Abstract
In this study, we evaluate dosimetric advantages of using patient‐specific aperture system with intensity‐modulated proton therapy (IMPT) for head and neck tumors at the shallow depth. We used four types of patient‐specific aperture system (PSAS) to irradiate shallow regions less than 4 g/cm2 with a sharp lateral penumbra. Ten head and neck IMPT plans with or without aperture were optimized separately with the same 95% prescription dose and same dose constraint for organs at risk (OARs). The plans were compared using dose volume histograms (DVHs), dose distributions, and some dose indexes such as volume receiving 50% of the prescribed dose (V50), mean or maximum dose (Dmean and Dmax) to the OARs. All examples verified in this study had decreased V50 and OAR doses. Average, maximum, and minimum relative reductions of V50 were 15.4%, 38.9%, and 1.0%, respectively. Dmax and Dmean of OARs were decreased by 0.3% to 25.7% and by 1.0% to 46.3%, respectively. The plans with the aperture over more than half of the field showed decreased V50 or OAR dose by more than 10%. The dosimetric advantage of patient‐specific apertures with IMPT was clarified in many cases. The PSAS has some dosimetric advantages for clinical use, and in some cases, it enables to fulfill dose constraints.
Keywords: aperture, IMPT, lateral penumbra, proton beam, spot scanning
1. INTRODUCTION
In recent years, accelerators, irradiation control systems, and treatment planning systems have been improved and, as a result of these improvements, scanning proton therapy systems have become more widely used.1 In spot scanning irradiation, a monoenergetic beam is delivered to a given spot at the target volume specified in the treatment plan and a full scan across the target volume and the dose distribution in the depth direction is formed by multiple monoenergetic beams.2 The energy generated by a commercially available accelerator for proton therapy has a lower limit of approximately 4 g/cm2 water equivalent penetration because of the stability of the accelerator, beam efficiency, and effective spot size. This limitation complicates the treatment planning so correspond to this limitation. One method to irradiate shallower regions less than 4 g/cm2 is to use an energy absorber (EA); however, the monoenergetic beam with an EA has a large spot size due to scattering and has a limited ability to make sharp lateral penumbra, worsening the dose distribution. Although in some cases the scanning method has some issues with the lateral penumbra, it has some advantages over the passive method and x‐ray treatment, and a number of studies using scanning proton beams reported dose comparisons of passive beam and x‐ray beam3, 4 or verification of the relative biological effectiveness (RBE).5 Flexibility of scanning technique allows for various irradiation methods, and these methods can improve the dose distribution. Developments in treatment planning systems (TPS), treatment machines, and treatment techniques permit the use of intensity‐modulated proton therapy (IMPT) for various tumor sites.6, 7
Figure 1 shows the archived spot sizes with or without EA that were retrieved from the Hitachi ProBeat III operating in the Nagoya Proton Therapy Center (NPTC),8 and these data were presented in a previous paper.9 Without the EA, the maximum spot size in air was 13.8 mm at the isocenter plane and the minimum range was 4 g/cm2. Regions below 4 g/cm2 could be irradiated using EA, but the spot size of minimum range was more than 26.7 mm. These bigger lateral penumbras mean a worse dose distribution.10, 11 To avoid these issues, a variety of useful methods, such as advanced optimize method (contour scanning),12 improvement of beam line,13 and various type of aperture or collimator,14, 15, 16, 17 have been proposed. In the same way, methods to reduce spot size owing to close the bolus or nozzle to patient were also reported.18, 19 These methods enable to achieve a sharp lateral penumbra, better dose distribution, and lower out‐of‐field dose. Although various useful methods were reported in recent years, we started scanning treatment with a patient‐specific aperture system (PSAS) in May 2016 to irradiate shallow regions and to obtain sharp lateral penumbras. The PSAS has some downsides that require patient‐specific manufacture and the weight of aperture is a burden to therapists. However, the treatment procedures and QA of the PSAS methods were the same as those with non‐PSAS methods. Furthermore, PSAS methods do not require complicate equipment and machine maintenance such as MLC, so it is easy to apply PSAS in a clinical setting. In the previous study, we presented verification results of the PSAS for the spot scanning beam and the results showed that PSAS reduced the lateral penumbra by 30%–70% in the simple case.9 The PSAS can be used with both single‐field uniform dose (SFUD) and IMPT, but the dosimetric advantage of the patient‐specific aperture for IMPT has not been clarified. In this study, we clarified the dosimetric advantage of the aperture for IMPT using DVH, dose distribution, and some dose indexes.
