Abstract
The identification of microRNAs (miRNAs/miRs) has enabled the improved understanding of the carcinogenesis and progression of hepatocellular carcinoma (HCC). miRNAs are small non-coding RNAs comprised of 19–24 nucleotides that regulate the expression of target genes. In the present study, miR-138 was demonstrated to be downregulated in human HCC tissues and cell lines. Restoration of miR-138 expression repressed the proliferation, migration and invasion of HCC cells. Furthermore, specificity protein 1 (SP1) was identified as a target gene of miR-138 in HCC using bioinformatics analysis, luciferase reporter assay, reverse transcription-quantitative polymerase chain reaction and western blot analysis. Knockdown of SP1 produced similar suppressive effects to those induced by miR-138 overexpression in HCC cells. These results indicate that miR-138 targeted SP1 to repress the growth, migration and invasion of HCC cells, and may therefore represent a therapeutic target in human HCC.
Keywords: microRNA-138, hepatocellular carcinoma, proliferation, migration, invasion, specificity protein 1
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent malignancy and the third-leading cause of cancer-associated mortality worldwide (1). It was reported that there were 782,500 new HCC cases and 745,500 incidences of mortality due to HCC worldwide during 2012, of which half were in China (2). Numerous risk factors for HCC patients have been identified, including chronic viral hepatitis B or C infection, chemical exposure, alcohol consumption, obesity, hereditary hemochromatosis and hepatosteatosis (3). At present, the primary therapeutic strategies for HCC are surgical resection, liver transplantation and ablation, and chemotherapy (4). For patients with early stage of HCC, the primary tumor can often be completely resected; however, patients with advanced-stage HCC have a poor prognosis due to the likelihood of intrahepatic recurrence and tumor metastasis (5,6). Furthermore, the cellular and molecular processes underlying the recurrence and metastasis of HCC remain poorly characterized. A complete understanding of the mechanisms that mediate HCC progression could aid the identification of novel therapeutic targets and consequently improve the prognosis for patients with HCC.
The identification of microRNAs (miRNAs/miRs) has enabled an improved understanding of carcinogenesis and HCC progression. miRNAs are small non-coding RNAs comprised of 19–24 nucleotides (7) that negatively regulate the expression of target genes at the post-transcriptional level through interaction with the 3′-untranslated regions (3′UTRs) of target mRNAs, resulting in either mRNA degradation or the inhibition of translation (8). Since the identification of the first miRNA, lin-4, in 1993, the existence of a large number of miRNAs has been validated (9). At the time of writing, ~1,000 miRNAs have been identified in humans (10). miRNAs are believed to modulate more than a third of human genes (11) and therefore serve notable functions in a large number of biological processes, including cell proliferation, cell cycle, apoptosis, differentiation, metabolism, migration and invasion (12–14). Furthermore, increasing evidence has demonstrated that the abnormal expression of miRNAs contributes to the initiation and development of human malignancies, including HCC (15–17). Although numerous miRNAs have been identified, their expression and roles in HCC carcinogenesis and progression, and the underlying mechanisms by which this occurs, remain largely unidentified.
Thus, the present study aimed to determine the level of miR-138 in HCC tissues and cells, its effects on cell proliferation, migration and invasion, and the mechanisms for the observed effects, including the potential targets of miR-138. miR-138 was demonstrated to be downregulated in HCC and inhibited cell proliferation, migration and invasion via directly targeting SP1. These findings provide supplementation to the general molecular mechanisms of carcinogenesis and progression in HCC and may aid the identification of novel targets for HCC treatment.
Materials and methods
Human tissue specimens
The present study was approved by the Ethics Committee of Qilu Hospital (Jinan, China) and each patient provided informed written consent. A total of 32 paired HCC tissues and corresponding adjacent non-tumor liver tissues were obtained by surgical resection from HCC patients (male, 23; female, 9; age range, 43–75 years; mean age 58 years) at Qilu Hospital. None of these HCC patients were treated with chemotherapy or radiotherapy prior to surgery. Fresh tissues were immediately frozen in liquid nitrogen and stored at −80°C.
