Abstract
Pancreatic cancer is the eighth-leading cause of cancer-associated mortality in males and the ninth-leading cause in females worldwide. Even when diagnosed early enough to be potentially resectable, the prognosis of invasive pancreatic cancer is poor. Galectin-9 (Gal-9) is a tandem-repeat type galectin that has recently been demonstrated to possess an anti-proliferative effect on cancer cells. Therefore, the present study evaluated the effects of Gal-9 on the proliferation of human pancreatic cancer cells and examined the microRNAs that are associated with the antitumor effects of Gal-9. Gal-9 suppressed the proliferation of multiple pancreatic cancer cell lines. In addition, Gal-9 treatment increased the levels of caspase-cleaved keratin 18 and the expression of cytochrome c in pancreatic cancer cell lines. This data suggests that Gal-9 induces intrinsic apoptosis in pancreatic cancer cell lines through the caspase-dependent and caspase-independent pathways. In addition, Gal-9 reduced the expression levels of phosphorylated epidermal growth factor receptor and numerous receptor tyrosine kinases (RTKs). In conclusion, Gal-9 may suppress the growth of human pancreatic cancer cells in vitro. These findings suggest that Gal-9 may be a new therapeutic agent for the treatment of pancreatic cancer.
Keywords: pancreatic cancer, galectin-9, apoptosis, caspase-cleaved keratin 18, cell cycle, microRNAs, cytochrome c
Introduction
Pancreatic cancer is the eighth-leading cause of cancer associated mortality in males and the ninth-leading cause in females worldwide (1). Pancreatic cancer has a rapid disease progression that is accompanied by the absence of specific symptoms, which largely precludes early diagnosis and curative treatment (2,3). The vast majority of patients with pancreatic cancer are not diagnosed until their cancer has metastasized, and so surgical treatment is the only curative treatment. However, due to this late diagnosis the majority of patients present in an advanced stage, and only a minority (10–20%) of these patients are amenable to surgical intervention (4,5). Even when diagnosed early enough to be potentially resectable, the prognosis of invasive pancreatic cancer is poor. Due to its high recurrence rate, post-operative patients with pancreatic cancer require adjuvant chemotherapy with or without radiotherapy, which provides a 5-year patient survival rate of 15–25% (6).
Galectin-9 (Gal-9) is a tandem-repeat type galectin with 2 carbohydrate-recognition domains (CRDs), and it was first identified as an eosinophil chemoattractant and activation factor (7–10). Similarly to other galectins, Gal-9 performs a role in cell aggregation and adhesion and in the apoptosis of tumor cells (10,11). Gal-9 can enhance antitumor immunity through the initial CRD-independent maturation of dendritic cells and the subsequent induction of Th1-mediated antitumor immunity (12). In addition, treatment with recombinant Gal-9 prolonged survival in a murine melanoma model, not only by increasing the numbers of CD8 cytotoxic T cells (CTLs) but also by increasing the number of natural killer (NK) cells and macrophages (13).
Previous studies have uncovered additional mechanisms by which T cell immunoglobulin mucin-3, a receptor for Gal-9, negatively regulates T cell responses by promoting CD8+ T cell exhaustion and inducing the expansion of myeloid-derived suppressor cells (14,15). Therefore, Gal-9 augments antitumor immunity by inducing a subset of macrophages and dendritic cells and activating tumor-specific CTLs and NK cells.
Recombinant Gal-9 induced apoptosis in various T cell leukemic cell lines in a dose-dependent and functional CRD-dependent manner (16,17). Additionally, several in vitro and in vivo studies have also indicated that Gal-9 inhibits the growth of multiple myeloma (18) and chronic myeloid leukemia (19). In hematologic malignancies, Gal-9 suppresses cellular proliferation and tumor growth in vitro and in vivo. However, in solid malignancies, the antitumor effect of Gal-9 remains unknown. Previously, it was reported that Gal-9 inhibits the growth of hepatocellular carcinoma and cholangiocarcinoma via apoptosis in vitro and in vivo (20,21).
However, less is known regarding the antitumor effects of Gal-9 on pancreatic cancer cells or the microRNAs (miRNAs) that are associated with these effects. The present study therefore evaluated the effects of Gal-9 on the growth of two pancreatic cancer cell lines, its mechanism of action, and the miRNAs that are associated with the antitumor effect of Gal-9 on pancreatic cancer cells.
Materials and methods
Cell lines and culture
The human pancreatic cancer PK-1 and PK-9 cell lines were obtained from the RIKEN cell bank (Ibarkai, Japan) and passaged in our laboratory for <6 months. The 2 cell lines were authenticated by the cell bank using short tandem repeat polymerase chain reaction. PK-1 and PK-9 cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum and 100 mg/l of penicillin-streptomycin in a humidified atmosphere with 5% CO2 at 37°C.
Chemicals and reagents
Recombinant mutant forms of human Gal-9 that lack linker peptides were expressed and purified as described in one of our previous studies (22). A cell counting kit (CCK-8) was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan), and all other chemicals were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell proliferation assay
A total of 5×103 cells were seeded in 100 µl of culture medium supplemented with 10% FBS into each well of a 96-well microplate and incubated at 37°C overnight. Following 24 h, cells were treated with 0.1, 0.3 or 1.0 µM of Gal-9 and the cells were cultured at 37°C for an additional 48 h. A total of 10 µl CCK-8 reagent was added to each well and the plates were incubated at 37°C for 3 h. The absorbance of each well was measured at 450 nm using an auto-microplate reader.
ELISA assay for apoptosis
Caspase-cleaved keratin 18 (CCK18) was evaluated using M30 Apoptosense ELISA kits obtained from PEVIVA AB (Bromma, Sweden) (23). Each cell line (cell density, 5×103 cells) was seeded into 96-well plates and cultured in 100 µl of culture medium for 24 h. Cells were then treated with 0.3 µM of Gal-9. The procedures of the assays were performed according to the manufacturer's protocol. The amounts of antigen in the control and treated samples were calculated by interpolation of a standard curve.