Figure 1.

Air spot sizes (1σ) in the plane of the isocenter versus range. Data are measured with (w/) or without (w/o) the EA. D is the distance from the EA to the isocenter.
2. MATERIALS AND METHODS
2.A. Scanning delivery system with PSAS
The spot size of the proton beam increases because of scattering by the materials in the beam nozzle and the distance from the exit of beam transport system to the isocenter. Each spot is controlled by scanning magnet and the maximum field size is 30 × 30 cm2 in the NPTC. Ninety‐five energies are available for the scanning treatment that are ranging from 71.6 to 221.4 MeV, resulting in water equivalent penetration depths of 4–30.6 g/cm2 at intervals of 0.1 g/cm2 at low energy beam and 0.6 g/cm2 at high energy beam. The irradiation system is able to use a range shifter from 0.1 to 0.5 g/cm2 for fine adjustment of the range of high or middle energy beams. The lateral penumbra is generally affected by the distance from the collimator to the surface, depth, beam energy, and energy absorber thickness. Thus, we designed four types of PSAS with different field sizes and distances from the isocenter to put the aperture closer to the patient's body surface. Small PSASs allowed access over the patient's body or patient's immobilization devices for small tumors. The EA had 4 g/cm2 water equivalent thickness. The maximum water equivalent penetration with PSAS was restricted to 15 g/cm2 because the patient‐specific aperture was made from 3‐cm thick brass. Figure 2 and Table 1 showed schematic view and fundamental parameters of the four types of PSASs. The field sizes of the large PSASs (Types 1 and 2) and small PSASs (Types 3 and 4) were 25 × 25 cm2 and 10 × 10 cm2, respectively. The distance from the isocenter to the aperture was 150–345 mm. As Types 2 and 4 had the same isocenter‐to‐aperture distance, the spot sizes were the same and the largest spot size was 18.9 mm. Type 1 shows the largest spot size that is 26.7 mm.
Figure 2.
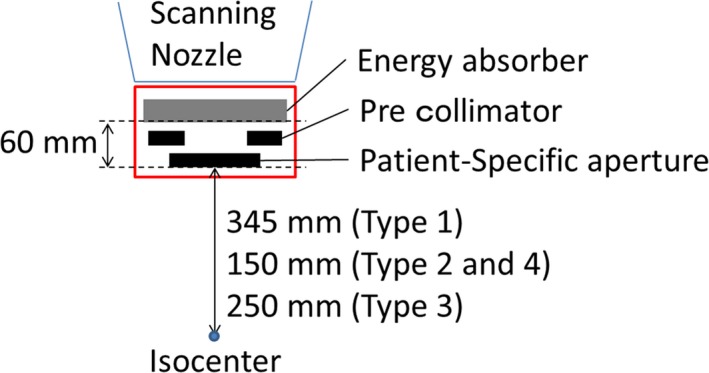
Schematic view of PSAS.
Table 1.
