Abstract
Background
Minimal change disease (MCD) is a kind of nephrotic syndrome (NS). In this study, we aimed to establish a mathematical diagnostic model based on biological parameters to classify MCD.
Methods
A total of 798 NS patients were divided into MCD group and control group. The comparison of biological indicators between two groups were performed with t-tests. Logistic regression was used to establish the diagnostic model, and the diagnostic value of the model was estimated using receiver operating characteristic (ROC) analysis.
Results
Thirteen indicators including Anti-phospholipase A2 receptor (anti-PLA2R) (P = 0.000), Total protein (TP) (P = 0.000), Albumin (ALB) (P = 0.000), Direct bilirubin (DB) (P = 0.002), Creatinine (Cr) (P = 0.000), Total cholesterol (CH) (P = 0.000), Lactate dehydrogenase (LDH) (P = 0.007), High density lipoprotein cholesterol (HDL) (P = 0.000), Low density lipoprotein cholesterol (LDL) (P = 0.000), Thrombin time (TT) (P = 0.000), Plasma fibrinogen (FIB) (P = 0.000), Immunoglobulin A (IgA) (P = 0.008) and Complement 3 (C3) (P = 0.019) were significantly correlated with MCD. Furthermore, the area under ROC curves of CH, HDL, LDL, TT and FIB were more than 0.70. Logistic analysis demonstrated that CH and TT were risk factors for MCD. According to the ROC of “CH+TT”, the AUC was 0.827, with the sensitivity of 83.0% and the specificity of 69.8% (P = 0.000).
Conclusion
The established diagnostic model with CH and TT could be used for classified diagnosis of MCD.
Keywords: Minimal change disease, Diagnostic model, Biological parameters
Introduction
Minimal change disease (MCD) is a common pathological type of nephrotic syndrome (NS), and its typical characteristic is the diffuse effacement of podocyte foot processes observed by electron microscope (Glick, 2007). The actin of foot process is linked with slit diaphragm, which is important for renal glomerular filtration function. MCD is commonly seen in child patients with NS. According to the reports, about 80% MCD cases are aged less than 10 years old. The incidence of MCD in adults is lower than that in children (Cameron, 1996; Haas et al., 1997; Kazi et al., 2009; Zech et al., 1982). In our country, the incidence of adult MCD patients in NS patients is less than 25% (Chu, Chen & Liu, 2014; Zhou et al., 2011). At present, renal biopsy, which is an invasive examination, is required for most glomerulopathy diagnoses. Although it can offer the diagnosis and testing information for the doctors, renal biopsy may cause complications, such as bleeding. Moreover, some patients can not accept the renal biopsy, leading to the absence of timely diagnosis (Fiorentino et al., 2016; Magistroni et al., 2015; Verde et al., 2012). Therefore, a non-invasive model is urgently needed to discriminate MCD.
It is reported that mathematical model, like classification and regression tree (CART) model can be used as a method to classify different diseases (Hu et al., 2011b; Yan, Lin & Liu, 2011). At present time, the diagnostic model based on data analysis has become the focus of disease diagnosis, and it also can be used in the noninvasive diagnosis (Azmak et al., 2015). Moreover, some reports have shown that the classification equations have been used in kidney diseases.
In present study, with the purpose of classifying MCD and other kidney diseases, we established a diagnostic model based on the clinical parameter. Additionally, we also conducted common statistical analyses, including Chi-square tests, logistic analysis and receiver operating characteristic (ROC) analysis.
Methods
Study object
This study was approved by the Medical Ethics Committee of the Chinese PLA General Hospital, and written consents were obtained from all patients. The inclusion criteria of the current research were listed as follows: (1) all the participants were the first time to be admitted into the Department of Nephrology of our hospital; (2) adult patients; (3) no one accepted the renal biopsy before entering our hospital; (4) no one accepted any treatments, including hypertension treatment or hyperlipidemia treatment; (5) no one suffered from any tumors, except hypertension, diabetes, hepatitis or lupus erythematosus; (6) all patients accepted renal biopsy during their hospitalization. The following exclusion criteria were applied in our study: (1) the patients could not accept renal biopsy; (2) no complete clinical data were provided. According to the inclusion and exclusion criteria, 798 patients were finally recruited, containing 47 MCD patients and 751 patients with other kidney diseases.
