Abstract
The angiopoietin 1 (Ang1)/angiopoietin receptor (Tie2) signaling pathway may have a notable role in the pathogenesis of inflammatory diseases. The abnormal expression of angiopoietin 1 and Tie2 has also been reported in various malignant tumors, including papillary thyroid carcinoma (PTC). However, the role and mechanism of the Ang1/Tie2 pathway in the progression of PTC remains unclear. Therefore, the aims of the present study were to clarify this. Significantly high expression levels of Ang1 and Tie2 were observed in PTC tissues and cell lines. Furthermore, MTT and wound-healing assays revealed that the Ang1-mediated stimulation of human PTC cells resulted in increased proliferation and migration. Conversely, the downregulation of Tie2 levels using short hairpin RNA targeted at Tie2 abrogated the Ang1-mediated effect on cell proliferation and migration. In studying the expression of phosphoinositide-3 kinase (PI3K)/RAC serine/threonine-protein kinase (Akt) pathway, the upregulation of Ang1/Tie2 was found to be associated with the activation of the PI3K/Akt pathway in PTC. In conclusion, the data from the present study indicated that the Ang1/Tie2 induces PTC oncogenesis via the PI3K/Akt pathway, providing novel insights into human PTC therapy.
Keywords: angiopoietin 1, angiopoietin receptor, papillary thyroid carcinoma, phosphoinositide-3 kinase/RAC serine/threonine-protein kinase pathway
Introduction
Papillary thyroid carcinoma (PTC) is the most prevalent histological thyroid carcinoma subtype, accounting for ~80% of cases (1,2). Although recent advances in diagnosis and treatment strategies have been made in clinical and experimental oncology, ≤30% of patients present with local/regional recurrence or distant metastasis within 10 years (2,3). Thus, the elucidation of the molecular mechanisms underlying PTC progression is urgently required in order to develop effective diagnostic, prognostic and therapeutic strategies.
Receptor tyrosine kinases are cell surface proteins that transduce signals from extracellular growth factors intracellularly, to elicit biological responses (4). Receptor tyrosine kinases act as potent oncoproteins and are abnormally expressed in a number of cancer types, including gliomas (5). Angiopoietin-1 (Ang1) receptor (TEK, also known as Tie2) is a receptor tyrosine kinase that was identified as an endothelial-cell-specific receptor with a critical role in the modulation of vasculogenesis and remodeling (6). Tie2 expression occurs, and is partially maintained, during differentiation in human neural stem cells (7), but not in mature neurons (8). Dysregulated Tie2 expression has also been observed in several tumor tissues, including oral squamous cell carcinoma, breast, gastric, leukemia and thyroid cancer (9–13). These studies revealed that Tie2 expression is associated with the identification and prognosis of these cancer types (12). Ang1 is a secreted ligand for Tie2 that maintains vascular plasticity, perturbations in which can contribute to abnormal vascular growth (4). Daly et al (6) revealed that Ang2 functions as a Tie2 agonist in tumor models, limiting the effects of VEGF inhibition (6). Mitsutake et al (13) revealed that Tie-2 and its ligand Ang1 were expressed in benign and malignant human thyroid tumor cells, as well as in hyperplastic regions of adenomatous goiter (13). However, the function and mechanism of the Ang1/Tie2 pathway in the proliferation and migration of PTC cells remains unclear. The aim of the present study was to determine the role and mechanism by which Ang1/Tie2 induces PTC oncogenesis.
Materials and methods
Tissue samples
A total of 30 paired PTC specimens and adjacent non-tumor tissues were obtained from patients (aged from 39 to 63, 16 female and 14 male, with no other diseases) admitted to Xiangya Hospital of Central South University (Changsha, China) between April 2012 and January 2015. The samples were immediately snap-frozen in liquid nitrogen. No patients received chemotherapy or radiotherapy prior to surgery. The present study was approved by the Ethical Committee for Scientific Studies at Xiangya Hospital of Central South University and informed consent was obtained from all patients.