Gel electrophoresis and western blotting
PK-1 cells (1.0×106/dish) were seeded in 100-mm culture dishes and cultured at 37°C for 24 h. Gal-9 was subsequently added, and the cells were cultured at 37°C for an additional 48 h. Cells were washed twice in PBS and lysed using a protease inhibitor cocktail (Proprep, Complete protease inhibitor mixture; Intron Biotechnology, Inc., Seongnam, Korea). Protein concentration was quantified using a NanoDrop 2000 fluorospectrometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Whole-cell lysates (10 µg) were separated using SDS-PAGE on 10% Tris-glycine gradient gels (24), and the proteins were transferred to nitrocellulose membranes. Subsequent to blocking with a solution containing 5% non-fat milk powder in TBS/Tween-20 at 37°C for 1 h, the membranes were incubated with primary antibodies at 4°C overnight and incubated with horseradish peroxidase (HRP) -conjugated secondary antibodies at 4°C for 1 h (25). The antibodies used were: Anti-β-actin monoclonal antibody (dilution, 1:3,000; cat. no. A5441; Sigma-Aldrich; Merck KGaA), cyclin D1 (dilution, 1:1,000; cat. no. RB-9041; Thermo Fisher Scientific Inc.), cyclin E (dilution, 1:1,000; cat. no. 870P1605B; Thermo Fisher Scientific, Inc.), Cdk (cyclin dependent kinase) 6 (dilution, 1:1,000; cat. no. sc-177; Santa Cruz Biotechnology, Inc., Dallax, TX, USA), Cdk4 (dilution, 1:1,000; cat. no. sc-749; Santa Cruz Biotechnology, Inc.), Cdk2 (dilution, 1:2,000; cat. no. sc-163; Santa Cruz Biotechnology, Inc.) and secondary horseradish peroxidase-linked anti-mouse (dilution, 1:2,000; cat no. 7076; Cell Signaling Technology, Inc., Danvers, MA, USA) and anti-rabbit Immunoglobulin G antibodies (dilution, 1:2,000; cat, no. 7074; Cell Signaling Technology, Inc.).
Immunoreactive proteins were visualized using an enhanced chemiluminescence detection system (Perkin Elmer Inc., Waltham, MA, USA) on X-ray film.
Antibody arrays of apoptosis-related proteins
A Human Apoptosis Antibody Array kit (R&D Systems, Inc., Minneapolis, MN, USA) was used according to the manufacturer's protocol.
Antibody arrays of phosphorylated receptor tyrosine kinase (p-RTK)
Human phospho-RTK was assayed using Human Phospho-RTK Array kits (R&D Systems, Inc.), according to the manufacturer's protocol. Each array membrane was exposed to X-ray film using a chemiluminescence detection system (Perkin-Elmer Co.).
Analysis of miRNA arrays
Total RNA was extracted from tumor samples using miRNeasy Mini kits (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol. RNA samples typically demonstrated A260/280 ratios between 1.9 and 2.1 when assayed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA, USA). PK-1 cells were cultured at 37°C with or without 0.3 µM Gal-9 for 24 h, with 5 replicates per sample. Following RNA measurement with an RNA 6000 Nano kit (Agilent Technologies, Inc.), the samples were labeled using a miRCURY LNMä microRNA Array Hi-Power Labeling kit (Exiqon A/S, Vedbaek, Denmark) and were hybridized to a human miRNA Oligo chip (v.21.0; Toray Industries). The chips were scanned with a 3-D Gene Scanner 3000 (Toray Industries, Inc., Tokyo, Japan), and the results were analyzed with 3D-Gene extraction version 1.2 software (Toray Industries, Inc., Tokyo, Japan). Differences in miRNA expression between Gal-9-treated and control samples were assessed by analyzing the raw data using GeneSpringGX v. 10.0 (Agilent Technologies, Inc). On the raw data that were above the background level, quantile normalization was performed. Differentially expressed miRNAs were determined using the Mann-Whitney U test. False discovery rate was computed with Benjamini_Hochberg method (26) as the correction for multiple testing. Hierarchical clustering was performed using the farthest neighbor method with the absolute uncentered Pearson's correlation coefficient as a metric. A heat map was produced with the relative expression intensity for each miRNA, in which the base-2 logarithm of the intensity was median-centered for each row.
Statistical analysis
All statistical analyses were performed using JMP 9.0 software (SAS Institute, Cary, NC, USA). Paired analysis between groups was performed using t-tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Gal-9 suppresses the proliferation of human pancreatic cancer cells
To evaluate the effect of Gal-9 on the growth activity of human pancreatic cells in vitro, the present study examined the effect of Gal-9 on the proliferation of the pancreatic cancer PK-1 and PK-9 cell lines. Cells were grown in 10% FBS and treated with 0.1, 0.3 or 1.0 µM Gal-9 or without Gal-9 (as a control) for 48 h. Gal-9 demonstrated strong, dose-dependent inhibition of cellular proliferation the two pancreatic cancer cell lines (Fig. 1).
Figure 1.
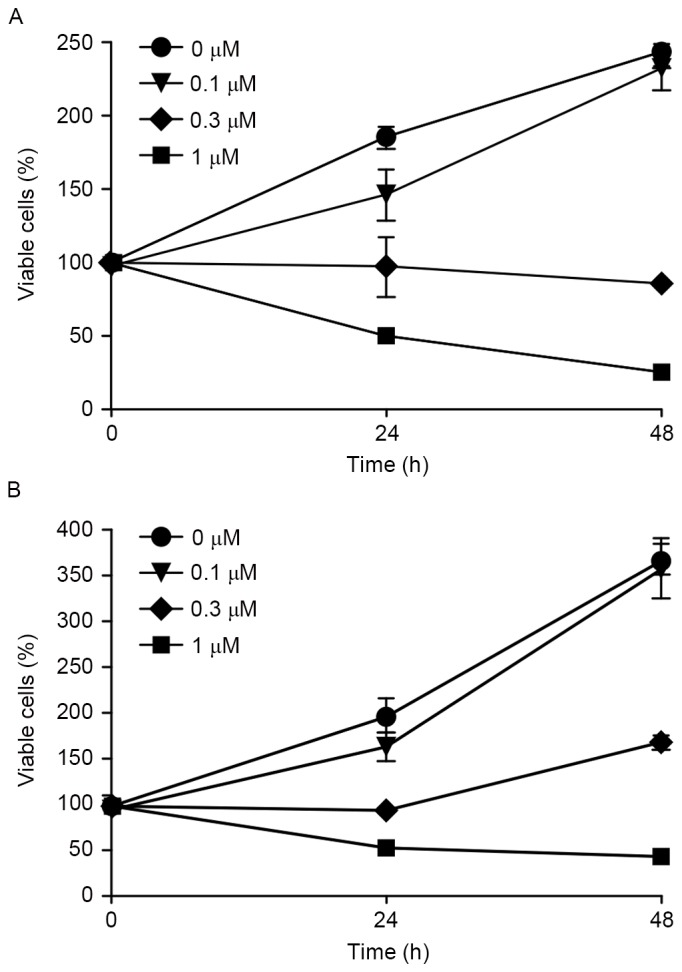
Gal-9 suppresses the proliferation of pancreatic cancer cell lines. (A) PK-1 and (B) PK-9 cells were seeded in 96-well plates. After 24 h, Gal-9 (0.1, 0.3 and 1 µM) was added to the culture medium. Subsequently, 24 h after the addition of these agents, a CCK-8 assay was conducted. PK-1 and PK-9 cells were seeded at 5,000 cells per well in a 96-well plate, and the agents were added at the 0-h time point. The data points represent mean cell numbers from 2 independent clusters, and the error bars represent the standard deviation. For PK-1 and PK-9 cells, the conditions at 24 and 48 h were significantly different from those of the control. Gal-9, galectin-9.