Samples and biological parameters
For all the 798 patients, their blood samples were collected on the second day after entering hospital. Then, blood coagulation test, blood routine examination and clinical biochemistry testing were performed. The demographic data as well as clinical and laboratory examination of all patients were recorded, including age, gender, presence of other diseases, physical examination, and so on.
Statistical analysis
In this study, all statistical analyses were performed using SPSS 19.0 and GraphPad Prism 5. The data were summarized and presented as means ± SD. The biological indicators of the two groups were assessed by using t-tests. Logistic regression was employed to establish the diagnostic model. The diagnostic value of the constructed model was examined via performing ROC analysis. P values less than 0.05 were considered to be statistically significant in this paper.
Results
The characteristics of patients
The tested biological parameters were all listed in Table 1, including Anti-phospholipase A2 receptor (anti-PLA2R), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Total protein (TP), Albumin (ALB), Total bilirubin (TB), Direct bilirubin (DB), Alkaline phosphatase (ALP), γ-Glutamyltransferase (GGT), Glucose (GLU), Urea nitrogen (UN), Creatinine (Cr), Uric acid (Ua), Total cholesterol (CH), Triglyceride (TG), Creatine kinase (CK), Lactate dehydrogenase (LDH), High density lipoprotein cholesterol (HDL), Low density lipoprotein cholesterol (LDL), Thrombin time (TT), Prothrombin time (PT), Plasma fibrinogen (FIB), D-dimer (D2), Immunoglobulin A (IgA), Immunoglobulin G (IgG), Immunoglobulin M (IgM), Immunoglobulin E (IgE), Complement 3 (C3), Complement 4 (C4) and Body mass index (BMI). Moreover, the reference ranges of them were also listed in the table. The demographic data and history of diseases of these two groups were recorded in Table 2. We found that the rates of hypertension and diabetes were declined in MCD patients compared with the patients with other kidney diseases. In MCD group, the numbers of patients less than 40 years old and more than 40 years old were about the same, and the similar result was found in the group of other kidney diseases. We observed more male patients than female patients in both groups. Besides, most patients of the two groups had no hypertension, diabetes or hepatitis. The BMI value was 25.03 ± 4.66 in MCD group, and the data for the group of other kidney disease was 25.45 ± 4.37.
Table 1. The biological parameters in this study.
The tested biological parameters.
| Index full name | Abbreviation | Reference range |
|---|---|---|
| Anti-phospholipase A2 receptor | Anti-PLA2R | |
| Alanine aminotransferase | ALT | 0–40 U/L |
| Aspartate aminotransferase | AST | 0–40 U/L |
| Total protein | TP | 55–80 g/L |
| Albumin | ALB | 35–50 g/L |
| Total bilirubin | TB | 0–21 µmol/L |
| Direct bilirubin | DB | 0–8.6 µmol/L |
| Alkaline phosphatase | ALP | 0–130 U/L |
| γ-Glutamyltransferase | GGT | 0–50 U/L |
| Glucose | GLU | 3.4–6.2 mmol/L |
| Urea nitrogen | UN | 1.8–7.5 mmol/L |
| Creatinine | Cr | 30–110 µmol/L |
| Uric acid | Ua | 104–444 µmol/L |
| Total cholesterol | CH | 3.1–5.7 mmol/L |
| Triglyceride | TG | 0.4–1.7 mmol/L |
| Creatine kinase | CK | 2–200 U/L |
| Lactate dehydrogenase | LDH | 40–250 U/L |
| High density lipoprotein cholesterol | HDL | 1–1.6 mmol/L |
| Low density lipoprotein cholesterol | LDL | 0–3.4 mmol/L |
| Thrombin time | TT | 16.0–18.0 s |
| Prothrombin time | PT | 11.0–15.0 s |
| Plasma fibrinogen | FIB | 200–400 mg/dL |
| D-dimer | D2 | 0.0–0.5 µg/L |
| Immunoglobulin A | IgA | 70–180 mg/dl |
| Immunoglobulin G | IgG | 700–1,600 mg/dl |
| Immunoglobulin M | IgM | 40–230 mg/dl |
| Immunoglobulin E | IgE | 0–100 IU/ml |
| Complement 3 | C3 | 90–180 mg/dl |
| Complement 4 | C4 | 10–40 mg/dl |
| Body mass index | BMI | 18.5–24.99 |
Table 2. Basic information of the two groups.
