Abstract
Background: Schizophrenia (SZ), bipolar disorder (BD), and major depressive disorder (MDD) are distinct diagnostic categories in current psychiatric nosology, yet there is increasing evidence for shared clinical and biological features in these disorders. No previous studies have examined brain structural features concurrently in these 3 disorders. The aim of this study was to identify the extent of shared and distinct brain alterations in SZ, BD, and MDD. We examined gray matter (GM) volume and white matter (WM) integrity in a total of 485 individuals (135 with SZ, 86 with BD, 108 with MDD, and 156 healthy controls [HC]) who underwent high-resolution structural magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) at a single site. Results: Significant 4-group (SZ, BD, MDD, and HC groups) differences (P < .05, corrected) in GM volumes were found primarily in the paralimbic and heteromodal corticies. Post hoc analyses showed that the SZ, BD, and MDD groups shared GM volume decreases in 87.9% of the total regional volume with significant 4-group differences. Significant 4-group differences in WM integrity (P < .05 corrected) were found in callosal, limbic-paralimbic-hetermodal, cortico-cortical, thalamocortical and cerebellar WM. Post hoc analyses revealed that the SZ and BD groups shared WM alterations in all regions, while WM alterations were not observed with MDD. Conclusions: Our findings of common alterations in SZ, BD, and MDD support the presence of core neurobiological disruptions in these disorders and suggest that neural structural distinctions between these disorders may be less prominent than initially postulated, particularly between SZ and BD.
Keywords: schizophrenia, bipolar disorder, major depressive disorder, gray matter volume, white matter integrity
Introduction
The Kraepelinian dichotomy, which classified dementia praecox and manic depressive psychosis as 2 distinct categories of endogenous psychosis, has been fundamental in the evolution of psychiatric nosology over the past century.1 This categorical approach has been highly influential in the development of the Diagnostic and Statistical Manual of Mental Disorders (DSM). In the DSM-III and its subsequent revisions (DSM-IIIR, DSM-IV, DSMIV-TR, and DSM-V), the major psychiatric manifestations are divided into 3 essential diagnostic categories, schizophrenia (SZ; dementia praecox), bipolar disorder (BD; manic depressive psychosis), and major depressive disorder (MDD). Subsequently, the field has approached SZ, BD, and MDD as distinct disease entities, and substantial efforts have been aimed at elucidating the biological basis for differentiation between these diagnostic categories. Yet ambiguity abounds within the field from both clinical and scientific perspectives. Clinically, SZ, BD, and MDD share symptoms such as depression, anxiety, and psychosis.2,3 Phenotypic similarities are particularly apparent among prodromal and first episode presentations of SZ, BD, and MDD.4 For example, clinical observations have included that the most frequent initial symptom in SZ and BD is depressed mood.5,6 Anxiety symptoms are ubiquitous among psychiatric patients with mood or SZ spectrum disorders, and in almost half of patients are reportedly severe.7
Genetic, molecular, and neuroimaging studies over recent decades have largely been unable to identify specific sets of genetic or neural abnormalities that differentiate SZ, BD, and MDD.8–11 In fact, the pathophysiology of SZ, BD, and MDD do not appear to simply reflect abnormalities within a single or finite set of genes or neural networks. Rather, a complex array of multiple genes and neural networks are implicated in SZ, BD, and MDD. A recent genome wide association study of 5 psychiatric disorders (SZ, BD, MDD, autism, and ADHD) found cross-diagnostic effects on polygenic risk scores to be most significant among SZ, BD, and MDD, indicating significant genetic commonalities among these 3 disorders.8 Other molecular studies support common neuropathophysiological mechanisms among SZ, BD, and MDD that may involve immune, endocrine, and mitochondrial function, as well as microglial activation, neuroinflammation, and alterations in highly conserved metabolic pathways in the brain.9,10,12–14 Furthermore, convergent lines of evidence support substantial regional brain structural and functional overlap in SZ, BD, and MDD, such as in the default mode and salience networks, suggesting that the 3 disorders share core neurobiological features.2,11 Prior neuroimaging studies have also demonstrated commonalities in structural and functional neural abnormalities among SZ, BD, and MDD,15,16 although there is evidence that white matter (WM) structural anomalies may be less prominent in MDD relative to other disorders.17 However, these studies have primarily consisted of 2-group comparisons to healthy controls (HC) and, to a lesser extent, direct comparisons between 2 of the 3 disorders (SZ and BD, BD and MDD, and SZ and MDD). To date, a trans-diagnostic neuroimaging study including SZ, BD, and MDD is absent in the literature.
Findings of neural commonalties among SZ, BD, and MDD have reinvigorated a longstanding debate in psychiatric nosology as to what biological basis gives rise to the various psychiatric manifestations (distinct disease processes vs a disease continuum) and challenge the biological validity of the Kraepelinian dichotomy, which Kraepelin himself questioned in years subsequent to its introduction.18 Despite implementation of advanced genetic, molecular, and neuroimaging techniques, resolution of these debates has thus far been elusive. Subsequently, the ambiguity surrounding the biological basis of psychiatric disorders poses a significant obstacle in psychiatric diagnostic and treatment advances. Trans-diagnostic studies of SZ, BD, and MDD could contribute to resolution of these long-standing questions by providing insight into the extent of shared and distinct neural abnormalities among these diagnostic categories.