Gal-9 induces apoptosis to suppresses cellular proliferation of pancreatic cancer
To clarify the mechanism of the inhibitory effect of Gal-9 on cancer cell growth, the present study first examined the induction of apoptosis by Gal-9. CCK18 was evaluated by ELISA to confirm whether apoptosis was involved in Gal-9-induced cell death. Gal-9 increased the levels of CCK18 in the two pancreatic cancer cell lines (Fig. 2).
Figure 2.
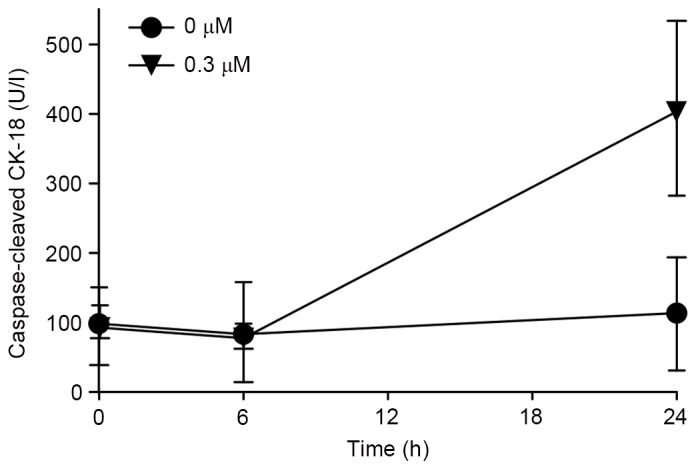
CCK18 produced specifically during apoptosis was identified by ELISA. Cells were incubated with or without 0.3 µM Gal-9. Gal-9 increased the levels of CCK18 in PK-1 cells, suggesting that the apoptotic process following phosphatidylserine exposure proceeds to cut the intermediate filaments of cells. Fold-change in CCK18 is shown as the mean ± standard deviation, with analysis by a paired t-test. For each cell line, the condition at 24 h was significantly different from that of the control. CCK18, caspase-cleaved cytokeratin-18; Gal-9, galectin-9.
The effects of Gal-9 on the cell cycle in PK-1
The effects of Gal-9 on the expression of various cell-cycle-related molecules in PK-1 cells were evaluated using western blot analysis. Cells were treated with 0 or 0.3 µM Gal-9 for 24–48 h. Assays for the expression of proteins associated with the G0-to-G1 transition demonstrated that cyclin E and Cdk4 were slightly decreased 48 h after the addition of 0.3 µM of Gal-9 (Fig. 3A). The expression of other proteins, including cyclin D1, Cdk2 and Cdk6 were not changed compared with the control.
Figure 3.
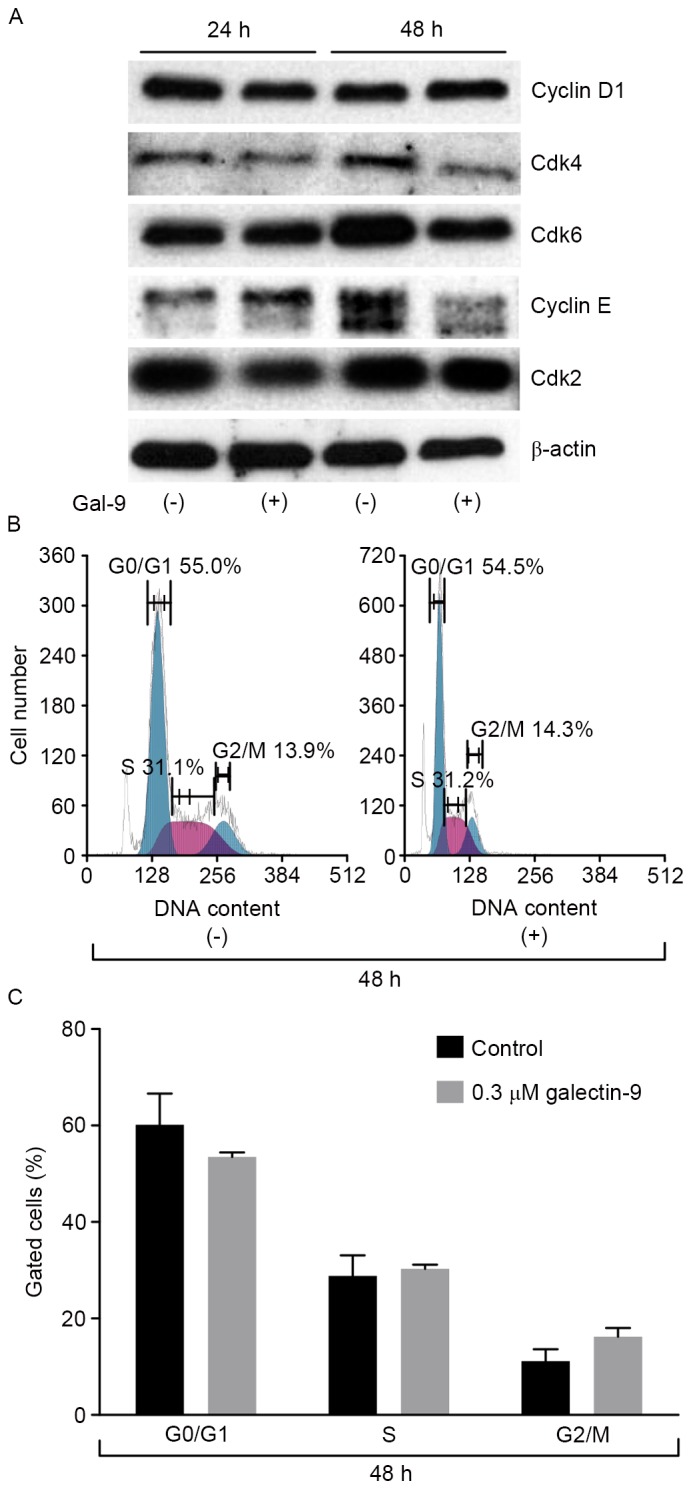
Western blot analysis and flow cytometric analysis of the cell cycle in PK-1 cells. (A) Expression levels of cell cycle-related proteins (cyclin D1, Cdk4, Cdk6, cyclin E and Cdk2) in PK-1 cells subsequent to the addition of 0.3 µM Gal-9 did not change compared with the β-actin control. (B) Flow cytometric analysis of proliferating PK-1 cells 48 h after the addition of 0.3 µM Gal-9. (C) PK-1 cells treated with Gal-9 demonstrated no change in the cell cycle. Cdk, cyclin dependent kinase; Gal-9, galectin-9.