Cell culture and transfection
Human TPC-1 and BCPAP PTC cell lines, human thyroid follicular epithelial cells (Nthy-ori 3-1) and 293T cells were purchased from the China Cell Culture Center (Shanghai, China) cultured in Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a humidified atmosphere containing 5% CO2. Cells were passaged every 2–3 days. The pcDNA3.1-Ang1 plasmid and Tie2 short hairpin (sh)RNA (5′-CACCGCTTCCTTCCTACCAGCTACTTTCAAGAGAAGTAGCTGGTAGGAAGGAAGC-3′) and shRNA-control (5′-CACCGCTTAGTAGCTGGTAGGAAGGTTCAAGAGACCTTCCTACCAGCTACTAAGC-3′) was purchased from Qiagen, Inc. (Valencia, CA, USA). The plasmids-control, pcDNA3.1-Ang1 plasmid (3 µg) and shRNA-control or Tie2-shRNA (3 µg; Qiagen, Inc., Valencia, CA, USA) were transfected into the PTC cells using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,) according to the manufacturer's protocol. The cells were collected for western blot analysis, qPCR and other experiments 24 h after transfection.
Western blot analysis
Cells were lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology, Haimen, China), supplemented with a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA), on ice for 30 min, followed by centrifugation at 12,000 × g for 10 min at 4°C. Next, cell lysates were collected and the protein concentration was determined. Proteins (30 µg) were separated via 10% SDS-PAGE and then transferred onto polyvinylidene fluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were blocked with 5% bovine serum albumin (Gibco; Thermo Fisher Scientific, Inc.) at room temperature for 1 h, and then incubated with the following primary antibodies: Anti-Ang1 (ab8451; 1:1,000), anti-Tie2 (ab8451; 1:1,000), anti-RAC serine/threonine protein kinase (Akt) (ab179463; 1:1,000), anti-phosphorylated (p)-Akt (ab81283; 1:1,000), and anti-phosphoinositide-3 kinase (PI3K; ab182651; 1:1,000); all from Abcam, Cambridge, MA, USA) at 4°C overnight. This was followed by incubation with horseradish peroxidase-conjugated secondary antibodies with a 1:2,500 dilution of secondary goat anti-rabbit IgG antibodies (sc-2007; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h. The protein signals were detected using an Enhanced Chemiluminescence kit (GE Healthcare Life Sciences) according to the manufacturer's protocol.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the TPC-1, BCPAP and Nthy-ori3-1 cells and tissues using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The first strand of cDNA was synthesized using the RT PrimeScript™ RT Reagent kit (Takara Bio, Inc., Otsu, Japan), according to the manufacturer's protocol, and the expression of microRNA (miRNA) was detected by qPCR analysis using the SYBR® Green detection system (Roche Applied Science, Penzberg, Germany). The cycling conditions for the qPCR were 95°C for 2 min followed by 45 cycles of 95°C for 15 sec and 60°C for 30 sec. The primer sequences used were as follows: U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′; and GAPDH forward, 5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse, 5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. U6 small nuclear RNA was used as the controls as miRNA and GAPDH was used as the controls of mRNA using the 2−ΔΔCq cycle threshold method (14). All tests were run in triplicate.
Cell proliferation assay
TPC-1 and BCPAP cell proliferation was measured using an MTT assay. Briefly, the cells were plated in 96-well plates at a density of 5×103 per well following transfection, and an MTT assay was conducted. The optical density was determined at 570 nm using an ELISA plate reader (Model 550; Bio-Rad Laboratories, Inc.).
Cell migration assay
A wound-healing assay was used to detect cell migration. TPC-1 and BCPAP cells (5×103) were seeded into 12-well plates for 24 h. Next, cells were scraped with 200 µl pipette tips and washed with PBS. Cell movement into the wound area was monitored and images were captured at 48 h using a light microscope. The migration distance between the leading edge of the migrating cells and the wound edge was compared, as previously described (15).