In this study, we examined whole brain gray matter (GM) volume and WM integrity in a large sample of individuals with SZ, BD, and MDD using voxel-based analysis (VBA). GM volume and WM integrity were selected because: (1) major psychiatric disorders have been examined across numerous independent neuroimaging studies and structural markers such as GM volume and WM integrity are relatively stable across time15 and (2) GM volume and WM integrity have been increasingly proposed as candidate endophenotypes and may provide trait measures of neuroanatomical abnormalities in these 3 conditions.16,19,20 Furthermore, our sample consisted of individuals in early stages of illness (duration of illness less than 5 y) and at relatively younger ages (ages 13–30 y) that coincide with critical periods for brain alterations in the development of SZ, BD, and MDD.21–23 The primary aim of this study was to examine the extent of shared and distinct alterations in GM volume and WM integrity across SZ, BD, and MDD.
Methods
Subjects
The study was conducted at a single site and included a total of 485 individuals ages 13–30 years: 135 with SZ, 86 with BD, 108 with MDD, and 156 HC. The study was approved by the Institutional Review Board of China Medical University. All participants provided written informed consent after receiving a detailed description of the study. All participants with SZ, BD, and MDD were recruited from the inpatient and outpatient services at Shenyang Mental Health Center and the Department of Psychiatry, First Affiliated Hospital of China Medical University, Shenyang, China. HC participants were recruited from the local community by advertisement. All participants were evaluated by 2 trained psychiatrists to determine the presence or absence of Axis I psychiatric diagnoses using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) in those 18 years old and older and the Schedule for Affective Disorders and Schizophrenia for School-Age Children-present and Lifetime Version (K-SADS-PL) in those younger than 18 years. SZ, BD, and MDD participants met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnostic criteria for SZ, BD and MDD, respectively, and did not meet criteria for any other Axis I disorder. Duration of illness was less than 5 years for the SZ, BD, and MDD groups. HC participants did not have current or lifetime Axis I disorder or history of psychotic, mood, or other Axis I disorders in first-degree relatives as determined by detailed family history. Participants were excluded if any of the following were present: (1) the existence of substance/alcohol abuse or dependence or concomitant major medical disorder, (2) any magnetic resonance imaging (MRI) contraindications, and (3) history of head trauma with loss of consciousness for ≥5 minutes or any neurological disorder.
For all participants, symptom measures were obtained using the Brief Psychiatric Rating Scale (BPRS), Hamilton Depression Rating Scale (HDRS), Hamilton Anxiety Rating Scale (HARS) and Young Mania Rating Scale (YMRS); cognitive function was evaluated by the Wisconsin Card Sorting Test (WCST). Detailed demographic and clinical data of participants are outlined in table 1.
Table 1.
Demographic, Clinical Characteristics and Cognitive Function of Healthy Controls, Schizophrenia, Bipolar Disorder, and Major Depressive Disorder
| Variable | Healthy Control (n = 156) | Schizophrenia (n = 135) | Bipolar Disorder (n = 86) | Major Depressive Disorder (n = 108) | F/χ2 Values | P Values |
|---|---|---|---|---|---|---|
| Demographic characteristic | ||||||
| Age at scan (y) | 22.25 (4.35) | 19.95 (4.52) | 21.97 (4.66) | 20.61 (4.91) | 7.473a | <.001 |
| Male | 63 (40%) | 59 (44%) | 35 (41%) | 38 (35%) | 4.769a | .57 |
| Right handedness | 150 (96%) | 128 (95%) | 85 (99%) | 102 (94%) | 11.678a | .23 |
| Clinical characteristic | ||||||
| Duration (mo) | — | 13.39 (17.34) | 20.64 (20.19) | 12.70 (15.19) | 5.483b | .005 |
| First episode, yes | — | 105 (78%) | 41 (48%) | 94 (87%) | 36.215b | <.001 |
| Medication, yes | — | 86 (64%) | 56 (65%) | 40 (37%) | 22.122b | <.001 |
| Anti-depressants | — | 11 (8%) | 19 (22%) | 34 (31%) | 21.366 b | <.001 |
| Antipsychotics | — | 74 (55%) | 35 (41%) | 5 (5%) | 68.615b | <.001 |
| Mood stabilizer | — | 5 (4%) | 41 (48%) | 0 | 110.586b | <.001 |
| HAMD-17 | (n = 138) | (n = 92) | (n = 77) | (n = 104) | ||
| 1.06 (1.41) | 9.54 (7.40) | 8.33 (8.51) | 20.15 (8.57) | 162.749a | <.001 | |
| HAMA | (n = 140) | (n = 83) | (n = 78) | (n = 88) | ||
| 0.72 (1.68) | 6.34 (7.15) | 6.69 (8.08) | 14.94 (8.67) | 86.899a | <.001 | |
| YMRS | (n = 136) | (n = 74) | (n = 81) | (n = 90) | ||
| 0.11 (0.42) | 3.85 (7.73) | 9.68 (10.53) | 1.28 (2.32) | 46.303a | <.001 | |
| BPRS | (n = 84) | (n = 129) | (n = 47) | (n = 38) | ||
| 18.23 (0.65) | 36.77 (15.21) | 25.77 (7.98) | 22.82 (4.71) | 56.521a | <.001 | |
| Cognitive function | ||||||
| WCST | (n = 101) | (n = 74) | (n = 45) | (n = 70) | ||
| Corrected responses | 32.31 (10.66) | 17.39 (12.17) | 25.44 (11.52) | 25.03 (11.48) | 24.531a | <.001 |
| Categories completed | 4.32 (1.97) | 1.72 (1.90) | 3.00 (2.03) | 3.00 (1.97) | 25.250a | <.001 |
| Total errors | 15.69 (10.66) | 30.62 (12.22) | 22.56 (11.52) | 23.29 (11.63) | 24.442a | <.001 |
| Perseverative errors | 5.76 (6.68) | 14.70 (13.02) | 9.22 (9.37) | 9.67 (9.31) | 12.218b | <.001 |
| Non-perseverative errors | 9.98 (5.58) | 15.93 (8.64) | 13.16 (7.04) | 13.64 (6.04) | 11.193b | <.001 |
Note: Data are n (%) or mean (SD). WCST, Wisconsin Card Sorting Test; HAMD, Hamilton Depression Scale; HAMA, Hamilton anxiety Scale; YMRS, young manic rating scale; BPRS, Brief Psychiatric Rating Scale.
