Abstract
Purpose
The objective of this study was to evaluate the association between depression and change in coronary heart disease (CHD) risk status by an analysis of examination data in the general Korean population.
Patients and methods
We examined 1,851 men and 1,689 women (aged 43–73 years) for the Korean Genome and Epidemiology Study Ansan between 2005 and 2012. The estimated CHD risk score of participants was calculated using the Framingham CHD risk score in baseline and after 8-year follow-up period. Among them, population with low Framingham CHD risk score (<10%) in baseline (n=1,582) was used for further analyses. The low Framingham CHD risk score participants were assigned to one of two groups based on the Beck depression inventory (BDI) score: no depression (BDI <10) and depression (BDI ≥10). Multivariate logistic regression was performed to test whether depression was associated with participants’ status change to intermediate or high CHD risk score (≥10%) in men and women, respectively, after 8-year follow-up period.
Results
Women with depression showed significant higher rates of changing to intermediate or high CHD risk score status when compared with women without depression even after adjusting for age, systolic blood pressure, high-density lipoprotein, and smoking (adjusted odds ratio [OR], 1.54; 95% CI, 1.08–2.03). However, depression was not associated with intermediate or high CHD risk score status in men (adjusted OR, 1.38; 95% CI, 0.95–1.82).
Conclusion
This general population-based cohort study provides evidence that depression can affect the risk of changing CHD risk score status in women.
Keywords: depressive symptom, beck depression inventory, Framingham coronary heart disease risk score, coronary heart disease risk factor
Introduction
Cardiovascular disease (CVD) and depression are currently the two most common causes of disability in high-income countries and are expected to become so for countries of all income levels by 2030.1 The key health system and economic indicators related to CVD and depression reveal rising medical costs, increased health service utilization, and lost productivity.2 There is no argument that depression is a risk marker for an increased incidence of new coronary heart disease (CHD).3,4 Conversely, patients with CHD are more likely to suffer from depression than the general population.5 Mild depression was identified in up to two-thirds of patients after acute myocardial infarction (MI),6 with major depression observed in 31.1% of MI patients.7 Furthermore, for depression to be causally related to the CHD incidence and prognosis, numerous clinical studies have demonstrated that depression is a “risk factor” rather than just a “risk marker.”8–11 However, despite ongoing efforts to improve the identification and treatment of depression, the association is still not explicable by known covariates.12
However, this relationship has not been investigated by monitoring the CHD risk scores of individuals. A large proportion of patients who suffer sudden cardiac death or nonfatal MI do not experience prior symptoms: indeed, as many as 50% of MIs occur in persons without a known history of symptomatic CHD. Therefore, it is important to identify individuals at risk of coronary events before they develop clinical symptoms. Regularly estimated CHD risk scores using biochemical data can influence clinical decisions about the type and intensity of therapies. Furthermore, a study of the main factors associated with CHD risk and their relationship to depression could solve the difficulty in identifying patients who ultimately suffer cardiovascular events.
In this study, we investigated whether depression can influence the movement of a low-risk CHD score population to an intermediate- or high-risk CHD score population using a population-based cohort design.