Statistical analysis
Each experiment was repeated at least three times. Data are shown as the mean ± standard deviation and analyzed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). All data were statistically analyzed using the Student's t-test or one-way analysis of variance followed by Tukey multiple comparison post-hoc analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Ang1 and Tie2 expression in PTC tissues and cell lines
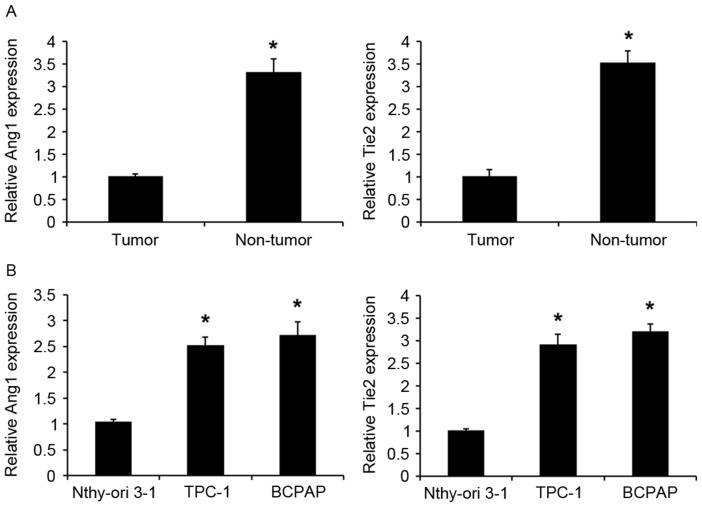
A previous study demonstrated that Ang1/Tie-2 system dysfunction may have an important role in thyroid carcinogenesis (16); however, the roles of Ang1 and Tie2 expression levels in PTC remain unclear. The present study detected miR-182 expression in 30 human PTC and adjacent normal tissues using RT-qPCR. Ang1 and Tie2 expression was significantly upregulated in PTC tissues compared with in the corresponding adjacent normal tissues (Fig. 1A). Similarly, the expression levels of Ang1 and Tie2 were significantly upregulated in PTC cell lines (TPC-1 and BCPAP) compared with in human thyroid follicular epithelial cells (Nthy-ori 3-1; Fig. 1B). Collectively, the findings of the present study indicate that the Ang1/Tie2 pathway is dysregulated in PTC and may have an important role in PTC carcinogenesis.
Figure 1.
Expression levels of Ang1 and Tie2 in PTC tissues and cell lines. (A) An RT-qPCR assay detected the expression of Ang1 and Tie2 in PTC and adjacent normal tissues. Data are presented as the mean ± standard deviation from three independent experiments. *P<0.01 vs. normal tissue. (B) An RT-qPCR assay detected the expression of Ang1 and Tie2 in PTC TPC-1 and BCPAP cell lines and human thyroid follicular epithelial cells (Nthy-ori 3-1). Data are presented as the mean ± standard deviation from three independent experiments. *P<0.01 vs. Nthy-ori 3-1 cell line. Ang1, angiopoietin 1; Tie2, Ang receptor; PTC, papillary thyroid carcinoma; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Ang1 induces PTC cell proliferation and migration via activating Tie2
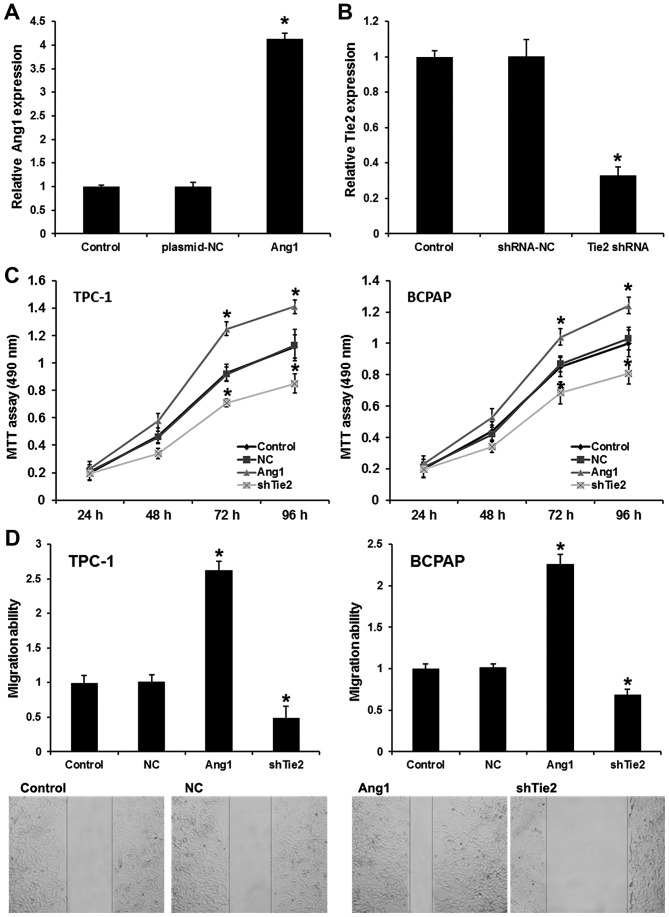
