral hygiene aids were used by the majority of the study participants [Table 1].
Table 1.
Distribution of study participants according to oral hygiene practices
Baseline evaluation
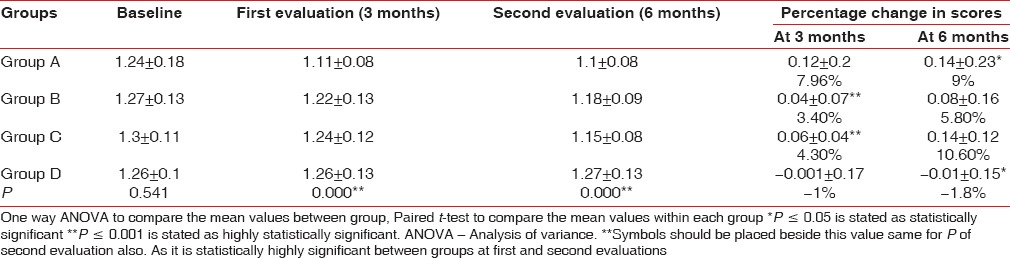
Analysis of clinical parameters recorded at baseline revealed a mean PlI and gingival index (GI) scores of 1.08 ± 0.08 and 1.24 ± 0.18 in participants of GroupA, 1.16 ± 0.13 and 1.27 ± 0.13 in participants of Group B, 1.15 ± 0.11 and 1.3 ± 0.11 in participants of Group C, and 1.09 ± 0.1 and 1.26 ± 0.1 in participants of Group D [Tables 2 and 3].
Table 2.
Comparison of mean plaque scores of the study participants at baseline, first follow-up and second follow-up evaluation
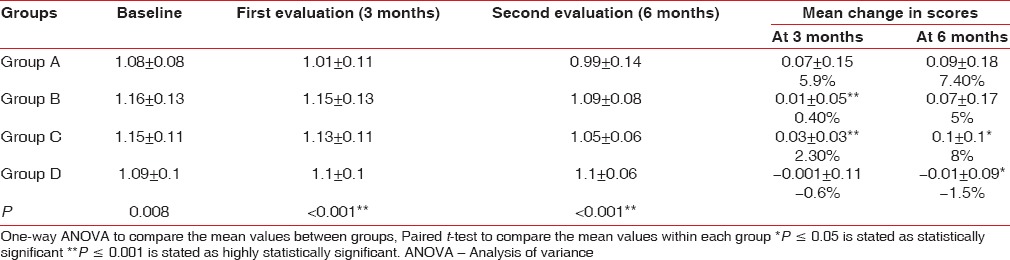
Table 3.
Comparison of mean gingival scores of the study participants at baseline, first follow-up and second follow-up evaluations
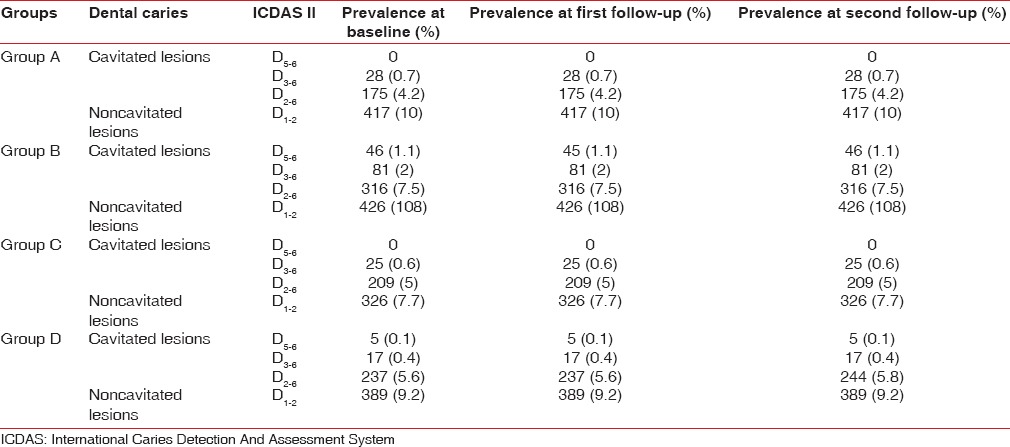
At baseline, ICDAS scores were divided into two categories cavitated (D5–6,D3–6,D2–6) and noncavitated (D1–2). Overall, the prevalence of decayed (cavitated) surfaces at baseline for Group A participants was D3–6-0.7%,D2–6- 4.2% and noncavitated surfaces was D1–2- 10%. Group B participants showed the prevalence of decayed (cavitated) surfaces D5–6- 1%,D3–6- 2%,D2–6- 7.5% and noncavitated surfaces was D1–2- 10%. Group C participants showed prevalence of decayed (cavitated) surfaces of D3–6- 0.6%, D2–6- 5%, and noncavitated surfaces were D1–2- 7.7%. Group D participants showed prevalence of decayed (cavitated) surfaces of D5-6- 0.1%,D3–6- 0.4%,D2–6- 5.6% and noncavitated D1–2- 9.2% at baseline [Table 4].
