Abstract
Background
Cancer-related cognitive impairment (CRCI) is often related to chemotherapy. Increased chronic inflammation is believed to play a key role in the development of CRCI related to chemotherapy but studies assessing this hypothesis specifically in patients receiving chemotherapy are rare.
Methods
We assessed several cognitive domains using the Cambridge Neuropsychological Test Automated Battery (CANTAB) in twenty-two breast cancer patients currently receiving chemotherapy. We also measured inflammatory cytokine and receptor (MCP-1, TNF-α, sTNFRI, sTNFRII) concentrations in patient sera using Luminex assays. These concentrations were log-transformed to obtain a normal distribution. Associations between log-transformed cytokines and cognition were evaluated using Pearson correlations and linear regression, taking into account relevant covariates.
Results
Increased concentrations of sTNFRI and sTNFRII were associated with poorer performance on the CANTAB Delayed Matching to Sample (DMS, tests visual memory). Increasing sTNFRI levels were negatively correlated with DMS percent correct (r=−0.47, p=0.029) and DMS percent correct after a 12 second (s) delay (r=−0.65, p=0.001). Increasing levels of sTNFRII negatively correlated with DMS percent correct after 12s delay (r=−0.57, p=0.006). After controlling for relevant demographic (i.e. age, education) and clinical variables (i.e. disease stage, regimen type), we found that increased sTNFRI remained significantly related to decline on the DMS at the 12 second delay (p=0.018).
Conclusion
This preliminary study shows a significant association between higher sTNFRI and lower scores on the short-term visual memory delayed match to sample test in breast cancer patients receiving chemotherapy, supporting the hypothesis that sTNFRI is involved in CRCI.
Keywords: breast cancer, chemotherapy, cancer-related cognitive impairment, inflammation, cytokines, cognitive testing
Introduction
Cancer-related cognitive impairment (CRCI) involves impairments in cognitive domains of memory, attention, executive functioning, and processing speed (reviewed in Wefel et al., 2015). CRCI has been reported to have a significant impact on quality of life, disrupting daily functioning as well as self-esteem, self-confidence, and perceived work ability of cancer survivors (Reid-Arndt et al., 2009, Von Ah et al., 2013, Wefel et al., 2004). While CRCI is likely to have a complex etiology, chemotherapy is most likely to initiate and exacerbate these problems, with up to 75% of patients reporting some kind of impairment during these treatments (reviewed in Janelsins et al., 2017, Janelsins et al., 2014) Several prospective longitudinal studies evaluating cognitive function prior to and up to one year after chemotherapy, have reported decreases in cognitive performance on tests of memory, executive function, processing speed, and attention. Approximately 17%–50% of breast cancer patients experience cognitive impairment for months or years after treatment completion (reviewed in Wefel et al., 2015). One study comparing the cognitive performance of breast cancer patients who had received adjuvant cyclophosphamide, methotrexate, and fluorouracil (CMF) chemotherapy found that these patients performed significantly worse than a healthy control group on cognitive testing that assessed immediate and delayed verbal memory, processing speed, executive functioning, and psychomotor speed, even 20 years after treatment (Koppelmans et al., 2012). With many patients surviving for decades following initial chemotherapy, mechanisms of cognitive impairment need to be investigated so that the etiology can be better understood, markers predictive of CRCI can be identified, and successful interventions can be developed.
The cognitive decline associated with chemotherapy is multifactorial, and likely involves diverse and complex biochemical pathways. While the etiology for the onset of CRCI is not clear, several hypothesized mechanisms have been proposed, including an inflammatory response resulting in altered circulating cytokine profiles. Cytokines are low molecular weight proteins and have specific soluble receptors that facilitate cellular communication through autocrine, paracrine, and endocrine mechanisms (Wilson et al., 2002). Chemotherapy, which interferes with cellular processes and cell division, can increase levels of cytokines during and after administration, and the extent to which this occurs may vary according to chemotherapy type (Janelsins et al., 2012, Mills et al., 2008, Pusztai et al., 2004). Janelsins et al. 2012 showed that anthracycline-containing regimens (i.e. doxorubicin plus cyclophosphamide (AC), or doxorubicin plus cyclophosphamide and fluorouracil (CAF)) are more inflammatory (increased interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and interleukin-8 (IL-8)) than non-anthracycline-containing regimens (i.e. cyclophosphamide, methotrexate, and fluorouracil (CMF)) (Janelsins et al., 2012).