aThe examination among the schizophrenia, bipolar disorder, major depressive disorder, and healthy control groups.
bThe examination among the schizophrenia, bipolar disorder, major depressive disorder groups.
MRI Acquisition
All participants underwent structural and diffusion tensor imaging (DTI). MRI data were acquired using a GE signa HDX 3.0T scanner with a standard 8-channel head coil at the First Affiliated Hospital of China Medical University, Shenyang, China. Three-dimensional, high-resolution, T1-weighted images was collected using a 3-D fast spoiled gradient-echo (FSPGR) sequence with the following parameters: TR/TE = 7.1/3.2 ms, image matrix = 240 × 240, field of view (FOV) = 240 × 240 mm2, 176 contiguous slices of 1 mm without gap, voxel size = 1.0 mm3. DTI was acquired using a single-short spin-echo planar imaging (EPI) sequence with the following parameters: TR/TE = 17 000/85.4 ms, image matrix = 120 × 120, FOV = 240 × 240 mm2, 65 contiguous slices of 2 mm without gap, 25 noncollinear directions (b = 1000 s/mm2), together with an axial acquisition without diffusion weighting (b = 0), voxel size = 2.0 mm3.
Data Processing
Whole-brain, high-resolution structural MRI data and DTI data were processed using standard procedures suggested by Ashburner24 and Cui.25
Specifically, structural brain images were processed using VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm8/), which was incorporated into the Statistical Parametric Mapping (SPM) software (SPM8; The Wellcome Department of Cognitive Neurology). VBM8 processing involves bias correction, tissue classification, and spatial normalization with Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL).26 According to the default parameters of VBM8, the images were spatially normalized to the Montreal Neurological Institute (MNI) space to obtain images with 1.5 mm3 voxels. The modulation process was performed using nonlinear deformations employed for normalization that allowed for comparison of the absolute amount of tissue, corrected for individual brain sizes. Finally, all images were smoothed with an isotropic Gaussian kernel of 8-mm full width at half maximum (FWHM). These segmented, normalized, modulated, and spatially smoothed GM images were then used for subsequent VBM statistical analysis.
DTI data were processed using Pipeline for Analyzing braiN Diffusion imAges (PANDA) (http://www.nitrc.org/projects/panda), a fully automated program for processing brain diffusion images. We used default program parameters to process DTI images. The voxel-wise diffusion tensor matrix was then calculated for each subject in the native space. Next, diagonalization was performed to yield 3 pairs of eigenvalues and eigenvectors. Based on the 3 eigenvalues, fractional anisotropy (FA) was computed on a voxel-by-voxel basis. Specifically, the FA image of each subject was nonlinearly registered to the FMRIB58_FA template in MNI space with 2 mm3 voxels. The mean of all aligned FA images was then calculated. FA images were then smoothed with a 6-mm FWHM Gaussian filter.
Statistical Analyses
Four-group (SZ, BD, MDD, and HC) analyses of GM volumes and FA values were performed in SPM8 using ANCOVA with diagnostic group as an independent factor, and age and gender as covariates. Correction for multiple comparisons was based on Monte Carlo simulation (AlphaSim, Analysis of Functional NeuroImages [AFNI]). Statistical significance was determined by corrected P < .05, corresponding to a threshold of 564 and 71 contiguous voxels with P < .01 (uncorrected) for GM volumes and FA values, respectively. Post hoc analyses were performed to determine the extent of shared alterations in GM volumes and FA values between the SZ, BD, and MDD groups. For each cluster with significant 4-group difference, GM volumes and FA values were extracted. Permutation t tests were then applied in pairwise fashion with the HC group as the common comparison (SZ vs HC, BD vs HC, and MDD vs HC). Similarities and differences among the SZ, BD, and MDD groups were then examined for significant clusters as determined by the permutation t tests. Statistical significance was determined by P < .05 (corrected, Benjamin Hochberg correction27). Analyses were also performed to test for effects of age, clinical, and cognitive variables on GM volumes and FA values for significant clusters from the 4-group comparison (supplementary material, pages 1–2).