Next, a flow cytometric analysis of the cell cycle was performed to evaluate the contribution of Gal-9 to cell cycle arrest during the suppression of pancreatic cancer cellular proliferation. PK-1 cells were treated with 0.3 µM Gal-9. In contrast to the expression levels of cyclin E and Cdk4, which decreased, Gal-9 did not alter the cell cycle of PK-1 cells (Fig. 3B and C). These results suggest that Gal-9 suppresses cell growth through tumor cell apoptosis but not through cell cycle arrest.
The effects of Gal-9 on apoptosis-associated proteins in PK-1 cells
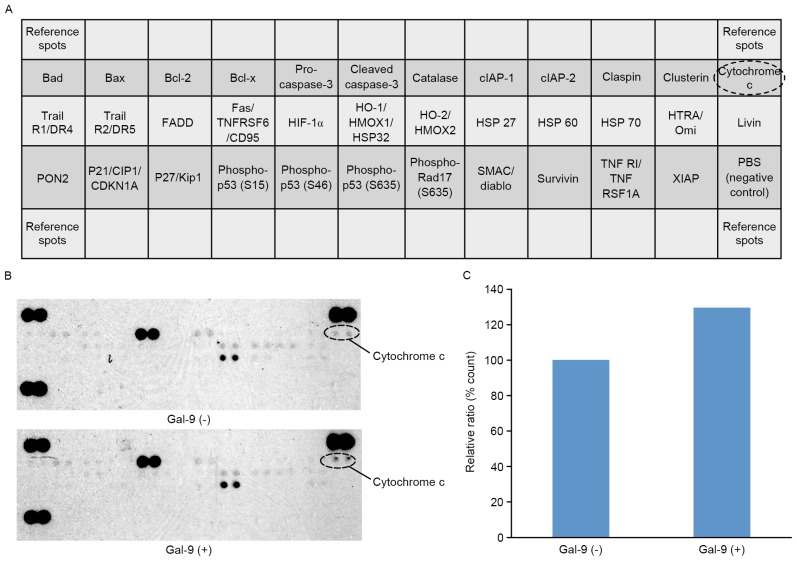
The present study used an apoptosis array system to identify apoptosis proteins that were associated with the antitumor effect of Gal-9. An antibody array enabled the screening of 35 apoptosis-associated proteins in PK-1 cells in the presence and absence of Gal-9 (Fig. 4A). Gal-9 increased the expression levels of cytochrome c (Fig. 4B). Densitometry analysis demonstrated that the intensity of cytochrome c spots from Gal-9-treated cells relative to untreated cells was 129.7% (Fig. 4C).
Figure 4.
The effects of Gal-9 on apoptosis-related proteins in PK-1 cells. (A) Template showing the locations of tyrosine kinase antibodies spotted onto a human apoptosis array. (B) Representative expression of various apoptosis-related proteins in PK-1 cells treated with or without Gal-9. (C) Densitometry analysis demonstrated that the intensity of cytochrome c spots from Gal-9-treated cells relative to untreated cells was 129.7%. Gal-9, galectin-9; Bad, Bcl-2 associated death promotor; Bax, Bcl-2 associated × protein; Bcl-2, B-cell lymphoma 2; Bcl-x, B cell lymphoma-extra-large; clAP, cellular inhibitor of apoptosis protein; Trail R1/DR4, Trail receptor 1/death receptor 4; FADD, Fas associated via death domain; TNFRSF6/CD95, tumor necrosis factor receptor superfamily member 6/cluster of differentiation 95; HIF-1α, hypoxia-inducible factor-1α; HO-1/HMOX1/HSP32, heme oxygenase-1/heme oxygenase (decycling) 1/heat shock protein 32; PON2, paraoxonase 2; P21/CIP1/CDKN1A, cyclin-dependent kinase inhibitor 1A; SMAC, second mitochondria-derived activator of caspases; TNF R1, tumor necrosis factor receptor 1; TNFRSF1A, TNF receptor superfamily member 1A; XIAP, X-linked inhibitor of apoptosis protein.
