Abstract
Introduction: Schizophrenia, bipolar disorder, and major depressive disorder (MDD) have all been associated with immune system dysfunction, including aberrant cerebrospinal fluid (CSF) levels of cytokines and tryptophan catabolites; however, the pattern of alterations has not been compared across disorders. We performed a meta-analysis of CSF cytokine and tryptophan catabolites in patients with these major psychiatric disorders. Methods: Articles were identified by searching Pub Med, PsycInfo, and Web of Science, and the reference lists of these studies. Results: Twenty-eight studies met the inclusion criteria (16 schizophrenia, 4 bipolar disorder, and 9 MDD). CSF levels of IL-1β and kynurenic acid were significantly increased in patients with schizophrenia and bipolar disorder compared to healthy controls (P < .001). CSF levels of IL-6 and IL-8 were significantly increased in patients with schizophrenia and MDD compared to healthy controls (P ≤ .013). Discussion: There is preliminary evidence for similarities in the pattern of CSF cytokine and tryptophan catabolite alterations across major psychiatric disorders, although findings must be interpreted with caution in light of small numbers of studies/subjects. Many CSF alterations are also concordant with those in the peripheral blood, particularly for schizophrenia. Findings have important implications for our understanding of the pathophysiology and treatment of major psychiatric disorders.
Keywords: schizophrenia, bipolar disorder, major depressive disorder, cerebrospinal fluid, cytokines, inflammation, meta-analysis
Introduction
In recent years, there has been increased attention towards a potential role of the immune system in the pathophysiology of major psychiatric disorders. This interest has been at least partially stimulated by our increased understanding of the interactions that occur between the immune system and the brain in other chronic medical disorders. Advances in molecular biology and genetics have led to the identification of associations between genes involved in the regulation of the immune system and increased risk of schizophrenia, bipolar disorder, and major depressive disorder (MDD).1–3 These disorders are also associated with abnormalities in blood immune cell numbers, inflammatory markers, tryptophan catabolites, and antibody titers.4–10 There is mixed evidence in major psychiatric disorders suggesting that adjunctive treatment with immunomodulatory agents may be associated with improvement in psychopathology.11–14 Taken together, these findings suggest we need to more extensively evaluate the hypothesis that immune dysfunction—manifested by an increase in pro-inflammatory and decrease in anti-inflammatory biomarkers, as well as alterations in tryptophan catabolites—may be involved in the pathogenesis of major psychiatric disorders in some individuals.
Cytokines are key regulators of inflammation that are produced by both immune and nonimmune cells. These signaling molecules coordinate innate and adaptive immunity by binding to specific cytokine receptors on various target cells, and they exert effects in both the periphery and the brain. Cytokine receptors also exist in soluble forms, which can inhibit (eg, soluble interleukin-2 receptor [sIL-2R]) or enhance [eg, sIL-6R]) the biological activity of cytokines. There are also endogenous cytokine receptor antagonists (eg, IL-1 receptor antagonist [IL-1RA]), which compete with cytokines for membrane receptors. Cytokines are key regulators of acute and chronic inflammation, a complex but vital biological response that impacts all organ systems. In addition to their roles in immune function, cytokines also play a role in a host of other physiological processes throughout the body.15,16
A recent hypothesis suggests that the activation of brain microglial cells is associated with increased production of pro-inflammatory cytokines and free radicals that cause neuronal degeneration, white matter abnormalities, and decreased neurogenesis associated with the pathophysiology of schizophrenia.17 Other studies have observed loss of glial elements in mood-relevant parts of the brain and suggest cytokine effects on glia may be important in the pathophysiology of mood disorders.18,19 Cytokines can also directly alter the activity of enzymes involved in tryptophan catabolism. Induction of the enzyme indoleamine 2,3-dioxygenase (IDO) by pro-inflammatory cytokines results in increased production of kynurenine (KYN), which is converted in astrocytes to the NMDA receptor antagonist kynurenic acid (KYNA) and to the NMDA receptor agonist quinolinic acid (QUIN).20,21 NMDA receptor hypofunction has been implicated in the pathophysiology of schizophrenia.22 Previous studies have found increased blood,23,24 cerebrospinal fluid (CSF),25–27 and postmortem brain28 levels of KYNA, as well as increased IDO activity in patients with schizophrenia.29 There is also evidence of abnormalities in tryptophan catabolites in the blood and CSF of patients with bipolar disorder and MDD.8,10,30
In a meta-analysis, we found that blood levels of 2 cytokines (IL-6, TNF-α), 1 soluble cytokine receptor (sIL-2R), and 1 cytokine receptor antagonist (IL-1RA) were significantly increased in acutely ill patients with schizophrenia, bipolar mania, and MDD compared to controls.31 The overall similarities in the pattern of blood cytokine alterations in schizophrenia, bipolar disorder, and MDD during acute and chronic phases of illness raises the possibility of common underlying pathways for immune dysfunction. However, measurements of peripheral blood cytokines and tryptophan catabolites may not necessarily reflect the immunological activity in the brain. At this time, we do not know if changes in these blood markers are mirrored in the CSF. Despite the evidence for cytokine alterations in major psychiatric disorders, there is tremendous between-study heterogeneity with respect to: specific cytokines; effects of potential confounding or moderating factors (eg, medications, smoking, body mass index, assay methodology, fasting status); and associations between immune markers and psychopathology. Meta-analysis is one approach that can bring increased clarity to an area of research with significant heterogeneity,32 and thus is well suited to the study of immune alterations in major psychiatric disorders. This article presents meta-analyses comparing and contrasting the patterns of CSF cytokine and tryptophan catabolite alterations across schizophrenia, bipolar disorder, and MDD. We chose to investigate tryptophan catabolites in addition to cytokines because of the evidence linking these pathways. In doing so, we extend our investigation of potential common underlying pathways for immune dysfunction across these disorders, identify important gaps in the literature, and discuss implications for the research agenda in this field.