Several cytokine or soluble receptors have been implicated in other neurocognitive disorders which has led to their investigation in relation to CRCI (Cheung et al., 2015, Ganz et al., 2013, Hayslip et al., 2015, Janelsins et al., 2014, Kesler et al., 2013, McAfoose and Baune, 2009, Patel et al., 2015, Reichenberg et al., 2001). Ganz et al. report higher soluble tumor necrosis factor receptor II (sTNFRII) levels in breast cancer patients post-chemotherapy treatment. The soluble receptor was significantly associated with increased complaints of memory dysfunction, and higher sTNFRII levels were also correlated with diminished brain metabolism in the inferior frontal cortex (Ganz et al., 2013). Kesler and colleagues showed that left hippocampal volumes and performance on memory testing were significantly negatively associated with elevated IL-6 and tumor necrosis factor alpha (TNF-α) concentrations in a cross-sectional study using high-resolution magnetic resonance images of 42 breast cancer patients post-chemotherapy (Kesler et al., 2013). Patel et al. also found an association between TNF and memory in patients prior to chemotherapy. However, further investigation into TNF cytokine/soluble receptors and cognition are needed, as sTNFRII levels were not greater in cancer patients compared to healthy control participants (Patel et al., 2015). These studies in breast cancer survivors post-treatment suggest that inflammation induced by chemotherapy may be a plausible avenue of investigation in understanding the etiology of CRCI and implicates cytokines/soluble receptors as possible contributors to CRCI. One recent study to date has assessed the relationships of cytokines (but not receptors) to CRCI in patients currently undergoing chemotherapy treatment (Lyon et al., 2016) and found that multiple cytokines may contribute to CRCI over time.
To acquire a greater understanding of the relationship of cytokine, chemokine, and relevant receptor levels and their relationship to cognitive function during chemotherapy, we designed a study evaluating the association of cytokines/receptors (sTNFRI, sTNFRII, TNF-α, MCP-1, IL-8, IL-6, and IL-6R) with cognitive performance during chemotherapy treatment in breast cancer patients receiving chemotherapy. These markers were chosen because previous studies have reported their association or implication with CRCI (Ganz et al., 2013, Janelsins et al., 2014). We hypothesized that greater serum concentrations of the cytokines and their soluble receptors would be associated with worse performance in cognitive testing across different domains of cognitive functioning: verbal recognition memory, visuospatial memory, and executive function, processing speed, and motor function. While the receptors themselves are not “inflammatory,” they may serve as markers to indicate inflammation and a relationship to cognitive decline, as preliminary data from the current literature suggests. Factors such as treatment type and disease stage, which may lead to varying levels of cytokine expression, as well as demographic factors such as education and age were also evaluated for their influence on inflammation and cognition.
Methods and Materials
Patient Population
Serum samples and cognitive data were collected from 22 female breast cancer patients (stages I-IV), currently receiving chemotherapy. These patients were enrolled in an ongoing Phase II clinical trial investigating interventions that may alleviate CRCI as well as the role of immune function (ClinicalTrials.Gov NCT01238120). Baseline (pre-intervention) data from the trial are presented herein. To be eligible, participants must have self-reported cognitive difficulties on a question adapted from the MD Anderson Symptom Inventory (Cleeland et al., 2000). Participants were asked “are you currently experiencing any cognitive problems (such as in your memory, attention, concentration, multi-tasking) since your cancer diagnosis?” Responses ranged from 0 (not present) to 10 (as bad as you can imagine). Participants with a score of 3 or greater were eligible for the study. Women currently receiving radiotherapy only, diagnosed with a neurodegenerative disease, metastatic disease to the central nervous system, or diagnosed with a major psychiatric illness within the last five years were ineligible. All women had a PET or CT scan that confirmed no brain metastases within 3 months of study enrollment (range 0 to 3 months). All proper IRB approvals were obtained, and patients were recruited from the James P. Wilmot Cancer Institute at the University of Rochester Medical Center (URMC). Cognitive assessments were performed during chemotherapy at cycle 2 or after (previous cycles were noted) and blood was drawn on the same day or within a week of the cognitive test. Cognitive assessments were done and blood was drawn prior to a chemotherapy session, never following the chemotherapy administration. Patients were actively undergoing chemotherapy treatments which were differentiated as either anthracycline-based or non-anthracycline-based.
Cytokine/Receptor Quantification
We measured the following cytokines and cytokine receptors from serum: IL-6, IL-6R, IL-8, MCP-1, TNF-α, sTNFRI, and sTNFRII. These markers were chosen because they have been implicated in CRCI (Cheung et al., 2015, Ganz et al., 2013, Hayslip et al., 2015, Janelsins et al., 2014, Kesler et al., 2013, McAfoose and Baune, 2009, Patel et al., 2015, Reichenberg et al., 2001). Serum samples collected from the 22 breast cancer patients were aliquoted into 1.5mL centrifuge tubes and stored in −80°C freezers until analysis. Samples were run in singlet and quantified using a BIOplex 200 (MCP-1, IL-6, IL-8, sTNFRI, and sTNFRII, URMC Human Immunology Core) or a Luminex Magpix (IL-6R, TNF-α, URMC Cancer Control and Psychoneuroimmunology Lab). The median of 50 beaded reactions per well was used to determine concentration per participant. Pre-mixed customized MILLIPLEX xMAP human cytokine and cytokine receptor immunoassay kits (catalog numbers: HSCRMAG-32K (IL-6R), HSCR-32K (sTNFRI, sTNFRII), HCYTOMAG-60K (MCP-1, TNF-α), HSCYTOMAG-60SK (IL-6, IL-8)) were used for the analysis and included beads, detection antibodies, standards, assay buffers, detection antibody diluents, and streptavidin-phycoerythrin.