The demographic data and history of diseases of these two groups.
| MCD group (n = 47) | Group of other kidney diseases (n = 751) | P value | |
|---|---|---|---|
| Age | 0.452 | ||
| <40 | 25 | 357 | |
| ≥40 | 22 | 394 | |
| Gender | 0.685 | ||
| Male | 30 | 457 | |
| Female | 17 | 294 | |
| Hypertension | 0.000 | ||
| Yes | 7 | 363 | |
| No | 40 | 388 | |
| Diabetes | 0.041 | ||
| Yes | 2 | 113 | |
| No | 45 | 638 | |
| Hepatitis | 0.050 | ||
| Yes | 0 | 57 | |
| No | 47 | 694 | |
| BMI | 25.03 ± 4.66 | 25.45 ± 4.37 | 0.895 |
The comparison of biochemical indicators between two groups
In order to explore the association between biochemical indicators and MCD, student’s t-test was performed. As shown in Table 3, the results showed that among the 28 biochemical indicators, 13 indicators including anti-PLA2R (P = 0.000), TP (P = 0.000), ALB (P = 0.000), DB (P = 0.002), Cr (P = 0.000), CH (P = 0.000), LDH (P = 0.007), HDL (P = 0.000), LDL (P = 0.000), TT (P = 0.000), FIB (P = 0.000), IgA (P = 0.008) and C3 (P = 0.019) were significantly different between the two groups.
Table 3. The comparison of serological parameters in the two groups.
| Parameter | MCD group (n = 47) (mean ± SD) | Group of other kidney diseases (n = 751) (mean ± SD) | P value |
|---|---|---|---|
| Anti-PLA2R | 2.00 ± 0.02 | 34.22 ± 114.96 | 0.000* |
| ALT | 27.00 ± 35.12 | 22.61 ± 21.99 | 0.201 |
| AST | 20.79 ± 8.80 | 18.82 ± 11.39 | 0.240 |
| TP | 43.32 ± 10.01 | 58.33 ± 12.02 | 0.000* |
| ALB | 22.68 ± 7.87 | 33.94 ± 9.04 | 0.000* |
| TB | 7.87 ± 3.64 | 8.63 ± 4.40 | 0.177 |
| DB | 1.39 ± 0.99 | 1.98 ± 1.32 | 0.002* |
| ALP | 69.36 ± 34.22 | 66.03 ± 31.66 | 0.835 |
| GGT | 41.43 ± 82.16 | 34.00 ± 51.21 | 0.398 |
| GLU | 4.62 ± 0.88 | 4.98 ± 1.75 | 0.102 |
| UN | 6.78 ± 4.30 | 6.30 ± 3.59 | 0.446 |
| Cr | 82.25 ± 23.15 | 104.31 ± 68.19 | 0.000* |
| Ua | 345.71 ± 104.69 | 366.78 ± 102.00 | 0.198 |
| CH | 8.81 ± 3.23 | 5.72 ± 2.21 | 0.000* |
| TG | 2.35 ± 1.60 | 2.22 ± 1.56 | 0.582 |
| CK | 97.12 ± 88.43 | 95.70 ± 99.36 | 0.756 |
| LDH | 204.66 ± 58.78 | 182.88 ± 59.31 | 0.007* |
| HDL | 1.74 ± 0.76 | 1.26 ± 0.84 | 0.000* |
| LDL | 6.31 ± 2.81 | 3.81 ± 1.84 | 0.000* |
| TT | 18.65 ± 2.52 | 16.56 ± 1.44 | 0.000* |
| PT | 12.85 ± 0.83 | 13.15 ± 1.55 | 0.243 |
| FIB | 5.46 ± 1.79 | 4.22 ± 1.50 | 0.000* |
| D2 | 1.07 ± 0.79 | 0.98 ± 1.84 | 0.721 |
| IgA | 220.47 ± 92.70 | 259.46 ± 117.18 | 0.008* |
| IgM | 142.22 ± 64.44 | 111.43 ± 126.53 | 0.099 |
| IgE | 445.22 ± 927.74 | 205.44 ± 894.09 | 0.090 |
| C3 | 116.86 ± 27.12 | 107.55 ± 26.38 | 0.019* |
| C4 | 28.15 ± 10.12 | 26.46 ± 9.49 | 0.238 |
Notes.
