Supplemental Digital Content is available in the text.
Key Words: neuropathic pain, spinal cord injury, clinical protocol, algorithms
Abstract
Objectives:
A clinical protocol was developed for clinicians to routinely assess and initiate treatment for patients with neuropathic pain (NP) in an acute care setting. The objectives of this study were to: (1) determine the incidence and onset of NP in patients with traumatic spinal cord injury during acute care and (2) describe how the implementation of a clinical protocol impacts the assessment and diagnosis of NP.
Materials and Methods:
The study was a cohort analysis with a pre-post-test utilizing a historical control. Data were retrospectively collected from a patient registry and charts. Participants were randomly selected in cohort 1 (control) and cohort 2 (NP clinical protocol).
Results:
The incidence of NP was 56% without significant difference between the cohorts (P=0.3). Onset of NP was 8 days (SD=14) across the study and >85% of the participants with NP were diagnosed within 2 weeks. Participants with incomplete injuries had a significant earlier onset than participants with complete injuries (6.2±12.8, 10.9±15.8 d; P=0.003). The mean number of days from hospital admission to initial assessment decreased with use of the NP clinical protocol (3.7±5.7 d; P=0.02).
Discussion:
This study demonstrates a high incidence and early onset of NP in traumatic spinal cord injury during acute hospital care, with an earlier emergence in participants with incomplete injury. The NP clinical protocol ensured continuous assessment and documentation of NP while decreasing the time to an initial screen, but did not impact diagnosis.
After a spinal cord injury (SCI), neuropathic pain (NP) can occur from damage to the neural tissues and nociceptive pain can result from damage to non-neural tissues.1 A combination of both types may be present and identification is important for treatment.1 The prevalence of NP among persons with SCI is high, ranging from 15% to 38% in acute care and up to 59% in the community,2–8 with NP impacting the prevalence of comorbidities such as insomnia, depressive syndromes, and anxiety.9 Among employed participants, 38% reported some level of work impairment and the total annual cost per participant in the community was US$26,270.9
Given the impact of NP on a person’s functioning, quality of life, and financial burden, accurate diagnosis and early treatment with appropriate medication is critical. Recognizing NP can be difficult for a clinician lacking the specialized training and clinical experience required to assess a patient’s history and perform a physical examination for definitive diagnosis.10 As a result, screening tools such as the DN4 or Leeds Assessment of Neuropathic Symptoms and Signs, were developed to help diagnose NP.10–12 Once diagnosed, treatments can include anticonvulsants (eg, gabapentin, pregabalin), tricyclic antidepressants (eg, amitriptyline, nortriptyline, desipramine), Serotonin Noradrenaline Reuptake Inhibitors, controlled-release opioids, cannabinoids, and other analgesics (eg, Selective Serotonin Reuptake Inhibitors, tramadol, lidocaine).13–15 The most recent Canadian guidelines for the pharmacological management of chronic NP provide a stepwise progression for the treatment of NP and emphasize an individualistic approach that considers efficacy, side-effects, and accessibility while recognizing nonpharmacological interventions (eg, physiotherapy, exercise, and psychological treatment) as essential.15
Pain experts at Vancouver General Hospital (VGH) suspected that there was a higher incidence of NP than was previously reported. Despite the need to promptly diagnose and treat NP, the hospital lacked a standardized protocol to differentiate nociceptive and NP, or facilitate early intervention in an acute setting. To address this gap, a group of pain experts at VGH developed and implemented a hospital-wide clinical protocol for all patients in acute care referred to as the Vancouver Acute Neuropathic Pain Treatment Guideline (VANPTG). Specifically, the VANPTG was developed for a general clinician to routinely assess acute patients for NP, prescribe initial treatment according to a clinical algorithm, and initiate a pain expert consultation.
The Acute Spine Unit (ASU) was selected as one of the first units to implement the protocol. There was interest in determining the incidence of NP in traumatic SCI patients and examining changes in pain management. The objectives of this study were to: (1) determine the incidence and onset of NP in patients with traumatic SCI in the acute phase of care and (2) describe how the implementation of a clinical protocol impacts the assessment and diagnosis of NP.