Results
Grey Matter Volume Findings
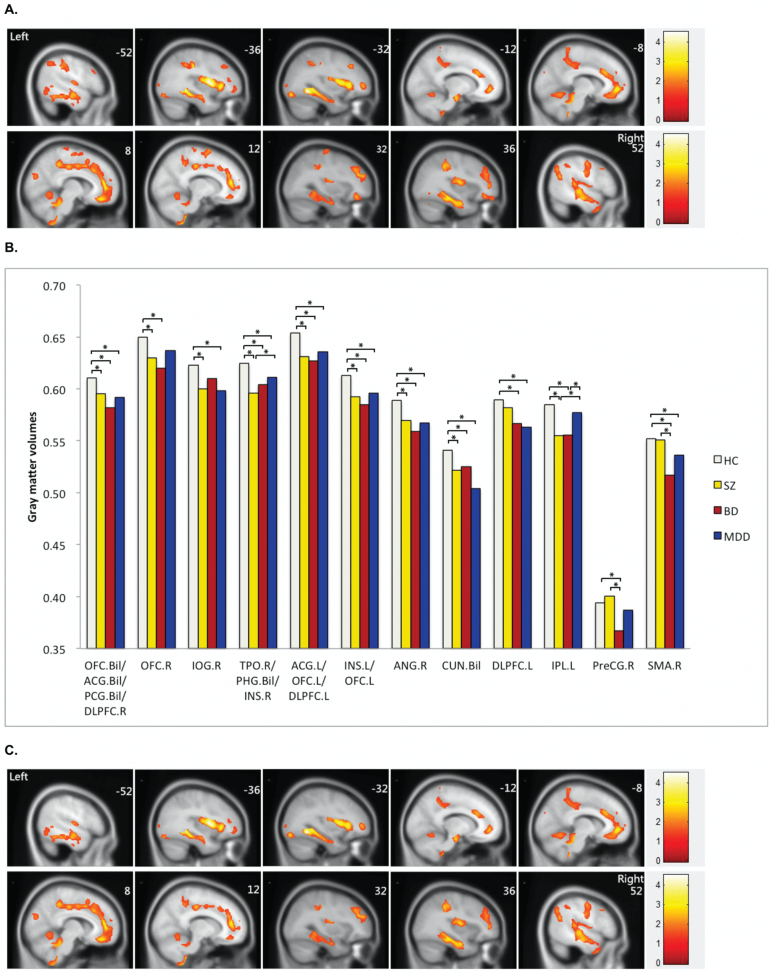
Four-group analysis of GM volumes showed 12 clusters with significant group differences. These clusters consisted of paralimbic regions, including right temporal pole, bilateral orbital frontal cortex, bilateral insula, bilateral parahippocampal and cingulate gyri. There were also findings in heteromodal cortical areas, including the bilateral dorsolateral prefrontal cortex (DLPFC), as well as the bilateral cuneus, right inferior occipital gyrus, right angular gyrus, left inferior parietal gyrus, right supplemental motor area and right pre-central gyrus (figure 1A and table 2). Post hoc analyses found 6 clusters of significantly decreased GM volume when compared to the HC group that were common to the SZ, BD, and MDD groups (figure 1B). These shared clusters represented 87.9% of the total regional volume that showed significant differences in the 4-group analysis. The clusters are located in paralimbic and heteromodal regions such as the temporal pole, orbital frontal cortex, insula, parahippocampal gyri, cingulate gyri, DLPFC, and angular gyri (figure 1C). Shared clusters were also found in the cuneus (figures 1B and 1C). In addition, significant GM decreases compared to the HC group were found common to the SZ and BD groups, but not the MDD group, in right orbital frontal cortex and left inferior parental cortex. The SZ and MDD groups, but not the BD group, had decreases compared to HC in the right inferior occipital cortex. Meanwhile, the BD and MDD groups, but not the SZ group, had decreases in left DLPFC and right supplementary motor area compared to HC (figure 1B). There were significant GM decreases in the right precentral gyrus in the BD group only when compared to the HC group (figure 1B).
Fig. 1.
(A) Significant differences in gray matter volumes among schizophrenia, bipolar disorder, major depressive disorder and healthy controls. Significant at P < .05 corrected by AlphaSim correction. (B) Gray matter volumes in regions showing significant differences among the participants with schizophrenia, bipolar disorder major depression disorder and healthy controls. (C) Shared alterations in gray matter volume in schizophrenia, bipolar disorder and major depression disorder compared with healthy controls. Significant at P < .05 corrected by AlphaSim correction. *Permutation t test for each disorder vs healthy controls (P < .05 corrected, Benjamin Hochberg correction). R, right; L, left; Bil, bilateral; OFC, orbital frontal cortex; ACG, anterior cingulate gyrus; PCG, posterior cingulate gyrus; TPO, temporal pole; PHG, parahippocampus gyrus; INS, insula; IOG, inferior occipital gyrus; DLPFC, dorsal lateral prefrontal cortex; CUN, cuneus; ANG, angular gyrus; IPL, inferior parietal lobe; SMA, supplementary motor area; PreCG, precentral gyrus.
Table 2.