Cognitive Assessment
The Cambridge Neuropsychological Test Automated Battery (CANTAB), a computer-based cognitive assessment consisting of five measures, was used to assess cognitive function. Tests included in the CANTAB battery used here assessed the domains that have been reported to be affected in cancer survivors including: visual memory, executive functioning, attention, verbal memory, and cognitive processing (Vardy et al., 2014, Vardy et al., 2015). Briefly, the first test was the latent motor screening test (MOT), which briefly screened motor function and speed. Second, the Delayed Matching to Sample (DMS) test was used to assess visual memory. The subject was shown a visual consisting of four patterns, each of a different multi-colored pattern, and then asked to identify the correct image from 4 possible selections either simultaneously or after a 0, 4, or 12s delay. Verbal recognition memory (VRM) tests were also administered. Different parameters on VRM tests were taken that measured free recall of correct words, recall of novel words (which indicated how many incorrect words the patients could recall). The Rapid Visual Information Processing (RVP) test evaluates visual sustained attention and processing speed through correct recognition of a number series. Executive function was examined using the One Touch Stockings of Cambridge (OTS) test, a spatial planning test which involved reorganizing balls within pockets to match a depicted organization. A complete description of each test measurement is provided in Table 2.
Table 2.
Description of study measures included in the CANTAB battery.
| Cognitive Domain (test) | Measure | Description | Directionality |
|---|---|---|---|
| (Motor Screening Task (MOT)) | Mean latency | Patients have to touch crosses that appear on the screen, latency is time take for the subject to touch the cross after it appeared. | Lower is better. |
| Mean error | Measure of the accuracy of the subject’s pointing. | Lower is better. | |
| Visual Memory (Delayed Matching to Sample Test (DMS)) | Percent correct | Percent of trials upon which a correct selection was made on the subject’s first response. | Higher is better. |
| Percent correct all delays, simultaneous, 0s, 4s, 12s, delays | Percentage of occasions upon which a subject selected the correct stimulus across all delays, the simultaneous, and 0s,4s,12s delays. | Higher is better. | |
| Mean correct latency | Mean latency from all trials where the subject selected the correct stimulus on their first response. | Lower is better. | |
| Mean correct latency all delays, simultaneous, 0s, 4s, 12s, delays | Mean latency from all trials where the subject selected the correct stimulus on their first choice after a delay. | Lower is better. | |
| Verbal Memory (Verbal Recognition Memory (VRM)) | Free recall of total correct words | Number of total correct words recalled from the list | Higher is better. |
| Free recall of total novel words | Number of total words recalled that are not on the list. | Lower is better. | |
| Free recall total perseverations | Number of total previously recalled words. | Lower is better. | |
| Recognition total correct | Number of words the subject correctly recognizes from the list. | Higher is better. | |
| Recognition total false positives | Number of times the subjects confirms the presence of a word that was not on the list. | Lower is better. | |
| Cognitive Processing (Rapid Visual Information Processing test (RVP)) | Total hits | Number of occasions upon which a target sequence is correctly responded to. | Higher is better. |
| Total misses | Number of occasions upon which a target sequence is not correctly responded to. | Lower is better. | |
| Probability of detecting target | Measure of how good the subject is at detecting target sequences. | Higher is better. | |
| Mean latency | Mean time taken to respond. | Lower is better. | |
| Executive function (One Touch Stockings of Cambridge test (OTS)) | Problems solved on first choice | Number of problem’s solved on the subject’s first choice. | Higher is better. |
| Mean choices to correct | Mean number of unique box choices that the subject made on each problem to make the correct choice. Lower is better. | Lower is better. | |
| Mean latency to correct | Mean time from appearance of pattern to correct choice. | Lower is better. | |
| Mean latency to first choice | Mean time from appearance of patter to first choice. | Lower is better. |
Statistical Analyses
SAS 9.4 (SAS Institute, Cary, NC) was used for all analyses. Cytokine concentrations were logarithmically-transformed to obtain a normal distribution to fit statistical test assumptions. With a relatively small sample size, potential influence of outliers (>3 SD from the mean) was also evaluated and none were found to be influential. Scatter plots were generated to ensure that cytokine and receptor levels would fit into a linear correlation model. Pearson correlations were then run on the log-transformed cytokine concentrations of patient sera and corresponding results from the CANTAB. Additionally, spearman rank correlations were performed on the untransformed cytokine concentrations, which showed similar results and are therefore not further presented here.