Table 4.
Comparison of prevalence of dental caries (International Caries Detection and Assessment System II) among study participants at baseline, first follow-up and second follow-up evaluation
First evaluation
The first evaluation was performed after 3 months of the start of intervention.
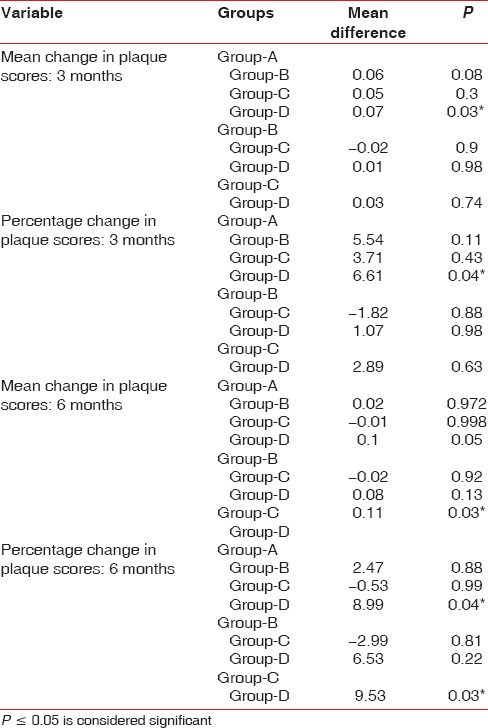
Group A showed 6% (mean difference = 0.07 ± 0.15) reduction in plaque scores at first evaluation. When group B and Group C were compared at the first evaluation with their baseline scores, 0.4% (mean difference = 0.01 ± 0.05) and 2.3% (mean difference = 0.03 ± 0.03) reduction in plaque scores was observed, respectively, which was found to be statistically highly significant (P < 0.001). However, for Group D participants, there was 0.6% increase in plaque scores (mean difference = −0.001 ± 0.11) [Table 2 and Figure 1].
Figure 1.
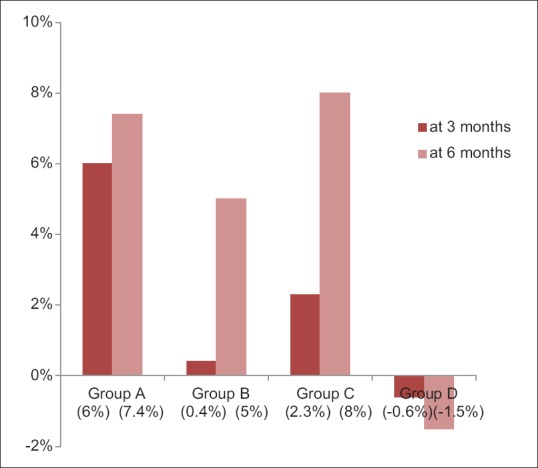
Percentage change in Plaque scores at first and second evaluations among subjects of four groups
Tukey post hoc analysis was done for between-group comparison; it revealed that Group A had significant improvement (P = 0.04) on plaque scores when compared to the other reference groups at 3 months evaluation [Table 5].
Table 5.
Mean plaque scores comparisons between groups using post hoc analysis
Group A showed 8% (mean difference = 0.12 ± 0.2) reduction in gingival scores at first evaluation. When group B and Group C were compared at the first evaluation with their baseline scores, 3.4% (mean difference = 0.04 ± 0.07) and 4.3% (mean difference = 0.06 ± 0.04) reduction was observed, respectively, which was found to be statistically highly significant P < 0.000. However, for Group D participants, there was 1% increase in gingival scores (mean difference = -0.001 ± 0.17) [Table 3 and Figure 2].
Figure 2.
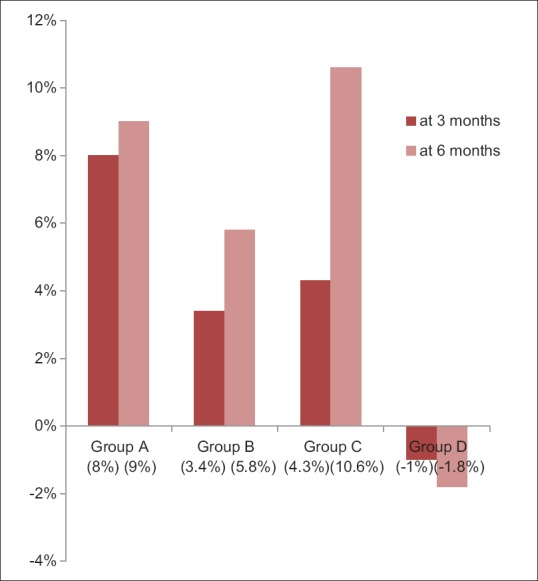
Percentage change in Gingival scores at first and second evaluations among subjects of four groups
Tukey post hoc analysis was done for between-group comparison; it revealed that Group A had significant improvement (P = 0.01) on gingival scores when compared to the other reference groups at 3 months evaluation [Table 6].