Materials and methods
Study population
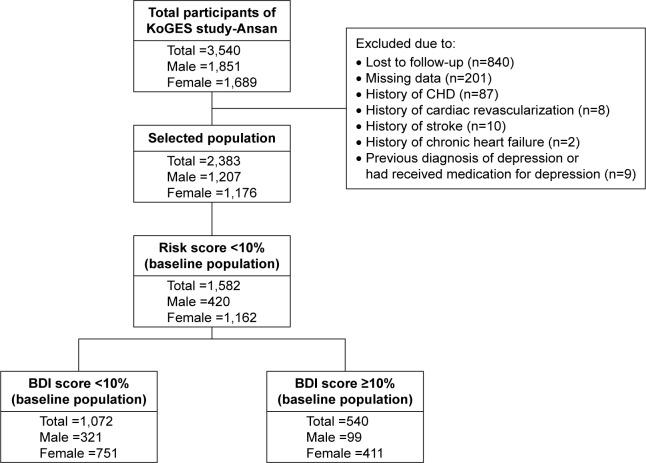
This population-based longitudinal study was part of the community-based Korean Genome Epidemiology Study (KoGES)-Ansan.13 This investigation was initiated in 2001 with the support of the Korean National Institute of Health. Our data accessed from the KoGES were freely available if researchers submitted an appropriate Institutional Review Board clearance to the Korea Centers for Disease Control and Prevention. The study protocol was approved by the Institutional Review Board of Seoul National University (IRB No E-1606-003-001). A baseline examination was performed on enrolled participants in 2001–2002, and biennial follow-up examinations were subsequently conducted. This study focused on 3,540 participants (age range 40–79 years) who had answered a questionnaire about their depression status in 2005. This retrospective cohort study included participants enrolled from 2005 to 2006 and followed until the fourth follow-up (8 years). Two thousand three hundred and eighty-three cohort members aged 40–79 years were analyzed after exclusion of 1,157 subjects who were lost to follow-up (n=840); had missing data (n=201); had a history of CHD (n=87), cardiac revascularization (n=8), stroke (n=10), or chronic heart failure (n=2); or had a previous diagnosis of depression or had been medicated for depression (n=9). Finally, we selected participants whose Framingham CHD risk score was in the low-risk score group at baseline (n=1,582; Figure 1), and we analyzed whether these participants moved to the intermediate or high CHD risk score group after the fourth follow-up.
Figure 1.
Flow chart of study participants.
Abbreviations: BDI, Beckman depression inventory; CHD, coronary heart disease; KoGES, Korean Genome Epidemiology Study.
Measurements
Demographic variables
Data in this study were from the KoGES. For assessment of cardiovascular risk, resting blood pressure (BP) was measured twice by trained technicians, and body mass index (BMI; kg/m2) was calculated. Serum high-density lipoprotein cholesterol (HDL), total cholesterol, and hemoglobin A1c (HbA1c) were determined from blood samples. Demographic, medical history (including brief medication information), health conditions, and family history of disease information were collected using interviewer-administered questionnaires.
Additionally, information about smoking (pack-year), alcohol (g/d), education status, annual income, and menopause status were collected. All examinations were administered by health professionals trained to follow standardized protocols.14
Assessment of depression
The Beck depression inventory (BDI), which has a 21-item self-report inventory, was used to assess the severity of depressive symptoms. The BDI is a well-validated measure of depressive symptoms and has high sensitivity and specificity for diagnosis of depression.15,16 Self-evaluative statements in the questionnaires were graded to measure the severity of depressive symptoms. Total scores on the BDI can range from 0 to 63, with higher scores reflecting greater levels of depressive symptoms. The presence of depression was defined by a BDI score ≥10.15
Calculation of CHD risk score
Estimated 10-year CHD risk score was calculated using Framingham risk scores.17 This calculation was based on the study of cardiovascular risk profile detailed in the online Framingham Heart Study. The following variables were used: gender, age, systolic blood pressure (SBP), HDL, total cholesterol, hypertension treatment (yes or no), and smoking (yes or no). Individuals were allocated points from each category, and then their 10-year Framingham CHD scores were calculated from their total points. We defined the intermediate or high CHD risk score cut-off point as a Framingham CHD score ≥10.18,19
Statistical analyses
The main outcome measure was the dichotomous variable indicating whether CHD risk score status of an individual changed to intermediate- or high-risk score status after 8 years. Descriptive values are presented as means (SD) for continuous variables and proportions for categorical variables. Comparisons of demographic variables were performed using Student t-tests for continuous variables and χ2 or Fisher’s exact tests for categorical variables. Multivariate logistic regression analyses to determine whether the low CHD risk score group of subjects changed to the intermediate or high CHD risk score group according to depression status was performed by analysis of the covariates: age, SBP, HbA1c, HDL, total cholesterol, smoking, BMI, alcohol, education, and annual income. Covariates for the multivariate model were selected on the basis of univariate correlation analysis and clinical relevance. In terms of covariates used in our multivariate logistic regression analysis, our outcome was whether participants moved to the intermediate or high CHD risk score group, which is not a Framingham CHD risk score itself. Furthermore, the risk of being in the intermediate- or high-score group after 8 years could be affected by the baseline Framingham risk score. Therefore, we adjusted for individuals’ risk factors in the baseline period that were used in calculating the baseline Framingham CHD risk score. Variables were reduced using backward elimination at α=0.15.20,21 Data were analyzed using the IBM SPSS statistical package (version 23.0; IBM Corp., Armonk, NY, USA). Probability values P<0.05 (two-sided) were considered statistically significant.