In bivariate analyses, age, education, disease stage, and chemotherapy regimen were correlated with some measures of cognitive function and therefore could be confounders of the association between inflammatory markers and cognitive function. To examine this possibility, five separate linear regression models were generated for each cytokine/receptor-test association that was statistically significant (Pearson correlation p<0.05): 1) a crude model with only the cytokine or receptor, 2) an age and education adjusted model, 3) a stage adjusted model, 4) a chemotherapy adjusted model (anthracycline use vs. not), and 5) an age, education, stage, and chemotherapy regimen adjusted model.
Results
Demographic Data and Cytokine and Receptor Measurements
Demographic and clinical data are presented in Table 1. Briefly, our subjects were 54 years of age on average (54.36±12.15 years), white (95.5%) and highly educated (86.3% with a 2 year-degree or more). The majority of subjects (9) were stage IV, with 5 stage III, 7 stage II and only 1 stage I. The distribution of anthracycline exposure was nearly even, with 57.1 percent of subjects on non-anthracycline based treatments. Overall descriptive statistics of cytokine and cytokine receptor concentrations can be found in Table 1.
Table 1.
Demographic and clinical characteristics of study participants (n=22) as well as descriptive statistics of cytokine and receptor concentrations.
| Characteristic | N (%) |
|---|---|
| Age (mean (S.D.)) | 54.36 (12.15) |
|
| |
| Ethnicity | |
| Caucasian | 21 (95.5) |
| Not Caucasian | 1 (4.5) |
|
| |
| Education1 | |
| No high school diploma or GED | 1 (4.8) |
| High school diploma or GED | 1 (4.8) |
| 2–4 year college degree | 12 (57.1) |
| 4 year college degree | 1 (4.8) |
| Graduate degree | 6 (28.5) |
|
| |
| Staging | |
| Stage I | 1 (4.6) |
| Stage II | 7 (31.8) |
| Stage III | 5 (22.7) |
| Stage IV | 9 (40.9) |
|
| |
| Treatment Regiment2 | |
| Non-anthracycline-based treatment | 12 (57.1) |
| Anthracycline-based treatment | 9 (42.9) |
|
| |
| Number of Previous Cycles (median (min, max)) | 2 (1,6) |
|
| |
| Cytokine concentration (median (IQR)) | Median (IQR) |
| MCP1 | 123.47 (364.11) |
| TNF-α | 6.43 (4.63) |
| sTNFRI | 1721.89 (708.83) |
| sTNFRII | 6815.29 (2975.38) |
One missing value for education,
one missing value for treatment regimen.
Cognitive Assessments
Figure 1a represents the mean and standard errors of the percent correct on the DMS at each delay. As expected, patients performed better during the simultaneous test compared to other delays. The mean percent correct increased slightly with increasing delay, however these differences were not significant (p=0.430), and participants remained below the simultaneous test performance at all delay times. The latency, or time between seeing the pattern and selecting the answer, increased with increasing delay which may account for this pattern of slightly increasing performance across all delays (Figure 1b). Overall, breast cancer patients performed in a similar pattern to a population of well-educated healthy adults from the same geographic area (van Wijngaarden et al., 2009).
Figure 1.
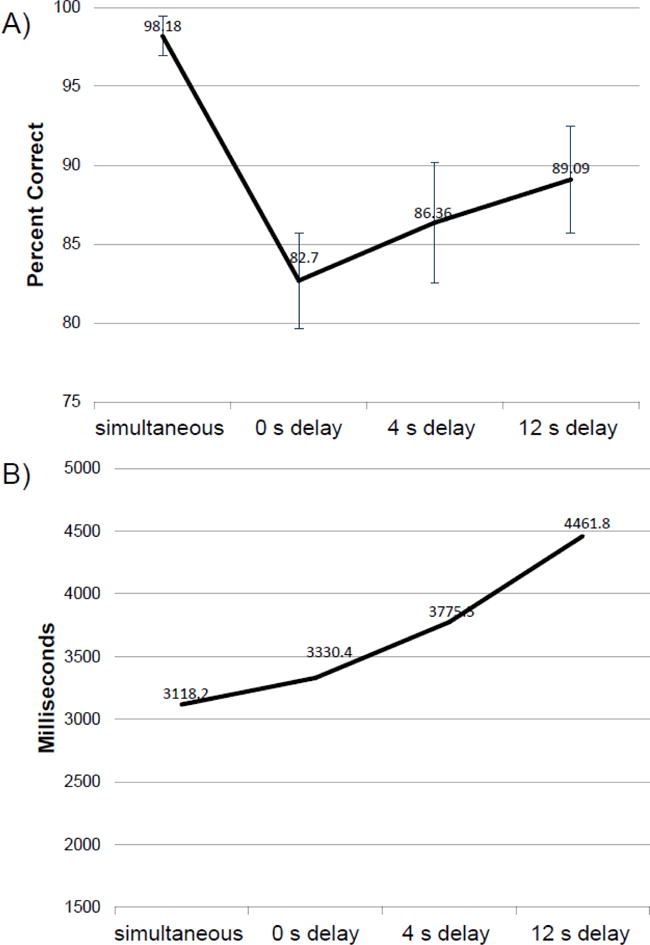
A) Mean and standard errors for the Delayed Matched to Sample Test across all delays. B) Mean latency for the Delayed Matched to Sample Test across all delays.