MATERIALS AND METHODS
SCI Care
In the province of British Columbia, the VGH ASU is the only specialized spine unit in a level 1 trauma center.16 The ASU has 32 beds that are staffed with 7 spine surgeons and a pain specialist, in addition to nursing and allied health staff with expertise in SCI management.17 Each year, this unit admits over 900 patients with complex trauma or disorders of the spinal column and/or cord. It also has an onsite Perioperative Pain Service, a Complex Pain and Addiction Service, and a physiatry consultation service.
NP Protocol
In 2010, the Perioperative Pain Advisory Committee at VGH, composed of anesthesia, psychiatry, palliative care, clinical pharmacy, and nursing disciplines was convened to develop the VANPTG (see Fig. S1, Supplemental Digital Content 1, http://links.lww.com/CJP/A447 for the Pain Assessment Record; Fig. S2, Supplemental Digital Content 2, http://links.lww.com/CJP/A448 for the VANPTG). The VANPTG was intended as a hospital-wide protocol for all acute care patients.
The VANPTG consists of a protocol for general clinicians to routinely assess all acute patients for pain using a Pain Assessment Record with questions involving NP symptoms, prescribe initial treatment with an algorithm, and initiate a pain expert consultation. According to the VANPTG, nurses in the ASU are required to assess patients with SCI every 12 hours using the Pain Assessment Record that incorporates items from the DN4 questionnaire.11
On the basis of input from Perioperative Pain Advisory Committee, the Pain Assessment Record was adjusted to include NP symptoms (ie, burning, painful cold, electrical shocks, tingling, pins and needles, numbness, itching) and allodynia so that the decision to consult a pain expert can be made without a physical examination. When a patient has at least 3 NP symptoms or significant allodynia (≥4/10 on a Numeric Rating Scale), treatment is initiated following an algorithm and a referral to a pain expert is made to confirm the diagnosis. The pain expert uses their experience, the patient’s history, and a physical examination that follows guidelines from the International Association for the Study of Pain and generally uses a grading system.18 See Figure 1 for a description of patient care flow in the ASU. For this study, an on-unit, fellowship-trained anesthesiologist, a psychiatrist with a specialty in pain management, and the Perioperative Pain Service anesthesiologists and physiatrists are defined as pain experts. Physicians from other disciplines and nurses are referred to as general clinicians.
FIGURE 1.
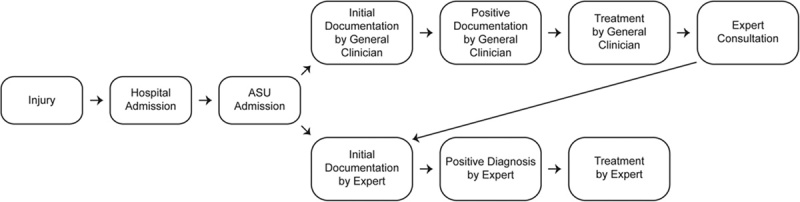
The flow of neuropathic pain management milestones. An overview of the 2 most common pathways for assessing and managing neuropathic pain in patients with traumatic SCI admitted to the ASU. ASU indicates acute spine unit; SCI, spinal cord injury.
Study Design and Sample
This is a cohort study with a pretest and posttest study design utilizing a historical control. Data were retrospectively collected from the Rick Hansen Spinal Cord Injury Registry (RHSCIR)19 and abstracted from patient charts. The study sample consisted of participants over 15 years of age, diagnosed with a new traumatic SCI who were admitted to the ASU and enrolled in RHSCIR from July 1, 2004 to March 31, 2012. The participants were divided into cohorts based on exposure to the NP protocol (cohort 1, historical control; cohort 2, VANPTG). It was not possible to conduct a chart review on the large number of eligible participants within the cohorts; therefore, a power analysis was conducted to determine the number of participants needed to answer the study objectives. The study sample was randomly selected using the simple random sampling function in Statistical Analysis Software, version 9.3.