Cell lines, cell culture and cell transfection
Immortalized human hepatocyte LO2 cells, and HepG2, Hep3B, SMMC-7721, Huh7 and BEL-7402 HCC cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). All cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin (all Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). An miR-138 mimic, the corresponding negative control (NC), small interfering RNA (siRNA) for SP1 (si-SP1) and si-NC were produced by Shanghai GenePharma Co., Ltd. (Shanghai, China). The miR-138 mimics sequence was 5′-AGCUGGUGUUGUGAAUCAGGCCG-3′ and the NC sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′. The si-SP1 sequence was 5′-GCAACAUGGGAAUUAUGAATT-3′ and the si-NC sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′. Cells were seeded in 6-well plates at 60–70% confluence and transfected with the oligonucleotides using Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cell lines using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The relative expression levels of miR-138 were measured using a One Step SYBR® PrimeScript™ miRNA RT-PCR kit (Takara Bio, Inc., Otsu, Japan) according to the manufacturer's protocol. The reaction conditions were as follows: 42°C for 5 min, 95°C for 10 sec, followed by 40 cycles of 95°C for 5 sec, 55°C for 30 sec and 70°C for 30 sec. To assess SP1 mRNA expression levels, cDNA was synthesized using M-MLV reverse transcriptase (Promega Corporation, Madison, WI, USA) and qPCR was performed using SYBR green I mix (Takara Bio, Inc.). The thermocycling conditions for qPCR were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The primers were designed as follows: miR-138, 5′-TCCGAGCCTGACTAAGTGTTGTGGTCGA-3′ (forward) and 5′-GTGCAGGGTCCGAGGT-3′ (reverse); U6, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ (forward) and 5′-CGCTTCACGAATTTGCGTGTCAT-3′ (reverse); SP1, 5′-TGGTGGGCAGTATGTTGT-3′ (forward) and 5′-GCTATTGGCATTGGTGAA-3′ (reverse); and GAPDH, 5′-GGAGTCAACGGATTTGGT-3′ (forward) and 5′-GTGATGGGATTTCCATTGAT-3′ (reverse). U6 small nuclear RNA and GADPH mRNA were used as internal controls for miR-138 and SP1 mRNA expression, respectively. All experiments were performed in triplicate, and data were analyzed with the 2−∆∆Ct method (18).
Cell proliferation assay
At 48 h after transfection, HepG2 and Huh7 cells were harvested and re-seeded into 96-well plates at a density of 3,000 cells per well. Cell proliferation was determined at 24, 48, 72 and 96 h after plating using Cell Counting Kit-8 (CCK8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) according to the manufacturer's protocol. In brief, cells were incubated with 10 µl CCK8 solution for 2 h in a humidified atmosphere containing 5% CO2 at 37°C. The optical density was measured at 450 nm using an ELISA reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each experiment was repeated at least three times.
Transwell cell migration and invasion assay
Cell migration and invasion were assessed using Transwell chambers (8-µm pore size; Corning, Inc., Corning, NY, USA) either with or without Matrigel (BD Biosciences, San Jose, CA, USA). At 48 h after transfection, HepG2 and Huh7 cells were harvested and re-seeded into the upper chamber at a density of 3×104 cells in 200 µl fetal bovine serum (FBS)-free culture medium. The bottom chamber was filled with 600 µl culture medium containing 20% FBS. Subsequent incubation for 48 h in a humidified atmosphere containing 5% CO2 at 37°C, the cells remaining on the top surface of the membrane were removed using a cotton swab. The migrated and invaded cells were fixed with 95% ethanol for 10 min at room temperature, stained with 0.5% crystal violet for 10 min at room temperature, and 5 random fields were counted under a light microscope (magnification, ×200).
miRNA target-gene prediction
TargetScan (http://www.targetscan.org/) and PicTar (http://pictar.bio.nyu.edu) were used to predict the potential target genes of miR-138.
Luciferase reporter assay
The wild-type [pMIR-SP1-3′UTR Wt 1 and Wt 2] and mutant (pMIR-SP1-3′UTR Mut 1 and Mut 2) SP1 3′UTR cloned in pMIR-REPORT were produced by Shanghai GenePharma Co., Ltd. HEK293T cells were seeded in a 24-well plate at a density of 40–50% confluence. Following incubation overnight, HEK293T cells were co-transfected with miR-138 mimics or NC, and pMIR-SP1-3′UTR Wt or pMIR-SP1-3′UTR Mut using Lipofectamine® 2000 reagent, following the manufacturer's recommendations. At 48 h after transfection, cells were harvested and firefly luciferase activity was detected using the Dual-Luciferase Reporter system (Promega Corporation) and normalized to that of Renilla luciferase, according to the manufacturer's protocol.
Western blot analysis
At 72 h after transfection, cells were lysed with ice-cold lysis buffer supplemented with protease inhibitor cocktail (Pierce; Thermo Fisher Scientific, Inc.). Total protein concentration was determined using the Enhanced BCA Protein Assay kit (Beyotime Institution of Biotechnology, Haimen, China). Protein samples (20 µg) were separated on a 10% SDS-PAGE and then transferred to a polyvinylidene fluoride membrane. Subsequent to blocking in 5% skimmed milk at room temperature for 2 h, the membranes were incubated overnight at 4°C with mouse anti-human SP1 monoclonal primary antibody (1:1,000, ab77441; Abcam, Cambridge, UK) and mouse anti-human GADPH monoclonal primary antibody (1:1,000, ab125247; Abcam) primary antibodies. Next, the membranes were incubated with the goat anti-mouse horseradish peroxidase-conjugated secondary antibody (1:3,000, ab6789; Abcam) for 1 h at room temperature. The membranes were visualized with Super Signal West Pico Chemiluminescent Substrate kit (Pierce; Thermo Fisher Scientific, Inc.). GADPH was used as a loading control.