Fundamental parameters of the four types of PSAS
| Type | Field size (cm2) | Distance from the isocenter (mm) | Maximum spot size (mm) | |
|---|---|---|---|---|
| To aperture | To absorber | |||
| 1 | 25 × 25 | 345 | 405 | 26.7 |
| 2 | 25 × 25 | 150 | 210 | 18.9 |
| 3 | 10 × 10 | 250 | 310 | 22.9 |
| 4 | 10 × 10 | 150 | 210 | 18.9 |
2.B. Treatment planning and patient selection
TPS used in this study was VQA ver. 3.0.1 (Hitachi Ltd.). The VQA is a commercially available TPS in Japan. The VQA was used for spot scanning with or without PSAS. The VQA system uses a pencil beam algorithm with the triple Gaussian (TG) kernel model to improve the accuracy of dose calculation for the scanning beam.20 In order to handle the aperture, a fluence dose model21 was used. The dose calculation with PSAS has sufficiently high‐accuracy and details of commissioning results were showed in our previous study.9
Ten head and neck (HN) tumor cases treated by IMPT technique with the PSAS in NPTC were examined. HN tumors are a suitable region for this scanning method because most HN tumors have a complex geometry and CTV close to organ at risks (OARs). In this study, the target volumes ranged from 14.33 to 260.02 cm3 and tumor types were nasal cavity, sinus cavity, mandible, base of skull, and oral cavity. Beam angles of each plan were selected considering clinical conditions such as tumor location, tumor size, and OARs. We created IMPT plans with PSASs for actual patient treatment and without apertures for plan comparison. These plans were optimized separately with the same D95 prescription dose and same dose constraint for OAR. The same number of fields and gantry angles were used for each plan and higher priority set to the D95 prescription dose. As mentioned earlier, some fields could not use the PSAS because the PSAS has mechanical limitations such as maximum penetration depth, field size, distance from the isocenter and collision with other devices or the patient. In this study, 12 fields could not use PSAS. These 12 fields consisted of four exceeded penetration depth limit fields and eight fields that have mechanical collision between outer frame of PSAS and patient or treatment couch. The treatment plan information is listed in Table 2. All PSAS fields including without aperture fields listed in Table 2 were using the EA. We used a worst case optimization22 for dose optimization to obtain the robust plan. Parameters of the worst case optimization were 3 mm for x, y, z direction and 3.5% for range uncertainty,23 so 9 scenarios were considered to the target and OARs. The value of 3 mm is the uncertainty of patient setup for HN tumor and machine variability in NPTC. The value of 3.5% is range uncertainty resulting from uncertainties of the range calculation, the acquisition of CT number, and CT number‐Stopping power conversion table. CT images were acquired and reconstructed with 1 mm slices. In the aperture field, the aperture margin was required to assure the marginal dose of the target. The aperture margin was computed by expanding of the maximum outline of the target from the beam's eye view and the margin set to the same value as the spot spacing. The spot spacing was affected by spot size, so the aperture margin was being from 7.2 mm to 11.2 mm at isocenter.
Table 2.
Plan information with fields, gantry angle, prescribed dose, CTV volume, and OAR
| ID | Gantry angle (°) | Aperture type | Field aperture/all | Energy range (MeV) | Prescribed dose (GyE) | CTV volume (cc) | OAR |
|---|---|---|---|---|---|---|---|
| 1 | 0 | Type 2 | 1/3 | 142.7–76.2 | 60.8 | 258.9 | Lens |
| 120 | Non | 161.1–71.6 | Optic nerve | ||||
| 240 | Non | 163.4–71.6 | Parotid | ||||
| 2 | 30 | Type 3 | 3/3 | 144.4–81.4 | 70.2 | 14.3 | Parotid |
| 90 | Type 3 | 135.8–76.2 | |||||