P < 0.05.
ROC analysis of related characteristics
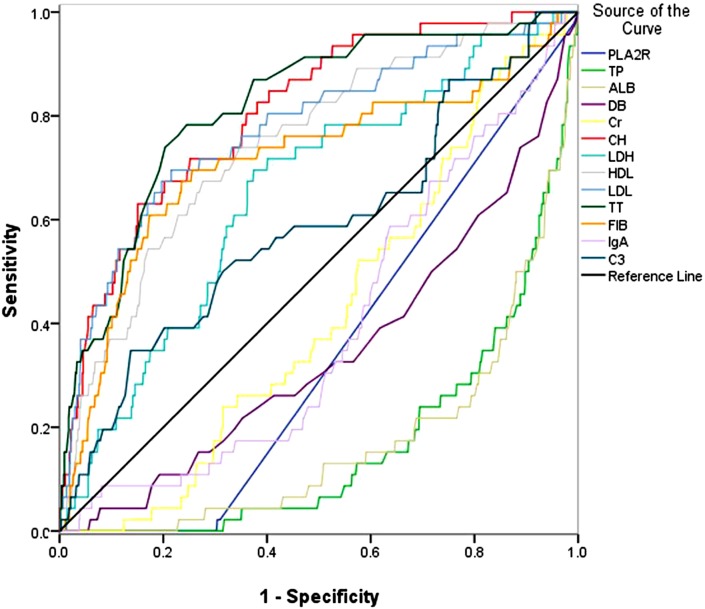
The ROC analysis was conducted to detect the diagnostic value of these 13 indicators, and the results were displayed in Fig. 1. We found that in Fig. 1, the area under the curves (AUCs) of CH, HDL, LDL, TT and FIB were more than 0.70, and the AUCs of them were 0.807, 0.746, 0.776, 0.817 and 0.713, respectively (P = 0.032, 0.037, 0.039, 0.032, and 0.046, respectively).
Figure 1. The ROC curves of anti-PLA2R, TP, ALB, DB, Cr, CH, LDH, HDL, LDL, TT, FIB, IgA and C3, the related indicators of MCD.
The ROC analysis was conducted to detect the diagnostic value of these 13 indicators.
Logistic analysis of the pre-selected parameters
In order to establish the classification models of MCD and other kidney diseases, the logistic analysis was carried out. From Table 4, we could see that CH and TT were risk factors for MCD, and the P values of them were both 0.000. Furthermore, the classification equation including CH and TT was as follows:
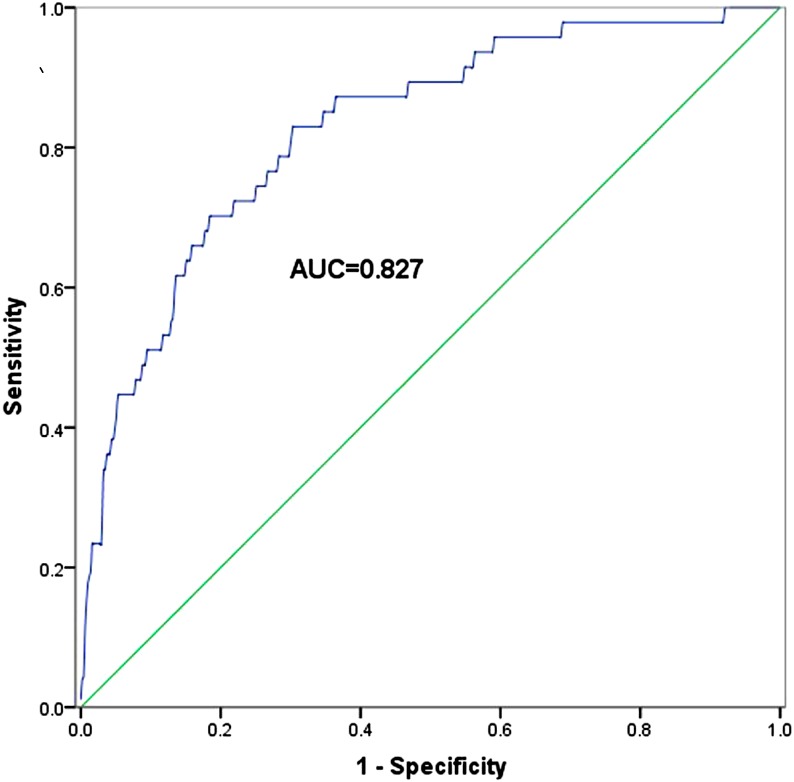
Then, based on logistic regression of the predicted probability (PRE), the ROC curve of “CH+TT” is presented in Fig. 2. From Fig. 2, we could see that the AUC of “CH+TT” was 0.827, with the sensitivity of 83.0% and the specificity of 69.8% (P = 0.000).