Statistical analysis
Data were expressed as mean ± standard deviation and compared with Student's t-test or one-way analysis of variance (ANOVA) using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). miR-138 expression in HCC cell lines were analyzed with ANOVA and other results were analyzed by Student's t-test. SNK was used as a post hoc test following ANOVA. P<0.05 was considered to indicate a statistically significant difference.
Results
miR-138 is downregulated in HCC tissues and cell lines
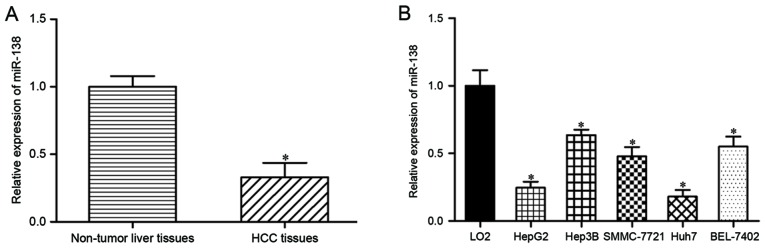
The expression levels of miR-138 were measured in HCC tissues and their corresponding adjacent non-tumor liver tissues using RT-qPCR. miR-138 was significantly downregulated in HCC tissues compared with that in adjacent non-tumor liver tissue (Fig. 1A; P<0.05). miR-138 expression levels were also assessed in HepG2, Hep3B, SMMC-7721, Huh7 and BEL-7402 HCC cells. As demonstrated in Fig. 1B, miR-138 expression levels were reduced in the HCC cell lines compared with the immortalized human hepatocyte LO2 cell line (P<0.05). Therefore, these results indicated that miR-138 was downregulated in HCC tissues and cell lines.
Figure 1.
miR-138 expression was downregulated in HCC tissues and cell lines. (A) Reverse transcription-quantitative polymerase chain reaction analysis measured the expression levels of miR-138 in HCC tissues and their corresponding nearby non-tumor liver tissues. (B) The expression of miR-138 in the immortalized human hepatocyte LO2 cell line and HepG2, Hep3B, SMMC-7721, Huh7 and BEL-7402 HCC cell lines. *P<0.05. miR-138, microRNA-138; HCC, hepatocellular carcinoma.
miR-138 suppresses HCC cell proliferation, migration and invasion
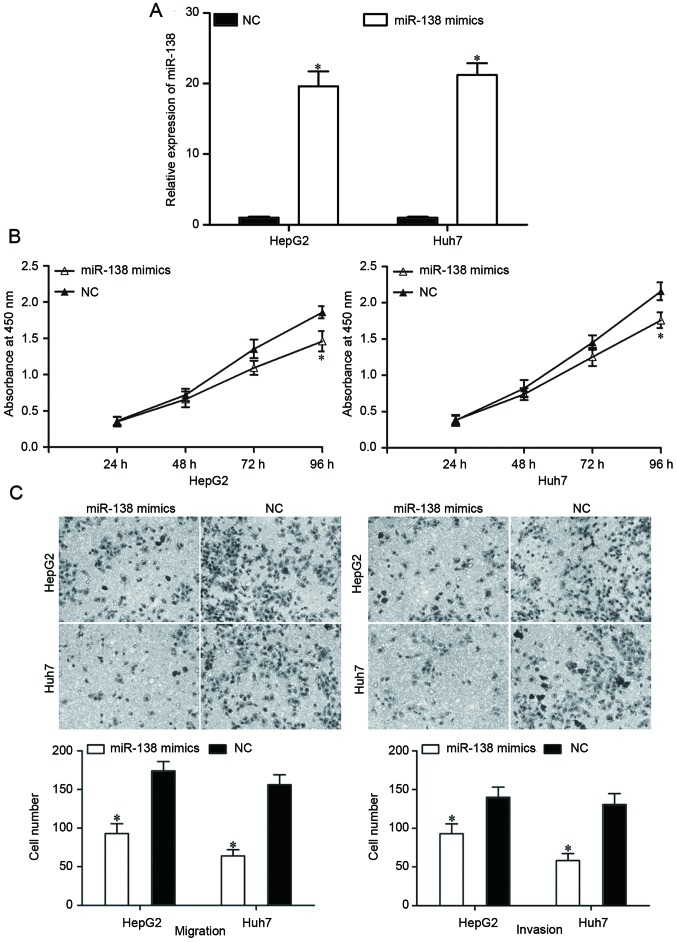
To determine the roles of miR-138 in HCC, an miR-138 mimic was transfected into HepG2 and Huh7 cells. Following transfection with the miR-138 mimic or an NC, the expression levels of miR-138 in HepG2 and Huh7 cells were evaluated by RT-qPCR. The expression in the miR-138 mimic-transfected cells was significantly increased (Fig. 2A, P<0.05).