| 140 | Type 4 | 146.3–77.9 | |||||
| 3 | 30 | Type 4 | 3/4 | 135.8–76.2 | 70.2 | 145.6 | Parotid |
| 65 | Type 3 | 130.5–76.2 | Tongue | ||||
| 105 | Type 1 | 130.5–77.0 | |||||
| 195 | Non | 173.3–107.3 | |||||
| 4 | 55 | Type 3 | 2/4 | 137.5–71.6 | 70.2 | 58.2 | Brain |
| 305 | Type 3 | 146.3–76.2 | Brain stem | ||||
| 115 | Non | 163.4–105.9 | Chiasm | ||||
| 260 | Non | 168.3–104.5 | |||||
| 5 | 0 | Type 3 | 1/3 | 148.3–76.2 | 70.2 | 260.0 | Chiasm |
| 130 | Non | 154.8–71.6 | Lens | ||||
| 230 | Non | 175.7–80.5 | Optic nerve | ||||
| 6 | 0 | Type 1 | 4/4 | 159–76.2 | 70.2 | 150.5 | Brain |
| 300 | Type 1 | 168.3–76.2 | Chiasm | ||||
| 100 | Type 4 | 146.3–76.2 | Eye | ||||
| 260 | Type 4 | 159–76.2 | Optic nerve | ||||
| 7 | 0 | Type 4 | 2/3 | 146.3–79.7 | 70.2 | 45.5 | Brain stem |
| 130 | Non | 170.8–112.7 | Chiasm | ||||
| 260 | Type 1 | 148.3–96.1 | Eye, optic nerve | ||||
| 8 | 0 | Type 4 | 2/3 | 154.8–77.9 | 70.2 | 60.7 | Brain stem |
| 110 | Type 3 | 165.7–93.5 | Chiasm | ||||
| 240 | Non | 163.4–81.4 | Eye, optic nerve | ||||
| 9 | 0 | Type 2 | 3/4 | 144.4–76.2 | 70.2 | 137.8 | Brain |
| 40 | Type 2 | 168.3–76.2 | Parotid | ||||
| 180 | Non | 175.7–107.3 | |||||
| 320 | Type2 | 168.3–76.2 | |||||
| 10 | 0 | Type 2 | 1/3 | 152.6–76.2 | 70.2 | 211.5 | Brain stem |
| 120 | Non | 168.3–71.6 | Chiasm | ||||
| 240 | Non | 168.3–71.6 | Eye, optic nerve |
2.C. Plan evaluation
We compared with‐ and without aperture plans using dose volume histogram (DVH), dose distribution, and some dose indexes. As mentioned earlier, we made with‐ or without aperture plans with the same target dose so that the 95% dose to the target was equal. In this study, we used relative reduction of some dose indexes such as volume receiving 50% of the prescribed dose (V50) and maximum dose (Dmax) or mean dose (Dmean) to the OARs. V50 was used for estimate to the out‐of‐field dose. The relevant OARs of each plan were varied so we evaluated the relevant OARs as described in Table 2. Lens, optic nerve, brain stem, chiasm, and eye were analyzed using Dmax. Parotid, tongue and brain were analyzed by Dmean.
3. RESULTS
Figure 3 shows examples of DVHs with and without aperture. The results show that the target doses were almost equal and some OARs doses were reduced. The most effective case was plan ID 6, in which V50 was decreased by 35.9%. In contrast, in plan ID 5, an ineffective case, V50 was decreased by only 3.6%. Figure 4 shows examples of dose distribution for both effective and ineffective cases. In the effective case of Fig. 4, all fields can use aperture and apertures are close to the patient surface, as results of which these plans have obviously sharp penumbras. Figure 5 shows the relative difference of V50 and Dmax or Dmean between with and without aperture. All examples verified in this study showed decreased V50 and OAR doses. The average, maximum, and minimum relative reductions of V50 were 15.4%, 38.9%, and 1.0%, respectively. The relative reductions of V50, Dmax, and Dmean are summarized in Fig. 5. Dmax and Dmean of OARs were decreased by 0.3% to 25.7% and by 1.0% to 46.3%, respectively. Plan IDs 2, 3, 6–9 used apertures for more than half of fields and decreased V50 or OAR doses more than 10%. In plan ID 6, Dmax of the chiasm decreased from 51.3 to 38.2 GyE (25.7%) and Dmean of the brain was decreased by 36.2%. The relative reduction of parotid Dmean in plan ID 3 was 45.3%. The dose constraints of the OARs of these plans were only fulfilled using aperture plans. At the same time, our PSASs have a maximum penetration depth and mechanical interference limitations so we could not use apertures for all fields. Plan IDs 1, 4, 5, and 10 could only use apertures for not more than half of fields because of the tumor depth or mechanical collision, so these cases showed only small effects.