Methods
Study Selection
Studies of CSF cytokine (and cytokine receptor or antagonist) and tryptophan catabolite levels in schizophrenia, bipolar disorder, and MDD were identified by a systematic search using Medline (PubMed, National Center for Biotechnology Information, US National Library of Medicine, Bethesda, Maryland), PsycInfo (via Ovid, American Psychological Association, Washington, DC), and Thomson Reuters (formerly ISI) Web of Science (Science Citation Index and Social Sciences Citation Index, Thomson Reuters, Charlottesville, Virginia) in June 2015 and again in March 2016. The primary search strategies were: (1) “(CSF OR “cerebrospinal fluid”) AND (inflammation OR cytokine OR interleukin OR interferon OR “tumor necrosis factor” OR kynurenine OR “kynurenic acid”) AND (schizophrenia OR psychosis)”, (2) “(CSF OR “cerebrospinal fluid”) AND (inflammation OR cytokine OR interleukin OR interferon OR “tumor necrosis factor” OR kynurenine OR “kynurenic acid”) AND (bipolar OR mania)”, and (3) “(CSF OR “cerebrospinal fluid”) AND (inflammation OR cytokine OR interleukin OR interferon OR “tumor necrosis factor” OR kynurenine OR “kynurenic acid”) AND (depression OR “major depressive disorder”), limiting results to human studies in English. From all sources, we identified 212 potential studies for schizophrenia, 49 for bipolar disorder, and 276 for MDD. The majority of initial matches were excluded because they were review articles, did not present CSF data, or were genetic studies related to cytokines or tryptophan catabolites.
The inclusion criteria were studies assessing CSF cytokine or tryptophan catabolite levels in patients with schizophrenia, bipolar mania, or MDD and healthy controls. The exclusion criteria were: (1) studies without a control group, (2) studies that did not present either mean and SDs or median and interquartile range (IQR) for cytokine levels (after attempting to contact the study authors), (3) significant overlap in study population, and (4) genetic studies related to cytokines or tryptophan catabolites. Due to the potential for low concentrations of some markers, the methods of the potential studies were reviewed to evaluate assay sensitivity. An individual marker was excluded if: (1) the mean concentration was less than the lower limit of assay detection, (2) concentrations were not detectable in >50% of subjects, or (3) either the intra-assay coefficient of variation (CV) was >10% or the inter-assay CV was >15%.
After independent searches, review of study methods by both authors (A.K.W. and B.J.M.) and attempts to contact other authors, 28 studies met the inclusion criteria (16 schizophrenia, 4 bipolar disorder, and 9 MDD—1 study reported on both schizophrenia and MDD).25–27,33–57 There was universal agreement on the included studies. A flow chart summarizing the study selection process is presented in supplementary material.
Data Extraction and Meta-analysis
Data were extracted (sample size, mean/SD or median/IQR for patients and controls), for every cytokine and tryptophan catabolite assessed in each study for each disorder. If necessary, we estimated the mean/SD from the median/IQR using the following formulas: (1) mean = (2m + a + b)/4, where m is the median and a and b are the 25th and 75th percentiles, respectively,58 and (2) IQR = 1.35 × SD.59 One author (A.K.W.) extracted all data, which was independently verified by another author (BJM). We then calculated effect size ES estimates (Standard Mean Difference [SMD]) for every marker in each study for each disorder, and these data are included in supplementary material. Fixed effects pooled ES estimates and 95% CIs were calculated using the method of Mantel and Haenszel. Separate meta-analyses were performed for each individual marker for each disorder (vs controls). P values were considered statistically significant at the α = .05 level. Given the preliminary nature of this study, we did not correct P-values for multiple comparisons. The statistical analyses were performed in Stata 10.0 (StataCorp LP).