Institutional approval for this study was obtained from the University of British Columbia (Clinical Research Ethics Board) and Vancouver Coastal Health (Research Operational Approval). On the basis of the Tri-council Policy Statement (TCPS2 2014), Article 3.7A, an alteration to the requirements for consent was approved by the Clinical Research Ethics Board for this study; therefore, participants were not required to provide written or verbal informed consent. This study was limited to the review and analysis of existing data that did not involve any therapeutic, clinical, or diagnostic intervention. The researchers took appropriate measures to protect the privacy of individuals and to safeguard identifiable information. Results presented in this manuscript are aggregated data devoid of identifiable information.
Data Collection
Data were retrospectively collected by one of the authors (S.E.P.) for the acute phase of care, which refers to the time a patient is admitted to an acute care hospital. The average length of stay for this population was 36 days (median, 27 d and range, 2 to 191 d), including both emergency and acute care. Injury and demographic data were obtained from RHSCIR. Data elements collected from participant charts included general clinician and pain expert assessment notes regarding NP, completion of the screening tool (yes/no), diagnosis of NP (yes/no), and NP medication records. Descriptors such as “burning, shooting pain” and “hypersensitive to touch” were included, as well as documentation of NP diagnostic terms such as “neuropathic pain” or “nerve pain,” to identify the presence of NP. The completion of the screening tool was only collected for cohort 2.
The gold standard for NP diagnosis was based on documentation by pain experts. To identify any false-negative diagnoses, the clinical nurse specialist (L.M.A.B.) examined the charts for documentation of NP symptoms for participants who had a negative NP diagnosis according to a general clinician (a clinician who is not classified as a pain expert) that was not confirmed by a pain expert.
Analysis
Comparison Between the Cohorts
Statistical Analysis Software version 9.3 was used for all analysis with P<0.05 as the threshold of statistical significance. Comparisons were made between cohorts 1 and 2 unless otherwise specified. The cohorts were compared using χ2 test, Fisher exact test, or t test depending on the type of variables to determine whether there was any difference in the distribution of demographic and injury variables.
NP Incidence, Documentation, and Onset
The incidence of NP was calculated by dividing the number of participants with a NP diagnosis by the total number of participants in the cohort. The percent of participants with documentation was calculated by dividing the number of participants with NP documentation by the total number of participants in the cohort. NP documentation refers to clinical documentation relating to NP either from chart notes or the screening tool—these data report the presence or absence of NP. NP diagnosis refers to the presence of NP. In cohort 1, NP data were only collected from the chart notes, in cohort 2 NP data were collected from the chart notes and the screening tool.
To compare the incidence and the documentation by the source of the data (general clinician or expert-derived), the incidence and documentation was assessed using the data from the general clinicians and the pain expert information separately. The incidence of NP was compared between the cohorts using χ2 test. As the gold standard was based on documentation by pain experts, the expert-derived incidence was used for determination of the NP incidence in this participant population.
The cohorts were then combined to determine whether there was any difference in the incidence and documentation of NP based on injury severity (completeness of the SCI). The same comparison was repeated for the onset of NP. NP onset was defined as the time from the injury to the earliest positive NP diagnosis either by a general clinician or a pain expert; however, only participants confirmed by a pain expert were included in the onset calculations. This was done so that the time it took for an expert consultation did not impact on the number of days to onset.
NP Protocol Adherence
The adherence to the VANPTG protocol was determined by calculating the percentage of documented screening using the Pain Assessment Record. Diagnostic accuracy of general clinicians was assessed by comparing the percentage of general clinician diagnoses that matched the pain expert diagnoses. Treatment was indicated by a prescription record for at least one of the following medications: gabapentin, nortriptyline, pregabalin, desipramine, and amitriptyline/ketamine cream. Treatment correction was calculated by examining the percentage of treatments initiated by general clinicians that were either stopped or had dosage adjustments by pain experts. χ2 test was used for comparisons.