Figure 3.

Examples of DVHs of an effective case (left) and an ineffective case (right). Left example is plan ID 6 and right example is plan ID 5. Solid lines are without (w/o) aperture and dashed lines are with (w/) aperture.
Figure 4.
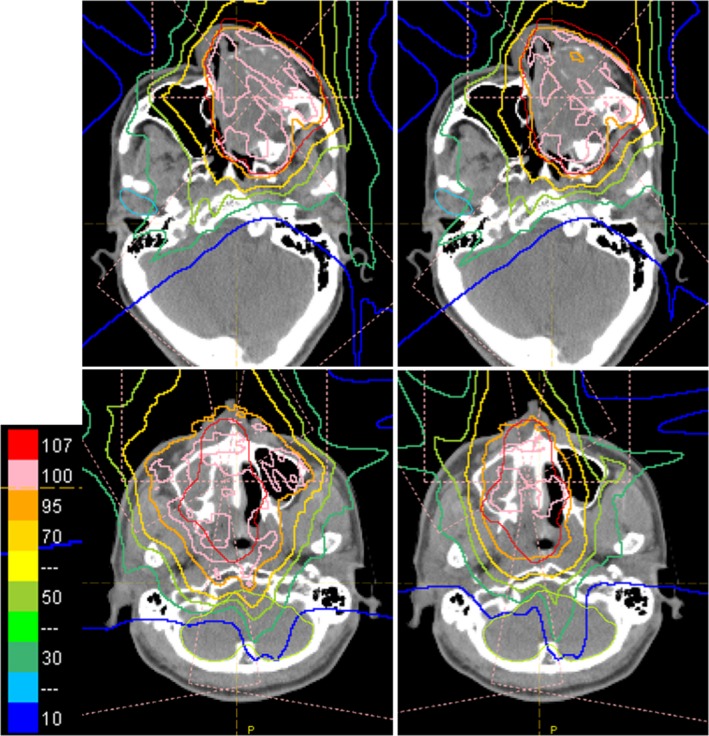
Examples of dose distribution of the ineffective case (upper) and effective case (lower). Upper example is plan ID 5 and lower example is plan ID 6. Right side of each case is with aperture and left side is without aperture. There are same plans shown in Fig. 3.
Figure 5.
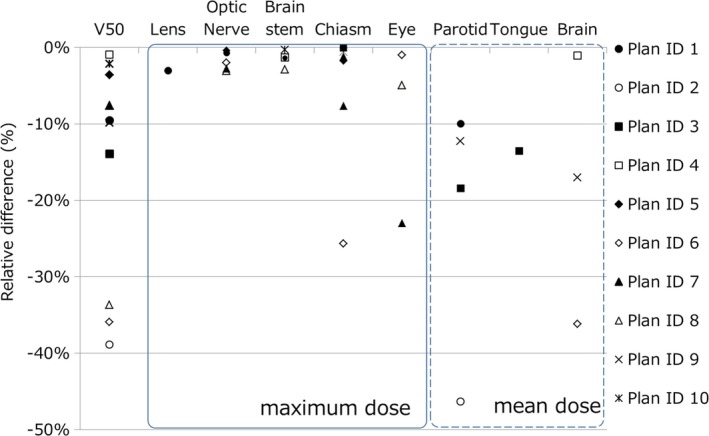
Relative reductions of V50, Dmax, and Dmean OAR doses between with and without aperture.
4. DISCUSSION
This study investigated the dosimetric advantages of the patient‐specific aperture to HN IMPT. The patient‐specific aperture reduced V50 and OAR doses as shown in Figs. 3, 4, 5 even when using IMPT. Plan dose constraints of ID 2, 6, 7 only fulfilled with the aperture plan and other plans can reduce unnecessary out‐of‐field dose. The decrease rate of Dmax in some cases was dramatic but other cases showed small effects because OARs were close to the CTV. In this study, we optimized plans while prioritizing the target dose so many maximum dose OARs showed minimal change. In addition, the aperture only collimates the outer line of the target from each beam's eye view and the current optimization algorithm of our TPS cannot consider OAR blocking when using the aperture. In the IMPT plan, the results might be improved by using OAR blocking with an aperture for each field. From the results of our study, using more than half of fields was needed to obtain effective results.