Table 6.
Mean gingival scores comparisons between groups using post hoc analysis
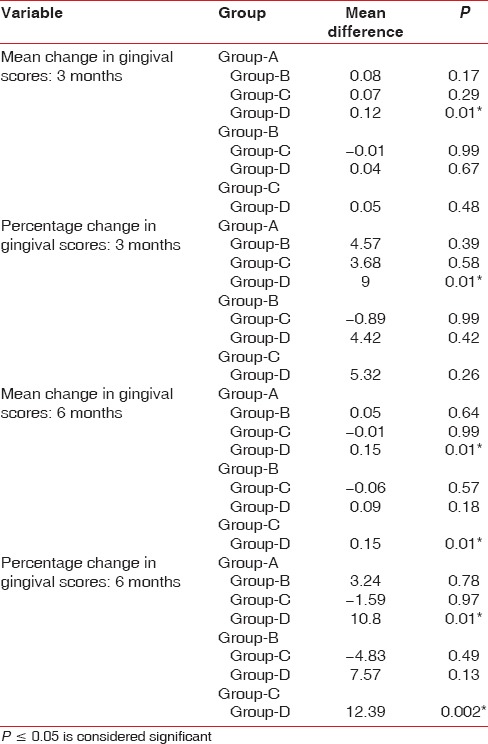
Analysis of ICDAS scores at 3 months indicated that values recorded were same as baseline for all the four groups [Table 4].
Second evaluation
The second evaluation was performed after 6 months of the start of the intervention.
Group A and Group B showed 7.4% (mean difference = 0.09 ± 0.18) and 5% (mean difference = 0.07 ± 0.17) reduction in plaque scores at second evaluation, respectively. When group C and Group D were compared at the second evaluation with their baseline scores, 8% (mean difference = 0.1 ± 0.1) reduction and 2.3% (mean difference = −0.01 ± 0.09) increase in plaque scores was observed, respectively, which was found to be statistically significant (P < 0.05) [Table 2 and Figure 1].
Tukey post hoc analysis was done for between-group comparison; it revealed that Group C had a significant improvement on and plaque scores when compared to the other reference groups at 6 months evaluation (P = 0.03) [Table 5].
Group B and C showed 5.8% (mean difference = 0.08 ± 0.16) and 10.6% (mean difference = 0.14 ± 0.12) reduction in gingival scores at second evaluation, respectively. When group A and Group D were compared at the second evaluation with their baseline scores, 9% (mean difference = 0.08 ± 0.16) reduction and 1.8% (mean difference = −0.01 ± 0.15) increase in gingival scores was observed, respectively, which was found to be statistically significant (P = 0.006) [Table 3 and Figure 2].
Tukey post hoc analysis was done for between-group comparison, it revealed that Group C had significant improvement on and gingival scores when compared to the other reference groups at 6 months evaluation (P = 0.002) [Table 6].
Analysis of ICDAS scores at 6 months indicated that values recorded were same as baseline for all the three groups except that for Group D there was increase in the prevalence of cavitated lesion D2-6 from 5.6% to 5.8% (the average number of decayed surfaces [n] increased from 237 to 244) [Table 4].
DISCUSSION
Once the study was completed the pharmacist of Narayana Pharmacy College who did the blinding and supplied the mouth rinses in coded assignments did the decoding to reveal the contents of the mouth rinses as follows:
Group A: chlorhexidine - 0.2%, Group B: Sodium fluoride - 0.05%, Group C: Stevia - 10%, Group D: Placebo.
There was not much difference in plaque and gingival scores of the study participants among all the groups at baseline because the present study was designed in such a way to simulate or reproduce the natural oral conditions; therefore, neither oral prophylaxis was performed nor were any oral hygiene instructions given. However, it is contradictory to many other studies were oral prophylaxis done before intervention with the purpose of making the dentition 100% free of plaque and calculus to make comparisons between groups effectively Van Strydonck DA et al.[7] Prevalence of dental caries which was recorded using ICDAS II criteria also did not show significant differences among all the four groups.