NP Management Practice
The effect of the VANPTG implementation in changing the NP management practice was examined by comparing any difference in time between the cohorts for the following data variables: injury, hospital admission, initial screening, first positive diagnosis (general clinician or pain expert), pain expert diagnosis, expert NP consultation, and treatment initiation. For all the milestones that used the first positive diagnosis, only participants that were confirmed by an expert were included. The cohorts were compared using the χ2 test and Wilcoxon Mann-Whitney test depending on the type of data.
RESULTS
Baseline Comparison of Study Sample
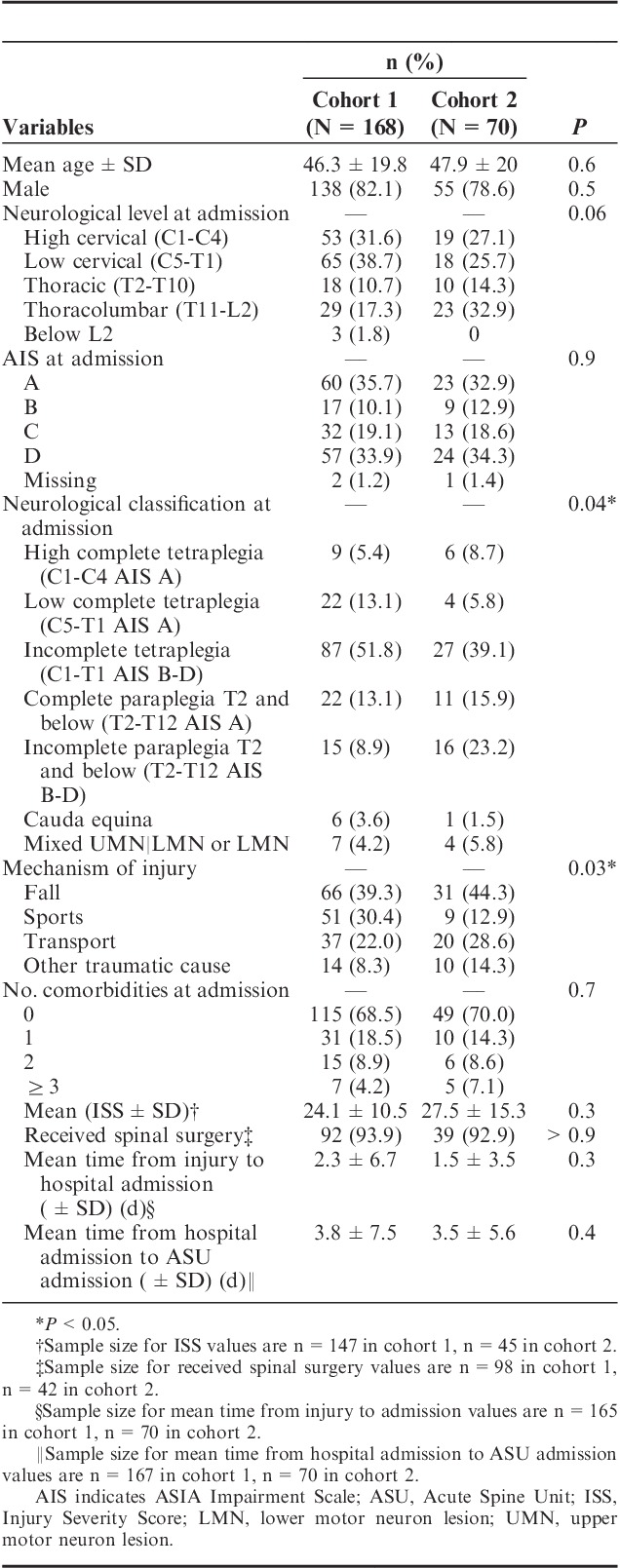
There were a large number of participants eligible for this study (n=594 in cohort 1, n=132 in cohort 2) and 276 participants were randomly selected for inclusion (Fig. 2). A detailed description of the demographic and injury variables for each cohort is shown in Table 1. There was no statistical difference among the cohorts except for mechanism of injury (P=0.03) and neurological classification at admission (P=0.04).