The meta-analysis procedure also calculates a χ2 value for the heterogeneity in ES estimates, which is based on Cochran’s Q-statistic,60 and I2, the proportion of the variation in ES attributable to between-study heterogeneity. Heterogeneity (χ2) was considered significant for P < .10.61 For many cytokines for each disorder, χ2 was significant, so we performed a sensitivity analysis. This was done by removing 1 study at a time and repeating the meta-analysis procedure, to examine its impact on the OR and between-study heterogeneity.62
Given the significant heterogeneity for many markers, we performed a series of meta-regressions for IL-6 in schizophrenia and MDD (the marker most frequently studied in each disorder) to explore possible moderating variables of age and sex. We were not able to perform meta-regression analyses for other markers due to the small number of available studies, nor other possible moderating variables (eg, illness duration, smoking, and body mass index) due to the absence of adequate data. Potential for publication bias was examined with Sterne’s funnel plot analysis63 and Egger’s regression intercept.64
Results
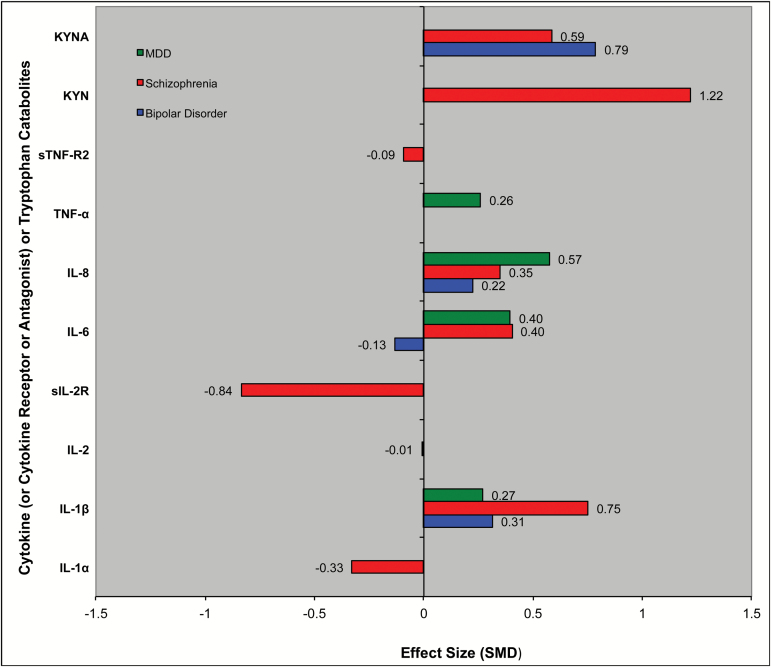
To summarize our findings: CSF levels of IL-6 (small–medium ES = 0.40) and IL-8 (small–medium ES, range 0.35–0.57) were significantly increased in patients with schizophrenia and MDD compared to controls (P ≤ .013 for each); IL-1β (small–medium ES, range 0.31–0.75) and KYNA (medium-large ES, range 0.59–0.79) were significantly increased in patients with schizophrenia and bipolar disorder compared to healthy controls (P ≤ .013 for each). CSF levels of KYN (large ES = 1.22) were significantly increased and CSF sIL-2R levels (large ES = −0.84) were significantly decreased in patients with schizophrenia compared to healthy controls (P < .02 for each). Findings for each disorder are presented in greater detail below (see also table 1 and figures 1 and 2).
Table 1.
CSF Cytokine and Tryptophan Catabolite Alterations in Patients With Schizophrenia, Bipolar Disorder, and MDD vs Controls
| Marker | N | Mean ES | 95% CI | P Value | Heterogeneity | I 2 | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Pt | Control | χ2 | P Value | |||||||
| Schizophrenia | |||||||||||
| IL-1α | 2 | 70 | 31 | −0.33 | −0.77 | 0.11 | .143 | 3.44 | .06 | 70.9 | 43,44 |
| IL-1β | 3 | 57 | 45 | 0.75 | 0.29 | 1.21 | .001 | 40.30 | <.01 | 95.0 | 33,38,54 |
| IL-2 | 4 | 114 | 52 | −0.01 | −0.35 | 0.33 | .954 | 6.14 | .11 | 51.2 | 33,35,43,44 |
| sIL-2R | 2 | 19 | 20 | −0.84 | −1.50 | −0.18 | .013 | 0.66 | .42 | 0.0 | 33,46 |
| IL-6 | 7 | 244 | 180 | 0.40 | 0.20 | 0.60 | <.001 | 6.12 | .53 | 0.0 | 27,33,37,38,53,54,56 |
| IL-8 | 3 | 112 | 101 | 0.35 | 0.07 | 0.63 | .013 | 1.01 | .60 | 0.0 | 27,37,54 |
| TNF-α | |||||||||||
| sTNF-R2 | 2 | 56 | 45 | −0.09 | −0.49 | 0.30 | .646 | 2.56 | .11 | 61.0 | 37,46 |
| KYN | 3 | 60 | 92 | 1.22 | 0.86 | 1.58 | <.001 | 14.66 | <.01 | 86.4 | 25,27,44 |
| KYNA | 4 | 148 | 141 | 0.59 | 0.34 | 0.83 | <.001 | 2.87 | .41 | 0.0 | 25–27,44 |
| Bipolar disorder | |||||||||||
| IL-1α | |||||||||||
| IL-1β | 2 | 151 | 101 | 0.31 | 0.05 | 0.58 | .020 | 27.51 | <.01 | 96.4 | 39,54 |
| IL-2 | |||||||||||
| sIL-2R | |||||||||||
| IL-6 | 2 | 151 | 101 | −0.13 | −0.39 | 0.12 | .310 | 12.94 | <.01 | 92.3 | 39,54 |
| IL-8 | 2 | 151 | 101 | 0.22 | −0.03 | 0.48 | .087 | 6.97 | <.01 | 85.7 | 39,54 |
| TNF-α | |||||||||||
| sTNF-R2 | |||||||||||
| KYN | |||||||||||
| KYNA | 2 | 74 | 35 | 0.79 | 0.36 | 1.21 | <.001 | 0.36 | .408 | 0.0 | 48,49 |
| MDD | |||||||||||
| IL-1α | |||||||||||
| IL-1β | 2 | 30 | 57 | 0.27 | −0.21 | 0.75 | .266 | 12.28 | <.01 | 91.9 | 41,45 |
| IL-2 | |||||||||||
| sIL-2R | |||||||||||
| IL-6 | 7 | 127 | 222 | 0.40 | 0.17 | 0.63 | .001 | 21.49 | <.01 | 67.4 | 34,41,45,47,50,53,57 |
| IL-8 | 2 | 38 | 114 | 0.57 | 0.20 | 0.95 | .003 | 2.12 | .15 | 52.9 | 45,57 |
| TNF-α | 3 | 48 | 82 | 0.26 | −0.10 | 0.63 | .161 | 1.18 | .55 | 0.0 | 41,45,47 |
| sTNF-R2 | |||||||||||
| KYN | |||||||||||
| KYNA | |||||||||||
Note: CSF, cerebrospinal fluid; MDD, major depressive disorder; KYN, kynurenine; KYNA, kynurenic acid. Bolded P values were significant at the P < .05 level.