To elucidate the role of Ang1 and Tie2 in the development and progression of PTC, TPC-1 cells were transfected with pcDNA3.1-Ang1 and Tie2-shRNA. As shown in Fig. 2A and B, pcDNA3.1-Ang1 markedly upregulated Ang1 expression and Tie2-shRNA markedly evidently silenced Tie2 expression (Fig. 2A and B). The MTT assay revealed that the overexpression of Ang1 markedly increased the proliferation of PTC cells; by contrast, Tie2-knockdown inhibited the proliferation of PTC cells (Fig. 2C). A wound-healing assay was used to reveal PTC cell migratory ability; the results indicated that Ang1 upregulation significantly promoted the migration of PTC cells compared with the plasmid control an effect that could be rescued by Tie2-knockdown suppression when the cells were transfected with Tie2-shRNA (Fig. 2D). Collectively, it can be concluded that Ang1 induces PTC cell proliferation and migration via Tie2.
Figure 2.
Ang1 induces PTC proliferation and migration via activating Tie2. (A) pcDNA3.1-Ang1 transfection upregulated Ang1 expression. (B) Tie2 shRNA silenced Tie2 expression. (C) An MTT assay was utilized to analyze the effect of Ang1 and Tie-2 on the proliferation of PTC cells. (D) A wound-healing assay was used to analyze the effect of expression Ang1 and Tie-2 on the migration of PTC cells. Ang1 group, PTC cells transfected with pcDNA3.1-Ang1. Tie2 shRNA group, PTC cells transfected with Tie2 shRNA. Data are presented as the mean ± standard deviation from three independent experiments. *P<0.01 vs. control group. Ang1, angiopoietin 1; Tie2, Ang receptor; PTC, papillary thyroid carcinoma; shRNA, short hairpin RNA.
Ang1/Tie2 activates the PI3K/AKT pathway in PTC
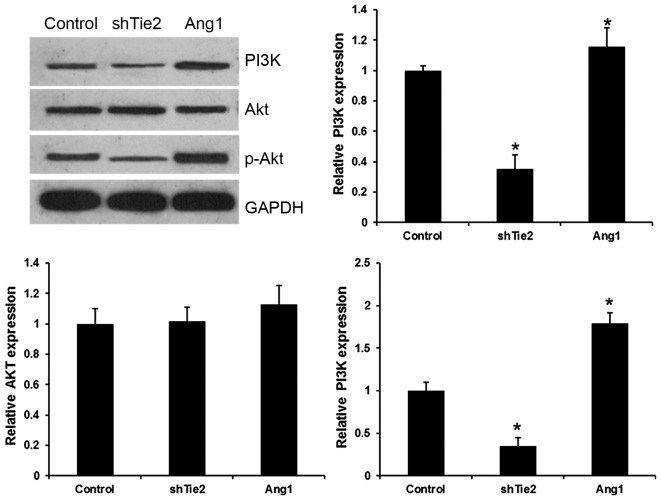
The PI3K/Akt pathway reportedly has an important role in the development of various cancer types. It was hypothesized that the Ang1/Tie2 system may regulate expression in the PI3K/AKT signaling pathway. Ang1 significantly increased the levels of PI3K and p-Akt, but did not affect Akt protein and mRNA expression in PTC cells (Fig. 3). However, Tie2-shRNA transfection significantly decreased PI3K and p-Akt expression levels in PTC cells. These results indicated that Ang1/Tie2 regulated the PI3K/Akt pathway in PTC.