Results
Demographic data
Data from 1,582 subjects aged 43–73 years, including 420 men and 1,162 women, were analyzed in this study. Demographic and clinical characteristics of the study participants according to the presence of depression are listed in Table 1. The mean age of all participants was 51.1±6.67 years. The mean ages of men with (BDI ≥10) and without (BDI <10) depression were 48.5 and 48.0 years, respectively, and were not significantly different (P=0.249); however, the mean ages of women with and without depression were significantly different at 53.4 and 51.6 years, respectively (P<0.001). The overall mean BDI scores were 7.81±6.68 (range 0–42), while scores for men with and without depression were 14.2 and 3.6, respectively, and the corresponding scores for women were 15.8 and 4.4, respectively. The mean Framingham CHD risk scores in men were 5.3% and 5.0% in those with and without depression, respectively (P=0.215), and were higher than those in women (1.7% and 1.5% for those with and without depression, respectively; P=0.014). The prevalence of menopause was higher in women with depression (60.6%) than in women without depression (46.5%; P<0.001).
Table 1.
Characteristics of the study population
| Variables | Men (N=420)
|
Women (N=1,162)
|
||||
|---|---|---|---|---|---|---|
| BDI <10 (N=321) | BDI ≥10 (N=99) | P-value | BDI <10 (N=751) | BDI ≥10 (N=411) | P-value | |
| BDI score | 3.6 (2.8) | 14.2 (5.2) | <0.001 | 4.4 (2.7) | 15.8 (6.1) | <0.001 |
| Age (years) | 48.0 (3.8) | 48.5 (4.6) | 0.249 | 51.6 (6.86) | 53.4 (7.34) | <0.001 |
| SBP (mmHg) | 113.3 (12.7) | 108.6 (16.3) | 0.003 | 108.0 (14.9) | 110.0 (14.4) | 0.09 |
| HbA1c (%) | 5.4 (0.6) | 5.5 (1.2) | 0.291 | 5.5 (0.8) | 5.5 (0.6) | 0.9 |
| HDL (mg/dL) | 44.7 (11.5) | 44.5 (11.6) | 0.929 | 47.0 (10.3) | 47.4 (9.9) | 0.563 |
| Total cholesterol (mg/dL) | 181.9 (31.5) | 177.2 (34.5) | 0.200 | 196.3 (34.6) | 199.5 (34.4) | 0.128 |
| Smoking (pack-year) | 8.7 (13.0) | 12.8 (15.5) | 0.009 | 0.1 (1.0) | 0.2 (1.7) | 0.320 |
| BMI (kg/m2) | 24.3 (3.3) | 23.9 (3.8) | 0.257 | 24.5 (3.4) | 24.4 (3.0) | 0.594 |
| Alcohol (g/d) | 17.2 (24.0) | 18.5 (26.0) | 0.650 | 1.4 (5.3) | 1.4 (5.2) | 0.984 |
| Education | ||||||
| Not high-school graduate (n, %) | 13 (4.0) | 12 (12.1) | 0.002 | 132 (17.6) | 92 (22.4) | <0.001 |
| High-school graduate (n, %) | 183 (57.0) | 65 (65.7) | 505 (67.2) | 291 (70.8) | ||
| College degree (n, %) | 125 (39.0) | 22 (22.2) | 114 (15.2) | 28 (6.8) | ||
| Annual income (US$) | ||||||
| <2,400 (n, %) | 122 (38.0) | 49 (49.5) | 0.001 | 414 (55.1) | 297 (72.3) | <0.001 |
| 2,400–3,600 (n, %) | 95 (29.6) | 29 (29.3) | 168 (22.4) | 58 (14.1) | ||
| >3,600 (n, %) | 104 (32.3) | 21 (21.2) | 169 (22.5) | 56 (13.6) | ||
| Menopause (n, %) | 349 (46.5) | 249 (60.6) | <0.001 | |||
| Framingham CHD risk score (%) | 5.0 (2.2) | 5.3 (2.3) | 0.215 | 1.5 (1.2) | 1.7 (1.4) | 0.014 |
Notes: Data are expressed as mean (SD) for continuous variables, or numbers (percentages) for categorical parameters. Variables were compared using the χ2 test.