Gray Matter Volumes in Brain Regions Demonstrating Significant Between-Group Differences
| Index | Brain Regions | Brodmann Area | Cluster Size | Peak MNI Coordinates | F Values* | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| GM-A | Bilateral orbital frontal cortex | 11/10/32/31/9 | 16 841 | 2 | 41 | −17 | 7.366 |
| Bilateral anterior cingulate gyri | |||||||
| Bilateral posterior cingulate gyri | |||||||
| Right dorsal lateral prefrontal cortex | |||||||
| GM-B | Right orbital frontal cortex | 11/47 | 581 | 33 | 39 | −15 | 3.846 |
| GM-C | Right inferior occipital gyrus | 19 | 682 | 41 | −80 | −14 | 4.703 |
| GM-D | Right insula | 38/21/20/19/37 | 27 279 | −29 | −54 | −11 | 14.429 |
| Right temporal pole | |||||||
| Bilateral para-hippocampal gyri | |||||||
| Bilateral hippocampus | |||||||
| Bilateral fusiform gyri | |||||||
| Bilateral middle temporal gyri | |||||||
| Left inferior temporal gyrus | |||||||
| Right superior temporal gyrus | |||||||
| Left middle occipital gyrus | |||||||
| Right supramarginal gyrus | |||||||
| Vermis | |||||||
| GM-E | Left anterior cingulate gyrus | 10/47/11/46 | 1182 | −27 | 54 | 3 | 4.070 |
| Left orbital frontal cortex | |||||||
| Left dorsal lateral prefrontal cortex | |||||||
| GM-F | Left insula | 13/47 | 3310 | −38 | 2 | 11 | 6.668 |
| Left orbital frontal cortex | |||||||
| GM-G | Right angular | 39/40 | 922 | 51 | −57 | 23 | 5.719 |
| Right supramarginal gyrus | |||||||
| GM-H | Bilateral cuneus | 19/18 | 651 | 5 | −84 | 25 | 5.650 |
| GM-I | Left dorsal lateral prefrontal cortex | 9/46 | 1571 | −30 | 27 | 39 | 4.214 |
| GM-J | Left inferior parietal lobe | 40/2/3/4 | 2610 | −38 | −27 | 39 | 7.908 |
| GM-K | Right precentral gyrus | 6 | 811 | 26 | −15 | 69 | 7.102 |
| GM-L | Right supplementary motor area | 6 | 647 | 15 | 3 | 72 | 6.129 |
Note: MNI, Montreal Neurological Institute; GM, gray matter.
*Significant at P < .05 corrected by AlphaSim correction.
WM Integrity Findings
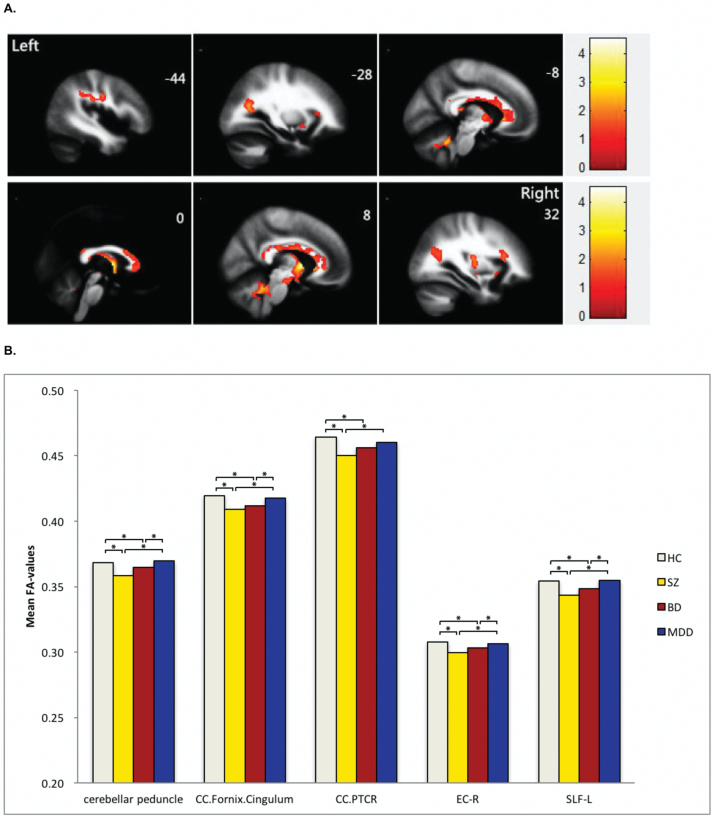
Four-group analysis of FA values found 5 clusters showing significant group differences. These clusters consisted of callosal, limbic-paralimbic-heteromodal, cortico-cortical, thalamocortical and cerebellar WM and included the genu, body and splenium of the corpus callosum, the uncinate fasciculus, fornix, cingulum, bilateral anterior limb of internal capsule, right external capsule, left superior longitudinal fasciculus, right cerebral peduncle, bilateral posterior thalamic radiation, and bilateral superior and middle cerebellar peduncles. Post hoc analyses revealed significantly decreased FA values, when compared to the HC group, that were common to the SZ and BD groups in all 5 clusters. No significant differences in FA values were observed between the MDD and HC groups in any of the 5 clusters (figure 2 and table 3).
Fig. 2.
(A) Significant differences in fractional anisotropy values among schizophrenia, bipolar disorder, major depressive disorder and healthy controls. Significant at P < .05 corrected by AlphaSim correction. (B) Fractional anisotropy values in white matter fibers showing significant differences among the participants with schizophrenia, bipolar disorder major depression disorder and healthy controls. *Permutation t test for each disorder vs healthy controls (P < .05 corrected; Benjamin Hochberg correction). CC, corpus callosum; PTCR, posterior thalamic radiations; EC-R, right external capsule; SLF-L, left superior longitudinal fasciculus.
Table 3.
Fractional Anisotropy in WM Showing Significant Between-Group Differences
| Index | WM Labels | Cluster Size | MNI Coordinates | F Value* | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| WM-A | Bilateral middle cerebellar peduncle | 569 | −6 | −48 | −28 | 15.823 |
| Bilateral superior cerebellar peduncle | ||||||
| WM-B | Genu of corpus callosum | 4872 | 6 | 0 | 0 | 14.251 |
| Body of corpus callosum | ||||||
| Splenium of corpus callosum | ||||||
| Fornix | ||||||
| Right cingulum | ||||||
| Bilateral anterior limb of internal capsule | ||||||
| Left posterior thalamic radiation | ||||||
| WM-C | Right cerebral peduncle | 277 | 34 | −14 | 12 | 8.499 |
| Splenium of corpus callosum | ||||||
| Right posterior thalamic radiation | ||||||
| WM-D | Right external capsule | 126 | 28 | −62 | 18 | 11.397 |
| WM-E | Left superior longitudinal fasciculus | 202 | −34 | −42 | 36 | 14.321 |
Note: WM lables are provided in accordance with the ICBM-DTI-81 White Matter Labels Atlas. MNI, Montreal Neurological Institute; WM, white matter.