FIGURE 2.
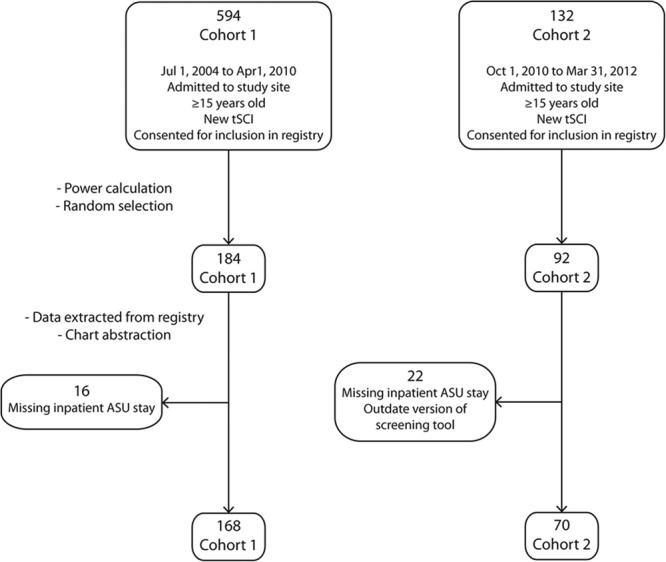
An overview of the participant selection process. This is a flow diagram that depicts the participant selection process and the final number of participants in each cohort. ASU indicates acute spine unit; tSCI, traumatic spinal cord injury.
TABLE 1.
Demographic and Injury Data Comparison of the Cohorts
NP Incidence, Documentation, and Onset
The comparison of NP incidence between the cohorts is shown in Figure 3A. The expert and general clinician–reported incidences both increased (7.2% and 3.5%, respectively); however, there was no significant difference in the incidence reported by either the expert (P=0.3), or the general clinician (P=0.6) following implementation of the VANPTG. The NP incidence over the entire study period was 56.3%, as reported by the experts and 68.9%, as reported by the general clinicians (Table S1, Supplemental Digital Content 3, http://links.lww.com/CJP/A449).
FIGURE 3.
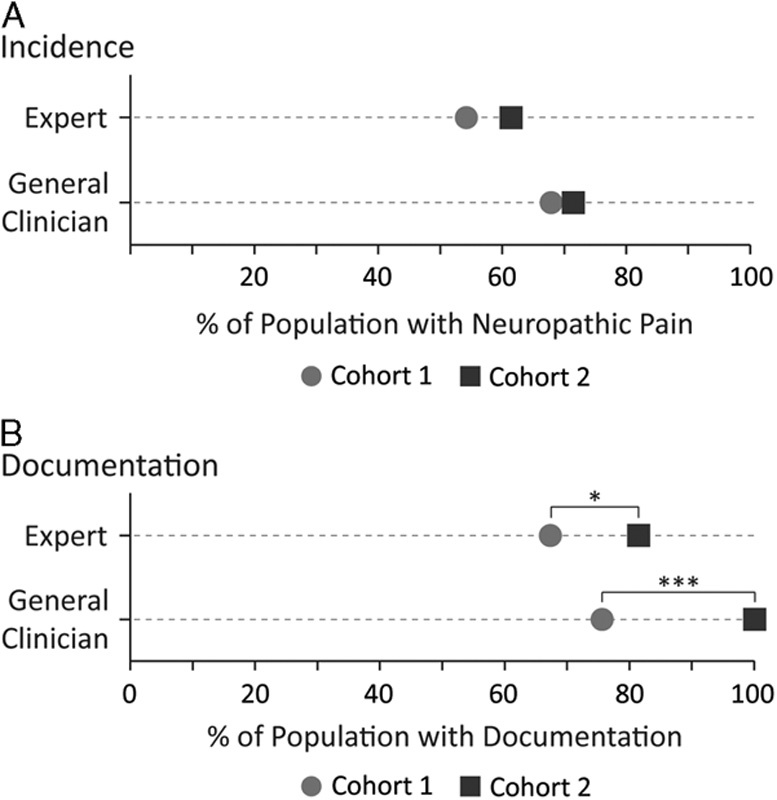
The dot plot comparisons of cohort 1, the control group, and cohort 2, the group with the implemented Vancouver Acute Neuropathic Pain Treatment Guidelines. A) The percent of the participant population diagnosed with neuropathic pain, as reported by the pain expert or the general clinician. B) The percent of the participant population with neuropathic pain documentation, as reported by the pain expert or the general clinician. *P<0.05; ***P<0.001.