Table 4. The multivariate logistic regression analysis for the model.
| B | S.E. | Wald | df | Sig. | Exp (B) | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| CH | −.270 | .058 | 21.720 | 1 | .000 | .763 | .682 | .855 |
| HDL | −.186 | .099 | 3.516 | 1 | .061 | .830 | .684 | 1.008 |
| TT | −.325 | .087 | 14.145 | 1 | .000 | .722 | .610 | .856 |
| Constant | 10.617 | 1.449 | 53.669 | 1 | .000 | 40,819.656 | ||
Figure 2. The ROC curve of “CH+TT” combination from logistic regression of the predicted probability for MCD patients.
Discussion
MCD is a kind of glomerular disease caused by lesions of the podocyte. Most MCDs are idiopathic nephrotic syndromes among children and adults. MCD is characterized by hypoalbuminemia, hyperlipidemia, proteinuria and edema (Braden et al., 2000; McGrogan, Franssen & De Vries, 2011). It has been reported that the outcome of MCD is correlated with some elements, such as virus infection, drugs, allergy and even tumors (Korzets et al., 1992; Meyrier et al., 1992). At present, the pathogenesis of MCD still remains unclear, but many scholars consider that it may be associated with podocyte injury. Now in clinic, renal biopsy centesis is still the golden standard for the diagnosis of nephropathy (Appel & Appel, 2009; Floege & Eitner, 2011). Renal biopsy centesis is safe and easy operation, but it is invasive with risks (Fisi et al., 2012). Because some patients have suffered from absolute and relative contraindications of renal biopsy (Mohamed & John, 2011; Whittier & Korbet, 2004), they refuse to undergo renal biopsy. Since the conditions of hospital are not adequate, renal biopsy can not be implemented in every patient who is suspected to have kidney diseases. Moreover, the histopathological diagnosis of renal biopsy is not stable (Lu et al., 2011). Consequently, the non-invasive methods with high accuracy are urgently needed for MCD diagnosis are needed.
The mathematical model based on the statistical analysis and computer technique has been used in clinic, such as computed tomography (CT) and nuclear magnetic resonance (NMR) (Bandak et al., 1995; Hu et al., 2011a). Levey et al. (2009) established a new equation of estimated glomerular filtration rate (eGFR), named CKD-EPI, which could assess the stages of kidney diseases (CKD stage) (Levey et al., 2009). Gao et al. (2011) also established a diagnostic model for IgA nephropathy with 6 serum biochemical parameters, including ALB, CK, Cr, HDL, CA125 and TB (Gao et al., 2011). Additionally, they certified that this model could be used in the auxiliary diagnosis of IgA nephropathy.