Figure 3.
Ang1/Tie2 activated the PI3K/Akt pathway in PTC. The expression of PI3K, Akt, p-Akt detected by western blotting and reverse transcription-quantitative polymerase chain reaction in PTC cells transfected with pcDNA3.1-Ang1 and Tie2 shRNA. *P<0.01 vs. the control group. Ang1, angiopoietin 1; shRNA, short hairpin RNA; shTie2, shRNA targeted at Ang receptor; PI3K, phosphoinositide-3 kinase; p-Akt, phosphorylated RAC serine/threonine-protein kinase; PTC, papillary thyroid carcinoma.
Discussion
PTC is the most prevalent histological subtype of thyroid carcinomas, accounting for ~80% of all cases (1,2). The present study revealed that Ang1 and Tie2 are overexpressed in PTC and revealed the role and mechanism of the Ang1/Tie2 pathway in the progression of PTC.
Ang proteins are a family of growth factors, which exhibit opposing actions in vascular endothelial cells. Ang1 is as a secreted ligand for Tie2 that stimulates Tie2 phosphorylation, with Tie2-mediated signal transduction being important for the survival of vascular endothelium and angiogenic sprouting (17–19). There is evidence that the angiopoietin/Tie2 system is dysregulated in various cancer types: For example, Tie2 and Ang2 are upregulated in tumor vessel cells (20); Ang1 overexpression was also detected in human breast cancer and squamous cell carcinoma cells (21,22). However, the expression levels of Ang1 and Tie2 in PTC remain unclear. Thus, the present study sought to investigate these. An RT-qPCR assay revealed that Ang1 and Tie2 were markedly upregulated in PTC tissues and TPC-1 and BCPAP cell lines, compared with in the adjacent normal tissues and the human Nthy-ori 3-1 thyroid follicular epithelial cells. To clarify the role of Ang1/Tie2 signaling in tumor progression, Ang1 was overexpressed or Tie2 was silenced by transfecting pcDNA3.1-Ang1 or Tie2-shRNA into PTC cells. Ang1 overexpression induced PTC proliferation and migration via Tie2. Consistent with the results of the present study, Ang1/Tie2 have also been observed to regulate cell growth and metastasis in several other types of cancer (21,23). Holopainen et al (23) revealed that systemic treatment with Ang1 promoted tumor metastasis, whereas Tie2 suppressed lung and lymphatic metastasis (23). Overexpression of Ang1 inhibited tumor growth in human breast cancer or squamous cell carcinoma cells (21,22), although its overexpression in cervical cancer cells promoted tumor angiogenesis (24). The Ang1/Tie2 signaling pathway is also important for tumor cell dissemination via lymphatic vessels and the establishment of tumor metastases in lymph nodes (4,5). The Ang1-induced increase in tumor metastasis could be suppressed by simultaneous treatment with Tie2 (25). This suppression demonstrates the role of the Ang1/Tie2 pathway in tumor progression, by regulating cell proliferation and migration in PTC.
Evidence indicates that the PI3K/Akt pathway regulates the epithelial-mesenchymal transition, cell cycle, angiogenesis and apoptosis, and has an important role in tumor progression (26–28). The PI3K/Akt pathway was reported to be activated in PTC and to be involved in cell proliferation and migration (29). PI3K/Akt has emerged as a critical downstream pathway of Tie2 that is required for cell survival, as well as for chemotaxis, the activation of endothelial nitric oxide synthase, and possibly for the anti-inflammatory effects mediated by Tie2 activation (30). The present study detected the effects of Ang1/Tie2 pathway on Akt/PI3K expression in PTC. The present study found that Ang1 markedly increased the expression levels of PI3K and p-Akt in PTC cells, which could be reversed by transfection with Tie2 shRNA. Combined, these results demonstrate that the Ang1/Tie2 pathway has important functions in PTC progression.
In summary, the present study identified that Ang1 and Tie2 expression is upregulated during PTC carcinogenesis. The current study provides evidence for the inductive role of Ang1-mediated Tie2 receptor activation in PTC growth and migration. In conclusion, the findings of the present study indicate that the Ang1/Tie2 pathway has a notable role in the progression of PTC and represents a potential novel target for the treatment of PTC.