Abbreviations: BDI, Beck depression inventory; BMI, body mass index; CHD, coronary heart disease; HbA1c, hemoglobinA1c; HDL, high-density lipoprotein; SBP, systolic blood pressure.
Changes in Framingham CHD risk scores
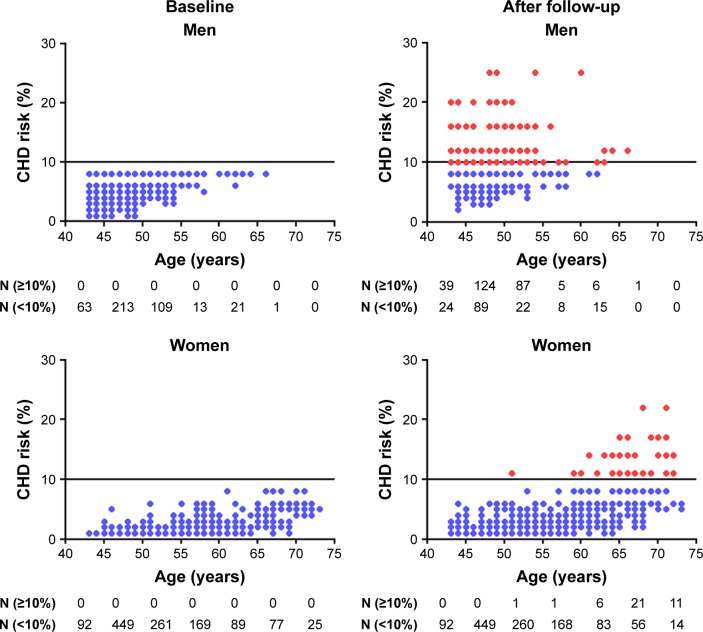
Changes in Framingham CHD risk score between baseline and 8-year follow-up according to age and gender of cohort participants are shown in Figure 2. At baseline, the number of participants with Framingham CHD risk scores <10% were lower among men (n=420) than women (n=1,162) for all age groups. The number of men older than 55 years was lower than that of men less than 55 years old at baseline because many were excluded as part of the study criteria (low Framingham risk score group). In contrast, women had relatively low risk score status even when they were older than 70 years. After the 8-year follow-up period, CHD risk scores became higher in men, regardless of their age; however, in women, CHD risk score status began to alter when they reached ages above 55 years.
Figure 2.
Change of CHD risk scores between baseline and 8-year follow-up in men and women.
Notes: A horizontal line indicates the Framingham CHD risk score with 10. ([img], low-risk group; [img], intermediate or high-risk group).
Abbreviation: CHD, coronary heart disease.
Association between depression and cardiac risk score
The results of univariate- and multivariate-adjusted analyses of the relationship between BDI and Framingham CHD risk score status are listed in Table 2. Univariate analysis demonstrated that both men (odds ratio [OR], 1.41; 95% CI, 1.05–1.78) and women (OR, 1.74; 95% CI, 1.37–2.12) exhibited an increased likelihood of being in the intermediate or high CHD risk score group according to their BDI category. After the univariate analysis, age, SBP, HbA1c, and smoking were associated with Framingham CHD risk scores in men, while age, SBP, HbA1c, HDL, smoking, BMI, education, annual income, and menopause status were associated with Framingham CHD risk scores in women.
Table 2.