Associations among Cytokines, Soluble Receptors and Cognition
Correlation plots of logarithmically-transformed cytokines and receptors significantly correlated with worse cognitive function can be seen in Figure 2. Results from multivariable linear regression analyses examining potential covariates can be found in Table 3. Significant correlations between the Delayed Matching to Sample (DMS) test (visual memory) and sTNFRI and sTNFRII were observed. Pearson correlations of the logarithmically-transformed sTNFRI revealed significance for their negative association to DMS tests for percent correct (r=−0.47, p=0.029), percent correct after all delays (r=−0.48, p=0.025), and percent correct after 12s delay (r=−0.65, p=0.001). These relationships remained statistically significant after adjustment for stage or anthracycline exposure; however, only the association between sTNFRI and percent correct after 12s delay remained significant after adjustment for age and education (p=0.006) or adjustment for all covariables (age, education, stage, anthracycline exposure, p=0.018). sTNFRII was also significantly correlated with percent correct after 12s delay (r=−0.57, p=0.006) which remained after adjustment for anthracycline exposure (p=0.009), stage (p=0.011), and age and education (p=0.028). However, statistical significance was lost after adjustment for all covariates (p=0.065). We also found a significant association between higher levels of MCP-1 and shorter mean correct latency for the 4s delay (r=−0.51, p=0.015), that remained after adjustment for age and education (p=0.026) and anthracycline exposure (p=0.024), but not after adjustment for stage or all covariates.
Figure 2.
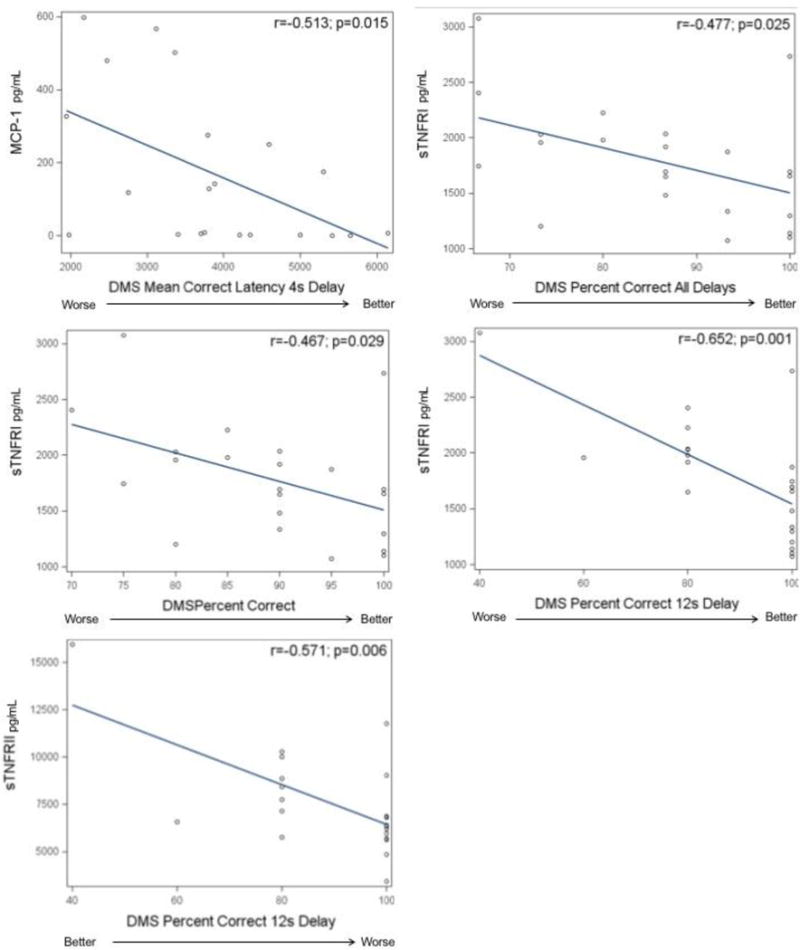
Correlations between select cytokines and receptors and worse scores on neuropsychological test measures.
Table 3.
