Abstract
Increased striatal dopaminergic activity and decreased prefrontal functioning have been reported in individuals at clinical high risk (CHR) for psychosis. Abnormal metabolic rate might affect resting-state cerebral blood flow (rCBF) in the respective regions. Here, we examined if striatal and prefrontal rCBF differ between patients with CHR, first-episode psychosis (FEP), chronic schizophrenia-spectrum disorder (SZ) and controls. Two cohorts with a total of 122 participants were included and analyzed separately: 32 patients with SZ and 31 healthy controls (HC) from the University Hospital of Psychiatry, and 59 patients from the Bern Early Recognition and Intervention Center (29 with CHR, 12 with FEP, and 18 clinical controls [CC]). Ultra-high risk criteria were assessed with the Structured Interview for Psychosis-Risk Syndromes, basic symptom criteria with the Schizophrenia Proneness Instrument. rCBF was measured with pseudo-continuous arterial spin labeling 3T-Magnetic Resonance Imaging. Striatal rCBF was significantly increased and prefrontal rCBF significantly decreased in the SZ group compared to HC group and in the CHR and FEP groups compared to CC group. Striatal rCBF correlated significantly with positive symptom scores in SZ and CHR. An inverse correlation between striatal and frontal rCBF was found in controls (HC, CC), but not in patient groups (SZ, FEP, CHR). This is the first study to demonstrate increased neuronal activity within the striatum, but reduced prefrontal activity in patients with CHR, FEP, and SZ compared to the respective controls. Our results indicate that alterations in striatal and prefrontal rCBF are reflecting metabolic abnormalities preceding the onset of frank psychosis.
Keywords: arterial spin labeling, cerebral blood flow, clinical high risk for psychosis, prefrontal cortex, first-episode psychosis, schizophrenia, striatum
Introduction
Early detection and intervention in individuals with initial signs of emerging psychosis is regarded as a promising strategy to reduce the burden of psychotic disorders.1 To this aim, 2 complementary sets of clinical high risk (CHR; henceforth used as superordinate term) criteria were developed2,3: One are the “ultra-high risk” (UHR) criteria4 that include attenuated and brief intermittent psychotic symptoms, as well as a combination of genetic risk factors and recent significant functional decline and aim to detect an imminent risk of transition to psychosis. The other are the “basic symptom” (BS) criteria5–7 “cognitive-perceptive basic symptoms” (COPER) and “cognitive disturbances” (COGDIS) that constitute of subtle subjective symptoms assumed to present the most immediate expression of the neurobiological processes underlying the development of psychoses and aim to detect a risk of psychoses as early as possible in the assumed prodromal phase.6
In vivo research on CHR states for psychosis indicated increased presynaptic striatal dopamine synthesis in patients with CHR as compared to controls.8–10 Furthermore, we recently reported an increase in abnormal involuntary movements in children and adolescents with CHR symptoms, which might reflect early striatal dysfunctions.11,12 An increase in the metabolic rate could also affect resting-state cerebral blood flow (rCBF) in the respective regions.13 The arterial spin labeling (ASL) magnetic resonance imaging (MRI) signal is directly linked to rCBF and provides a quantitative and absolute measure of rCBF, reflecting the level of neuronal activity.14,15 The ASL technique has successfully been used to measure global differences in rCBF between healthy individuals and patients with schizophrenia,16–18 to characterize psychotic psychopathological phenomena19–25 and to analyze functional connectivity.16,26–28 Recently, increased rCBF in the basal ganglia was reported in patients with chronic schizophrenia18 and with CHR.29
The striatum has extensive structural and functional connections to the frontal cortex30 via cortico-striato-thalamo-cortical circuitries and is particularly involved in motor and complex cognitive functioning.31,32 Decreased prefrontal activity33,34 paralleled by neurocognitive impairments35 and abnormal prefrontal rCBF24,28 have consistently been demonstrated in chronic schizophrenia. Importantly, dopaminergic activity in the striatum has been inversely related to prefrontal functioning in patients with schizophrenia36 and individuals with CHR.37 However, the interaction between striatal and prefrontal cortex (PFC) neuronal activity in individuals at the beginning of the psychotic disorder, namely individuals with CHR, is still poorly understood.
In the present study, our first aim was to investigate whether rCBF in the striatum serves as a biomarker for psychosis. For this, we tracked rCBF abnormalities in the striatum in patients with CHR, first-episode psychosis (FEP), and chronic schizophrenia-spectrum patients (SZ), expecting to find an increase in striatal rCBF in all 3 groups as compared to the respective clinical (CC) and healthy controls (HC). We assumed that abnormal rCBF in striatum would be correlated in particular with CHR and positive symptoms in patients with psychosis and increased psychosis risk.38,39 Our second aim was to investigate fronto-striatal interactions by additionally measuring the rCBF in the PFC. Here, we tested if abnormal prefrontal rCBF was already present in FEP and CHR and hypothesized to find negative correlations between rCBF in striatal and prefrontal areas in patient groups and controls.
Methods
Sample and Assessments
Two sample groups with a total of 122 participants with ASL-MR scans were investigated (table 1). The first sample (n = 63) of SZ (n = 32) and HC (n = 31) from the University Hospital of Psychiatry, Bern; and the second sample of 59 patients from the Bern Early Recognition and Intervention Center (FETZ Bern), consisting of patients with CHR (n = 29), FEP (n = 12), and nonpsychotic/non-CHR CC (n = 18). The local ethics committee approved the study. Written informed consent was provided by all participants and, by parents of minors in the FETZ sample (n = 28). All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation, and with the Helsinki Declaration of 1975, as revised in 2008.
Table 1.
Sociodemographic and Clinical Characteristics of Samples
| Sample 1 | Sample 2 | ||||||
|---|---|---|---|---|---|---|---|
| SZ, n = 32 | HC, n = 31 | CHR, n = 29 | FEP, n = 12 | CC, n = 18 | Test-Value | P | |
| Age in years; mean ± SD | 41.6 ± 13.4 | 39.4 ± 12.3 | 19.3 ± 4.8 | 20.7 ± 6.4 | 19.0 ± 5.4 | F(4,121) = 33.9 | <.001* |
| Sex; % male | 56% | 48% | 41% | 50% | 67% | χ2 (4) = 3.3 | .514 |
| rCBF GM; mean ± SD | 67.8 ± 7.4 | 69.7 ± 6.8 | 71.9 ± 15.0 | 71.1 ± 13.2 | 69.9 ± 12.1 | F(4,121) = 0.58 | .677 |
| CPZ; mean ± SD | 4.97 ± 2.1 | 0 ± 0 | 0.14 ± 0.67 | 0.28 ± 0.89 | 0.16 ± 0.66 | F(4,121) = 72.9 | <.001* |
| SOFAS; mean ± SD | — | — | 66.4 ± 10.9 | 48.8 ± 13.8 | 63.4 ± 9.5 | F(2,58) = 10.05 | .001* |
| PANSS total; mean ± SD | 76.6 ± 17.4 | — | — | — | — | — | — |
| PANSS positive score; mean ± SD | 19.6 ± 6.7 | — | — | — | — | — | — |
| PANSS negative score; mean ± SD | 19.7 ± 6.0 | — | — | — | — | — | — |
| PsyRats; mean ± SD | 40.0 ± 11.3 | — | — | — | — | — | — |
| SIPS total; mean ± SD | — | — | 27.9 ± 10.5 | — | — | — | — |
| SIPS positive score; mean ± SD | — | — | 8.8 ± 4.0 | — | — | — | — |
| SIPS negative score; mean ± SD | — | — | 8.3 ± 6.0 | — | — | — | — |
Note: SZ, schizophrenia, HC, healthy controls, CHR, clinical high-risk, FEP, first-episode psychosis, CC, clinical controls; rCBF GM, resting-state cerebral blood flow in gray matter in ml/100g/min; SIPS, Structured Interview for Prodromal Syndromes; SOFAS, Social and Occupational Functioning Assessment Scale, CPZ, chlorpromazine equivalents (in mg × 100); PANSS, Positive and Negative Symptom Scale; PsyRats, Psychotic symptom rating scale; SIPS, Structured Interview for Psychosis-Risk Syndromes. Test value referring to differences across all groups.