Acknowledgements
The present study was funded by The Health and Family Planning Commission of Hunan province (grant no. 132015-010) and National Natural Science Foundation of China (grant no. 81372860).
References
- 1.Liu X, He M, Hou Y, Liang B, Zhao L, Ma S, Yu Y, Liu X. Expression profiles of microRNAs and their target genes in papillary thyroid carcinoma. Oncol Rep. 2013;29:1415–1420. doi: 10.3892/or.2013.2263. [DOI] [PubMed] [Google Scholar]
- 2.He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, et al. The role of microRNA genes in papillary thyroid carcinoma; Proc Natl Acad Sci USA; 2005; pp. 19075–19080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu H, Fang J, Zhang J, Zhao Z, Liu L, Wang J, Xi Q, Gu M. miR-182 targets CHL1 and controls tumor growth and invasion in papillary thyroid carcinoma. Biochem Biophys Res Commun. 2014;450:857–862. doi: 10.1016/j.bbrc.2014.06.073. [DOI] [PubMed] [Google Scholar]
- 4.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/S0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee OH, Xu J, Fueyo J, Fuller GN, Aldape KD, Alonso MM, Piao Y, Liu TJ, Lang FF, Bekele BN, Gomez-Manzano C. Expression of the receptor tyrosine kinase Tie2 in neoplastic glial cells is associated with integrin beta1-dependent adhesion to the extracellular matrix. Mol Cancer Res. 2006;4:915–926. doi: 10.1158/1541-7786.MCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 6.Daly C, Eichten A, Castanaro C, Pasnikowski E, Adler A, Lalani AS, Papadopoulos N, Kyle AH, Minchinton AI, Yancopoulos GD, Thurston G. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res. 2013;73:108–118. doi: 10.1158/0008-5472.CAN-12-2064. [DOI] [PubMed] [Google Scholar]
- 7.Parati EA, Bez A, Ponti D, de Grazia U, Corsini E, Cova L, Sala S, Colombo A, Alessandri G, Pagano SF. Human neural stem cells express extra-neural markers. Brain Res. 2002;925:213–221. doi: 10.1016/S0006-8993(01)03291-7. [DOI] [PubMed] [Google Scholar]
- 8.Ward NL, Putoczki T, Mearow K, Ivanco TL, Dumont DJ. Vascular-specific growth factor angiopoietin 1 is involved in the organization of neuronal processes. J Comp Neurol. 2005;482:244–256. doi: 10.1002/cne.20422. [DOI] [PubMed] [Google Scholar]
- 9.Kitajima D, Kasamatsu A, Nakashima D, Miyamoto I, Kimura Y, Saito T, Suzuki T, Endo-Sakamoto Y, Shiiba M, Tanzawa H, Uzawa K. Tie2 regulates tumor metastasis of oral squamous cell carcinomas. J Cancer. 2016;7:600–607. doi: 10.7150/jca.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortes-Santiago N, Hossain MB, Gabrusiewicz K, Fan X, Gumin J, Marini FC, Alonso MM, Lang F, Yung WK, Fueyo J, Gomez-Manzano C. Soluble Tie2 overrides the heightened invasion induced by anti-angiogenesis therapies in gliomas. Oncotarget. 2016;7:16146–16157. doi: 10.18632/oncotarget.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Zeng X, Kleibeuker E, Buffa F, Barberis A, Leek RD, Roxanis I, Zhang W, Worth A, Beech JS, et al. Paracrine effect of GTP cyclohydrolase and angiopoietin-1 interaction in stromal fibroblasts on tumor Tie2 activation and breast cancer growth. Oncotarget. 2016;7:9353–9367. doi: 10.18632/oncotarget.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He YF, Wang CQ, Yu Y, Qian J, Song K, Sun QM, Zhou J. Tie2-expressing monocytes are associated with identification and prognoses of hepatitis B virus related hepatocellular carcinoma after resection. PLoS One. 2015;10:e0143657. doi: 10.1371/journal.pone.0143657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsutake N, Namba H, Takahara K, Ishigaki K, Ishigaki J, Ayabe H, Yamashita S. Tie-2 and angiopoietin-1 expression in human thyroid tumors. Thyroid. 