Examining the influence of relevant demographic and clinical covariables on the associations between cytokine/receptors and cognitive tests. Results for independent multivariable linear regression models.
| Test measure (Outcome) | Cytokine or Soluble Receptor | Crude | Age and Education Adjusted1 | Stage Adjusted2 | Anthracycline Adjusted | Age, Education, Stage, and Anthracycline Adjusted1,2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p-value | β | p-value | β | p-value | β | p-value | β | p-value | ||
| Delayed Matching to Sample | |||||||||||
| Percent Correct | sTNFRI | −34.84 | 0.029 | −25.30 | 0.194 | −38.18 | 0.033 | −35.11 | 0.038 | −28.80 | 0.202 |
| Percent Correct All Delays | sTNFRI | −45.72 | 0.025 | −34.39 | 0.172 | −48.90 | 0.033 | −44.05 | 0.042 | −37.44 | 0.208 |
| Percent Correct 12s Delay | sTNFRI | −83.59 | 0.001 | −89.91 | 0.006 | −87.11 | 0.002 | −83.67 | 0.002 | −92.06 | 0.018 |
| Percent Correct 12s Delay | sTNFRII | −64.82 | 0.006 | −61.77 | 0.028 | −63.68 | 0.011 | −63.72 | 0.009 | −62.57 | 0.065 |
| Mean Correct Latency 4s Delay | MCP-1 | −573.54 | 0.015 | -738.72 | 0.026 | −548.45 | 0.052 | -599.83 | 0.024 | −725.85 | 0.063 |
| Rapid Visual Information Processing | |||||||||||
| Total Misses | TNF-α | −3.87 | 0.049 | -4.61 | 0.026 | −3.57 | 0.086 | −1.67 | 0.276 | −2.29 | 0.393 |
| Probability of detecting a Target | TNF-α | 0.040 | 0.037 | 0.047 | 0.024 | 0.039 | 0.054 | 0.021 | 0.193 | 0.03 | 0.258 |
| One Touch Stockings of Cambridge | |||||||||||
| Problems Solved on the First Choice | MCP-1 | 1.43 | 0.036 | 2.11 | 0.030 | 1.09 | 0.189 | 1.94 | 0.008 | 1.67 | 0.109 |
| Mean Choices to Correct | MCP-1 | −0.146 | 0.033 | −0.235 | 0.014 | −0.137 | 0.104 | −0.201 | 0.006 | −0.20 | 0.059 |
Age: age at enrollment,
Stage categorized as stage 4, stage 3, and stage 1 or 2.
Higher levels of MCP-1 were significantly associated with better performance on the One Touch Stockings of Cambridge test (executive function). MCP-1 was significantly correlated with problems solved on the first choice (r=0.45, p=0.036) and mean choices to correct (r=−0.46, p=0.033). These associations remained after adjustment for age and education (p=0.030 and p=0.014 respectively) and anthracycline exposure (p=0.008 and p=0.006 respectively), but not after adjustment for stage or all covariates together (see Table 3). Lastly, higher levels of TNF-α were associated with better performance on the Rapid Visual Processing test. TNF-α was significantly associated with the number of total misses (r=−0.42, p=0.049) and the probability of the correct choice (r=0.45, p=0.037), both of which remained statistically significant after adjustment for age and education (p=0.026 and p=0.024 respectively), but not after adjustment for stage, anthracycline exposure, or all covariates together. Interestingly, anthracycline exposure significantly, independently, predicted these associations (p=0.002 and p=0.008 respectively, data not shown). There were no other significant results for other measures in this study. In summary, the only statistically significant (p<0.05) association after adjustment for all covariates (age, education, stage, and anthracycline exposure) was between sTNFRI and the DMS percent correct 12s delay.
Discussion
In this pilot study, we showed an association between increasing sTNFRI concentrations and declines in short-term visual memory, even after adjustment for demographic and clinical covariables. We also report a similar trend for sTNFRII. This is the first study to find associations between these cytokine receptors and worse short-term visual memory in breast cancer patients currently receiving chemotherapy. This is an important finding as many studies investigating inflammation and cognition have focused on cancer survivors who have completed chemotherapy, yet many patients report more severe CRCI during chemotherapy, and CRCI is more prevalent during chemotherapy treatment. Examining the associations between inflammatory markers during chemotherapy may provide unique insights into potential mechanisms and guide intervention development.
Relatively few studies have examined the association between inflammatory markers and CRCI in breast cancer. A study of 174 breast cancer patients evaluated prior to chemotherapy reported that increased concentrations of sTNFRI and sTNFRII were associated with worse memory (Patel et al. 2015). Another study of 49 breast cancer patients evaluated after chemotherapy completion also found that elevated levels of sTNRII were associated with more memory complaints. Further, longitudinal data from these patients suggested that decreases in sTNFRII are associated with decreases in memory complaints (Ganz et al., 2013). Similarly, we reported that worse performance on visual memory was correlated with increased levels of sTNFRI and sTNFRII. Collectively, our results combined with these previous findings implicate sTNFRI and sTNFRII in memory deficits.