Fig. 1.
Cerebrospinal fluid (CSF) cytokine and tryptophan catabolite alterations in patients with schizophrenia, bipolar disorder, and major depressive disorder vs controls.
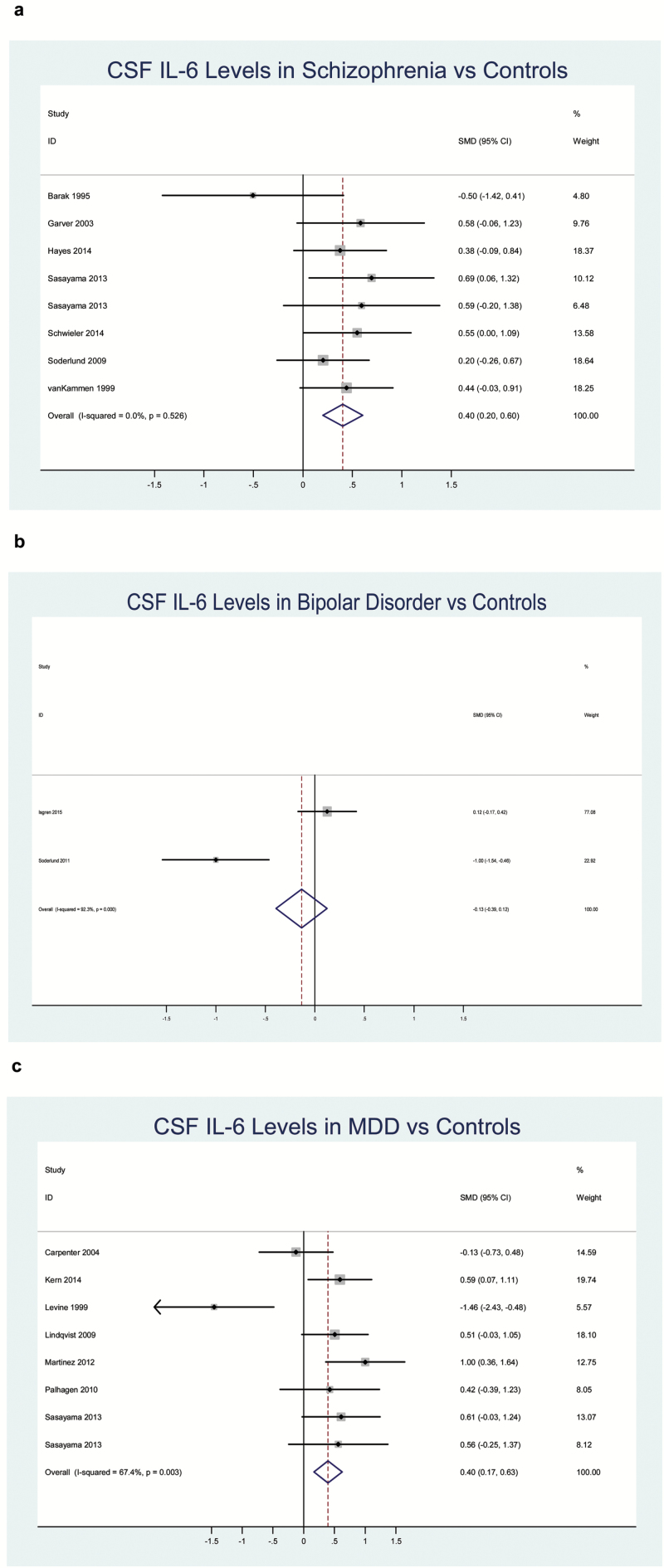
Fig. 2.
Forest plots of cerebrospinal fluid (CSF) IL-6 alterations in patients with schizophrenia, bipolar disorder, and major depressive disorder (MDD) vs controls. (a) Schizophrenia, (b) bipolar disorder, and (c) MDD.
Schizophrenia
CSF levels of IL-1β, IL-6, IL-8, KYN, and KYNA were all significantly increased (P < .02 for each), and CSF levels of sIL-2R were significantly decreased (P < .02) in schizophrenia vs controls. There was not a significant ES difference for IL-1α, IL-2, or sTNFR2. Between-study heterogeneity was significant for IL-1β and KYN. In sensitivity analyses, heterogeneity was no longer significant, but the ES remained significant after removing 1 study for KYN.45 A funnel plot and results of Egger’s test showed no evidence for publication bias for IL-6 (P > .05). In meta-regression analyses, age and sex were both unrelated to IL-6 in schizophrenia (P > .05 for each).
Bipolar Disorder
CSF levels of IL-1β (P = .02) and KYNA (P < .001) were both significantly increased in patients with bipolar disorder vs controls, and IL-8 was increased at the trend level (P = .087). There was not a significant ES difference for IL-6. Between-study heterogeneity was significant for IL-1β, IL-6, and IL-8, but not for KYNA. Sensitivity analysis was not possible for any markers due to the small number of studies.
Major Depressive Disorder
CSF levels of IL-6 and IL-8 were both significantly increased in MDD patients vs controls (P < .01). There was not a significant ES difference for IL-1β and TNF-α. Between-study heterogeneity was significant for IL-1β and IL-6, but not for IL-8 or TNF-α. In a sensitivity analysis, heterogeneity was no longer significant, but the ES remained significant, after removing 1 study for IL-6.42 Sensitivity analysis was not possible for IL-8 due to the small number of studies. A funnel plot and results of Egger’s test showed no evidence for publication bias for IL-6 (P > .05). In meta-regression analyses, age and sex were both unrelated to IL-6 in MDD (P > .05 for each).