Figure 2.
Increased miR-138 expression repressed the proliferation, migration and invasion of HCC cells. (A) Transfection of miR-138 mimics into HepG2 and Huh7 increased the expression levels of miR-138. (B) Cell proliferation assay revealed a significant reduction of cell proliferation in miR-138-transfected cells compared with an NC in HepG2 and HuH7 cells. (C) miR-138 exerted an inhibitory effect on the migration and invasion capacity of HepG2 and HuH7 cells. *P<0.05. miR-138, microRNA-138; HCC, hepatocellular carcinoma; NC, negative control.
A cell proliferation assay was performed to investigate the effect of miR-138 on HCC cell proliferation. The results revealed that the restoration of miR-138 expression suppressed the proliferation of HepG2 and Huh7 cells (Fig. 2B; P<0.05). A Transwell migration and invasion assay was performed to investigate the effects of miR-138 on the migration and invasion capacity of HCC cells. As demonstrated in Fig. 2C, the migratory and invasive abilities of HepG2 and Huh7 cells transfected with the miR-138 mimic were significantly reduced compared with cells transfected with the NC (P<0.05). These findings indicated that miR-138 can act as a potential tumor suppressor in HCC.
miR-138 directly targets SP1 in HCC
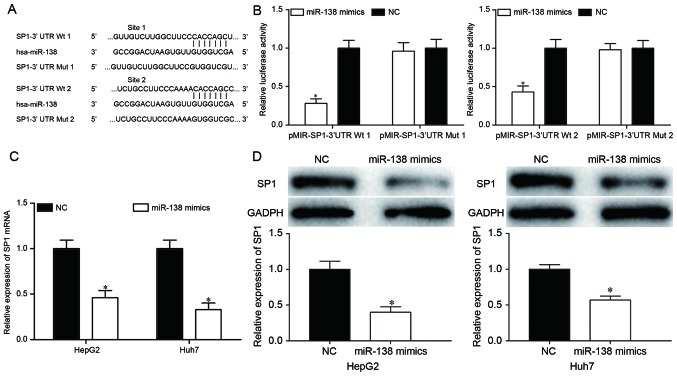
To investigate the mechanisms of miR-138-induced cell proliferation, migration and invasion inhibition, the target genes of miR-138 were searched for using TargetScan and PicTar. Bioinformatics analysis demonstrated that SP1 may be a target of miR-138, based on putative target sequences at the SP1 3′UTR (Fig. 3A). To confirm whether SP1 was a target of miR-138, a dual-luciferase reporter assay was performed, which identified that miR-138 overexpression markedly decreased the luciferase activities with pMIR-SP1-3′UTR Wt 1 and Wt 2, but not pMIR-SP1-3′UTR Mut 1 and Mut 2, indicating that miR-138 directly targeted the 3′UTR of SP1 (Fig. 3B; P<0.05). Furthermore, RT-qPCR and western blot analysis revealed that ectopic miR-138 expression suppressed SP1 expression compared with transfection with the NC in HepG2 and Huh7 cells at the mRNA (Fig. 3C; P<0.05) and protein (Fig. 3D; P<0.05) levels. These results suggested that SP1 was a direct target gene of miR-138 in HCC.
Figure 3.
miR-138 targeted the 3′UTR of SP1 to decrease its expression in HCC. (A) Bioinformatic analysis of the predicted interactions of miR-138 in the 3′UTR of SP1. (B) miR-138 overexpression reduced the luciferase activity of cells transfected with pMIR-SP1-3′UTR Wt 1 and Wt 2, but not pMIR-SP1-3′UTR Mut 1 and Mut 2. (C) Transfection with an miR-138 mimic decreased the expression levels of SP1 mRNA in HepG2 and Huh7 cells. (D) Transfection with an miR-138 mimic reduced SP1 protein levels in HepG2 and Huh7 cells. *P<0.05. miR-138, microRNA-138; HCC, hepatocellular carcinoma; NC, negative control; SP1, specificity protein 1; 3′UTR, 3′-untranslated region; Wt, wild type; Mut, mutant.