Effects of Gal-9 on p-RTKs in PK-1
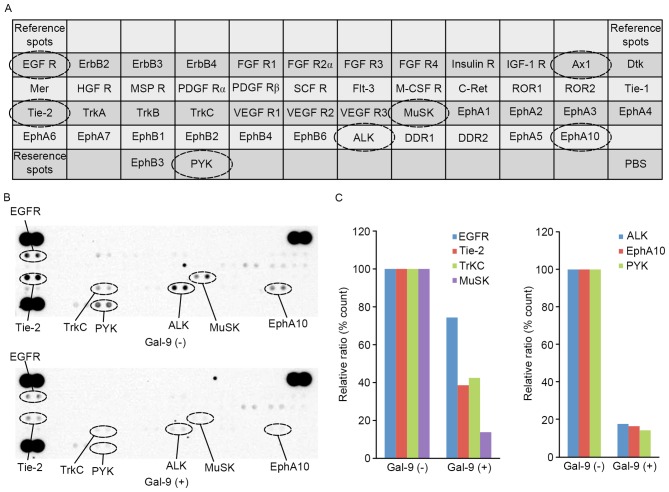
The present study used a p-RTK array system to identify key RTKs that were associated with the antitumor effect of Gal-9. An antibody array enabled the screening of 49 activated RTKs in PK-1 cells in the presence and absence of Gal-9 (Fig. 5A). Gal-9 reduced the expression levels of phosphorylated epidermal growth factor receptor (p-EGFR) and phosphorylated tyrosine kinase with immunoglobulin-like and EGF-like domains 2 (p-Tie-2), and it also reduced the expression of tropomyosin receptor kinase C (TrKC), muscle-Specific Kinase (MuSK), anaplastic lymphoma kinase (ALK), erythropoietin-producing human hepatocellular carcinoma cell (EphA10) and receptor-like tyrosine kinase (RYK) (Fig. 5B).
Figure 5.
The effects of Gal-9 on pRTKs in PK-1 cells. (A) Template showing the locations of tyrosine kinase antibodies spotted onto a human pRTK array. (B) Representative expression of various phosphorylated tyrosine kinase receptors in PK-1 cells treated with or without Gal-9. (C) Densitometry analysis demonstrated that the intensities of p-EGFR, p-Tie-2, p-TrKC, p-MuSK, p-ALK, p-EphA10 and p-RYK spots from Gal-9-treated cells relative to untreated cells were 74.4, 38.6, 42.5, 13.8, 17.7, 16.5 and 14.3%, respectively. Gal-9, galectin-9; pRTK, phospho-receptor tyrosine kinase; p-EGFR, phosphor-epidermal growth factor; p-Tie-2, phospho-tyrosine kinase with immunoglobulin-like and EGF-like domains-2; p-TrKC, phospho-tropomyosin receptor kinase C; p-MuSK, phospho-muscle-specific kinase; p-ALK, phospho-anaplasic tyrosine kinase; p-EphA10, phospho-erythropoietin-producing human hepatocellular receptors 10; p-RYK, phosphor-receptor-like tyrosine kinase; ErbB2, human epidermal growth factor 2; FGF R1, fibroblast growth factor receptor 1; IGF-1R, insulin-like growth factor 1 receptor; Ax1, tyrosine-protein kinase receptor UFO; Dtk, Dtk receptor tyrosine kinase; Mer, Mer receptor tyrosine kinase; HGFR, hepatocellular growth factor receptor; MSP R, macrophage-stimulating protein receptor; PDGF Rα, platelet-derived growth factor receptor α; SCF R, stem cell factor receptor; Flt-3, Fms-like tyrosine kinase 3; M-CSF R, monocyte colony stimulating factor; C-Ret, C-Te receptor tyrosine kinase; ROR1, receptor tyrosine kinase-like orphan receptor 1; Tie-1, tyrosine kinase with immunoglobulin-like and EGF-like domains-1; Trk, tropomyosin receptor kinase; VEGF R1, vascular endothelial growth factor receptor 1; Musk, muscle-specific kinase; EphA1, erythropoietin-producing human hepatocellular receptor A1; ALK, anaplasic tyrosine kinase; DDR1, discoidin domain receptor 1.
Densitometry analysis demonstrated that the intensities of p-EGFR, p-Tie-2, p-TrKC, p-MuSK, p-ALK, p-EphA10 and p-RYK spots from Gal-9-treated cells relative to untreated cells were 74.4, 38.6, 42.5, 13.8, 17.7, 16.5 and 14.3%, respectively (Fig. 5C).
The effects of Gal-9 on the in vitro miRNA expression of cells treated with and without Gal-9
Using a custom microarray platform, the present study analyzed the in vitro expression levels of 2,555 miRNA probes in tumorous tissues that were treated with and without Gal-9. In PK-1 cells treated with Gal-9, there were 13 upregulated and 6 downregulated miRNAs among the 2,555 miRNAs (Table I).
Table I.
Statistical results and chromosomal locations of miRNAs in PK-1 cells treated with and without Gal-9.
| miRNA | Fold-change (treated/non-treated) | P-value | FDR | Chromosomal localization |
|---|---|---|---|---|
| Upregulated | ||||
| hsa-miR-6807-5p | 2.27 | 0.00016 | 0.0045 | 19 |
| hsa-miR-6784-5p | 2.25 | 0.00016 | 0.0045 | 17 |
| hsa-miR-4739 | 2.18 | 0.00016 | 0.0045 | 17 |
| hsa-miR-6845-5p | 2.04 | 0.00016 | 0.0045 | 8 |
| hsa-miR-3196 | 1.91 | 0.00016 | 0.0045 | 20 |
| hsa-miR-3649 | 1.88 | 0.00016 | 0.0045 | 12 |
| hsa-miR-1202 | 1.88 | 0.00016 | 0.0045 | 6 |
| hsa-miR-762 | 1.72 | 0.00016 | 0.0045 | 16 |
| hsa-miR-204-3p | 1.67 | 0.00016 | 0.0045 | 9q21.12 |
| hsa-miR-4689 | 1.63 | 0.00016 | 0.0045 | 1 |
| hsa-miR-575 | 1.55 | 0.00031 | 0.0071 | 4q21.22 |
| hsa-miR-6765-5p | 1.46 | 0.00031 | 0.0071 | 14 |
| hsa-miR-4655-5p | 1.33 | 0.00016 | 0.0045 | 7 |
| Downregulated | ||||
| hsa-miR-301a-3p | 0.46 | 0.00031 | 0.0071 | 17q22 |
| hsa-miR-503-5p | 0.56 | 0.00016 | 0.0045 | Xq26.3 |
| hsa-miR-422a | 0.63 | 0.00016 | 0.0045 | 15q22.31 |
| hsa-miR-30a-3p | 0.66 | 0.00031 | 0.0071 | 6q13 |
| hsa-miR-1913 | 0.68 | 0.00016 | 0.0045 | 6 |
| hsa-miR-1285-3p | 0.69 | 0.00016 | 0.0045 | 7q21-q22 |
miRNA, microRNA; Gal-9, galectin-9; FDR, false discovery rate; hsa-miR, human microRNA.