Discussion
Overall, there is preliminary evidence for some similarities in the ES direction and, to a lesser extent, magnitude of alterations in CSF cytokine and tryptophan catabolite levels in schizophrenia, bipolar disorder, and MDD compared to controls. Levels of 3 cytokines (IL-1β, IL-6, and IL-8) and 1 tryptophan catabolite (kynurenic acid) were significantly elevated in 2 of 3 syndromes. In addition, levels of kynurenine were significantly increased and levels of sIL-2R were significantly decreased in patients with schizophrenia vs controls.
We found some evidence of concordance between cytokine alterations in the CSF identified in the present study with those found in our previous meta-analysis of cytokine alterations in the peripheral blood,31 particularly for schizophrenia (table 2). Both blood and CSF IL-1β, IL-6, and IL-8, but not IL-2, are increased in patients with schizophrenia vs controls.31 By contrast, CSF sIL-2R levels were significantly decreased, whereas blood levels are significantly increased in schizophrenia.31 In bipolar disorder, increased blood and CSF IL-1β was a concordant finding. By contrast, blood IL-6 levels were significantly increased in bipolar disorder, whereas CSF IL-6 levels were nonsignificantly decreased. In MDD, increased blood and CSF IL-6 and nonsignificant alterations in IL-1β were concordant findings. Blood and CSF findings were discordant for IL-8 and TNF-α in MDD. However, the majority of findings for blood and CSF cytokines are based on independent studies. We identified 4 studies with available data on blood and CSF cytokines in the same patients. Sasayama et al31 found significantly higher CSF than blood IL-6 levels in schizophrenia, and a trend for higher levels in MDD. However, blood and CSF IL-6 levels were not correlated. van Kammen et al56 found lower CSF than blood IL-6 levels in schizophrenia, but did not report on the correlation between these measures. Katila et al40 found lower CSF than blood IL-1β levels in schizophrenia, and no correlation between the 2 measures. Lindqvist et al46 found lower levels of IL-1β, IL-6, and TNF-α, and higher IL-8 levels in CSF than blood in suicide attempters with depression. Blood and CSF levels were not correlated for any of the cytokines.
Table 2.
Comparison of Blood and CSF Cytokine Alterations in Patients with Schizophrenia, Bipolar Disorder, and MDD vs Controls
| Marker | Blood31 | CSF | ||
|---|---|---|---|---|
| First-Episode | Acutely Ill | Outpatient | ||
| Schizophrenia | ||||
| IL-1β | ↑ | ↑ | ↑ | ↑ |
| IL-2 | NS | NS | NS | NS |
| sIL-2R | ↑ | ↑ | ↑ | ↑ |
| IL-6 | ↑ | ↑ | ↑ | ↑ |
| IL-8 | ↑ | ↑ | ↑ | ↑ |
| TNF-α | ↑ | ↑ | ↑ | |
| Bipolar disorder | ||||
| IL-1β | ↑ | ↑ | ||
| IL-2 | ||||
| sIL-2R | ||||
| IL-6 | ↑ | ↑ | NS | |
| IL-8 | NS | |||
| TNF-α | ↑ | NS | ||
| MDD | ||||
| IL-1β | NS | NS | NS | |
| IL-2 | NS | ↑ | ||
| sIL-2R | NS | |||
| IL-6 | ↑ | ↑ | ↑ | |
| IL-8 | NS | ↑ | ||
| TNF-α | ↑ | NS | NS | |
Note: CSF, cerebrospinal fluid; MDD, major depressive disorder; NS, not significant; ↑, Significantly increased in patients vs controls; ↓, Significantly decreased in patients vs controls.
One potential explanation for discordant results is that the CSF findings are generally based on much smaller sample sizes than those for blood. Another potential contributing factor is that we were unable to control for the clinical status of subjects in the analysis of CSF cytokines, whereas previous findings for blood cytokines were stratified based on this variable (eg, first-episode, acutely ill, and chronically ill subjects).31 Alternatively, it is possible that there are differences in regulatory mechanisms in the CNS compared to the periphery which contribute to the observed discrepancies. There is evidence for discordant findings for blood and CSF cytokine levels in other disorders, including neuropsychiatric lupus and multiple sclerosis.65,66
It is intriguing that all 3 cytokines that were elevated in patients (IL-1β, IL-6, and IL-8) are modulated through the Nuclear Factor-kappa B (NF-κB) signaling pathway that is commonly activated in inflammatory and autoimmune disease.67–69 Elevations in the CSF cytokines IL-1β and IL-6 suggest the possibility of microglial activation, which has been reported in patients with schizophrenia.70–72 These findings are broadly consistent with elevations in CSF kynurenic acid in schizophrenia and bipolar disorder, as cytokines can modulate the activity of tryptophan catabolism in astrocytes and microglia. However, none of the included studies reported correlative data between levels of cytokines and tryptophan catabolites.