Knockdown of SP1 produces similar effects to miR-138 overexpression in HCC cells
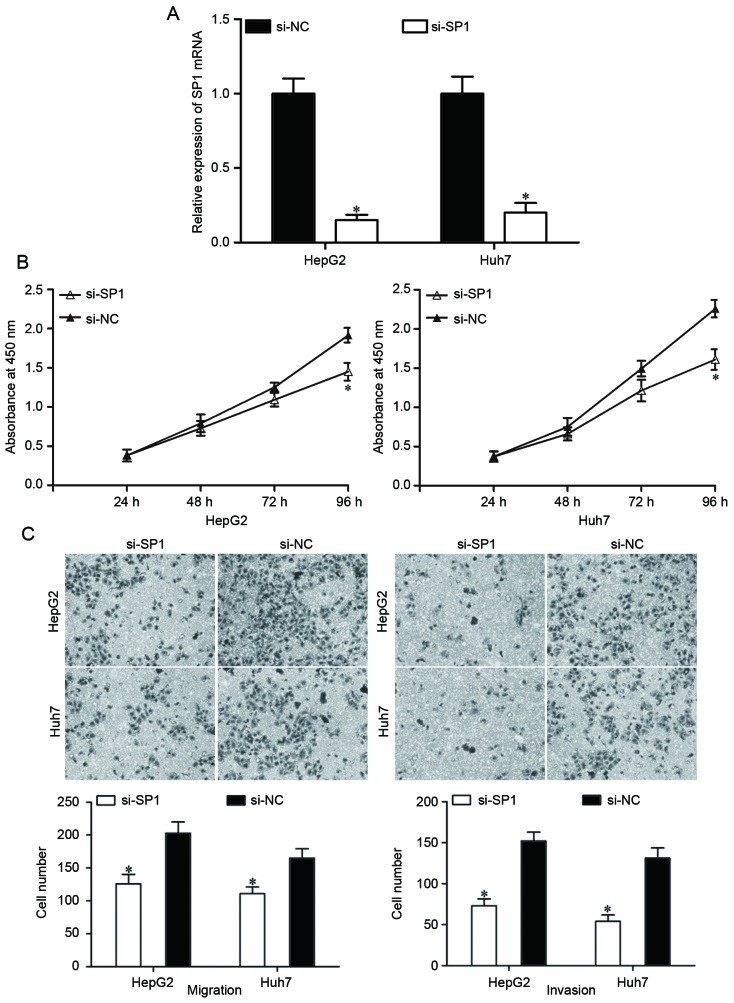
SP1 expression was knocked down using si-SP1 to assess whether SP1 suppressed HCC cell proliferation, migration and invasion as induced by miR-138 overexpression. At 48 h after transfection, the expression of SP1 mRNA was determined using RT-qPCR (Fig. 4A; P<0.05). Based on the results of cell proliferation and Transwell migration and invasion assays, knockdown of SP1 resulted in the suppression of HepG2 and Huh7 cell proliferation (Fig. 4B; P<0.05), migration and invasion (Fig. 4C; P<0.05), which were similar to the effects of miR-138 overexpression in HepG2 and Huh7 cells. These results demonstrated that miR-138 repressed proliferation, migration and invasion of HCC cells by downregulating SP1.
Figure 4.
Knockdown of SP1 repressed the proliferation, migration and invasion of HCC cells. (A) Transfection of si-SP1 into HepG2 and Huh7 cells reduced SP1 mRNA expression. (B) SP1 underexpression inhibited HepG2 and Huh7 proliferation. (C) SP1 underexpression inhibited HepG2 and Huh7 migration and invasion abilities. *P<0.05. si-SP1, small interfering RNA targeting specificity protein 1; HCC, hepatocellular carcinoma.
Discussion
miRNAs have emerged as critical regulators of HCC progression and tumorigenesis (19,20). In the present study, RT-qPCR was performed to measure miR-138 expression in HCC tissues and cell lines. The data revealed that miR-138 expression levels were reduced in HCC tissues and cell lines, compared with corresponding adjacent non-tumor liver tissues, and an immortalized human hepatocyte cell line, respectively. Further experiments demonstrated that miR-138 also suppressed HCC cell proliferation, migration and invasion. SP1 was validated to be a direct target gene of miR-138 in HCC. miR-138/SP1-based targeted therapy could therefore represent a promising therapeutic strategy for HCC patients.