*Significant at P < .05.
Sample 1: The SZ and HC Groups
In the SZ group, 29 were diagnosed with chronic schizophrenia and 3 with chronic schizoaffective disorder according to the International Classification of Diseases (ICD) 10. Inclusion criteria were a clinical diagnosis of schizophrenia or schizoaffective disorder according to ICD-10 (F20, F25), age between 18 and 65 years, right-handedness and medication-resistant auditory verbal hallucinations. Diagnoses of schizophrenia or schizoaffective disorder were established on the basis of unstructured clinical interviews and review of psychiatric history by 2 independent psychiatrists. All patients had medication-resistant positive psychotic symptoms (eg, auditory hallucinations, delusions, and ego disturbances). Therapy refractoriness was defined as having no response to at least 2 antipsychotic treatments in recommended dosages, each administered for at least 8 weeks. Medications remained unchanged since 2 weeks prior to the study. Exclusion criteria were history of epileptic seizures, signs of elevated neuronal activity by electroencephalography, MR contraindications and medical disorders other than schizophrenia or schizoaffective disorder. Substance misuse in the 4 weeks before treatment was ruled out by a urinary drug screen before treatment. Psychopathological assessments consisted of the Positive and Negative Symptom Scale (PANSS),40 and the Psychotic Symptom Rating Scale (PsyRats).41 Patients with SZ were on antipsychotic medication in recommended dosages and were moderately ill with a mean PANSS score of 76.6 (table 1). The 31 HC were matched for age and sex; thus, no differences between SZ and HC groups were found in age or sex (supplementary table S1a).
Sample 2: The FEP, CHR, and CC Groups
A CHR state was defined either by the presence of any UHR and/or BS criteria. For UHR criteria, the Structured Interview for Psychosis-Risk Syndromes (SIPS)42 was used to assess the presence of attenuated psychotic symptoms (APS; any SIPS positive (P) item with a score between 3 and 5), brief intermittent psychotic symptoms (BIPS; any SIPS P item with a score of 6), and genetic risk and functional decline. For BS criteria, the Schizophrenia Proneness Instrument, Adult version (SPI-A),43 was used for adults, the Schizophrenia Proneness Instrument, Child & Youth Version (SPI-CY),44 for minors. All 14 BS included in COPER and COGDIS were evaluated. Of the 29 patients with CHR, 1 fulfilled BIPS, 20 APS, 14 COGDIS, and 19 COPER criteria in various combinations (supplementary table S1b). The 12 patients with FEP were diagnosed with brief psychotic disorder (F23, n = 4), paranoid schizophrenia (F20.0, n = 4), schizophreniform disorder (F20.8, n = 2), and unspecified psychosis (F29, n = 2). The CC who did not fulfill CHR criteria or have psychosis were mainly diagnosed with affective disorders (44%) according to the Mini-International Neuropsychiatric Interview for adults and its version for children (supplementary table S3).45 The symptom-independent current global level of psychosocial functioning was estimated using the Social and Occupational Functioning Assessment Scale (SOFAS).46 Interviewers received an intensive 3-month training prior to the start of the study. Further supervision of ratings was provided by F.S.-L.
With regard to neurocognitive domains frequently reported to be impaired in CHR states and linked to conversion,47,48 verbal memory and learning were examined with the German version of the Auditory Verbal Learning Test,49 spatial working memory with the Subject-Ordered Pointing Task,50 verbal fluency with the Regensburger Word Fluency Test,51 processing speed with the Digit Symbol Test,52 and the Trail-Making Test (TMT), Part A.53 Part B of the TMT was used to assess set-shifting as one of the domains of executive functions. Premorbid verbal intelligence was assessed with the Peabody Picture Vocabulary Test.54 Percentiles on age-adjusted norms were used in all subjects. Neurocognitive deficits were defined relative to normative data provided for each test as (1) more than 1 SD below the mean, (2) a t score below 40, or (3) a percentile below 16.
No significant differences among the CHR, FEP, and CC groups were detected in age and sex; however, patients with FEP showed significant lower psychosocial functioning as measured with the SOFAS (supplementary table S1b). The cognitive test results did not reveal differences between groups (supplementary table S2).
No difference among the groups was detected in chlorpromazine equivalents (100 mg/d) within Sample 2 (supplementary table S1b); however, a difference was detected within Sample 1 (supplementary table S1a) and across all groups (F = 72.9; P < .001) due to the higher score in the SZ group (see supplementary table S4 for comedications of each subject). Additionally, in Sample 2, evaluation of handedness, level of nicotine, alcohol and cannabis consumption (0 = no use, 1 = experimental use, 2 = occasional use, 3 = moderate use, 4 = severe use) revealed no group differences (χ2(6) ≤ 5.1, P ≥ .54).
MRI Data Assessment and Analysis
MRI was conducted on 3.0-Tesla whole-body Siemens MRI systems (Magnetom Trio [Sample 1] and Magnetom Verio [Sample 2], Siemens Medical Systems) with a standard 12-channel radio frequency head coil. Participants were told to rest in the MR scanner and stay awake with their eyes closed. High-resolution 3-dimensional (3D) structural MRI and ASL were acquired in 1 session. T1-weighted 3D modified driven equilibrium Fourier transform (MDEFT) scans were recorded (number of slices, 176; matrix, 256 × 256; slice thickness, 1 mm; voxel size, 1 × 1 × 1 mm3), and served as high-resolution 3D anatomical templates for co-registration with the functional data. A pseudocontinuous ASL (pCASL) technique was used to measure rCBF.55 In this gradient-echo echo-planar imaging sequence, interleaved images with and without labeling were acquired (field of view, 220 mm2; matrix, 64 × 64; flip angle, 25°; tagging duration, 1600 ms; post-labeling delay, 1250 ms; TR/TE, 4000 ms/13 ms; 100 volumes). Fourteen axonal slices with 6 mm thickness and 1.5 mm gap were placed parallel to the anterior-posterior commissure line covering the whole brain.