We clarified the dosimetric advantage of patient‐specific apertures with IMPT in many cases. However, PSAS requires patient‐specific manufacture, mounting during treatment and storage of the aperture after treatment, so it was not the best way from the view point of patient throughput and a burden on therapists, too. In addition, some fields could not use PSAS due to mechanical collision and limitation of penetration depth. To overcome these limitations, it is assumed that a movable nozzle and MLC system would improve the lateral penumbra and treatment throughput. Some additional methods have been investigated, for example, to use dynamic collimation with MLC (layer‐by‐layer collimation)24 or dynamic collimation (spot‐by‐spot collimation).16, 25 These methods will come into practical and might improve the dose distribution more. But at present, PSAS system showed dosimetric advantage for shallow region treatment, and in some case, it enables to fulfill dose constraints.
5. CONCLUSION
We herein reported improvement of dose distribution by using patient‐specific apertures with IMPT for shallow depth tumor. The PSAS has some dosimetric advantages for clinical use and is easy to use because it does not require complex machine or control mechanism. Using the PSAS has some demerits from the viewpoint of patient throughput and usability; however, it is useful for clinical application.
CONFLICT OF INTEREST
The authors have no conflicts of interest directly relevant to the content of this article.
ACKNOWLEDGMENTS
We thank the members of NPTC and Hitachi Ltd. who cooperated with this study.
REFERENCES
- 1. ICRU . Prescribing, recording, and reporting proton‐beam therapy. ICRU Report No. 78. Bethesda, MD, 2007. [Google Scholar]
- 2. Kanai T, Kawachi K, Kumamoto Y, et al. Spot scanning system for proton radiotherapy. Med Phys. 1980;7:365–369. [DOI] [PubMed] [Google Scholar]
- 3. Hashimoto S, Shibamoto Y, Iwata H, et al. Whole‐pelvic radiotherapy with spot‐scanning proton beams for uterine cervical cancer: a planning study. J Radiat Res. 2016;57:524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tran A, Zhang J, Woods K, et al. Treatment planning comparison of IMPT, VMAT and 4π radiotherapy for prostate cases. Radiat Oncol. 2017;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Iwata H, Ogino H, Hashimoto S, et al. Spot scanning and passive scattering proton therapy: relative biological effectiveness and oxygen enhancement ratio in cultured cells. Int J Radiat Oncol Biol Phys. 2016;95:95–102. [DOI] [PubMed] [Google Scholar]
- 6. Lomax AJ. Intensity modulation methods for proton radiotherapy. Phys Med Biol. 1999;44:185–205. [DOI] [PubMed] [Google Scholar]
- 7. Lomax AJ, Boehringer T, Coray A, et al. Intensity modulated proton therapy: a clinical example. Med Phys. 2001;28:317–324. [DOI] [PubMed] [Google Scholar]
- 8. Toshito T, Omachi C, Kibe Y, et al. A proton therapy system in Nagoya proton therapy center. Australas Phys Eng Sci Med. 2016;39:645–654. [DOI] [PubMed] [Google Scholar]
- 9. Yasui K, Toshito T, Omachi C, et al. A patient‐specific aperture system with an energy absorber for spot scanning proton beams: verification for clinical application. Med Phys. 2015;42:6999–7010. [DOI] [PubMed] [Google Scholar]
- 10. Moteabbed M, Yock TI, Depauw N, Madden TM, Kooy HM, Paganetti H. Impact of spot size and beam‐shaping devices on the treatment plan quality for pencil beam scanning proton therapy. Int J Radiat Oncol Biol Phys. 2016;95:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van de Water T, Lomax AJ, Bijl HP, Schilstra C, Hug EB, Langendijk JA. Using a reduced spot size for intensity‐modulated proton therapy potentially improves salivary gland‐sparing in oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2012;82:313–319. [DOI] [PubMed] [Google Scholar]