Downregulation of miR-138 has been reported in diverse types of human cancer, including anaplastic thyroid carcinoma (21), head and neck squamous cell carcinoma (22), clear cell renal cell carcinoma (23), ovarian cancer (24), glioblastoma (25), colorectal cancer (26), non-small cell lung cancer (NSCLC) (27), pancreatic cancer (28) and breast cancer (29). Furthermore, reduced miR-138 expression was associated with a number of cancer characteristics. For example, in NSCLC, low miR-138 expression levels were significantly associated with an advanced clinical stage and positive lymph node metastasis (27). In addition, the overall survival of NSCLC patients with low miR-138 expression was significantly shorter than those with high miR-138 expression (27). Zhang et al (29) reported that low miR-138 expression levels were associated with lymph node metastasis and invasion in breast cancer. Another study revealed that in HCC, miR-138 was associated with an advanced clinical stage, the presence of portal vein invasion and lymph node metastasis (30). These studies suggested that deregulation of miR-138 may be implicated in carcinogenesis and progression of human cancer, and may be a promising prognostic biomarker in these types of human cancer.
Studies have also revealed that the dysregulation of miR-138 is associated with the carcinogenesis and progression of diverse types of human cancer. Zhang et al (29) reported that miR-138 regulated metastasis and the epithelial-mesenchymal transition in breast cancer cells. In NSCLC, miR-138 suppressed cell proliferation, migration, in vitro cisplatin sensitivity and in vivo tumor growth (31–33). The restoration of miR-138 expression in NSCLC cells was found to reverse gefitinib resistance (34). Xu et al (35) revealed that miR-138 overexpression suppressed oral squamous cell carcinoma (36) and gallbladder carcinoma cell growth. Yang et al (26) found that miR-138 expression resulted in a significant inhibition of colorectal cancer cell migration and invasion in vitro and in vivo. In ovarian cancer, miR-138 overexpression repressed cancer cell invasion and metastasis (24). In nasopharyngeal carcinoma, miR-138 markedly decreased cell proliferation and colony formation in vitro, and tumorigenesis in vivo (37). In head and neck squamous cell carcinoma, ectopic transfection with miR-138 inhibited cell invasion, and enhanced cell-cycle arrest and apoptosis (22). The evidence from these prior studies, together with the findings of the present study, indicates that miR-138 acts as a tumor suppressor in cancer.
Evidence suggests that miRNAs can exert functions as oncogenes or tumor suppressors in carcinogenesis and progression of human cancer depending on the roles of their target genes (38–40). Therefore, identification of miR-138 target genes involved in HCC tumorigenesis and progression may provide valuable insight for the diagnosis and therapeutic treatments of patients with HCC. Numerous genes are directly regulated by miR-138, including vimentin in breast cancer (29), zinc finger E-box-binding homeobox 2 in bladder cancer (41), ARF GTPase-activating protein GIT1, semaphoring 4C (42) and G1/S-specific cyclin-D3 (33) in NSCLC, transcriptional coactivator YAP1 in oral squamous cell carcinoma (36) and BAG family molecular chaperone regulator 1 in gallbladder carcinoma (35). In the present study, SP1 was identified as a novel direct target gene for miR-138. Bioinformatic analysis revealed that there are two putative binding sites of miR-138 in the 3′UTR of SP1. A dual-luciferase reporter assay demonstrated that miR-138 could directly bind to the 3′UTR of the SP1 gene. RT-qPCR and western blot analysis further indicated that the restoration of miR-138 expression decreased the expression of SP1 mRNA and protein in HCC cells. Notably, knockdown of SP1 resulted in the suppression of HCC cell proliferation, migration and invasion, similar to the effects of miR-138 overexpression in HCC cells. Collectively, these findings indicate that miR-138 represses HCC cell proliferation, migration and invasion via the negative regulation of endogenous SP1 expression at the transcriptional and translational levels.
The present study demonstrated that miR-138 was significantly downregulated in HCC tissues and cell lines and that miR-138 overexpression repressed HCC cell proliferation, migration and invasion. Furthermore, SP1 was identified as a direct target gene of miR-138 in HCC. These results indicate that miR-138 may be associated with the tumorigenesis and progression of HCC, and may therefore represent a potential target for HCC treatment.