ASL data analysis was performed as described in Homan et al.56 Briefly, we used aslm,56 based on MATLAB (MATLAB version 8, release 14; MathWorks, Inc.) and statistical parametric mapping (SPM 8, Wellcome Department of Imaging Neuroscience, London, England; www.fil.ion.ucl.ac.uk/spm8). All data were screened for excessive motion and magnetic resonance imaging artifacts. We calculated a flow-time series by subtracting the labeling images from the control images and subsequently computed mean rCBF images for each subject.57 Each subject’s T1 anatomy was segmented into gray matter (GM) and white matter (WM). The mean ASL images were realigned and co-registered to the GM-segmented T1 images. Motion parameters of rCBF data demonstrated no group differences (supplementary table S5). T1, GM, WM, and ASL images were normalized to the SPM Montreal Neurologic Institute (MNI) T1 template. ASL images were spatially smoothed with a 3D 8-mm full-width at the half-maximum Gaussian kernel. A correction for GM volume was performed using GM segments as inclusive masks. Data were z-transformed [z = (voxel rCBF − global GM rCBF)/SD across individual brain voxels] to remove sources of variance caused by differences in the global mean rCBF, and a correction for GM was performed using GM segments as inclusive masks. The MRI analysis for the CHR, FEP, and CC groups and for the SZ and HC groups was performed as described above. Owing to the potentially influential sample differences in age,58 chlorpromazine equivalents,59 and the known inter-scanner variability,60,61 no direct comparison between the 2 samples was calculated.
Statistics
For behavior and sample characteristics, the frequencies and percentages were compared by chi-square tests, the means of normally distributed interval data by ANOVA or independent t tests, and the non-normally distributed interval or ordinal data by Mann-Whitney U tests, using SPSS 21.0.
For the MRI analysis, the SZ and HC groups were compared voxel-wise, in independent t tests, small volume corrected (SVC) for the regions of interest (ROI 1 striatum = caudate and putamen; ROI 2 PFC = superior, middle, inferior, medial frontal gyrus and anterior cingulate; WFU Pickatlas, supplementary figure S1), and family-wise error (FWE) corrected at P < .05.
For the comparison of the CHR, FEP, and CC groups, a whole-brain, voxel-wise, 1-way ANOVA with group (CHR, FEP, and CC) as covariate was calculated to reveal group effects. Here, statistical significance was set at P < .001, uncorrected, cluster size (CS) > 50. In the main analysis, the CHR, FEP, and CC groups were compared independent t tests, SVC for the ROIs and FWE corrected at P < .05. Finally, striatal rCBF was extracted, and ANOVA and subsequent post hoc t tests were calculated using SPSS 21.0.
To evaluate the impact of age on striatal and prefrontal rCBF, the groups were split into individuals with age < 16 years and those with ≥16 years. Independent t test was calculated with rCBF as dependent variable and age group as an independent variable for the total sample (n = 59) and separated for diagnostic groups (CHR, FEP, and CC).
For the SZ group, the association between striatal rCBF and PANSS positive, negative and general symptoms were evaluated with Spearman rank correlations (Bonferroni-corrected). For the CHR group, the association between striatal rCBF and SIPS subscale scores, ie, positive, negative, disorganized and general symptoms, was evaluated with Spearman rank correlations (Bonferroni-corrected).
Finally, associations between striatal and prefrontal rCBF (areas with most significant differences between patient groups and controls) values were tested with Spearman rank correlations in both samples. Results were reported as significance at P < .05, 2-sided.
Results
Striatal rCBF in SZ and HC Groups
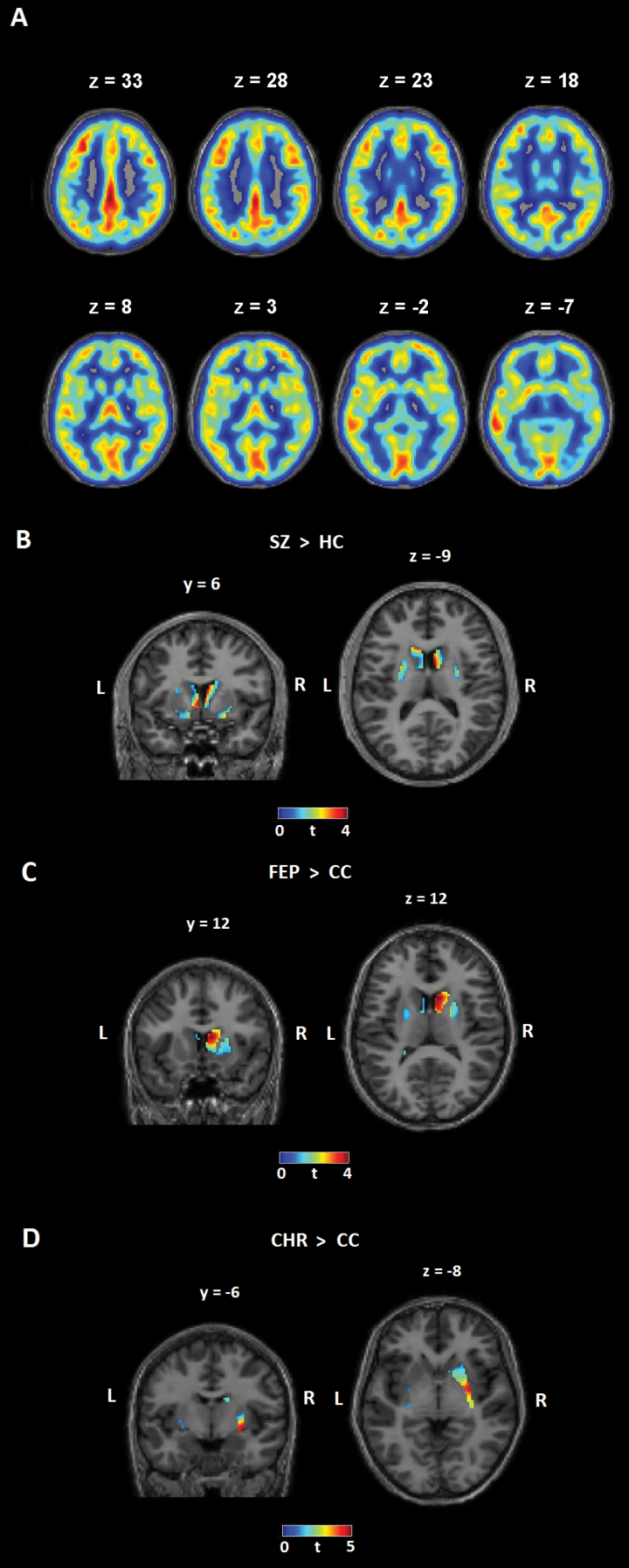
Total gray matter rCBF was not different among the groups (table 1, figure 1A). The comparison of SZ vs HC groups demonstrated significantly increased striatal rCBF in the SZ group (P = .010, figure 1B, table 2, SVC FWE corrected). rCBF values in the striatum did not correlate with chlorpromazine equivalents in the SZ group (r = .083, P = .662).