*Significant at P < .05 corrected by Alphasim correction.
The details of the effects of age, clinical, or cognitive variables on GM volumes or FA values were in the supplementary material (supplementary figures S1–S3 and supplementary tables S1–S4).
Discussion
This is the first study that examines whole brain GM volumes and WM integrity concurrently in SZ, BD, and MDD at relatively large sample scale. Prior to this study, insight regarding the neural similarities and differences in SZ, BD, and MDD primarily stemmed from individual comparisons to HC or direct comparisons between 2 of the 3 disorders (SZ and BD, BD and MDD, and MDD and SZ). In addition, the study sample consisted of individuals at younger ages (ages 13–30 y) and during relatively early stages of their psychiatric illnesses (less than 5 y for illness duration). Thus, confounding effects of aging and illness chronicity were minimized.
In the study, significant group differences in GM volumes were found among the SZ, BD, MDD, and HC groups mainly in the paralimbic and heteromodal cortical regions. Interestingly, the SZ, BD, and MDD groups were found to share GM volume decreases in 87.9% of the total regional volumes with significant group differences in the 4-group analysis, suggesting that the paralimbic and heteromodal regions may represent shared neural substrates for psychopathophysiology.
The paralimbic system is thought to link the brain’s internal milieu and the external world.23 It consists of highly interconnected structures that include the orbital frontal cortex, insular, and temporal pole, parahippocampal gyrus, and cingulate cortex. These structures are situated between the autonomic, limbic, and heteromodal cortical regions and represent a gradual transitive cytoarchitectonic zone between the primitive allocortex and primary sensory-motor areas within the neocortex.28 Paralimbic sulcogyral morphology exhibits substantial inter-individual variability, likely reflecting the significant influence of neurodevelopmental processes such as neuronal migration, local neuronal connection, synaptic development, and lamination and formation of cytoarchitecture on paralimbic structures.29,30 Further, paralimbic structures develop in concert to form the neural systems critical for emotional processing and regulation, motivation, memory, and higher autonomic control.23,28,31 These are all functions that appear to be impaired in SZ, BD, and MDD.3,32,33 Lesions within the paralimbic system have resulted in affective and psychotic symptoms similar to SZ, BD, and MDD, as well as deficits in cognition and emotional regulation with lesions in paralimbic components such as the orbital frontal cortex, anterior cingulate and temporal regions.34,35 Our previous work has demonstrated structural alterations within the paralimbic system in SZ36 and adolescents with BD.37 A recent meta-analysis study examined structural brain abnormalities across 6 diagnostic groups (SZ, BD, MDD, substance use disorder, obsessive-compulsive disorder, and anxiety). The study found convergence of GM abnormalities across all diagnostic groups within the anterior insula and dorsal anterior cingulate regions, which are key components of the paralimbic system.11 This finding is in line with our results, and suggest that paralimbic anatomical alterations are common across multiple major Axis I disorders. Alterations in paralimbic cortices may underlie shared clinical features in SZ, BD, and MDD, such as deficits in emotional processing and regulation. Although we were unable to identify any correlations between clinical symptoms and paralimbic regional volumes in the present study, future research designed to carefully characterize emotional processing features in these disorders may be better positioned to elucidate the relationship between GM volume and WM integrity in paralimbic regions and emotional processing features across these diagnoses.
Heteromodal cortical regions are believed to have developed more recently in evolution and are considered part of the neocortex. They represent a group of inter-connected higher-order neural circuits that receive input from multiple sensory and motor areas and other heteromodal cortices. Heteromodal cortices integrate associative elaboration of perceptual and cognitive processing and include the DLPFC, and inferior parietal lobule regions, such as the supramarginal and angular gyri.28,38 These regions undergo significant myelination and synaptic reorganization during adolescence and young adulthood,38 which are also critical periods for the symptomatic onset of SZ, BD, and MDD. Prior structural and functional neuroimaging studies in SZ, BD and MDD converge on the DLPFC and angular gyri in the neuropathophysiology of these disorders, demonstrating alterations in neuronal morphology, functional activation, and connectivity in these regions.38–40 In conjunction with these prior observations, our trans-diagnostic findings in the paralimbic system and heteromodal cortices underscore how neurobiological commonalities may be underappreciated in current conceptualizations of psychiatric disorders. Our findings suggest that neural circuitries involving paralimbic structures and heteromodal cortices may have significant involvement in the pathophysiology of not just one particular psychiatric illness, but in psychopathology across several diagnostic categories more generally.