Although opposite of the hypothesized direction, we reported higher levels of MCP-1 were associated with better performance on tests of executive function and visual memory. Similarly, in a previous study of chemotherapy-treated breast cancer survivors, increased concentrations of MCP-1 were associated with fewer cognitive complaints (Janelsins et al., 2012). MCP-1 has been implicated in studies of mild cognitive impairment in Alzheimer’s patients (Galimberti et al., 2006, Stuart and Baune, 2014), therefore, it remains unclear why MCP-1 would be associated with better cognitive function. One possible hypothesis is that MCP-1 is shared risk factor for CRCI, possibly a mediator of additional inflammatory pathways influencing CRCI, or that some levels of MCP-1 may be beneficial for some domains of cognitive function. We also report increased concentrations of TNF-α were associated with better processing speed, however, anthracyline exposure was a strong independent predictor of the association between TNF-α and processing speed outcomes. The addition of anthracyline exposure to the model eliminated the significant effect of TNF-α and decreased its effect size by 56.8%. This may indicate that TNF-α may be a mediating factor in other inflammatory pathways stimulated by anthracycline exposure. The results of MCP-1 and TNF-α and better cognitive function should be interpreted with caution, as the association was no longer significant after adjustment for stage of disease.
Lastly, consistent with our results, several studies have reported no significant associations between IL-8 or IL-6 and CRCI and cognitive function in breast, colon, and hematological malignancy populations (Cheun, 2015, Vardy et al., 2014, Zimmer et al., 2015). Although our results add to the existing literature and build upon previous findings, they are preliminary and exploratory in nature. Future, larger, comprehensive studies of the association between pro-inflammatory markers and CRCI during chemotherapy treatment are needed to confirm these findings.
Clinical implications of this study and related studies in the future can provide the incipient measures in creating assessments that can predict the onset and development of CRCI across different demographic groups. Pronounced cognitive deficits are also seen in geriatric patients and thus, our results may be related to studies of aging and CRCI (Mandilaras et al., 2013, Williams et al., 2015). With a greater understanding of the association between inflammation and cognition across different age groups, races, and chemotherapy regimen, we may be able to eventually identify which chemokines, cytokines, or cytokine receptors may be useful in CRCI risk modeling.
Our results add to the literature supporting the hypothesis that pro-inflammatory pathways are involved in cognitive dysfunction (McAfoose and Baune, 2009, Reichenberg et al., 2001). While the majority of chemotherapeutic agents are not thought to cross an intact blood brain barrier due to their molecular size, they can indirectly culminate in toxicity of the brain by the penetration of cytokines that bind to endothelial receptors, eliciting inflammatory responses through oxidative and nitrosative processes, or other related pathways (Myers, 2008). Along with affecting neuronal and glial cell functioning, pro-inflammatory cytokines play a role in the metabolism of dopamine and serotonin, which are both vital neurotransmitters in the maintenance of cognitive function (Ahles and Saykin, 2007). We report here evidence supporting a role of pro-inflammatory pathways in impairment of the visual memory domain.
Our study has many strengths, including being one of the first studies to assess the association between multiple chemokines, cytokines, and soluble receptors and multiple measures of objective cognitive function in breast cancer patients currently receiving chemotherapy. This is also one of the first studies to assess levels of cytokines and their cognate receptors simultaneously. We also utilized a group of patients currently reporting cognitive difficulties which may have increased our ability to detect associations between CRCI and inflammation. Additionally, this is also one of the first studies of this type to take into account the influence of demographic and clinical covariables. However, there are a number of limitations; the primary ones are small sample size and diversity in stage of disease, education, and anthracycline exposure. Perhaps with a larger sample size we would have been able to detect significant influences of covariables on the associations between pro-inflammatory markers and CRCI. We also had a large number of stage IV patients. It is very unlikely that participants had brain metastases, as scans within 3 months of enrollment were negative for all participants, but it would have been ideal to have scans on all participants closer to the time of cognitive assessment. Additionally, measures of cognitive reserve or IQ were unavailable and could not be accounted for as a predictor in analyses. Importantly, this is a cross-sectional study; therefore, cause and effect cannot be established.
Future research studies need to assess these and other immune markers in larger prospective studies investigating breast cancer patients prior to, during, and after chemotherapy to better understand their anti- and pro-inflammatory processes and the role of inflammation in the trajectory of CRCI. These studies should be powered to examine the influence of demographic and clinical covariables, as the nature of CRCI is likely multi-factorial. In exploratory analyses we examined the correlations of each cytokine and each cognitive test scores with depression (using the Center for Epidemiologic Studies Depression scale) and saw no significant correlations. However, this observation needs to be interpreted cautiously due to the overall small sample size and further clarifies the need for studies that are powered to address the relationships between cytokines, cognition, and other psychosocial factors.