Fig. 1.
Cerebral blood flow in a representative subject and results of group comparisons Results of the Arterial Spin Labeling Analysis: (A) Gray matter resting-state cerebral blood flow in a representative subject; (B) T-contrast, patients with schizophrenia (SZ, n = 32) > healthy controls (HC, n = 31); (C) T-contrast, patients with first-episode psychosis (FEP, n = 12) > clinical controls (CC, n = 18); (D) T-contrast, clinical high-risk patients (CHR, n = 29) > clinical controls (CC, n = 18); small volume- and FWE-corrected for the region of interest (striatum).
Table 2.
Striatal Cerebral Blood Flow in Patients with Schizophrenia, Clinical High Risk, First-Episode Psychosis and Controls
| Voxel-Wise Analysis, Small Volume (Striatum) and FWE-Corrected | |||||
|---|---|---|---|---|---|
| Contrast | x/y/z | CS | Location | t (df) | P |
| SZ > HC | 4/4/6 | 9 | Caudate head and body | 4.4 (61) | .010 |
| SZ < HC | — | — | — | — | — |
| FEP > CC | 8/12/12 | 55 | Caudate head | 3.81 (28) | .043 |
| FEP < CC | — | — | — | — | — |
| CHR > CC | 30/-6/8 | 46 | Putamen | 4.6 (45) | .004 |
| CHR < CC | — | — | — | — | — |
| FEP vs CHR | — | — | — | — | — |
Note: Significant differences in resting-state cerebral blood flow (rCBF, z-transformed) between patients with Schizophrenia (SZ) and Healthy Controls (HC), and between Clinical High Risk (CHR), First-Episode Psychosis (FEP) and Clinical Controls (CC); t tests. rCBF in SZ, CHR and FEP groups was increased as compared to controls in the striatum (Caudate Head, Body, and Putamen). CS, cluster size, t, t value; df, degrees of freedom; P = P value, x/y/z coordinates according to MNI stereotactic space, Cluster size > 5; FWE-corrected.
Striatal rCBF in CHR, FEP, and CC Groups
Whole-brain voxel-wise analysis revealed significant group effects (P < .001, CS > 50, uncorrected) in the right putamen, insula, superior temporal gyrus, and left inferior and middle frontal gyrus in Sample 2 (supplementary figure S2, supplementary table S6). The direct voxel-wise comparisons revealed significantly increased striatal rCBF in FEP group (P = .043, figure 1C, table 2) and in CHR group (P = .004, figure 1D, table 2) compared to CC group, respectively (SVC FWE corrected). There was no significant difference between the CHR and FEP groups (no significant voxels).
The ROI ANOVA confirmed a significant group effect (F(59,2) = 8.45, P = .001) for the striatum, with post hoc t tests demonstrating increased striatal rCBF in the CHR group (t(45) = 4.2, P < .001) and in FEP group (t(28) = 2.4, P = .023) compared to the CC group, respectively. No difference between CHR and FEP groups was observed (t(39) = 0.9, P = .375, supplementary figure S3). Seventeen patients in Sample 2 were younger than 16 years, 42 were older than 16 years. Age group had no significant impact on striatal rCBF, neither in the total sample nor when separated as diagnostic groups (CHR, FEP, and CC; supplementary table S7A).
Correlations Between Striatal rCBF and Symptoms in SZ and CHR Groups
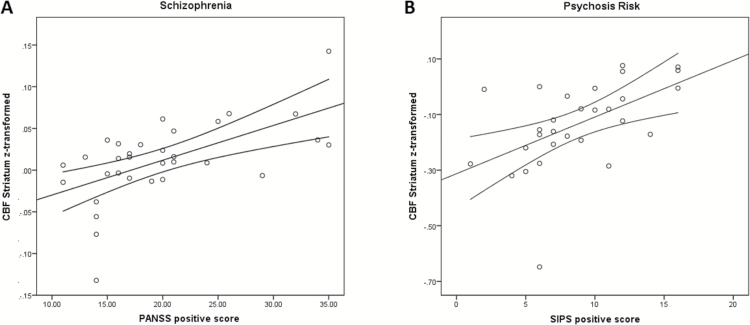
A significant positive association was detected in SZ group between striatal rCBF and PANSS positive scores (ρ = 0.617, P < .001, figure 2), but not with PANSS negative scores (ρ = 0.323, P = .071) or general symptoms (ρ = 0.110, P = .549). A significant positive association was also detected in CHR group between striatal rCBF and SIPS positive symptom score (Spearman ρ = 0.602, P = .001, figure 2), but not with scores of negative (ρ = −0.129, P = .506), disorganized (ρ = 0.026, P = .894) or general symptoms (ρ = 0.012, P = .973). Furthermore, no significant correlation was found between striatal rCBF and COPER (ρ = −0.208, P = .287) and COGDIS sum score (ρ = −0.309, P = .110).
Fig. 2.
Correlation of symptoms and striatal cerebral blood flow in patients with schizophrenia and those with clinical high risk for psychosis Results of the correlation analysis between Positive and Negative Symptom Scale (PANSS) positive scores and mean resting-state cerebral blood flow (rCBF, z-transformed) in the striatum of patients with schizophrenia (A) and Structured Interview for Prodromal Syndromes (SIPS) positive score and rCBF in the striatum of patients at clinical high risk for psychosis (B). A significant positive correlation was detected between rCBF and positive symptom scores (Schizophrenia: Spearman ρ = 0.617, n = 32, P < .001; Psychosis Risk: Spearman ρ = 0.602, P = .001, n = 29, 2-sided). Lines represent the linear effect and 95% CIs.