This study also found significant alterations in WM integrity common to both the SZ and BD groups in callosal, limbic-paralimbic-hetermodal, cortico-cortical, thalamocortical and cerebellar WM. The MDD group was distinct from the SZ and BD groups in that no significant alterations in WM integrity were observed in these regions compared to the HC group. These observations suggest that intact WM integrity may differentiate MDD from BD and SZ. Moreover, they indicate that abnormalities in brain connectivity may be more significant in the neuropathophysiology of SZ and BD than MDD. Consistent with our WM findings, a large sample, 2-site MRI study by Skudlarski, et al. found decreased FA in multiple WM regions including callosal, limbic-paralimbic-hetermodal, cortico-cortical, thalamocortical and cerebellar WM in SZ and BD (n = 125 and n = 82, respectively) when compared to HC (n = 104).16 Other studies directly compared SZ and BD, albeit with relatively smaller sample sizes (<50 participant per group), have demonstrated shared WM abnormalities in multiple regions, including callosal, anterior and posterior thalamic/optic, paralimbic, fronto-occipital, uncinate and posterior corona radiata regions.41–43 Previous neuroimaging studies of either SZ or BD alone also support similarities in WM abnormalities in the 2 disorders relative to control participants.44,45 Furthermore, significant decreases in FA in most major WM tracts have been shown in the early stages of SZ and BD, suggesting that WM integrity alterations are implicated in the pathophysiology of axonal migration or myelination in both disorders. Interestingly, a DTI study utilizing both VBA and tract-based spatial statistics (TBSS) failed to find significant alteration in WM integrity in a large sample of medication-free MDD patients.46 A recent TBSS study also did not find significant differences in FA between MDD and HC.47 These findings further suggest that WM integrity may differentiate MDD from SZ and BD.
There were several limitations in this study. Firstly, approximately 50% of the participants with SZ, BD, and MDD were taking psychotropic medications at the time of the study. There were no significant effects were detected for the presence or absence of psychotropic medication on the regions showing significant 4-group differences in each patient group. However, when primary analyses with controlling medication subclasses were performed, GM volumes/FA values in some regions showing significant group differences without controlling medication subclasses, lost significance (supplementary material). Hence, our findings might in part be attributed to treatment differences. Future studies in medication naïve patients or with focus on specific psychotic medication are needed to clarify these issues. Secondly, we only detected the negative correlation between illness duration and GM values in orbital frontal cortex, cingulate gyri and right DLPFC in the entire patients. Because our sample was relatively early in their illness course, it could be that relationships between clinical features and neuroanatomy are not yet present, but would develop over time. Additionally, there was also a relatively wide age range in the present sample (13–30 y). This broad age range and cross sectional design may limit the interpretation of our findings. Finally, because a diagnosis of SZ or BD may be preceded by a depressive episode before first admission, the SZ and BD groups in the present study may have experienced more psychiatric symptoms prior to their first diagnosis, which could potentially influence our findings. Clearly, longitudinal, transdiagnostic research is needed to better understand the complex relationships between neuroanatomy, behavior, and clinical symptoms across the illness course.
In summary, the present study found significant commonalities between SZ, BD, and MDD in GM alterations and between SZ and BD in WM abnormalities. Alterations in WM integrity common to SZ and BD were not present in the MDD group. Altogether, our findings implicate disruptions in the paralimbic system and heteromodal cortices that are core neurobiological features shared among SZ, BD, and MDD. Furthermore, while WM integrity may differentiate MDD from SZ and BD, our findings also indicate that broad alterations in WM integrity are shared features in SZ and BD. The findings herein emphasize the importance of considering the extent of commonalities between SZ, BD, and MDD in our diagnostic, treatment, and research approaches.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
National Natural Science Foundation of China (81271499 and 81571311 to Y.T., 81571331 to F.W.); Liaoning Pandeng Scholar (to F.W.); National Key Research and Development Program (2016YFC0904300 to F. W.); National Key Research and Development Program (2016YFC1306900 to Y.T.); National High Tech Development Plan (863) (2015AA020513 to F.W.).
Supplementary Material
Acknowledgments
We thank all the participants for their cooperation and we are grateful for the support of Shenyang Mental Health Center, Department of Psychiatry and Radiology, First Affiliated Hospital of China Medical University. The authors declare no conflict of interest.