Acknowledgements
This study was supported by the Natural Science Foundation of Shandong (grant nos. ZR2012HM064 and ZR2009CM126).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Li Y, Wang R, Qin S, Liu J, Su F, Yang Y, Zhao F, Wang Z, Wu Q. MiR-130a-3p regulates cell migration and invasion via inhibition of Smad4 in gemcitabine resistant hepatoma cells. J Exp Clin Cancer Res. 2016;35:19. doi: 10.1186/s13046-016-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindrat I, Tryndyak V, de Conti A, Shpyleva S, Mudalige TK, Kobets T, Erstenyuk AM, Beland FA, Pogribny IP. MicroRNA-152-mediated dysregulation of hepatic transferrin receptor 1 in liver carcinogenesis. Oncotarget. 2016;7:1276–1287. doi: 10.18632/oncotarget.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen PJ, Furuse J, Han KH, Hsu C, Lim HY, Moon H, Qin S, Ye SL, Yeoh EM, Yeo W. Issues and controversies of hepatocellular carcinoma-targeted therapy clinical trials in Asia: Experts' opinion. Liver Int. 2010;30:1427–1438. doi: 10.1111/j.1478-3231.2010.02292.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 6.Pelus LM, Fukuda S. Peripheral blood stem cell mobilization: The CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp Hematol. 2006;34:1010–1020. doi: 10.1016/j.exphem.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Cortez MA, Calin GA. MicroRNA identification in plasma and serum: A new tool to diagnose and monitor diseases. Expert Opin Biol Ther. 2009;9:703–711. doi: 10.1517/14712590902932889. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee RC, Feinbaum RL, Ambros V. The C. Elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-Y. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008;36(Database issue):D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 12.He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe SW, Hannon GJ, Hammond SM. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JG, Shi Y, Hong DF, Song M, Huang D, Wang CY, Zhao G. MiR-148b suppresses cell proliferation and invasion in hepatocellular carcinoma by targeting WNT1/β-catenin pathway. Sci Rep. 2015;5:8087. doi: 10.1038/srep08087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng B, Liang L, Wang C, Huang S, Cao X, Zha R, Liu L, Jia D, Tian Q, Wu J, et al. MicroRNA-148a suppresses tumor cell invasion and metastasis by downregulating ROCK1 in gastric cancer. Clin Cancer Res. 2011;17:7574–7583. doi: 10.1158/1078-0432.CCR-11-1714. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Bo L, Zhao X, Chen Q. MicroRNA-133a inhibits cell proliferation, colony formation ability, migration and invasion by targeting matrix metallopeptidase 9 in hepatocellular carcinoma. Mol Med Rep. 2015;11:3900–3907. doi: 10.3892/mmr.2015.3232. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Liu K, Liu S, Ji B, Wang Y, Liu Y. MicroRNA-133a functions as a tumor suppressor by targeting IGF-1R in hepatocellular carcinoma. Tumour Biol. 2015;36:9779–9788. doi: 10.1007/s13277-015-3749-8. [DOI] [PubMed] [Google Scholar]
- 17.Dong Y, Zou J, Su S, Huang H, Deng Y, Wang B, Li W. MicroRNA-218 and microRNA-520a inhibit cell proliferation by downregulating E2F2 in hepatocellular carcinoma. Mol Med Rep. 2015;12:1016–1022. doi: 10.3892/mmr.2015.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Chen J, Li F, Lin Y, Zhang X, Lv Z, Jiang J. MiR-214 inhibits cell growth in hepatocellular carcinoma through suppression of β-catenin. Biochem Biophys Res Commun. 2012;428:525–531. doi: 10.1016/j.bbrc.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 21.Mitomo S, Maesawa C, Ogasawara S, Iwaya T, Shibazaki M, Yashima-Abo A, Kotani K, Oikawa H, Sakurai E, Izutsu N, et al. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci. 2008;99:280–286. doi: 10.1111/j.1349-7006.2007.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Jiang L, Wang A, Yu J, Shi F, Zhou X. MicroRNA-138 suppresses invasion and promotes apoptosis in head and neck squamous cell carcinoma cell lines. Cancer Lett. 2009;286:217–222. doi: 10.1016/j.canlet.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang J, Zhang Y, Jiang G, Liu Z, Xiang W, Chen X, Chen Z, Zhao J. MiR-138 induces renal carcinoma cell senescence by targeting EZH2 and is downregulated in human clear cell renal cell carcinoma. Oncol Res. 2013;21:83–91. doi: 10.3727/096504013X13775486749218. [DOI] [PubMed] [Google Scholar]
- 24.Yeh YM, Chuang CM, Chao KC, Wang LH. MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1α. Int J Cancer. 2013;133:867–878. doi: 10.1002/ijc.28086. [DOI] [PubMed] [Google Scholar]