Frontal rCBF in SZ and HC Groups
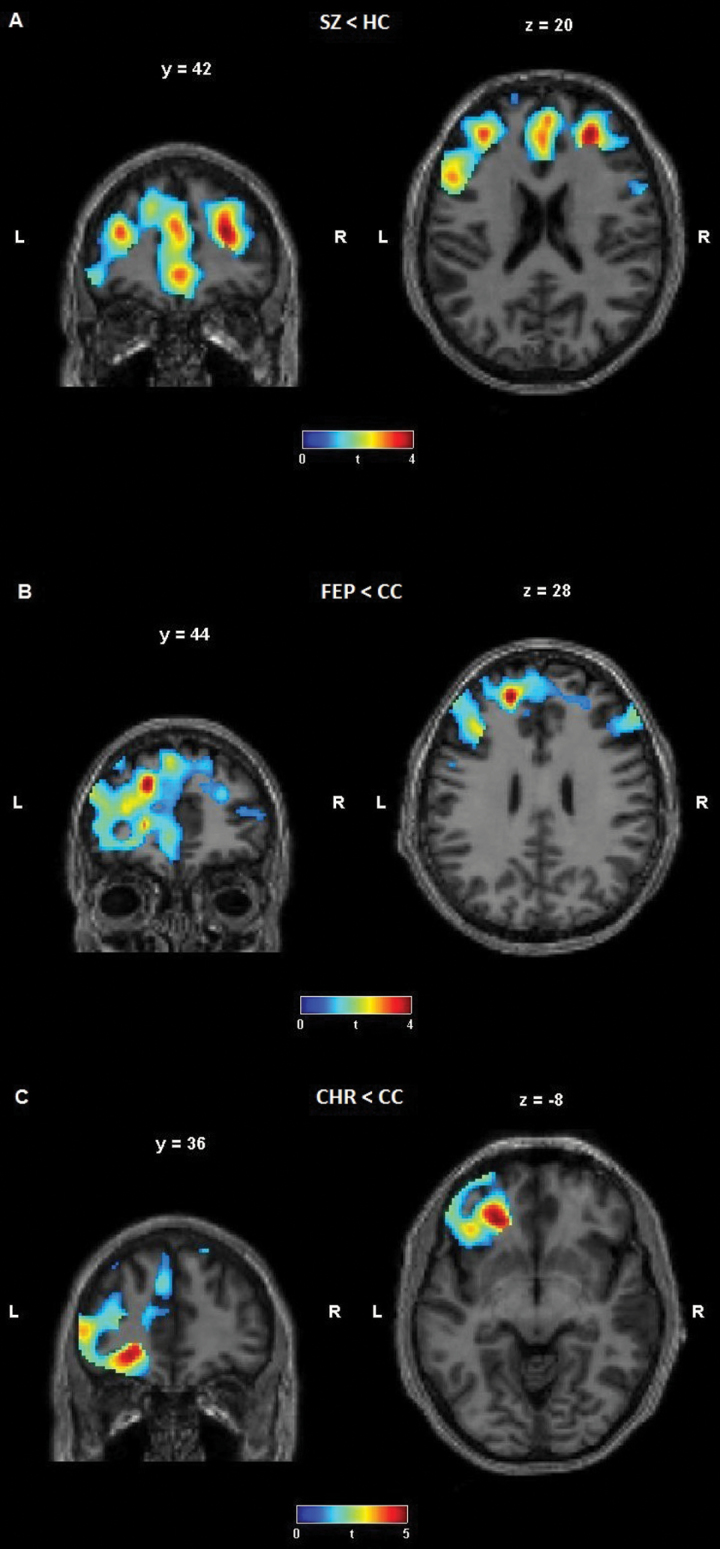
The direct voxel-wise comparisons revealed significantly reduced rCBF in SZ group in widespread frontal areas, including the medial frontal gyrus (P = .012), extending to the anterior cingulate, the superior frontal gyrus (P = .027) and the middle frontal gyrus (P = .041), extending to the inferior frontal gyrus as compared to HC (SVC FWE corrected, figure 3A, table 3).
Fig. 3.
Cerebral blood flow in the frontal cortex – group comparisons (A) T-contrast, patients with schizophrenia (SZ, n = 32) < healthy controls (HC, n = 31); (B) T-contrast, patients with first-episode psychosis (FEP, n = 12) < clinical controls (CC, n = 18); (C) T-contrast, clinical high-risk patients (CHR, n = 29) < clinical controls (CC, n = 18); small volume corrected for the region of interest (frontal cortex).
Table 3.
Frontal Cerebral Blood Flow in Patients with Schizophrenia, Clinical High Risk, First-Episode Psychosis and Controls
| Voxel-Wise Analysis, Small Volume (Frontal Cortex) and FWE-Corrected | |||||
|---|---|---|---|---|---|
| Contrast | x/y/z | CS | Location | t (df) | P |
| SZ < HC | −2/50/−2 | 269 | Medial frontal gyrus, Anterior cingulate, BA 10, BA 32, left | 4.3 (61) | .012 |
| SZ < HC | 28/42/20 | 215 | Superior frontal gyrus, Middle frontal gyrus, BA 9, BA 10, right | 4.5 (61) | .027 |
| SZ < HC | −44/14/32 | 89 | Middle and Inferior frontal gyrus, BA 9, BA 46, left | 3.7 (61) | .041 |
| SZ > HC | — | — | — | — | — |
| FEP < CC | −18/44/28 | 40 | Middle and Superior frontal gyrus, left | 4.3 (28) | .012 |
| FEP > CC | — | — | — | — | — |
| CHR < CC | −24/36/−8 | 135 | Inferior and Middle frontal gyrus, BA 11, BA 47, left | 4.6 (45) | .034 |
| CHR > CC | — | — | — | — | — |
| FEP vs CHR | — | — | — | — | — |
Note: Significant differences in resting-state cerebral blood flow (rCBF, z-transformed) between patients with Schizophrenia (SZ) and Healthy Controls (HC), and between Clinical High Risk (CHR), First-Episode Psychosis (FEP) and Clinical Controls (CC); t tests. rCBF in SZ, CHR and FEP groups was decreased as compared to controls in the prefrontal cortex. CS, cluster size; t, t value; df, degrees of freedom; BA, Brodmann area; P, P-value; x/y/z coordinates according to MNI stereotactic space; FWE-corrected.
Frontal rCBF in CHR, FEP, and CC Groups
Significantly reduced rCBF was found in FEP group in the middle and superior frontal gyrus (P = .012, figure 3B, table 3) and in CHR group in the inferior and middle frontal gyrus (P = .034, figure 3C, table 3) compared to CC group, respectively (SVC FWE corrected). There was no significant difference between the CHR and FEP groups. Age group had no significant impact on prefrontal rCBF, neither in the total sample nor when separated as diagnostic groups (CHR, FEP, and CC; supplementary table S7B).
Correlations Between Striatal and Frontal rCBF
A significant inverse correlation between striatal rCBF and frontal rCBF was found in sample 1 (total group, ρ = −0.376, P = .002, supplementary figure S4A), and when separated into diagnostic subgroups, in HC (ρ = −0.489, P = .005), but not in SZ (ρ = −0.076, P = .678). Additionally, a significant inverse correlation was also found in sample 2 (total group, ρ = −0.322, P = .013, supplementary figure S4B), and when separated, in CC (ρ = −0.564, P = .015), but not in FEP (ρ = −0.014, P = .966) or CHR (ρ = 0.114, P = .557).
Discussion
This is the first study to demonstrate increased rCBF in the striatum and decreased rCBF in the PFC of CHR, FEP and SZ groups.