- 12. Meier G, Leiser D, Besson R, et al. Contour scanning for penumbra improvement in pencil beam scanned proton therapy. Phys Med Biol. 2017;62:2397–2416. [DOI] [PubMed] [Google Scholar]
- 13. Kralik JC, Xi L, Solberg TD, Simone CB, Lin L. Comparing proton treatment plans of pediatric brain tumors in two pencil beam scanning nozzles with different spot sizes. J Appl Clin Med Phys. 2015;16:5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charlwood FC, Aitkenhead AH, Mackay RI. A Monte Carlo study on the collimation of pencil beam scanning proton therapy beams. Med Phys. 2016;43:1462–1472. [DOI] [PubMed] [Google Scholar]
- 15. Hyer DE, Hill PM, Wang D, Smith BR, Flynn RT. A dynamic collimation system for penumbra reduction in spot‐scanning proton therapy: proof of concept. Med Phys. 2014;41:1701. [DOI] [PubMed] [Google Scholar]
- 16. Moignier A, Gelover E, Wang D, et al. Theoretical benefits of dynamic collimation in pencil beam scanning proton therapy for brain tumors: dosimetric and radiobiological metrics. Int J Radiat Oncol Biol Phys. 2016;95:171–180. [DOI] [PubMed] [Google Scholar]
- 17. Dowdell SJ, Clasie B, Depauw N, et al. Monte Carlo study of the potential reduction in out‐of‐field dose using a patient‐specific aperture in pencil beam scanning proton therapy. Phys Med Biol. 2012;57:2829–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jelen U, Bubula ME, Ammazzalorso F, Engenhart‐Cabillic R, Weber U, Wittig A. Dosimetric impact of reduced nozzle‐to‐isocenter distance in intensity‐modulated proton therapy of intracranial tumors in combined proton‐carbon fixed‐nozzle treatment facilities. Radiat Oncol. 2013;8:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Both S, Shen J, Kirk M, et al. Development and clinical implementation of a universal bolus to maintain spot size during delivery of base of skull pencil beam scanning proton therapy. Int J Radiat Oncol Biol Phys. 2014;90:79–84. [DOI] [PubMed] [Google Scholar]
- 20. Hirayama S, Takayanagi T, Fujii Y, et al. Evaluation of the influence of double and triple Gaussian proton kernel models on accuracy of dose calculations for spot scanning technique. Med Phys. 2016;43:1437. [DOI] [PubMed] [Google Scholar]
- 21. Schaffner B, Pedroni E, Lomax A. Dose calculation models for proton treatment planning using a dynamic beam delivery system: An attempt to include density heterogeneity effects in the analytical dose calculation. Phys Med Biol. 1999;44:27–41. [DOI] [PubMed] [Google Scholar]
- 22. Pflugfelder D, Wilkens JJ, Oelfke U. Worst case optimization: a method to account for uncertainties in the optimization of intensity modulated proton therapy. Phys Med Biol. 2008;53:1689–1700. [DOI] [PubMed] [Google Scholar]
- 23. Park PC, Zhu XR, Lee AK, et al. A beam‐specific planning target volume (PTV) design for proton therapy to account for setup and range uncertainties. Int J Radiat Oncol Biol Phys. 2012;82:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kanematsu N, Endo M, Futami Y, et al. Treatment planning for the layer‐stacking irradiation system for three‐dimensional conformal heavy‐ion radiotherapy. Med Phys. 2002;29:2823–2829. [DOI] [PubMed] [Google Scholar]
- 25. Moignier A, Gelover E, Smith BR, et al. Toward improved target conformity for two spot scanning proton therapy delivery systems using dynamic collimation. Med Phys. 2016;43:1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]