The primary strength of our study is the comparison of CSF cytokine and tryptophan catabolite alterations across 3 major psychiatric disorders, which has not been previously performed. There are several limitations of the present work. Our findings should be interpreted with caution in light of small numbers of studies and cumulative sample size available for many of the markers, including cytokines that were not studied in some of the disorders.13 of the 17 markers were reported in 3 or fewer studies. This is itself an important finding: the need to replicate many of the observed alterations, as well as studies of specific markers in order to facilitate comparisons across disorders. A second limitation is that one cannot account for either the clinical status or the type/length of pharmacologic intervention employed, as there were an insufficient number of studies to conduct subgroup analyses. One also needs to acknowledge the problem of important potential confounding, and some potentially moderating, factors such as smoking, BMI, medical comorbidities, level and type of psychopathology, genetic heterogeneity, sample collection and processing, time of day, type of assay employed and how long samples are stored before being analyzed.73–78 Subjects were medicated in the majority of studies included in the present analysis. There is evidence that antipsychotics (as a class) increase sIL-2R and decrease IL-1β and interferon-gamma (IFN-γ) levels in the blood in patients with schizophrenia.78 In vitro studies of the effects of antipsychotics on cytokines are heterogeneous, with evidence for the same antipsychotic showing pro- and anti-inflammatory effects across different studies.79 Furthermore, in vitro studies have found that among antidepressants, fluoxetine and clomipramine tend to decrease IL-6, IFN-γ, and TNF-α production, whereas mirtazapine and venlafaxine may increase production of these cytokines.79 However, the effects of psychotropic medications on CSF cytokines have not been characterized. Although we did not find evidence for moderating effects of age or sex on IL-6 levels in either schizophrenia or MDD, this does not preclude moderating effects between such clinical and demographic factors and other markers. These factors should be carefully considered regarding the significance of our meta-analytic findings. Based on the lack of available data, we were also not able to perform a meta-analysis of other molecules/markers that are involved in immune/inflammatory mechanisms (eg, chemokines).
Our findings raise many questions. What are the most important CSF markers to measure across disorders? We suggest that some of the most important CSF cytokines for further study include IL-1β, sIL-2R, IL-6, IL-8, and TNF-α, because of (1) existing evidence of alterations in the peripheral blood and/or CSF, (2) some of these markers have not been studied in the CSF in some psychiatric disorders (eg, TNF-α in schizophrenia and bipolar disorder, sIL-2R in bipolar disorder and MDD), and/or (3) the opportunity to address discordant findings (eg, blood vs CSF). Other issues in such an approach include statistical considerations of multiple comparisons due to the potential for type 1 error, and whether investigators should focus on a composite “inflammatory score” rather than individual markers. Studies measuring both cytokines and tryptophan catabolites would be informative regarding the relationship of these pathways across disorders. What is the relationship of CSF markers to demographic and clinical features (eg, psychopathology and cognition)? Relationships between cytokine levels and psychopathology have not been considered in the majority of previous studies. It would therefore be informative to investigate if there are any symptom dimensions, which may cut across diagnostic categories, which may be more strongly associated with inflammation. Studies in first-episode drug-naïve subjects would clarify whether alterations in cytokines and tryptophan catabolites are more attributable to the disorder itself or medication effects. Studies of changes in these CSF markers across the course of the disorder, including acute illness episodes and treatment-resistant illness would also be particularly informative. Is there a level of concordance in findings that would justify preferential use of peripheral blood vs CSF markers, particularly if findings in blood were coupled with brain imaging?
In conclusion, there is some preliminary evidence for similarities in the pattern of CSF cytokine and tryptophan catabolite alterations in subjects with schizophrenia, bipolar disorder and MDD most consistent with an inflammatory profile, although many findings are based on a small number of studies/subjects and there was substantial between-study heterogeneity. Our findings do not rule out the possibility of common underlying pathways for the expression of immune dysfunction in patients with these disorders, and this hypothesis warrants further evaluation. Many CSF alterations are also concordant with those in the peripheral blood, particularly for schizophrenia. The moderate effect sizes observed most likely reflects that fact that immune system involvement occurs in only a subset of patients with each of these syndromes, which is an important consideration for future studies of immune function and anti-inflammatory therapies. The results from this meta-analysis reflect a need to more rigorously evaluate how and what we measure in the immune system. More extensive and systematic investigation in this area is clearly warranted.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
A.K.W. received funding from the Augusta University Dean’s Medical Scholars program.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Goran Engberg, Lindsay Hayes, and Silke Kern for sharing data and/or information about their studies. B.J.M. has nothing to disclose for this study. In the past 12 months, B.J.M. received research support from the National Institute of Mental Health, National Institutes of Health Clinical Loan Repayment Program, NARSAD, the Stanley Medical Research Institute, and Augusta University; and Honoraria from Psychiatric Times.