The striatum is among the most intensively studied brain areas in schizophrenia research and is in the center of the “dopamine hypothesis.”62 A recent version of the dopamine hypothesis suggests that interaction among multiple neurodevelopmental incidents results in dopamine dysregulation via increased presynaptic dopamine synthesis that is linked to psychosis spectrum disorders, and results in modification of appraisal of stimuli and aberrant salience.39 More importantly, studies have demonstrated the direct relation between striatal dopaminergic activity and rCBF.13,63,64 Our results replicate and extend previous findings29 by showing that striatal rCBF is consistently increased from CHR stages to FEP and chronic schizophrenia. Additionally, we found a strong association between SIPS positive scores and striatal rCBF of patients with CHR, supporting the link between striatal dopaminergic activity, rCBF and positive psychotic symptoms.39
One recent study reported increased rCBF in the striatum, midbrain, and the hippocampus in individuals with CHR.29 However, compared to that study, we also evaluated BS. Several studies have reported benefits of combining UHR and BS criteria resulting in earlier detection and increased predictive value.65,66 Despite their clinical recognition, neurobiological research has only just begun considering the neural correlates of BS.6 Our study did not detect an association among rCBF and COPER or COGDIS scores when focusing on striatal regions. As BS are a heterogeneous set of symptoms comprising perception, affect, drive, and cognition, future research should investigate neurophysiological signatures of BS on large-scale brain networks.
Furthermore, by including FEP and chronic SZ, potential longitudinal trajectories of striatal rCBF in the development from pre-psychotic risk stages to the full-blown disease can be reasoned. The results of the present study support the notion29 that striatal rCBF is impaired independent of the clinical stage. The specificity of a psychosis biomarker demands that it effectively differentiates patients with psychosis from healthy controls but also from patients with other neuropsychiatric disorders. The non-CHR clinical controls differed from controls in the above-cited study,29 as they were seeking for help for mental health problems. Besides CHR status the clinical controls were closely matched to the CHR group with respect to chlorpromazine equivalents, nicotine use, additional psychiatric diagnoses, cognition and concomitant medication—all of them factors that potentially influence rCBF.
Several studies suggested that striatal activity and PFC dysfunction are related in SZ and CHR conditions.36,37,67,68 Decreased rCBF in frontal areas including the dorsolateral prefrontal cortex (DLPFC) and the anterior cingulate have been demonstrated in schizophrenia.69 Here, we could replicate these findings in an independent chronic schizophrenia cohort (sample 1). Additionally, we report for the first time that rCBF decreases in PFC can be backtracked to FEP and CHR stages and thus precede the onset of full psychosis.
However, there were also differences between psychosis groups with respect to localization of CBF abnormalities. In SZ and FEP the local maximum was detected in the caudate head and body, whereas in CHR it was located in the putamen. These differences are of interest, as the striatum is somatotopically organized with specific regions of the striatum projecting to specific frontal areas.70 The caudate is heavily connected with the DLPFC and thus responsible for executive functioning, incentive behavior and action evaluation.71 The putamen is connected with sensorimotor areas and involved in sensory and motor processes, but also in social and language-related functions.71
With regard to prefrontal rCBF, a reduction was found in SZ and FEP in areas covering the DLPFC, whereas in CHR rCBF decreases were mainly localized in the orbitofrontal cortex. The latter is involved in decision making, reward processing, planning, reasoning and encoding long-term memory information.72 Thus our data broadly confirm early reductions of neuronal activity in the PFC but also suggest that DLPFC abnormalities unfold later in disease development.
Importantly, the fronto-striatal rCBF abnormalities detected in the present study correspond to previously described networks.71 Increased rCBF in caudate, concurrently with reduced rCBF in DLPFC as seen in our FEP and SZ patients potentially interact to produce executive dysfunctions. Increased rCBF in putamen in combination with inferior and orbitofrontal rCBF decreases could contribute to deficits in social cognition, language-related dysfunctions and motor symptoms as reported in subjects with CHR.11,47,73 Future longitudinal studies will have to clarify whether these rCBF abnormalities shift in localization prior to psychosis conversion and, if so, a precise definition of the time point of this shift would be of interest.
Finally, we calculated correlations between frontal and striatal areas with significant differences in rCBF between patient and controls groups. Consistent with previous findings,36 we detected negative correlations between frontal and striatal rCBF (in total samples 1 and 2). However, contrary to our expectations, these negative correlations were not found in patient groups, but only in controls. Therefore, low striatal neuronal activity might have beneficial effects enabling optimal PFC functioning (and vice versa) in controls, whereas striatal and prefrontal areas become disconnected with higher striatal activity and, thus, in psychosis and CHR groups—in a nonlinear threshold effect. Nevertheless, based on the present data we cannot exclude the possibility that frontal and striatal rCBF abnormalities in our patient groups are due to basically unrelated pathological mechanisms.
As the CHR stages might evolve years before the first incidence peak in young adulthood, it is important to improve knowledge on pathophysiological alterations in minors with CHR. A recent paper suggested an age cut-off for APS of 16 years,74 with younger individuals reporting more perceptive APS with less functional impairment. If the clinical significance and conversion rate2,75 are lower, the association between APS and psychosis risk biomarkers might be weaker in the young. Nonetheless, our study did not find significant differences in rCBF when separated for age groups. This suggests that striatal and prefrontal rCBF are markers unaffected by age.
Despite the strengths of our study, such as the inclusion of different stages of psychosis as well as healthy and clinical controls, and the use of basic symptom criteria, some limitations have to be considered. One is the sample size, which, albeit comparable to other neuroimaging studies,9,10,76,77 did not allow for further subgroup analyses, eg, between CHR groups with and without basic symptom criteria. Moreover, the 2 samples were investigated with identical MRI sequences but on different scanners (Sample 1 on Magnetom Trio, Sample 2 on Magnetom Verio), which limits the comparability between the 2 samples. Furthermore, antipsychotic use might have influenced rCBF, although no differences in chlorpromazine equivalents among CHR, FEP, and CC groups were detected in Sample 2. In Sample 1, patients with SZ were on standard doses of antipsychotic medication while HCs were treatment-naive. Nevertheless, a recent study indicated that increased striatal rCBF in patients with SZ is not related to antipsychotic medication in schizophrenia,18 which is in line with the presented study. The age range is quite wide (18–65) for the SZ study. Age can influence mean total grey matter CBF with younger individuals showing higher CBF.78,79 However, CBF data where z-transformed to adjust for mean CBF and there were no age differences between groups within sample 1 and sample 2. Another limitation might be the cross-sectional design of the study that strictly limits statements related to the course of the disorder that is best studied in a longitudinal intra-subject design.
To summarize, our results provide the first evidence of increased neuronal activity within the striatum but decreased neuronal activity in the PFC from the CHR stage via FEP to chronic schizophrenia-spectrum disorder. The increase in striatal rCBF is related to more severe positive symptoms in CHR and SZ. Given replication in future longitudinal studies, abnormal striatal and prefrontal rCBF might, therefore, be considered as additional predictors of psychosis in patients with CHR, reflecting abnormal metabolic activity in CHR stages.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Funding
The study (Sample 1) was supported by the Swiss National Science Foundation (SNSF) projects grant (SNSF: 32003B-112578). The FETZ Bern (Sample 2) is a cooperation of the University Hospitals of Psychiatry and Psychotherapy, and of Child and Adolescent Psychiatry and Psychotherapy, University of Bern, and the Soteria Bern.