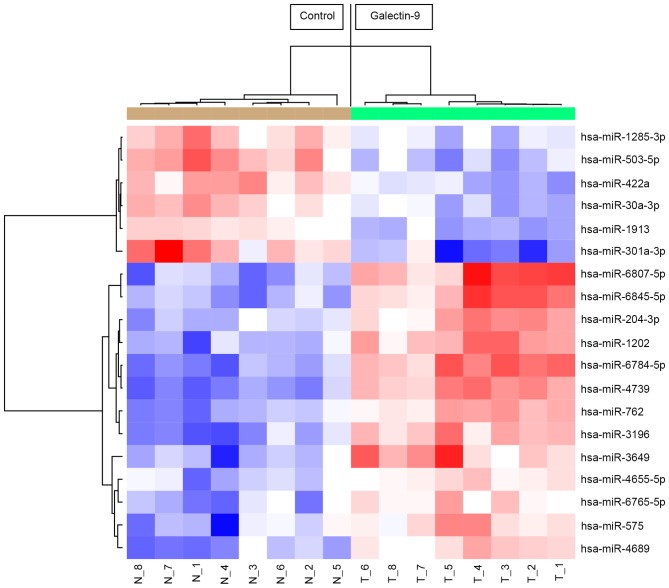
Unsupervised hierarchical clustering analysis using Pearson's correlation demonstrated that in vivo, tumorous tissues treated with Gal-9 clustered together and separated from untreated cell lines (Fig. 6).
Figure 6.
Hierarchical clustering of PK-1 cell tumor tissue with and without Gal-9. Tumor tissues clustered according to the expression profiles of 49 miRNAs that were differentially expressed in PK-1 cells with and without Gal-9. The analyzed samples are shown in the columns, and the miRNAs are presented in the rows. The miRNA clustering color scale shown at the top indicates the relative expression levels of miRNAs, with red and blue representing high and low expression levels, respectively. Gal-9, galectin-9; hsa-miR, human microRNA.
Discussion
Pancreatic cancer has the worst 5-year survival rate of all malignancies due to its aggressive progression and resistance to therapy. Worldwide, the incidence of all types of pancreatic cancer ranges between 1 and 10 cases per 100,000 individuals, and it is generally higher in developed countries and among men (1). However, there is no effective screening tool to detect asymptomatic premalignant or early malignant tumors, and >90% of patients who receive a diagnosis of pancreatic cancer die from the disease (27). Thus, there is strong demand for new curative approaches to pancreatic cancer therapy.
The present data revealed that Gal-9 suppressed the cellular proliferation and tumor growth of human pancreatic cancer cell lines in vitro. The antitumor effect of Gal-9 in T cell hemostasis, cell aggregation and metastasis is well known (14,15). Previous findings suggest that Gal-9 inhibits the proliferation of hematologic malignancies, including multiple myeloma (18) and chronic myeloid leukemia (19), and significantly retards the tumor growth of myeloma xenografts in mice (18). Cell surface-associated Gal-9 triggered the aggregation of melanoma cells, indicative of Gal-9-mediated cellular adhesion and inhibition of cell detachment (28). In hematologic malignancies, Gal-9 may suppress cellular proliferation and tumor growth in vitro and in vivo. On the other hand, in solid malignancies, breast cancer cell lines with high levels of endogenous Gal-9 had a strong tendency to aggregate, whereas cells with low levels of Gal-9 did not (29). Importantly, ectopic expression of endogenous Gal-9 and treatment with recombinant Gal-9 triggered the formation of tight cellular clusters (28,29). Therefore, Gal-9 directly suppresses cellular proliferation and tumor growth and has therapeutic potential for several solid tumors.
Recombinant Gal-9 induces apoptosis and cell death through an apoptotic signaling pathway (18,19). Such apoptotic signaling was caspase-dependent and was induced by activation of the mitogen activated protein kinases, c-Jun N-terminal kinases and p38 in multiple myeloma cells (18). In addition, Gal-9 induced the proapoptotic Bcl-2 family member Noxa via activation of transcription factor 3, leading to the death of chromic myeloma cells (19). Various hematological malignancies are sensitive to apoptotic elimination by recombinant Gal-9. Cleavage of cytokeratin 18 (CK18) occurs as an early event during apoptosis following activation of apoptosis executioners, particularly effector caspases (30). However, CK18 remains intact during other types of cell death, including autophagy or necrosis (30). Several studies have made use of this phenomenon to detect cellular apoptosis at its early phase (31–33). Our data suggested that Gal-9 increases the levels of CCK18 in human pancreatic cancer cell lines. Additionally, using an apoptosis array, the present study revealed that the expression of cytochrome c was increased in Gal-9-treated pancreatic cancer cell lines. Cytochrome c release from damaged mitochondria is an early event in the intrinsic apoptosis pathway and contributes to caspase-9 activation. The present data suggest that Gal-9 may induce the apoptosis of pancreatic cancer cell lines in the intrinsic apoptosis pathway through caspase-dependent and caspase-independent pathways.
A previous study demonstrated that Gal-9 suppresses cellular proliferation and tumor growth in hepatocellular carcinoma and cholangiocarcinoma by inducing apoptosis but not cell cycle arrest (20,21). Although the expression levels of certain cell cycle-related proteins (Cdk4 and cyclin E) decreased 48 h after the addition of Gal-9, flow cytometry demonstrated that Gal-9 did not affect PK-1 pancreatic cancer cells at the G0-to-G1 transition in vitro. These data suggest that the antitumor effect of Gal-9 may not be associated with the reduction of various cell cycle-related proteins.
Since the discovery of these proteins, RTKs have been investigated as key regulators of the proliferation, differentiation and metastasis of cancer cells (34). Gal-9 reduced the expression levels of p-EGFR and phosphorylated Tie-2, TrKC, MuSK, ALK, EphA10 and RYK, according to the pRTK array. The EGFR family and their ligands are frequently overexpressed in pancreatic cancer (35), and EGFR activity correlates with the prognosis of the patient (36).