Logistic regression analysis of the association between depression and change of CHD risk score status
| Variable | Men (n=420)
|
Women (n=1,162)
|
||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
| BDI | ||||
| Control | Ref | Ref | Ref | Ref |
| Depression | 1.41 (1.05–1.78) | 1.38 (0.95–1.82) | 1.74 (1.37–2.12) | 1.54 (1.08–2.03) |
| Age | ||||
| Per 10 years | 2.16 (1.58–2.77) | 3.40 (2.57–4.29) | 4.52 (3.59–5.53) | 4.53 (3.50–5.64) |
| SBP | ||||
| Per 10 mmHg | 1.17 (1.02–1.32) | 1.32 (1.13–1.51) | 1.42 (1.24–1.61) | 1.41 (1.12–1.71) |
| HbA1c | ||||
| <6.5% | Ref | Ref | Ref | Ref |
| ≥6.5% | 1.46 (1.59–3.61) | 0.96 (0.33–2.75) | 4.59 (2.02–10.40) | 1.97 (0.69–5.61) |
| HDL | ||||
| Per 10 mg/dL | 0.85 (0.97–1.01) | 0.47 (0.26–0.69) | 0.54 (0.21–0.87) | 0.44 (0.02–0.80) |
| Total cholesterol | ||||
| Per 10 mg/dL | 1.00 (0.99–1.01) | – | 1.01 (0.92–1.10) | – |
| Smoking | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 4.18 (2.78–6.30) | 5.77 (2.86–11.62) | 5.61 (1.57–20.08) | 9.66 (1.39–67.20) |
| BMI | ||||
| Per 1 kg/m2 | 1.04 (0.98–1.10) | – | 1.16 (1.06–1.27) | 1.10 (0.96–1.26) |
| Alcohol | ||||
| No | Ref | – | Ref | – |
| Yes | 1.39 (0.90–2.13) | – | 0.54 (0.23–1.31) | – |
| Education | ||||
| Not high-school graduate | Ref | – | Ref | Ref |
| High-school graduate | 0.98 (0.26–3.73) | – | 0.59 (0.20–1.69) | 0.64 (0.17–2.43) |
| College degree | 0.71 (0.48–1.07) | – | 0.17 (0.06–0.49) | 0.42 (0.03–5.74) |
| Annual income (US$) | ||||
| <2,400 | Ref | – | Ref | Ref |
| 2,400–3,600 | 0.98 (0.26–3.73) | – | 0.17 (0.09–0.33) | 0.81 (0.35–1.88) |
| >3,600 | 0.71 (0.48–1.07) | – | 0.03 (0.01–0.25) | 0.56 (0.06–5.67) |
| Menopause | ||||
| No | – | – | Ref | Ref |
| Yes | – | – | 39.28 (5.37–286.88) | 1.05 (0.12–9.33) |
Abbreviations: BDI, Beck depression inventory; BMI, body mass index; CHD, coronary heart disease; HbA1c, hemoglobinA1c; HDL, high-density lipoprotein; OR, odds ratio; SBP, systolic blood pressure.
After multivariate adjustment, BDI category was not significantly associated with being intermediate or high CHD risk score group in men (adjusted OR, 1.38; 95% CI, 0.95–1.82). In contrast, BDI category was statistically significantly associated with belonging to the intermediate or high CHD risk score group, after adjustment for age, SBP, HDL, and smoking in women (adjusted OR, 1.54; 95% CI, 1.08–2.03).
Discussion
To the best of our knowledge, this is the first report to demonstrate that depression is associated with a risk of moving from a low-risk CHD group to an intermediate- or a high-risk CHD group. The CHD risk score is reliable and has been used in numerous observational studies.22 The variables for CHD risk prediction can be obtained using blood samples and questionnaire information. Additionally, BDI scores have been used in many studies and have been shown to have good test–retest reliability and validity.23–25 By analyzing and adjusting for each risk factor, we could identify to what extent each risk factor was correlated with the BDI scores. Additionally, through the analysis of these robust data, we assessed the overall association between CHD risk score and depression.