References
- 1. Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci. 2010;8:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The International Schizophrenia Consortium ; Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia that overlaps with bipolar disorder. Nature. 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barbosa IG, Machado-Vieira R, Soares JC, Teixeira AL. The immunology of bipolar disorder. Neuroimmunomodulation. 2014;21:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ezeoke A, Mellor A, Buckley P, Miller BJ. A systematic quantitative review of blood autoantibody elevations in schizophrenia. Schizophr Res. 2013;150:245–251. [DOI] [PubMed] [Google Scholar]
- 6. Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. 2013;8:900–920. [DOI] [PubMed] [Google Scholar]
- 7. Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2013;73:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Müller N. Immunology of major depression. Neuroimmunomodulation. 2014;21:123–130. [DOI] [PubMed] [Google Scholar]
- 9. Pearlman DM, Najjar S. Meta-analysis of the association between N-methyl-d-aspartate receptor antibodies and schizophrenia, schizoaffective disorder, bipolar disorder, and major depressive disorder. Schizophr Res. 2014;157:249–258. [DOI] [PubMed] [Google Scholar]
- 10. Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS J. 2012;279:1375–1385. [DOI] [PubMed] [Google Scholar]
- 11. Ayorech Z, Tracy DK, Baumeister D, Giaroli G. Taking the fuel out of the fire: evidence for the use of anti-inflammatory agents in the treatment of bipolar disorders. J Affect Disord. 2015;174:467–478. [DOI] [PubMed] [Google Scholar]
- 12. Köhler O, Benros ME, Nordentoft M, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. [DOI] [PubMed] [Google Scholar]
- 13. Nitta M, Kishimoto T, Müller N, et al. Adjunctive use of nonsteroidal anti-inflammatory drugs for schizophrenia: a meta-analytic investigation of randomized controlled trials. Schizophr Bull. 2013;39:1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue, structure, function, and factors that influence bone cells. BioMed Res Int. 2015;2015:421746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ingman WV, Robertson SA. The essential roles of TGFB1 in reproduction. Cytokine Growth Factor Rev. 2009;20:233–239. [DOI] [PubMed] [Google Scholar]
- 17. Monji A, Kato T, Kanba S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci. 2009;63:257–265. [DOI] [PubMed] [Google Scholar]
- 18. Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. [DOI] [PubMed] [Google Scholar]
- 19. Ongur D, Drevets WC, Price JL. Glial Reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA. 1998;95:13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muller N, Weidinger E, Leitner B, Schwarz MJ. The role of inflammation in schizophrenia. Front Neurosci. 2015;9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Myint AM, Kim YK. Network beyond IDO in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:304–313. [DOI] [PubMed] [Google Scholar]
- 22. Gaspar PA, Bustamante ML, Silva H, et al. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111:891–900. [DOI] [PubMed] [Google Scholar]
- 23. Chiappelli J, Pocivavsek A, Nugent KL, et al. Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA Psychiatry. 2014;71:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ravikumar A, Deepadevi KV, Arun P, et al. Tryptophan and tyrosine catabolic pattern in neuropsychiatric disorders. Neurology India. 2000;48:231–238. [PubMed] [Google Scholar]
- 25. Kegel ME, Bhat M, Skogh E, et al. Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res. 2014;7:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nilsson LK, Linderholm KR, Engberg G, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–322. [DOI] [PubMed] [Google Scholar]
- 27. Schwieler L, Larsson MK, Skogh E, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia – significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;2:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sathyasaikumar KV, Stachowski EK, Wonodi I, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barry S, Clarke G, Scully P, et al. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–294. [DOI] [PubMed] [Google Scholar]
- 30. Bay-Richter C, Linderholm KR, Lim CK, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-asparate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–117. [DOI] [PubMed] [Google Scholar]
- 31. Goldsmith D, Rapaport MH, Miller BJ. Meta-analysis of cytokine alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Molecular Psychiatry. 2016;21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. [DOI] [PubMed] [Google Scholar]
- 33. Barak V, Barak Y, Levine J, Nisman B, Roisman I. Changes in interleukin-1 beta and soluble interleukin-2 receptor levels in CSF and serum of schizophrenic patients. J Basic Clin Physiol Pharmacol. 1995;6:61–69. [DOI] [PubMed] [Google Scholar]
- 34. Carpenter LL, Heninger GR, Malison RT, Tyrka AR, Price LH. Cerebrospinal fluid interleukin (IL)-6 in unipolar major depression. J Affect Disord. 2004;79:285–289. [DOI] [PubMed] [Google Scholar]
- 35. el-Mallakh RS, Suddath RL, Wyatt RJ. Interleukin-1 alpha and interleukin-2 in cerebrospinal fluid of schizophrenic subjects. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:383–391. [DOI] [PubMed] [Google Scholar]
- 36. Erhardt S, Kim CK, Linderholm KR, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garver DL, Tamas RL, Holcomb JA. Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology. 2003;28:1515–1520. [DOI] [PubMed] [Google Scholar]
- 38. Hayes LN, Severance EG, Leek JT, et al. Inflammatory molecular signature associated with infectious agents in psychosis. Schizophr Bull. 2014;40:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Isgren A, Jakobsson J, Pålsson E, et al. Increased cerebrospinal fluid Interleukin-8 in bipolar disorder patients associated with lithium and antipsychotic treatment. Brain Behav Immun. 2015;43:198–204. [DOI] [PubMed] [Google Scholar]
- 40. Katila H, Hurme M, Wahlbeck K, Appelberg B, Rimón R. Plasma and cerebrospinal fluid interleukin-1 beta and interleukin-6 in hospitalized schizophrenic patients. Neuropsychobiology. 1994;30:20–23. [DOI] [PubMed] [Google Scholar]
- 41. Kern S, Skoog I, Börjesson-Hanson A, et al. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav Immun. 2014;41:55–58. [DOI] [PubMed] [Google Scholar]