PlI and gingival scores of study participants at first evaluation (3 months) and second evaluation (6 months) were compared with baseline. It was found that there was a significant reduction in mean PlI and GI scores for chlorhexidine, sodium fluoride and stevia groups. However, there was increase in PlI and GI scores among the participants who used placebo rinse.
Chlorhexidine had significant improvement on plaque and gingival scores when compared to the other reference groups at 3 months evaluation as similar to studies reported by Löe and Schiott,[8] Flötra et al.,[9] Löe et al.,[10] Lang et al.,[11] Jenkins et al.[12]
This study showed that stevia mouthrinse is superior in reducing in mean PlI and GI scores at the end of 6 months as compared to chlorhexidine, sodium fluoride and placebo groups because Stevia contains tannins, xanthines (theobromine and caffeine) and flavonoids that have antiplaque activity as similar to studies reported by Menaker,[13] Yabu et al.[14] Moreover Stevia is heat-stable, resistant to acid hydrolysis, and nonfermentable by oral bacteria reported by Kinghorn et al.[15]
Interestingly in this study, the initial lesions which were diagnosed according to ICDAS II criteria remained the same throughout the study period which signifies that chlorhexidine, sodium fluoride and stevia mouthrinses were effective in arresting the carious lesions at the initial stages.
CONCLUSION
This study documented that among experimental groups stevia was more effective in reduction of plaque and gingivitis when compared to other reference groups. Furthermore, this study highlights the effectiveness of stevia on plaque and gingivitis when used over a period of 6 months as there are no other studies reported in literature. Further studies for investigation of the effect of stevia use on dental caries are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements:
I would like to express my gratitude to Dr. Nusrath Fareed, Professor of Public Health Dentistry for guiding me to select this topic and Bhoopathi srinivasan for helping me to in statistical analysis.
REFERENCES
- 1.Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988;15:488–98. doi: 10.1111/j.1600-051x.1988.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 2.Zanela NL, Bijella MF, Rosa OP. The influence of mouthrinses with antimicrobial solutions on the inhibition of dental plaque and on the levels of mutans streptococci in children. Pesqui Odontol Bras. 2002;16:101–6. doi: 10.1590/s1517-74912002000200002. [DOI] [PubMed] [Google Scholar]
- 3.Slavutzky SM. Stevia and sucrose effect on plaque formation. J Verbr Lebensm. 2010;5:213–6. [Google Scholar]
- 4.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 5.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 6.Ismail AI, Sohn W, Tellez M, Amaya A, Sen A, Hasson H, et al. The International Caries Detection and Assessment System (ICDAS): An integrated system for measuring dental caries. Community Dent Oral Epidemiol. 2007;35:170–8. doi: 10.1111/j.1600-0528.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 7.Van Strydonck DA, Timmerman MF, van der Velden U, van der Weijden GA. Plaque inhibition of two commercially available chlorhexidine mouthrinses. J Clin Periodontol. 2005;32:305–9. doi: 10.1111/j.1600-051X.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 8.Löe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5:79–83. doi: 10.1111/j.1600-0765.1970.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 9.Flötra L, Gjermo P, Rölla G, Waerhaug J. A 4-month study on the effect of chlorhexidine mouth washes on 50 soldiers. Scand J Dent Res. 1972;80:10–7. doi: 10.1111/j.1600-0722.1972.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 10.Löe H, Schiött CR, Karring G, Karring T. Two years oral use of chlorhexidine in man. I. General design and clinical effects. J Periodontal Res. 1976;11:135–44. doi: 10.1111/j.1600-0765.1976.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 11.Lang NP, Hotz P, Graf H, Geering AH, Saxer UP, Sturzenberger OP, et al. Effects of supervised chlorhexidine mouthrinses in children. A longitudinal clinical trial. J Periodontal Res. 1982;17:101–11. doi: 10.1111/j.1600-0765.1982.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins S, Addy M, Newcombe RG. Dose response of chlorhexidine against plaque and comparison with triclosan. J Clin Periodontol. 1994;21:250–5. doi: 10.1111/j.1600-051x.1994.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 13.Menaker L. Caries Dentarias: Bases Biológicas. Rio de Janeiro: Guanabara Koogan; 1989. p. 461. [Google Scholar]
- 14.Yabu M, Takase M, Toda K, Tanimoto K, Yasutake A. Studies on stevioside, natural sweetener. Effect on the growth of some oral microorganisms (author's transl) Hiroshima Daigaku Shigaku Zasshi. 1977;9:12–7. [PubMed] [Google Scholar]
- 15.Kinghorn AD, Kaneda N, Baek NI, Kennelly EJ, Soejarto DD. Noncariogenic intense natural sweeteners. Med Res Rev. 1998;18:347–60. doi: 10.1002/(sici)1098-1128(199809)18:5<347::aid-med5>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]