The prevalence of depression in Korea ranges from 4.3% to 9.1%, while that of depressive symptoms is between 9.1% and 33.0%.26 In our study population, depression (BDI ≥10) occurred in 23.6% (99 of 420) and 35.4% (411 of 1,162) of the men and women with low CHD risk scores, respectively. Consistent with a previous report,27 we found that women had inferior depression scores to men. In general, the CHD incidence rate in men is higher than that in women.28 In agreement with this, our results indicate that the baseline CHD risk scores of men (5.1%±0.1%) were higher than those of women (1.6%±0.4%). In this study, women older than 55 years showed a change in the CHD risk score status. In the Adult Treatment Panel III, researchers also reported an age of 55 years or older as a positive risk factor for their intermediate- or high-risk score status in women.29 In our study, the incident rate for a change in the CHD risk score status was 62.4% (262 of 420) in men and 3.4% (40 of 1,162) in women after the 8-year follow-up period.
Depression increased the likelihood of women, but not men, being in an either the intermediate or high CHD risk score group after the follow-up period. In terms of covariates used in our multivariate logistic regression analysis, our outcome was whether participants moved to the intermediate or high CHD risk score group, which is not a Framingham CHD risk score itself. Furthermore, the risk of being in the intermediate- or high-score group after 8 years could be affected by the baseline Framingham risk score. Therefore, we adjusted for individuals’ risk factors in the baseline period that were used in calculating the baseline Framingham CHD risk score. However, because of the large effect of age on the Framingham CHD risk score,30,31 the effect of depression may have been downsized. One study did an age-grouped analysis to solve this problem.32 Therefore, we did a subgroup analysis in men older than 45 years and in women older than 55 years. The age cutoff was determined in the Adult Treatment Panel III final report.29 The result shows that the BDI category still has a significant value in terms of belonging to the intermediate or high CHD risk score group after adjusting for age, SBP, HbA1c, and smoking in women (adjusted OR, 2.11; 95% CI, 1.06–4.19) but not in men (adjusted OR, 1.26; 95% CI, 0.73–2.19).
There have been several reports regarding the differences in CHD outcomes between men and women with CVD and comorbid depression. Women were reported to be susceptible to CHD when depressed;10 however, the Danish Glostrup study found that gender did not affect the association of increased depression with increased risk of MI and mortality, despite the prevalence of depression among women being higher than that among men.8,33 Another study showed that depressed women did not have a significant increase in risk, while depressed men did for CHD mortality.9 This difference could be due to some unknown physiological disorders that may occur due to depression.27 These disorders can cause autonomic dysfunction, hypercortisolemia, elevated catecholamines and inflammatory markers, endothelial dysfunction, and platelet activation.34 They are also related to the hyperactivity of the hypothalamic–pituitary–adrenal axis and defective serotonin signaling, which result in the dysfunction of the amygdala.34
Generally, the effects of depression can be revealed by examining the behavior of individuals as well as by their physiological measures. In our population, depressed men were more likely to have a higher SBP and smoking than that of the nondepressed men at baseline. Men have been reported to be more likely than women to show changes in traditional physiological measures, such as a high BP, and depression in men has a key role in cigarette smoking.35,36 After the follow-up period, SBP and smoking were also associated with the change in CHD risk score status, regardless of gender, similar to a number of previous reports.35–37 Menopause is an important physiological factor because of its hormonal effects in women; however, menopause was not significantly associated with belonging to the intermediate or high CHD risk score group after a multivariate analysis. We did an additional subgroup analysis of postmenopausal women, and depression was significantly related to being in the intermediate or high CHD risk score group (adjusted OR, 1.69; 95% CI, 1.17–2.23) after adjusting for age, SBP, HDL, and smoking. In another study of postmenopausal women, depression was also significantly associated with an increased risk of cardiovascular death and all-cause mortality.38 Education and annual income were different between the low and high BDI groups; however, they were not associated with the change in CHD risk score status. Another study of depressed people also found no relationship between socioeconomic status and CHD outcomes.39
This study has several limitations. First, we controlled for baseline risk factor status and lifestyle variables that affected the CHD risk score in our analyses; additionally, we had no data on adherence to medication. It is possible that participants who reported depressive symptoms were less adherent to their medication, which would put them at a greater risk of CHD events. Further study is required regarding how antidepressants and medication compliance affect the change in CHD risk score status. Additionally, there was a study showing that the Framingham risk function overestimates the risk of CHD in the Korean population, in which the CHD incidence is low;30 however, this method of scoring has been the basis of CHD risk assessment in numerous clinical settings including Korea.18,32
The strengths of this study include the use of cohort data with a large sample size as well as a relatively long follow-up period. We were also able to analyze data separately for women and men. Of particular interest, the results of this study indicate that although other variables are already used to estimate the CHD risk score, depression also affects the likelihood of developing an intermediate or high risk of CHD, especially among women, indicating that it is a significant relevant risk factor. Another strength of this study was our ability to control for numerous cardiac risk factors and demographic variables.