- 42. Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. [DOI] [PubMed] [Google Scholar]
- 43. Levine J, Barak Y, Chengappa KRN, Rapoport A, Antelman SM, Barak V. Low CSF soluble interleukin 2 receptor levels in acute depression. J Neural Transm. 1999;106:1011–1015. [DOI] [PubMed] [Google Scholar]
- 44. Licinio J, Seibyl JP, Altemus M, Charney DS, Krystal JH. Elevated CSF levels of interleukin-2 in neuroleptic-free schizophrenic patients. Am J Psychiatry. 1993;150:1408–1410. [DOI] [PubMed] [Google Scholar]
- 45. Linderholm KR, Skogh E, Olsson SK, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lindqvist D, Janelidze S, Hagell P, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. [DOI] [PubMed] [Google Scholar]
- 47. Lu S. Interleukin level in CSF of patients with first-episode psychosis. Zhongguo Xin Li Wei Sheng Za Zhi. 2003;17:206. [Google Scholar]
- 48. Martinez JM, Garakani A, Yehuda R, Gorman JM. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2012;29:32–38. [DOI] [PubMed] [Google Scholar]
- 49. Olsson SK, Samuelsson M, Saetre P, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J Psychiatry Neurosci. 2010;35:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Olsson SK, Sellgren C, Engberg G, Landén M, Erhardt S. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipolar Disord. 2012;14:719–726. [DOI] [PubMed] [Google Scholar]
- 51. Pålhagen S, Qi H, Mårtensson B, Wålinder J, Granérus AK, Svenningsson P. Monoamines, BDNF, IL-6 and corticosterone in CSF in patients with Parkinson’s disease and major depression. J Neurol. 2010;257:524–532. [DOI] [PubMed] [Google Scholar]
- 52. Rapaport MH, McAllister CG, Pickar D, Tamarkin L, Kirch DG, Paul SM. CSF IL-1 and IL-2 in medicated schizophrenic patients and normal volunteers. Schizophr Res. 1997;25:123–129. [DOI] [PubMed] [Google Scholar]
- 53. Sasayama D, Hattori K, Wakabayashi C, et al. Increased cerebrospinal fluid Interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res. 2013;47:401–406. [DOI] [PubMed] [Google Scholar]
- 54. Söderlund J, Schröder J, Nordin C, et al. Activation of brain interleukin-1beta in schizophrenia. Mol Psychiatry. 2009;14:1069–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Söderlund O, Olsson SK, Samuelsson M, et al. Elevation of cerebrospinal fluid interleukin-1β in bipolar disorder. J Psychiatry Neurosci. 2011;36:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Kammen DP, McAllister-Sistilli CG, Kelley ME, Gurklis JA, Yao JK. Elevated interleukin-6 in schizophrenia. Psychiatry Res. 1999;87:129–136. [DOI] [PubMed] [Google Scholar]
- 57. Vawter MP, Dillon-Carter O, Issa F, Wyatt RJ, Freed WJ. Transforming growth factors beta 1 and beta 2 in the cerebrospinal fluid of chronic schizophrenic patients. Neuropsychopharmacology. 1997;16:83–87. [DOI] [PubMed] [Google Scholar]
- 58. Hernandez AV, Guarnizo M, Miranda Y, et al. Association between insulin resistance and breast carcinoma: a systematic review and meta-analysis. PLoS One. 2014;9:e99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Higgins J, Deeks J. Selecting studies and collecting data. Cochrane Handbook for Systematic Reviews of Interventions Version 510 (updated March 2011) The Cochrane Collaboration 2001. www.cochrane-handbook.org. Accessed March 8, 2016.
- 60. Cochran WB. The comparison of percentages in matched samples. Biometrika. 1950;37:256–266. [PubMed] [Google Scholar]
- 61. Song F, Sheldon TA, Sutton AJ, Abrams KR, Jones DR. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof. 2001;24:126–151. [DOI] [PubMed] [Google Scholar]
- 62. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 9.7. Sensitivity analyses. The Cochrane Collaboration 2011. www.cochrane-handbook.org. Accessed March 8, 2016.
- 63. Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. [DOI] [PubMed] [Google Scholar]
- 64. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Brit Med J. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dellalibera-Joviliano R, Dos Reis ML, Cunha Fde Q, Donadi EA. Kinins and cytokines in plasma and cerebrospinal fluid of patients with neuropsychiatric lupus. J Rheumatol. 2003;30:485–492. [PubMed] [Google Scholar]
- 66. Maimone D, Gregory S, Arnason BGW, Reder AT. Cytokine levels in the cerebrospinal fluid and serum of patients with multiple sclerosis. J Neuroimmunol. 1991;32:67–74. [DOI] [PubMed] [Google Scholar]
- 67. Pace TW, Miller AH. Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci. 2009;1179:86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thomas R. The TRAF6-NF kappa B signaling pathway in autoimmunity: not just inflammation. Arthritis Res Ther. 2005;7:170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology. 2013;38:2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Banati R, Hickie IB. Therapeutic signposts: using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. Med J Aust. 2009;190:S26–S32. [DOI] [PubMed] [Google Scholar]
- 71. Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. [DOI] [PubMed] [Google Scholar]
- 72. van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 Positron Emission Tomography Study. Biol Psychiatry. 2008;64:820–822. [DOI] [PubMed] [Google Scholar]
- 73. Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. J Gerontol A Biol Sci Med Sci. 2008;63:879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. O’Connor MF, Bower JE, Cho HJ, et al. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. De Berardis D, Conti CM, Serroni N, et al. The effect of newer serotonin-noradrenalin antidepressants on cytokine production: a review of the current literature. Int J Immunopathol Pharmacol. 2010;23:417–422. [DOI] [PubMed] [Google Scholar]
- 76. Młodzikowska-Albrecht J, Steinborn B, Zarowski M. Cytokines, epilepsy and epileptic drugs–is there a mutual influence?Pharmacol Rep. 2007;59:129–138. [PubMed] [Google Scholar]
- 77. Raghavendra PB, Lee E, Parameswaran N. Regulation of macrophage biology by lithium: a new look at an old drug. J Neuroimmune Pharmacol. 2014;9:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tourjman V, Kouassi É, Koué MÈ, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013;151:43–47. [DOI] [PubMed] [Google Scholar]
- 79. Baumeister D, Ciufolini S, Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment?Psychopharmacology (Berl). 2016;233:1575–1589. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.