While the interaction of soluble receptors and cytokines still need to be understood in the context of inflammation, our results suggest that sTNFRI and sTNFRII are adversely associated with cognitive performance in sustained visual memory. As investigators continue to gather preliminary data on potential inflammatory markers and pathways involved in CRCI, further experimental, longitudinal studies need to be designed that not only attempt to elucidate physiological processes with more clarity, but can also take a more translational research approach, standardizing biomarkers of inflammation in order to evaluate risk factors and further investigate these mechanisms in animal models of CRCI.
Acknowledgments
The authors would like to thank the participating patients for making this study possible and the URMC Human Immunology Core and the Cancer Control and Psychoneuroimmunology Lab for analysis of the cytokines and their receptors. This study was funded by the NIH by grants DP2CA195765, K07CA168886, and UG1 CA189961.
Footnotes
The authors do not have any financial disclosures or conflicts of interest.
References
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nature reviews Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort studydagger. Ann Oncol. 2015;26:1446–51. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Bouwman F, Venturelli E, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Archives of neurology. 2006;63:538–43. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, et al. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(Suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayslip J, Dressler EV, Weiss H, Taylor TJ, Chambers M, Noel T, et al. Plasma TNF-alpha and Soluble TNF Receptor Levels after Doxorubicin with or without Co-Administration of Mesna-A Randomized, Cross-Over Clinical Study. PloS one. 2015;10:e0124988. doi: 10.1371/journal.pone.0124988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Heckler CE, Peppone LJ, Kamen C, Mustian KM, Mohile SG, et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. Journal of Clinical Oncology. 2017;35:506–14. doi: 10.1200/JCO.2016.68.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. International review of psychiatry (Abingdon, England) 2014;26:102–13. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Mustian KM, Palesh OG, Mohile SG, Peppone LJ, Sprod LK, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:831–9. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(Suppl):S109–16. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MM, Boogerd W, Seynaeve C, Gundy C, Schagen SB. Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:1080–6. doi: 10.1200/JCO.2011.37.0189. [DOI] [PubMed] [Google Scholar]
- Lyon DE, Cohen R, Chen H, Kelly DL, McCain NL, Starkweather A, et al. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. Journal of neuroimmunology. 2016;301:74–82. doi: 10.1016/j.jneuroim.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandilaras V, Wan-Chow-Wah D, Monette J, Gaba F, Monette M, Alfonso L. The impact of cancer therapy on cognition in the elderly. Frontiers in pharmacology. 2013;4:48. doi: 10.3389/fphar.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–66. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Ancoli-Israel S, Parker B, Natarajan L, Hong S, Jain S, et al. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain, behavior, and immunity. 2008;22:98–104. doi: 10.1016/j.bbi.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JS. Proinflammatory cytokines and sickness behavior: implications for depression and cancer-related symptoms. Oncology nursing forum. 2008;35:802–7. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]
- Patel SK, Wong AL, Wong FL, Breen EC, Hurria A, Smith M, et al. Inflammatory Biomarkers, Comorbidity, and Neurocognition in Women With Newly Diagnosed Breast Cancer. Journal of the National Cancer Institute. 2015;107 doi: 10.1093/jnci/djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25:94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Reid-Arndt SA, Yee A, Perry MC, Hsieh C. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. Journal of psychosocial oncology. 2009;27:415–34. doi: 10.1080/07347330903183117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart MJ, Baune BT. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neuroscience and biobehavioral reviews. 2014;42:93–115. doi: 10.1016/j.neubiorev.2014.02.001. [DOI] [PubMed] [Google Scholar]
- van Wijngaarden E, Campbell JR, Cory-Slechta DA. Bone lead levels are associated with measures of memory impairment in older adults. Neurotoxicology. 2009;30:572–80. doi: 10.1016/j.neuro.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy J, Dhillon HM, Pond GR, Rourke SB, Xu W, Dodd A, et al. Cognitive function and fatigue after diagnosis of colorectal cancer. Ann Oncol. 2014;25:2404–12. doi: 10.1093/annonc/mdu448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy JL, Dhillon HM, Pond GR, Rourke SB, Bekele T, Renton C, et al. Cognitive Function in Patients With Colorectal Cancer Who Do and Do Not Receive Chemotherapy: A Prospective, Longitudinal, Controlled Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33:4085–92. doi: 10.1200/JCO.2015.63.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. European journal of oncology nursing: the official journal of European Oncology Nursing Society. 2013;17:236–41. doi: 10.1016/j.ejon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123–38. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–9. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Williams AM, Janelsins MC, van Wijngaarden E. Cognitive function in cancer survivors: analysis of the 1999–2002 National Health and Nutrition Examination Survey. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2015 doi: 10.1007/s00520-015-2992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition–the case for a head-to-toe inflammatory paradigm. Journal of the American Geriatrics Society. 2002;50:2041–56. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Zimmer P, Mierau A, Bloch W, Struder HK, Hulsdunker T, Schenk A, et al. Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leukemia & lymphoma. 2015;56:347–52. doi: 10.3109/10428194.2014.915546. [DOI] [PubMed] [Google Scholar]