Supplementary Material
Acknowledgment
The authors report no biomedical financial interests or potential conflicts of interest relevant to this project.
References
- 1. WHO. Prevention of Mental Disorders: Effective Interventions and Policy Options. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- 2. Schultze-Lutter F, Michel C, Schmidt SJ, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30:405–416. [DOI] [PubMed] [Google Scholar]
- 3. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 5. Klosterkötter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58:158–164. [DOI] [PubMed] [Google Scholar]
- 6. Schultze-Lutter F, Debbané M, Theodoridou A, et al. Revisiting the basic symptom concept: toward translating risk symptoms for psychosis into neurobiological targets. Front Psychiatry. 2016;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schultze-Lutter F, Ruhrmann S, Fusar-Poli P, Bechdolf A, Schimmelmann BG, Klosterkötter J. Basic symptoms and the prediction of first-episode psychosis. Curr Pharm Des. 2012;18:351–357. [DOI] [PubMed] [Google Scholar]
- 8. Howes O, Bose S, Turkheimer F, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16:885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Egerton A, Chaddock CA, Winton-Brown TT, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74:106–112. [DOI] [PubMed] [Google Scholar]
- 10. Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kindler J, Schultze-Lutter F, Michel C, et al. Abnormal involuntary movements are linked to psychosis-risk in children and adolescents: results of a population-based study. Schizophr Res. 2016;174:58–64. [DOI] [PubMed] [Google Scholar]
- 12. Mittal VA, Daley M, Shiode MF, Bearden CE, O’Neill J, Cannon TD. Striatal volumes and dyskinetic movements in youth at high-risk for psychosis. Schizophr Res. 2010;123:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sander CY, Hooker JM, Catana C, et al. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci U S A. 2013;110:11169–11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jann K, Koenig T, Dierks T, Boesch C, Federspiel A. Association of individual resting state EEG alpha frequency and cerebral blood flow. Neuroimage. 2010;51:365–372. [DOI] [PubMed] [Google Scholar]
- 15. Jann K, Kottlow M, Dierks T, Boesch C, Koenig T. Topographic electrophysiological signatures of FMRI Resting State Networks. PLoS One. 2010;5:e12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kindler J, Jann K, Homan P, et al. Static and dynamic characteristics of cerebral blood flow during the resting state in schizophrenia. Schizophr Bull. 2015;41:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheef L, Manka C, Daamen M, et al. Resting-state perfusion in nonmedicated schizophrenic patients: a continuous arterial spin-labeling 3.0-T MR study. Radiology. 2010;256:253–260. [DOI] [PubMed] [Google Scholar]
- 18. Pinkham A, Loughead J, Ruparel K, et al. Resting quantitative cerebral blood flow in schizophrenia measured by pulsed arterial spin labeling perfusion MRI. Psychiatry Res. 2011;194:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kindler J, Homan P, Jann K, et al. Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biol Psychiatry. 2013;73:518–524. [DOI] [PubMed] [Google Scholar]
- 20. Homan P, Kindler J, Hauf M, Hubl D, Dierks T. Cerebral blood flow identifies responders to transcranial magnetic stimulation in auditory verbal hallucinations. Transl Psychiatry. 2012;2:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Homan P, Kindler J, Hauf M, Walther S, Hubl D, Dierks T. Repeated measurements of cerebral blood flow in the left superior temporal gyrus reveal tonic hyperactivity in patients with auditory verbal hallucinations: a possible trait marker. Front Hum Neurosci. 2013;7:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Walther S, Federspiel A, Horn H, et al. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res. 2011;192:117–124. [DOI] [PubMed] [Google Scholar]
- 23. Pinkham AE, Liu P, Lu H, Kriegsman M, Simpson C, Tamminga C. Amygdala Hyperactivity at Rest in Paranoid Individuals With Schizophrenia. Am J Psychiatry. 2015;172:784–792. [DOI] [PubMed] [Google Scholar]
- 24. Walther S, Schappi L, Federspiel A, et al. Resting-state hyperperfusion of the supplementary motor area in Catatonia. Schizophr Bull. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stegmayer K, Strik W, Federspiel A, Wiest R, Bohlhalter S, Walther S. Specific cerebral perfusion patterns in three schizophrenia symptom dimensions. Schizophr Res. 2017. [DOI] [PubMed] [Google Scholar]
- 26. Orosz A, Jann K, Federspiel A, et al. Reduced cerebral blood flow within the default-mode network and within total gray matter in major depression. Brain Connect. 2012;2:303–310. [DOI] [PubMed] [Google Scholar]
- 27. Jann K, Gee DG, Kilroy E, et al. Functional connectivity in BOLD and CBF data: similarity and reliability of resting brain networks. Neuroimage. 2015;106:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu J, Zhuo C, Qin W, et al. Altered resting-state cerebral blood flow and its connectivity in schizophrenia. J Psychiatr Res. 2015;63:28–35. [DOI] [PubMed] [Google Scholar]
- 29. Allen P, Chaddock CA, Egerton A, et al. Resting Hyperperfusion of the Hippocampus, Midbrain, and Basal Ganglia in People at High Risk for Psychosis. Am J Psychiatry. 2016;173:392–399. [DOI] [PubMed] [Google Scholar]
- 30. Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. [DOI] [PubMed] [Google Scholar]
- 31. Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: A [(11)C] raclopride PET study. Neuroimage. 2006;33:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. [DOI] [PubMed] [Google Scholar]
- 34. Kindler J, Weickert CS, Schofield PR, Lenroot R, Weickert TW. Raloxifene increases prefrontal activity during emotional inhibition in schizophrenia based on estrogen receptor genotype. Eur Neuropsychopharmacol. 2016;26:1930–1940. [DOI] [PubMed] [Google Scholar]
- 35. Weinberg D, Lenroot R, Jacomb I, et al. Cognitive Subtypes of Schizophrenia Characterized by Differential Brain Volumetric Reductions and Cognitive Decline. JAMA Psychiatry. 2016;73:1251–1259. [DOI] [PubMed] [Google Scholar]
- 36. Meyer-Lindenberg A, Miletich RS, Kohn PD, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271. [DOI] [PubMed] [Google Scholar]
- 37. Fusar-Poli P, Howes OD, Allen P, et al. Abnormal frontostriatal interactions in people with prodromal signs of psychosis: a multimodal imaging study. Arch Gen Psychiatry. 2010;67:683–691. [DOI] [PubMed] [Google Scholar]
- 38. Dandash O, Fornito A, Lee J, et al. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 2014;40:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 41. Woodward TS, Jung K, Hwang H, et al. Symptom dimensions of the psychotic symptom rating scales in psychosis: a multisite study. Schizophr Bull. 2014;40(suppl 4):S265–S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McGlashan T, Walsh B, Woods S. The Psychosis-Risk Syndrome. Handbook for Diagnosis and Follow-up. New York, NY: Oxford University Press; 2010. [Google Scholar]