- 25.Qiu S, Huang D, Yin D, Li F, Li X, Kung HF, Peng Y. Suppression of tumorigenicity by microRNA-138 through inhibition of EZH2-CDK4/6-pRb-E2F1 signal loop in glioblastoma multiforme. Biochim Biophys Acta. 2013;1832:1697–1707. doi: 10.1016/j.bbadis.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Long L, Huang G, Zhu H, Guo Y, Liu Y, Huo J. Down-regulation of miR-138 promotes colorectal cancer metastasis via directly targeting TWIST2. J Transl Med. 2013;11:275. doi: 10.1186/1479-5876-11-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han L, Zhang G, Zhang N, Li H, Liu Y, Fu A, Zheng Y. Prognostic potential of microRNA-138 and its target mRNA PDK1 in sera for patients with non-small cell lung cancer. Med Oncol. 2014;31:129. doi: 10.1007/s12032-014-0129-y. [DOI] [PubMed] [Google Scholar]
- 28.Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X, Deng Y, Jiang J, Sun C. MicroRNA-138-5p regulates pancreatic cancer cell growth through targeting FOXC1. Cell Oncol (Dordr) 2015;38:173–181. doi: 10.1007/s13402-014-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Liu D, Feng Z, Mao J, Zhang C, Lu Y, Li J, Zhang Q, Li Q, Li L. MicroRNA-138 modulates metastasis and EMT in breast cancer cells by targeting vimentin. Biomed Pharmacother. 2016;77:135–141. doi: 10.1016/j.biopha.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Huang B, Li H, Huang L, Luo C, Zhang Y. Clinical significance of microRNA 138 and cyclin D3 in hepatocellular carcinoma. J Surg Res. 2015;193:718–723. doi: 10.1016/j.jss.2014.03.076. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Zhang H, Zhao M, Lv Z, Zhang X, Qin X, Wang H, Wang S, Su J, Lv X, et al. MiR-138 inhibits tumor growth through repression of EZH2 in non-small cell lung cancer. Cell Physiol Biochem. 2013;31:56–65. doi: 10.1159/000343349. [DOI] [PubMed] [Google Scholar]
- 32.Ye XW, Yu H, Jin YK, Jing XT, Xu M, Wan ZF, Zhang XY. miR-138 inhibits proliferation by targeting 3-phosphoinositide-dependent protein kinase-1 in non-small cell lung cancer cells. Clin Respir J. 2015;9:27–33. doi: 10.1111/crj.12100. [DOI] [PubMed] [Google Scholar]
- 33.Han LP, Fu T, Lin Y, Miao JL, Jiang QF. MicroRNA-138 negatively regulates non-small cell lung cancer cells through the interaction with cyclin D3. Tumour Biol. 2016;37:291–298. doi: 10.1007/s13277-015-3757-8. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Fan X, Li W, Ping W, Deng Y, Fu X. miR-138-5p reverses gefitinib resistance in non-small cell lung cancer cells via negatively regulating G protein-coupled receptor 124. Biochem Biophys Res Commun. 2014;446:179–186. doi: 10.1016/j.bbrc.2014.02.073. [DOI] [PubMed] [Google Scholar]
- 35.Ma F, Zhang M, Gong W, Weng M, Quan Z. MiR-138 Suppresses Cell Proliferation by Targeting Bag-1 in Gallbladder Carcinoma. PLoS One. 2015;10:e0126499. doi: 10.1371/journal.pone.0126499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu R, Zeng G, Gao J, Ren Y, Zhang Z, Zhang Q, Zhao J, Tao H, Li D. miR-138 suppresses the proliferation of oral squamous cell carcinoma cells by targeting Yes-associated protein 1. Oncol Rep. 2015;34:2171–2178. doi: 10.3892/or.2015.4144. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Lv XB, Wang XP, Sang Y, Xu S, Hu K, Wu M, Liang Y, Liu P, Tang J, et al. MiR-138 suppressed nasopharyngeal carcinoma growth and tumorigenesis by targeting the CCND1 oncogene. Cell Cycle. 2012;11:2495–2506. doi: 10.4161/cc.20898. [DOI] [PubMed] [Google Scholar]
- 38.Sarver AL, Li L, Subramanian S. MicroRNA miR-183 functions as an oncogene by targeting the transcription factor EGR1 and promoting tumor cell migration. Cancer Res. 2010;70:9570–9580. doi: 10.1158/0008-5472.CAN-10-2074. [DOI] [PubMed] [Google Scholar]
- 39.Gong C, Yao Y, Wang Y, Liu B, Wu W, Chen J, Su F, Yao H, Song E. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem. 2011;286:19127–19137. doi: 10.1074/jbc.M110.216887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Liu S, Shi R, Zhao G. miR-27 promotes human gastric cancer cell metastasis by inducing epithelial-to-mesenchymal transition. Cancer Genet. 2011;204:486–491. doi: 10.1016/j.cancergen.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Sun DK, Wang JM, Zhang P, Wang YQ. MicroRNA-138 regulates metastatic potential of bladder cancer through ZEB2. Cell Physiol Biochem. 2015;37:2366–2374. doi: 10.1159/000438590. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Wang Q, Wen R, Liang J, Zhong X, Yang W, Su D, Tang J. MiR-138 inhibits cell proliferation and reverses epithelial-mesenchymal transition in non-small cell lung cancer cells by targeting GIT1 and SEMA4C. J Cell Mol Med. 2015;19:2793–2805. doi: 10.1111/jcmm.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]