Conclusion
Our results suggest that clinical depression can lead to a change in CHD risk score status in Korean women. These findings may be useful for determining appropriate disease management or therapeutic lifestyle changes of depressed populations. The assessment, treatment, and monitoring each risk factors will have an important effect on clinical care, research investigation, and treatment guidelines. In addition, research into whether changes to intermediate or high CHD risk score status are reversible through pharmacologic or psychotherapeutic interventions is required.
Acknowledgments
Data in this study were from the Korean Genome and Epidemiology Study (4851-302), National Research Institute of Health, Centres for Disease Control and Prevention, Ministry for Health and Welfare, Republic of Korea.
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC15C1045).
Footnotes
Author contribution
HYJ contributed to the study concept and design, managed KoGES data, performed statistical analyses, and drafted and revised the manuscript. YKS, JHK, MGK, and NH contributed to the study concept and design, analysis and interpretation of the data, and performed critical revision of the manuscript for important intellectual content. HYL performed the critical revision of the manuscript for important intellectual content. JMO and IWK performed critical revision of the manuscript for important intellectual content and supervised data analysis. All authors contributed toward data analysis and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization . The Global Burden of Disease: 2004 Update. Geneva: WHO Press; 2008. [Google Scholar]
- 2.Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry. 2007;29(5):409–416. doi: 10.1016/j.genhosppsych.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Colquhoun DM, Bunker SJ, Clarke DM, et al. Screening, referral and treatment for depression in patients with coronary heart disease. Med J Aust. 2013;198(9):483–484. doi: 10.5694/mja13.10153. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27(23):2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 5.Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. 2014;35(21):1365–1372. doi: 10.1093/eurheartj/eht462. [DOI] [PubMed] [Google Scholar]
- 6.Cay EL, Vetter N, Philip AE, Dugard P. Psychological status during recovery from an acute heart attack. J Psychosom Res. 1972;16(6):425–435. doi: 10.1016/0022-3999(72)90068-2. [DOI] [PubMed] [Google Scholar]
- 7.Thombs BD, Bass EB, Ford DE, et al. Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med. 2006;21(1):30–38. doi: 10.1111/j.1525-1497.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ariyo AA, Haan M, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102(15):1773–1779. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 9.Ferketich AK, Schwartzbaum JA, Frid DJ, Moeschberger ML. Depression as an antecedent to heart disease among women and men in the NHANES I study. Arch Intern Med. 2000;160(9):1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- 10.Hybels CF, Pieper CF, Blazer DG. Sex differences in the relationship between sub-threshold depression and mortality in a community sample of older adults. Am J Geriatr Psychiatry. 2002;10:283–291. [PubMed] [Google Scholar]
- 11.Unutzer J, Patrick DL, Marmon T, Simon GE, Katon WJ. Depressive symptoms and mortality in a prospective study of 2,558 older adults. Am J Geriatr Psychiatry. 2002;10(5):521–530. doi: 10.1097/00019442-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Tully PJ, Baumeister H. Collaborative care for comorbid depression and coronary heart disease: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2015;5(12):e009128. doi: 10.1136/bmjopen-2015-009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H, Yun CH, Thomas RJ, et al. Obstructive sleep apnea as a risk factor for cerebral white matter change in a middle-aged and older general population. Sleep. 2013;36(5):709B–715B. doi: 10.5665/sleep.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YH, Kim SH, Lim SY, et al. Relationship between depression and subclinical left ventricular changes in the general population. Heart. 2012;98(18):1378–1383. doi: 10.1136/heartjnl-2012-302180. [DOI] [PubMed] [Google Scholar]