- 43. Schultze-Lutter F, Addington J, Ruhrmann S, Klosterkotter J. Schizophrenia Proneness Instrument, Adult version (SPI-A). Rome, Italy: Giovanni Fioriti Editore; 2007. [Google Scholar]
- 44. Schultze-Lutter F, Koch E. Schizophrenia Proneness Instrument, Child & Youth version (SPI-CY). Rome, Italy: Giovanni Fioriti Editore; 2010. [Google Scholar]
- 45. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- 46. APA, ed. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 47. Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69:562–571. [DOI] [PubMed] [Google Scholar]
- 48. Michel C, Ruhrmann S, Schimmelmann BG, Klosterkötter J, Schultze-Lutter F. A stratified model for psychosis prediction in clinical practice. Schizophr Bull. 2014;40:1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Helmstaedter C, Lendt M, Lux S. Verbaler Lern- und Merkfähigkeitstest. Göttingen, Germany: Beltz Test GmbH; 2001. [Google Scholar]
- 50. Petrides M, Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. [DOI] [PubMed] [Google Scholar]
- 51. Aschenbrenner S, Tucha O, Lange K. Regensburger-Wortflüssigkeits-Test (RWT) (Regensburg Word Fluency Test). Göttingen, Germany: Hogrefe; 2000. [Google Scholar]
- 52. Petermann F, Petermann U. HAWIK-IV. 3rd ed. Bern, Switzerland: Huber; 2010. [Google Scholar]
- 53. Reitan RM. Trail making test results for normal and brain-damaged children. Percept Mot Skills. 1971;33:575–581. [DOI] [PubMed] [Google Scholar]
- 54. Dunn LM, Dunn DM. PPVT-4: Peabody picture vocabulary test. 4th ed. Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- 55. Wang J, Rao H, Wetmore GS, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci U S A. 2005;102:17804–17809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Homan P, Kindler J, Hubl D, Dierks T. Auditory verbal hallucinations: imaging, analysis, and intervention. Eur Arch Psychiatry Clin Neurosci. 2012;262(suppl 2):S91–S95. [DOI] [PubMed] [Google Scholar]
- 57. Federspiel A, Müller TJ, Horn H, Kiefer C, Strik WK. Comparison of spatial and temporal pattern for fMRI obtained with BOLD and arterial spin labeling. J Neural Transm (Vienna). 2006;113:1403–1415. [DOI] [PubMed] [Google Scholar]
- 58. Schmid Daners M, Knobloch V, Soellinger M, et al. Age-specific characteristics and coupling of cerebral arterial inflow and cerebrospinal fluid dynamics. PLoS One. 2012;7:e37502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Handley R, Zelaya FO, Reinders AA, et al. Acute effects of single-dose aripiprazole and haloperidol on resting cerebral blood flow (rCBF) in the human brain. Hum Brain Mapp. 2013;34:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Huppertz HJ, Kröll-Seger J, Klöppel S, Ganz RE, Kassubek J. Intra- and interscanner variability of automated voxel-based volumetry based on a 3D probabilistic atlas of human cerebral structures. Neuroimage. 2010;49:2216–2224. [DOI] [PubMed] [Google Scholar]
- 61. Friedman L, Glover GH, Krenz D, Magnotta V; FIRST BIRN Reducing inter-scanner variability of activation in a multicenter fMRI study: role of smoothness equalization. Neuroimage. 2006;32:1656–1668. [DOI] [PubMed] [Google Scholar]
- 62. Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. Lancet. 2014;383:1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sander CY, Hooker JM, Catana C, Rosen BR, Mandeville JB. Imaging agonist-induced D2/D3 receptor desensitization and internalization in vivo with PET/fMRI. Neuropsychopharmacology. 2016;41:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohlin KE, Sebastianutto I, Adkins CE, Lundblad C, Lockman PR, Cenci MA. Impact of L-DOPA treatment on regional cerebral blood flow and metabolism in the basal ganglia in a rat model of Parkinson’s disease. Neuroimage. 2012;61:228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schultze-Lutter F, Ruhrmann S, Berning J, Maier W, Klosterkötter J. Basic symptoms and ultrahigh risk criteria: symptom development in the initial prodromal state. Schizophr Bull. 2010;36:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry. 2015;30:388–404. [DOI] [PubMed] [Google Scholar]
- 67. Fusar-Poli P, Howes OD, Allen P, et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry. 2011;16:67–75. [DOI] [PubMed] [Google Scholar]
- 68. Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. [DOI] [PubMed] [Google Scholar]
- 69. Zhuo C, Zhu J, Qin W, Qu H, Ma X, Yu C. Cerebral blood flow alterations specific to auditory verbal hallucinations in schizophrenia. Br J Psychiatry. 2017;210:209–215. [DOI] [PubMed] [Google Scholar]
- 70. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 71. Pauli WM, O’Reilly RC, Yarkoni T, Wager TD. Regional specialization within the human striatum for diverse psychological functions. Proc Natl Acad Sci U S A. 2016;113:1907–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. [DOI] [PubMed] [Google Scholar]
- 73. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological Test Performance to Enhance Identification of Subjects at Clinical High Risk for Psychosis and to Be Most Promising for Predictive Algorithms for Conversion to Psychosis: A Meta-Analysis. J Clin Psychiatry. 2017;78:e28–e40. [DOI] [PubMed] [Google Scholar]
- 74. Schimmelmann BG, Michel C, Martz-Irngartinger A, Linder C, Schultze-Lutter F. Age matters in the prevalence and clinical significance of ultra-high-risk for psychosis symptoms and criteria in the general population: Findings from the BEAR and BEARS-kid studies. World Psychiatry. 2015;14:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cornblatt BA, Carrión RE, Auther A, et al. Psychosis Prevention: A Modified Clinical High Risk Perspective From the Recognition and Prevention (RAP) Program. Am J Psychiatry. 2015;172:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cho KI, Shenton ME, Kubicki M, et al. Altered Thalamo-Cortical White Matter Connectivity: Probabilistic Tractography Study in Clinical-High Risk for Psychosis and First-Episode Psychosis. Schizophr Bull. 2016;42:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Allen P, Chaddock CA, Egerton A, et al. Functional outcome in people at high risk for psychosis predicted by thalamic glutamate levels and prefronto-striatal activation. Schizophr Bull. 2015;41:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Satterthwaite TD, Shinohara RT, Wolf DH, et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc Natl Acad Sci U S A. 2014;111:8643–8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu Y, Zhu X, Feinberg D, et al. Arterial spin labeling MRI study of age and gender effects on brain perfusion hemodynamics. Magn Reson Med. 2012;68:912–922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.