In our study, a diagnostic model was established for MCD, and the enrolled patients were divided into two groups, which were MCD group and group of other kidney diseases. The present paper enrolled 47 MCD patients and 751 patients with other kidney diseases. In the two groups, the numbers of patients older than 40 years old and younger than 40 years old were almost equal. The ratio of male patients and female patients was about 2:1 in both groups, and very few patients suffered from hypertension, diabetes or hepatitis. Besides, the BMI of the two groups also had no significant differences. The t-test was implemented to analyze the serum biological indicators, and anti-PLA2R, TP, ALB, DB, Cr, CH, LDH, HDL, LDL, TT, FIB, IgA and C3 were significantly different between the two groups. Like the previous study by Gao and his colleagues, which also found 15 significant different serological indicators between IgAN patients and non-IgAN patients, the ROC analysis was performed to further assess the diagnostic value of the collected parameters (Gao et al., 2012). In our study, ROC analysis for the 13 indicators revealed that the AUCs of five indicators, CH, HDL, LDL, TT and FIB were more than 0.70. Logistic analysis was performed with CH, HDL, LDL, TT and FIB. The results suggested that CH and TT were risk factors for MCD. Based on logistic regression of the predicted probability on the two indicators, the results of ROC curve revealed that “CH +TT” the AUC of “CH +TT” was 0.827, with the sensitivity of 83.0% and the specificity of 69.8%.
In this study, we found that the combined diagnostic value of “CH +TT” was significantly higher than either of them alone. The combined diagnostic model might help improve the diagnosis of MCD, especially for those challenging case. However, the present study has some limitations. Firstly, the sample size of MCD group is not large enough. Moreover, all the patients were collected from a single institution and population, which might cause bias to the final results. Secondly, the specificity was not high in our study, leading to high false positive rate. The diagnostic accuracy of the combined model was 70.4%. In order to improve the diagnosis specificity, we could prioritize diagnosis sensitivity based on the trade-off between diagnosis false positive cases versus omitting true positive cases. According to ROC analysis, the diagnostic specificity was adjusted as 70%, and the sensitivity was 80.9%, while the diagnostic accuracy was 70%, which also hold great potential for clinical application. Thirdly, the diagnostic performance of the constructed models was only verified in the populations collected in the original analysis. A cross-validation was not set to investigate the diagnostic performance of the combined model for MCD in clinic. Additionally, all the MCD cases collected in our study were adults. However, MCD is frequently diagnosed among children, and the adult cases only account for about 10%–15%. The main reasons for childhood MCD include congenital anomalies and inherited disorders, but the diagnostic value of the combined model for the childhood MCD cases remained unknown (Downie et al., 2017; Ingelfinger, Kalantar-Zadeh & Schaefer, 2016). The distinct etiologies may lead to various clinical symptoms, biological parameters, and therapeutic responses. Thus, further investigations are needed to determinate whether the selected biological parameters exerted advantages in diagnosing MCD among children. Further related research will be carried out to address the above issues.
Conclusion
This study has established a diagnostic model based on the clinical parameters to classify MCD and other kidney diseases. The combined diagnostic model with CH and TT could effectively distinguish MCD from other nephrotic syndrome.
Supplemental Information
The data used in ROC-1 (Fig. 1).
Raw data used in ROC-2 (Fig. 2).
Raw data used in the logistic analysis.
Funding Statement
This study was supported by the National Key R&D Program of China (2016YFC1305500); the National Natural Science Foundation of China (No. 61671479); the National Natural Science Foundation of China (No. 61471399); Innovation Nursery Fund of PLA General Hospital (No. 15KMZ04); and the National Natural Science Foundation of China (No. 81401719). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Dong Zhang, Email: dongzhang301301@126.com.
Yong Wang, Email: wangyong301@263.net.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Hanyu Zhu and Qiuxia Han performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Dong Zhang and Yong Wang conceived and designed the experiments.
Jing Gao analyzed the data.
Wenjia Geng and Xiaoli Yang contributed reagents/materials/analysis tools, wrote the paper.
Xiangmei Chen reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The code is included in the Results section of the manuscript.