- 15.Beck AT. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 16.Ohayon MM, Hong SC. Prevalence of insomnia and associated factors in South Korea. J Psychosom Res. 2002;53:593–600. doi: 10.1016/s0022-3999(02)00449-x. [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 18.Kim WJ, Kwon CH, Han S, et al. Role of coronary artery calcium scoring in detection of coronary artery disease according to Framingham risk score in populations with low to intermediate risks. J Korean Med Sci. 2016;31(6):902–908. doi: 10.3346/jkms.2016.31.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryoo JH, Ha EH, Kim SG, Ryu S, Lee DW. Apolipoprotein B is highly associated with the risk of coronary heart disease as estimated by the Framingham risk score in healthy Korean men. J Korean Med Sci. 2011;26(5):631–636. doi: 10.3346/jkms.2011.26.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 21.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 22.Bitton A, Gaziano TA. The Framingham Heart Study’s impact on global risk assessment. Prog Cardiovasc Dis. 2010;53(1):68–78. doi: 10.1016/j.pcad.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim NH, Kim HC, Lee JY, Lee JM, Suh I. Association between environmental tobacco smoke and depression among Korean women. BMJ Open. 2015;5(6):e007131. doi: 10.1136/bmjopen-2014-007131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1997;1:385–401. [Google Scholar]
- 25.Sun XY, Li YX, Yu CQ, Li LM. Reliability and validity of depression scales of Chinese version: a systematic review. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2017;38(1):110–116. doi: 10.3760/cma.j.issn.0254-6450.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Cho MJ, Seong SJ, Park JE, et al. Prevalence and correlates of DSM-IV mental disorders in South Korean adults: the Korean epidemiologic catchment area study 2011. Psychiatry Investig. 2015;12(2):164–170. doi: 10.4306/pi.2015.12.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moller-Leimkuhler AM. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin Neurosci. 2007;9(1):71–83. doi: 10.31887/DCNS.2007.9.1/ammoeller. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation. 2011;124(19):2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 30.Jee SH, Jang Y, Oh DJ, et al. A coronary heart disease prediction model: the Korean Heart Study. BMJ Open. 2014;4(5):e005025. doi: 10.1136/bmjopen-2014-005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex differences in age-related cardiovascular mortality. PLoS One. 2013;8(5):e63347. doi: 10.1371/journal.pone.0063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karmali KN, Goff DC, Jr, Ning H, Lloyd-Jones DM. A systematic examination of the 2013 ACC/AHA pooled cohort risk assessment tool for atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2014;64(10):959–968. doi: 10.1016/j.jacc.2014.06.1186. [DOI] [PubMed] [Google Scholar]
- 33.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93(11):1976–1980. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 34.Bradley SM, Rumsfeld JS. Depression and cardiovascular disease. Trends Cardiovasc Med. 2015;25(7):614–622. doi: 10.1016/j.tcm.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Korhonen T, Broms U, Varjonen J, et al. Smoking behaviour as a predictor of depression among Finnish men and women: a prospective cohort study of adult twins. Psychol Med. 2007;37(5):705–715. doi: 10.1017/S0033291706009639. [DOI] [PubMed] [Google Scholar]
- 36.Samad Z, Boyle S, Ersboll M, et al. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J Am Coll Cardiol. 2014;64(16):1669–1678. doi: 10.1016/j.jacc.2014.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCaffery JM, Papandonatos GD, Stanton C, Lloyd-Richardson EE, Niaura R. Depressive symptoms and cigarette smoking in twins from the National Longitudinal Study of Adolescent Health. Health Psychol. 2008;27(3S):S207–S215. doi: 10.1037/0278-6133.27.3(suppl.).s207. [DOI] [PubMed] [Google Scholar]
- 38.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI) Arch Intern Med. 2004;164(3):289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 39.Liu RT, Hernandez EM, Trout ZM, Kleiman EM, Bozzay ML. Depression, social support, and long-term risk for coronary heart disease in a 13-year longitudinal epidemiological study. Psychiatry Res. 2017;251:36–40. doi: 10.1016/j.psychres.2017.02.010. [DOI] [PubMed] [Google Scholar]