2002;12:95–99. doi: 10.1089/105072502753522310. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Liang CC, Park AY, Guan JL. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 16.Niedźwiecki S, Stepień T, Kopeć K, Kuzdak K, Komorowski J, Krupiński R, Stepień H. Angiopoietin 1 (Ang-1), angiopoietin 2 (Ang-2) and Tie-2 (a receptor tyrosine kinase) concentrations in peripheral blood of patients with thyroid cancers. Cytokine. 2006;36:291–295. doi: 10.1016/j.cyto.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 18.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 19.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 20.Peters KG, Kontos CD, Lin PC, Wong AL, Rao P, Huang L, Dewhirst MW, Sankar S. Functional significance of Tie2 signaling in the adult vasculature. Recent Prog Horm Res. 2004;59:51–71. doi: 10.1210/rp.59.1.51. [DOI] [PubMed] [Google Scholar]
- 21.Hawighorst T, Skobe M, Streit M, Hong YK, Velasco P, Brown LF, Riccardi L, Lange-Asschenfeldt B, Detmar M. Activation of the tie2 receptor by angiopoietin-1 enhances tumor vessel maturation and impairs squamous cell carcinoma growth. Am J Pathol. 2002;160:1381–1392. doi: 10.1016/S0002-9440(10)62565-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes AJ, Huang WQ, Yu J, Maisonpierre PC, Liu A, Kern FG, Lippman ME, McLeskey SW, Li LY. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000;83:1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holopainen T, Huang H, Chen C, Kim KE, Zhang L, Zhou F, Han W, Li C, Yu J, Wu J, et al. Angiopoietin-1 overexpression modulates vascular endothelium to facilitate tumor cell dissemination and metastasis establishment. Cancer Res. 2009;69:4656–4664. doi: 10.1158/0008-5472.CAN-08-4654. [DOI] [PubMed] [Google Scholar]
- 24.Shim WS, Teh M, Bapna A, Kim I, Koh GY, Mack PO, Ge R. Angiopoietin 1 promotes tumor angiogenesis and tumor vessel plasticity of human cervical cancer in mice. Exp Cell Res. 2002;279:299–309. doi: 10.1006/excr.2002.5597. [DOI] [PubMed] [Google Scholar]
- 25.Lin P, Buxton JA, Acheson A, Radziejewski C, Maisonpierre PC, Yancopoulos GD, Channon KM, Hale LP, Dewhirst MW, George SE, Peters KG. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2; Proc Natl Acad Sci USA; 1998; pp. 8829–8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boufraqech M, Zhang L, Jain M, Patel D, Ellis R, Xiong Y, He M, Nilubol N, Merino MJ, Kebebew E. miR-145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr Relat Cancer. 2014;21:517–531. doi: 10.1530/ERC-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang G, Liu Z, Xu H, Yang Q. miR-409-3p suppresses breast cancer cell growth and invasion by targeting Akt1. Biochem Biophys Res Commun. 2016;469:189–195. doi: 10.1016/j.bbrc.2015.11.099. [DOI] [PubMed] [Google Scholar]
- 28.Bai H, Li H, Li W, Gui T, Yang J, Cao D, Shen K. The PI3K/AKT/mTOR pathway is a potential predictor of distinct invasive and migratory capacities in human ovarian cancer cell lines. Oncotarget. 2015;6:25520–25532. doi: 10.18632/oncotarget.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minna E, Romeo P, Dugo M, De Cecco L, Todoerti K, Pilotti S, Perrone F, Seregni E, Agnelli L, Neri A, et al. miR-451a is underexpressed and targets AKT/mTOR pathway in papillary thyroid carcinoma. Oncotarget. 2016;7:12731–12747. doi: 10.18632/oncotarget.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findley CM, Cudmore MJ, Ahmed A, Kontos CD. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol. 2007;27:2619–2626. doi: 10.1161/ATVBAHA.107.150482. [DOI] [PubMed] [Google Scholar]