References
- Appel & Appel (2009).Appel AS, Appel GB. An update on the use of mycophenolate mofetil in lupus nephritis and other primary glomerular diseases. Nature Clinical Practice Nephrology. 2009;5:132–142. doi: 10.1038/ncpneph1036. [DOI] [PubMed] [Google Scholar]
- Azmak et al. (2015).Azmak O, Bayer H, Caplin A, Chun M, Glimcher P, Koonin S, Patrinos A. Using big data to understand the human condition: the ‘kavli’ HUMAN project. Big Data. 2015;3:173–188. doi: 10.1089/big.2015.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandak et al. (1995).Bandak FA, Vander Vorst MJ, Stuhmiller LM, Mlakar PF, Chilton WE, Stuhmiller JH. An imaging-based computational and experimental study of skull fracture: finite element model development. Journal of Neurotrauma. 1995;12:679–688. doi: 10.1089/neu.1995.12.679. [DOI] [PubMed] [Google Scholar]
- Braden et al. (2000).Braden GL, Mulhern JG, O’Shea MH, Nash SV, Ucci Jr AA, Germain MJ. Changing incidence of glomerular diseases in adults. American Journal of Kidney Diseases. 2000;35:878–883. doi: 10.1016/S0272-6386(00)70258-7. [DOI] [PubMed] [Google Scholar]
- Cameron (1996).Cameron JS. Nephrotic syndrome in the elderly. Seminars in Nephrology. 1996;16:319–329. [PubMed] [Google Scholar]
- Chu, Chen & Liu (2014).Chu F, Chen G, Liu Y. Pathological patterns of primary nephrotic syndrome in Central China: a retrospective study of 627 cases. Renal Failure. 2014;36:514–519. doi: 10.3109/0886022X.2014.882736. [DOI] [PubMed] [Google Scholar]
- Downie et al. (2017).Downie ML, Gallibois C, Parekh RS, Noone DG. Nephrotic syndrome in infants and children: pathophysiology and management. Paediatrics and International Child Health. 2017;37:1–11. doi: 10.1080/20469047.2017.1374003. [DOI] [PubMed] [Google Scholar]
- Fiorentino et al. (2016).Fiorentino M, Bolignano D, Tesar V, Pisano A, Van Biesen W, D’Arrigo G, Tripepi G, Gesualdo L. Renal biopsy in 2015–from epidemiology to evidence-based indications. American Journal of Nephrology. 2016;43:1–19. doi: 10.1159/000444026. [DOI] [PubMed] [Google Scholar]
- Fisi et al. (2012).Fisi V, Mazak I, Degrell P, Halmai R, Molnar GA, Feher E, Nemeth K, Pinter I, Kovacs T, Wittmann I. Histological diagnosis determines complications of percutaneous renal biopsy: a single-center experience in 353 patients. Kidney & Blood Pressure Research. 2012;35:26–34. doi: 10.1159/000329939. [DOI] [PubMed] [Google Scholar]
- Floege & Eitner (2011).Floege J, Eitner F. Current therapy for IgA nephropathy. Journal of the American Society of Nephrology. 2011;22:1785–1794. doi: 10.1681/ASN.2011030221. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2011).Gao J, Cui J, Wang Y, Dong Z, Tian Y, Xu Y. Identification of potential predictors for subtype IgA nephropathy through analyses of blood biochemical indicators. Clinica Chimica Acta. 2011;412:441–445. doi: 10.1016/j.cca.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Gao et al. (2012).Gao J, Wang Y, Dong Z, Yan Z, Jia X, Tian Y. A novel differential diagnostic model based on multiple biological parameters for immunoglobulin A nephropathy. BMC Medical Informatics and Decision Making. 2012;12:58. doi: 10.1186/1472-6947-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick (2007).Glick AM. Focal segmental glomerulosclerosis: a case study with review of pathophysiology. Nephrology Nursing Journal. 2007;34:176–182. [PubMed] [Google Scholar]
- Haas et al. (1997).Haas M, Meehan SM, Karrison TG, Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. American Journal of Kidney Diseases. 1997;30:621–631. doi: 10.1016/S0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2011b).Hu W, O’Leary RA, Mengersen K, Low Choy S. Bayesian classification and regression trees for predicting incidence of cryptosporidiosis. PLOS ONE. 2011b;6:e23903. doi: 10.1371/journal.pone.0023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2011a).Hu J, Qiao J, Kang D, Liu B. Analysis on the distinguishing features of traditional Chinese therapeutics and related statistical issues. Frontiers in Medicine. 2011a;5:203–207. doi: 10.1007/s11684-011-0138-6. [DOI] [PubMed] [Google Scholar]