The miRNAs that are associated with the antitumor effects of Gal-9 were assessed using miRNA expression arrays. miRNAs are small, endogenous, non-coding siRNAs that are 21–30 nucleotides in length and that modulate the expression of various target genes at the post-transcriptional and translational levels (37). miRNAs take part in fundamental molecular processes associated with pancreatic cancer initiation and progression, including the cell cycle, DNA repair, apoptosis, invasion and metastasis (38). Cluster analyses clearly demonstrated that Gal-9 treatment affected the extent of miRNA expression in pancreatic cancer cell lines. The present study identified 19 miRNAs that were differentially expressed in the cluster. These miRNAs are potential candidates to gauge the effectiveness of Gal-9 treatment, and provide clues regarding the molecular basis of the anti-cancer effects of Gal-9, particularly those mediated by miRNAs. Notably, miR301a was downregulated in a Gal-9-treated pancreatic cancer cell line. The overexpression of miR-301a has been previously demonstrated in pancreatic tumor tissue (39), hepatocellular carcinoma (40) and breast cancer (41). Additionally, miR-301a acts as a nuclear factor-κB activator in pancreatic cancer (42) and promotes proliferation and invasion in breast cancer. Thus, the present data suggest that miR-301a may be a candidate target for new therapeutic approaches to pancreatic cancer.
In conclusion, our results reveal that Gal-9 inhibits human pancreatic cancer cellular proliferation, possibly by inducing apoptosis through cytochrome c release, which is associated with the alteration of miRNAs. These findings suggest that Gal-9 may be a new therapeutic agent for the treatment of pancreatic cancer.
Acknowledgements
The authors would like to thank Ms. Kana Ogawa, Ms. Kayo Endo, Ms. Fuyuko Kokado, Ms. Keiko Fujikawa and Ms. Noriko Murao for providing technical assistance.
Glossary
Abbreviations
- Gal-9
Galectin-9
- CRDs
carbohydrate-recognition domains
- CTLs
cytotoxic T cells
- NK cells
natural killer cells
- miRNAs
microRNAs
- CCK-8
cell counting kit
- CCK18
Caspase-cleaved keratin 18
- phospho-RTK
phosphorylated receptor tyrosine kinases
- EGFR
epidermal growth factor receptor
- CRDs
carbohydrate-recognition domains
- Tie-2
tyrosine kinase with immunoglobulin-like and EGF-like domains 2
- TrKC
tropomyosin receptor kinase C
- MuSK
muscle-Specific Kinase
- ALK
anaplastic lymphoma kinase
- Eph
erythropoietin-producing human hepatocellular carcinoma cell
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Stathis A, Moore MJ. Advanced pancreatic carcinoma: Current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 3.Schneider G, Siveke JT, Eckel F, Schmid RM. Pancreatic cancer: Basic and clinical aspects. Gastroenterology. 2005;128:1606–1625. doi: 10.1053/j.gastro.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Gudjonsson B. Pancreatic cancer: Survival, errors and evidence. Eur J Gastroenterol Hepatol. 2009;21:1379–1382. doi: 10.1097/MEG.0b013e328323aab7. [DOI] [PubMed] [Google Scholar]
- 5.Wray CJ, Ahmad SA, Matthews JB, Lowy AM. Surgery for pancreatic cancer: Recent controversies and current practice. Gastroenterology. 2005;128:1626–1641. doi: 10.1053/j.gastro.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 6.Loos M, Kleeff J, Friess H, Büchler MW. Surgical treatment of pancreatic cancer. Ann NY Acad Sci. 2008;1138:169–180. doi: 10.1196/annals.1414.024. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto R, Matsumoto H, Seki M, Hata M, Asano Y, Kanegasaki S, Stevens RL, Hirashima M. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998;273:16976–16984. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita N, Nishi N, Seki M, Matsumoto R, Kuwabara I, Liu FT, Hata Y, Nakamura T, Hirashima M. Requirement of divalent galactoside-binding activity of ecalectin/galectin-9 for eosinophil chemoattraction. J Biol Chem. 2000;275:8355–8360. doi: 10.1074/jbc.275.12.8355. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto R, Hirashima M, Kita H, Gleich GJ. Biological activities of ecalectin: A novel eosinophil-activating factor. J Immunol. 2002;168:1961–1967. doi: 10.4049/jimmunol.168.4.1961. [DOI] [PubMed] [Google Scholar]
- 10.Saita N, Goto E, Yamamoto T, Cho I, Tsumori K, Kohrogi H, Maruo K, Ono T, Takeya M, Kashio Y, et al. Association of galectin-9 with eosinophil apoptosis. Int Arch Allergy Immunol. 2002;128:42–50. doi: 10.1159/000058002. [DOI] [PubMed] [Google Scholar]
- 11.Asakura H, Kashio Y, Nakamura K, Seki M, Dai S, Shirato Y, Abedin MJ, Yoshida N, Nishi N, Imaizumi T, et al. Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol. 2002;169:5912–5918. doi: 10.4049/jimmunol.169.10.5912. [DOI] [PubMed] [Google Scholar]
- 12.Dai S, Nakagawa R, Itoh A, Murakami H, Kashio Y, Abe H, Katoh S, Kontani K, Kihara M, Zhang SL, et al. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J Immunol. 2005;175:2974–2981. doi: 10.4049/jimmunol.175.5.2974. [DOI] [PubMed] [Google Scholar]
- 13.Nobumoto A, Oomizu S, Arikawa T, Katoh S, Nagahara K, Miyake M, Nishi N, Takeshita K, Niki T, Yamauchi A, Hirashima M. Galectin-9 expands unique macrophages exhibiting plasmacytoid dendritic cell-like phenotypes that activate NK cells in tumor-bearing mice. Clin Immunol. 2009;130:322–330. doi: 10.1016/j.clim.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Wiersma VR, de Bruyn M, Helfrich W, Bremer E. Therapeutic potential of Galectin-9 in human disease. Med Res Rev. 2013;33(Suppl 1):E102–E126. doi: 10.1002/med.20249. [DOI] [PubMed] [Google Scholar]
- 15.Fujihara S, Mori H, Kobara H, Rafiq K, Niki T, Hirashima M, Masaki T. Galectin-9 in cancer therapy. Recent Pat Endocr Metab Immune Drug Discov. 2013;7:130–137. doi: 10.2174/1872214811307020006. [DOI] [PubMed] [Google Scholar]