References
- 1. Kraepelin E. Manifestation of insanity. Z Gesamte Neurol Psychiatr. 1920;62:1–29. [Google Scholar]
- 2. Barch DM, Sheffield JM. Cognitive impairments in psychotic disorders: common mechanisms and measurement. World Psychiatry. 2014;13:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee RS, Hermens DF, Naismith SL et al. . Neuropsychological and functional outcomes in recent-onset major depression, bipolar disorder and schizophrenia-spectrum disorders: a longitudinal cohort study. Transl Psychiatry. 2015;5:e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosen C, Marvin R, Reilly JL et al. . Phenomenology of first-episode psychosis in schizophrenia, bipolar disorder, and unipolar depression: a comparative analysis. Clin Schizophr Relat Psychoses. 2012;6:145–151. [DOI] [PubMed] [Google Scholar]
- 5. Häfner H, Maurer K, Trendler G, an der Heiden W, Schmidt M, Könnecke R. Schizophrenia and depression: challenging the paradigm of two separate diseases–a controlled study of schizophrenia, depression and healthy controls. Schizophr Res. 2005;77:11–24. [DOI] [PubMed] [Google Scholar]
- 6. Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry 2003;64:161–174. [PubMed] [Google Scholar]
- 7. Karpov B, Joffe G, Aaltonen K et al. . Anxiety symptoms in a major mood and schizophrenia spectrum disorders. Eur Psychiatry. 2016;37:1–7. [DOI] [PubMed] [Google Scholar]
- 8. Cross-Disorder Group of the Psychiatric Genomics C. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016;21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia-Rizo C, Kirkpatrick B, Fernandez-Egea E, Oliveira C, Bernardo M. Abnormal glycemic homeostasis at the onset of serious mental illnesses: a common pathway. Psychoneuroendocrinology. 2016;67:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goodkind M, Eickhoff SB, Oathes DJ et al. . Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Network, Pathway Analysis Subgroup of Psychiatric Genomics C. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2015;18:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Réus GZ, Fries GR, Stertz L et al. . The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. [DOI] [PubMed] [Google Scholar]
- 14. Toker L, Agam G. Mitochondrial dysfunction in psychiatric morbidity: current evidence and therapeutic prospects. Neuropsychiatr Dis Treat. 2015;11:2441–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jovicich J, Marizzoni M, Sala-Llonch R et al. ; PharmaCog Consortium. Brain morphometry reproducibility in multi-center 3T MRI studies: a comparison of cross-sectional and longitudinal segmentations. Neuroimage. 2013;83:472–484. [DOI] [PubMed] [Google Scholar]
- 16. Skudlarski P, Schretlen DJ, Thaker GK et al. . Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry. 2013;170:886–898. [DOI] [PubMed] [Google Scholar]
- 17. Kempton MJ, Salvador Z, Munafò MR et al. . Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. [DOI] [PubMed] [Google Scholar]
- 18. Pearlson GD. Etiologic, phenomenologic, and endophenotypic overlap of schizophrenia and bipolar disorder. Annu Rev Clin Psychol. 2015;11:251–281. [DOI] [PubMed] [Google Scholar]
- 19. Ivleva EI, Bidesi AS, Keshavan MS et al. . Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Won E, Ham BJ. Imaging genetics studies on monoaminergic genes in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:311–319. [DOI] [PubMed] [Google Scholar]
- 21. Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114(Pt 5):2037–2049. [DOI] [PubMed] [Google Scholar]
- 22. Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. [DOI] [PubMed] [Google Scholar]
- 23. Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. [DOI] [PubMed] [Google Scholar]
- 24. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. [DOI] [PubMed] [Google Scholar]
- 25. Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 27. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological). 1995;57:289–300. [Google Scholar]
- 28. Mesulam MM. Principles of Behavioral and Cognitive Neurology. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 29. Rakic P, Goldman-Rakic PS, Gallager D. Quantitative autoradiography of major neurotransmitter receptors in the monkey striate and extrastriate cortex. J Neurosci. 1988;8:3670–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armstrong JL, Reid M, Bigrigg A. Scare over oral contraceptives. Effect on behaviour of women attending a family planning clinic. BMJ. 1995;311:1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol. 1984;230:465–496. [DOI] [PubMed] [Google Scholar]
- 32. Hoertnagl CM, Hofer A. Social cognition in serious mental illness. Curr Opin Psychiatry. 2014;27:197–202. [DOI] [PubMed] [Google Scholar]
- 33. Takizawa R, Fukuda M, Kawasaki S et al. ; Joint Project for Psychiatric Application of Near-Infrared Spectroscopy (JPSY-NIRS) Group Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. 2014;85(Pt 1):498–507. [DOI] [PubMed] [Google Scholar]
- 34. Devinsky O. Postictal psychosis: common, dangerous, and treatable. Epilepsy Curr. 2008;8:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaw P, Mellers J, Henderson M, Polkey C, David AS, Toone BK. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: timing and risk factors. J Neurol Neurosurg Psychiatry. 2004;75:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liao J, Yan H, Liu Q et al. . Reduced paralimbic system gray matter volume in schizophrenia: correlations with clinical variables, symptomatology and cognitive function. J Psychiatr Res. 2015;65:80–86. [DOI] [PubMed] [Google Scholar]
- 37. Wang F, Kalmar JH, Womer FY et al. . Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain. 2011;134:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex?Neuropsychop harmacology. 1996;14:1–17. [DOI] [PubMed] [Google Scholar]
- 39. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. [DOI] [PubMed] [Google Scholar]
- 40. Lyoo IK, Sung YH, Dager SR et al. . Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. [DOI] [PubMed] [Google Scholar]
- 41. Kumar J, Iwabuchi S, Oowise S, Balain V, Palaniyappan L, Liddle PF. Shared white-matter dysconnectivity in schizophrenia and bipolar disorder with psychosis. Psychol Med. 2015;45:759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li J, Kale Edmiston E, Chen K et al. . A comparative diffusion tensor imaging study of corpus callosum subregion integrity in bipolar disorder and schizophrenia. Psychiatry Res. 2014;221:58–62. [DOI] [PubMed] [Google Scholar]
- 43. McIntosh AM, Muñoz Maniega S, Lymer GK et al. . White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–1092. [DOI] [PubMed] [Google Scholar]
- 44. Canu E, Agosta F, Filippi M. A selective review of structural connectivity abnormalities of schizophrenic patients at different stages of the disease. Schizophr Res. 2015;161:19–28. [DOI] [PubMed] [Google Scholar]
- 45. Marlinge E, Bellivier F, Houenou J. White matter alterations in bipolar disorder: potential for drug discovery and development. Bipolar Disord. 2014;16:97–112. [DOI] [PubMed] [Google Scholar]
- 46. Choi KS, Holtzheimer PE, Franco AR et al. . Reconciling variable findings of white matter integrity in major depressive disorder. Neuropsychopharmacology. 2014;39:1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Olvet DM, Delaparte L, Yeh FC et al. . A comprehensive examination of white matter tracts and connectometry in major depressive disorder. Depress Anxiety. 2016;33:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.