- Ingelfinger, Kalantar-Zadeh & Schaefer (2016).Ingelfinger JR, Kalantar-Zadeh K, Schaefer F. Averting the legacy of kidney disease: focus on childhood. International Journal of Organ Transplantation Medicine. 2016;7:61–68. doi: 10.1016/j.kint.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazi et al. (2009).Kazi JI, Mubarak M, Ahmed E, Akhter F, Naqvi SA, Rizvi SA. Spectrum of glomerulonephritides in adults with nephrotic syndrome in Pakistan. Clinical and Experimental Nephrology. 2009;13:38–43. doi: 10.1007/s10157-008-0075-0. [DOI] [PubMed] [Google Scholar]
- Korzets et al. (1992).Korzets Z, Golan E, Manor Y, Schneider M, Bernheim J. Spontaneously remitting minimal change nephropathy preceding a relapse of Hodgkin’s disease by 19 months. Clinical Nephrology. 1992;38:125–127. [PubMed] [Google Scholar]
- Levey et al. (2009).Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro 3rd AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Annals of Internal Medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu et al. (2011).Lu J, Tam LS, Lai FM, Kwan BC, Choi PC, Li EK, Chow KM, Li PK, Szeto CC. Repeat renal biopsy in lupus nephritis: a change in histological pattern is common. American Journal of Nephrology. 2011;34:220–225. doi: 10.1159/000330356. [DOI] [PubMed] [Google Scholar]
- Magistroni et al. (2015).Magistroni R, D’Agati VD, Appel GB, Kiryluk K. New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney International. 2015;88:974–989. doi: 10.1038/ki.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan, Franssen & De Vries (2011).McGrogan A, Franssen CF, De Vries CS. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrology, Dialysis, Transplantation. 2011;26:414–430. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- Meyrier et al. (1992).Meyrier A, Delahousse M, Callard P, Rainfray M. Minimal change nephrotic syndrome revealing solid tumors. Nephron. 1992;61:220–223. doi: 10.1159/000186877. [DOI] [PubMed] [Google Scholar]
- Mohamed & John (2011).Mohamed N, John R. Use of renal biopsy in the elderly. International Urology and Nephrology. 2011;43:593–600. doi: 10.1007/s11255-010-9874-9. [DOI] [PubMed] [Google Scholar]
- Verde et al. (2012).Verde E, Quiroga B, Rivera F, Lopez-Gomez JM. Renal biopsy in very elderly patients: data from the Spanish Registry of Glomerulonephritis. American Journal of Nephrology. 2012;35:230–237. doi: 10.1159/000336307. [DOI] [PubMed] [Google Scholar]
- Whittier & Korbet (2004).Whittier WL, Korbet SM. Renal biopsy: update. Current Opinion in Nephrology and Hypertension. 2004;13:661–665. doi: 10.1097/00041552-200411000-00013. [DOI] [PubMed] [Google Scholar]
- Yan, Lin & Liu (2011).Yan FR, Lin JG, Liu Y. Sparse logistic regression for diagnosis of liver fibrosis in rat by using SCAD-penalized likelihood. Journal of Biomedicine and Biotechnology. 2011;2011 doi: 10.1155/2011/875309. Article 875309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech et al. (1982).Zech P, Colon S, Pointet P, Deteix P, Labeeuw M, Leitienne P. The nephrotic syndrome in adults aged over 60: etiology, evolution and treatment of 76 cases. Clinical Nephrology. 1982;17:232–236. [PubMed] [Google Scholar]
- Zhou et al. (2011).Zhou FD, Shen HY, Chen M, Liu G, Zou WZ, Zhao MH, Wang HY. The renal histopathological spectrum of patients with nephrotic syndrome: an analysis of 1,523 patients in a single Chinese centre. Nephrology, Dialysis, Transplantation. 2011;26:3993–3997. doi: 10.1093/ndt/gfr166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The data used in ROC-1 (Fig. 1).
Raw data used in ROC-2 (Fig. 2).
Raw data used in the logistic analysis.
Data Availability Statement
The following information was supplied regarding data availability:
The code is included in the Results section of the manuscript.