- 16.Kashio Y, Nakamura K, Abedin MJ, Seki M, Nishi N, Yoshida N, Nakamura T, Hirashima M. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J Immunol. 2003;170:3631–3636. doi: 10.4049/jimmunol.170.7.3631. [DOI] [PubMed] [Google Scholar]
- 17.Lu LH, Nakagawa R, Kashio Y, Ito A, Shoji H, Nishi N, Hirashima M, Yamauchi A, Nakamura T. Characterization of galectin-9-induced death of Jurkat T cells. J Biochem. 2007;141:157–172. doi: 10.1093/jb/mvm019. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi T, Kuroda J, Ashihara E, Oomizu S, Terui Y, Taniyama A, Adachi S, Takagi T, Yamamoto M, Sasaki N, et al. Galectin-9 exhibits anti-myeloma activity through JNK and p38 MAP kinase pathways. Leukemia. 2010;24:843–850. doi: 10.1038/leu.2010.25. [DOI] [PubMed] [Google Scholar]
- 19.Kuroda J, Yamamoto M, Nagoshi H, Kobayashi T, Sasaki N, Shimura Y, Horiike S, Kimura S, Yamauchi A, Hirashima M, Taniwaki M. Targeting activating transcription factor 3 by Galectin-9 induces apoptosis and overcomes various types of treatment resistance in chronic myelogenous leukemia. Mol Cancer Res. 2010;8:994–1001. doi: 10.1158/1541-7786.MCR-10-0040. [DOI] [PubMed] [Google Scholar]
- 20.Fujita K, Iwama H, Sakamoto T, Okura R, Kobayashi K, Takano J, Katsura A, Tatsuta M, Maeda E, Mimura S, et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int J Oncol. 2015;46:2419–2430. doi: 10.3892/ijo.2015.2941. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K, Morishita A, Iwama H, Fujita K, Okura R, Fujihara S, Yamashita T, Fujimori T, Kato K, Kamada H, et al. Galectin-9 suppresses cholangiocarcinoma cell proliferation by inducing apoptosis but not cell cycle arrest. Oncol Rep. 2015;34:1761–1770. doi: 10.3892/or.2015.4197. [DOI] [PubMed] [Google Scholar]
- 22.Nishi N, Itoh A, Fujiyama A, Yoshida N, Araya S, Hirashima M, Shoji H, Nakamura T. Development of highly stable galectins: Truncation of the linker peptide confers protease-resistance on tandem-repeat type galectins. FEBS Lett. 2005;579:2058–2064. doi: 10.1016/j.febslet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 23.Schutte B, Henfling M, Kölgen W, Bouman M, Meex S, Leers MP, Nap M, Björklund V, Björklund P, Björklund B, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004;297:11–26. doi: 10.1016/j.yexcr.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications; Proc Natl Acad Sci USA; 1979; pp. 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roval Statis Soc. 1995;57:289–300. [Google Scholar]
- 27.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 28.Kageshita T, Kashio Y, Yamauchi A, Seki M, Abedin MJ, Nishi N, Shoji H, Nakamura T, Ono T, Hirashima M. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int J Cancer. 2002;99:809–816. doi: 10.1002/ijc.10436. [DOI] [PubMed] [Google Scholar]
- 29.Irie A, Yamauchi A, Kontani K, Kihara M, Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H, Hirashima M. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res. 2005;11:2962–2968. doi: 10.1158/1078-0432.CCR-04-0861. [DOI] [PubMed] [Google Scholar]
- 30.Kramer G, Erdal H, Mertens HJ, Nap M, Mauermann J, Steiner G, Marberger M, Bivén K, Shoshan MC, Linder S. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64:1751–1756. doi: 10.1158/0008-5472.CAN-03-2455. [DOI] [PubMed] [Google Scholar]
- 31.Linder S. Cytokeratin markers come of age. Tumour Biol. 2007;28:189–195. doi: 10.1159/000107582. [DOI] [PubMed] [Google Scholar]
- 32.Cummings J, Ranson M, Butt F, Moore D, Dive C. Qualification of M30 and M65 ELISAs as surrogate biomarkers of cell death: Long term antigen stability in cancer patient plasma. Cancer Chemother Pharmacol. 2007;60:921–924. doi: 10.1007/s00280-007-0437-4. [DOI] [PubMed] [Google Scholar]
- 33.Scott LC, Evans TR, Cassidy J, Harden S, Paul J, Ullah R, O'Brien V, Brown R. Cytokeratin 18 in plasma of patients with gastrointestinal adenocarcinoma as a biomarker of tumour response. Br J Cancer. 2009;101:410–417. doi: 10.1038/sj.bjc.6605175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morishita A, Gong J, Masaki T. Targeting receptor tyrosine kinases in gastric cancer. World J Gastroenterol. 2014;20:4536–4545. doi: 10.3748/wjg.v20.i16.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohenuram M, Saif MW. Epidermal growth factor receptor inhibition strategies in pancreatic cancer: Past, present and the future. JOP. 2007;8:4–15. [PubMed] [Google Scholar]
- 36.Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K, Mochizuki H. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: Poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–e8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 37.Morishita A, Masaki T. miRNA in hepatocellular carcinoma. Hepatol Res. 2015;45:128–141. doi: 10.1111/hepr.12386. [DOI] [PubMed] [Google Scholar]
- 38.Marin-Muller C, Li D, Bharadwaj U, Li M, Chen C, Hodges SE, Fisher WE, Mo Q, Hung MC, Yao Q. A tumorigenic factor Interactome connected through tumor suppressor MicroRNA-198 in human pancreatic cancer. Clin Cancer Res. 2013;19:5901–5913. doi: 10.1158/1078-0432.CCR-12-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi W, Gerster K, Alajez NM, Tsang J, Waldron L, Pintilie M, Hui AB, Sykes J, P'ng C, Miller N, et al. MicroRNA-301 mediates proliferation and invasion in human breast cancer. Cancer Res. 2011;71:2926–2937. doi: 10.1158/0008-5472.CAN-10-3369. [DOI] [PubMed] [Google Scholar]
- 42.Lu Z, Li Y, Takwi A, Li B, Zhang J, Conklin DJ, Young KH, Martin R, Li Y. miR-301a as an NF-κB activator in pancreatic cancer cells. EMBO J. 2011;30:57–67. doi: 10.1038/emboj.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]