The comparison of the NP documentation between the cohorts is shown in Figure 3B. The percent of participants with expert and general clinician–reported documentation both increased significantly. The percent of participants with expert documentation significantly increased (P=0.03) by 14.1% to 81.4% with the implementation of VANPTG. The percent of participants with general clinician documentation significantly increased (P<0.0001) by 32.1% to 100% with the implementation of VANPTG (Table S2, Supplemental Digital Content 4, http://links.lww.com/CJP/A450).
No statistical significance was found among the cohorts with respect to NP onset (9±17 and 6±6 d; P=0.7) and the mean onset over both cohorts was 8±14 days. The cumulative distribution of NP onset is shown in Figure 4. The shape of the graph shows a sharp increase in the number of NP cases diagnosed within the first week of injury with 71% and 87% of all the participants with NP diagnosed by 7 and 14 days, respectively.
FIGURE 4.
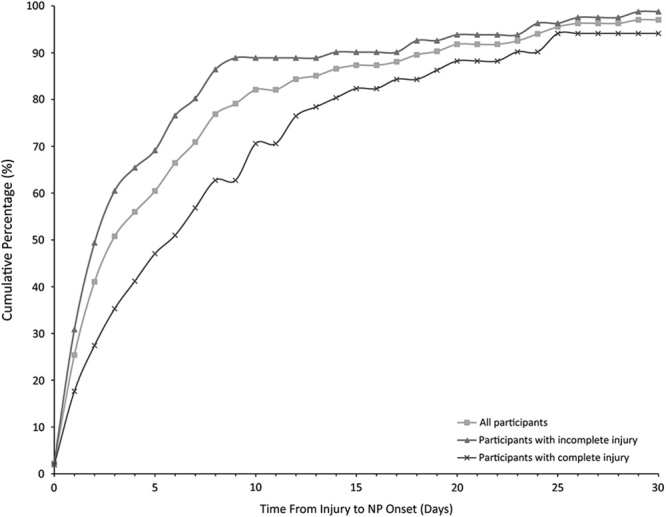
The cumulative distribution of NP onset. The cumulative percentage of participants diagnosed with NP according to the date of onset. The percentages represent the proportion of only those who have NP during their acute stay as confirmed by a pain expert. NP indicates neuropathic pain.
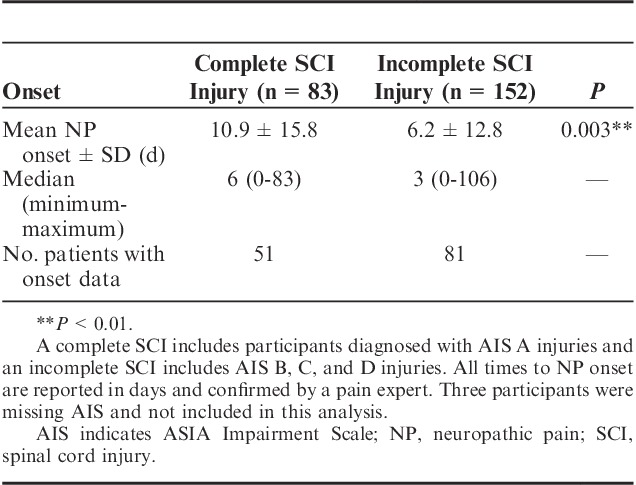
When the cohorts were pooled and participants with complete SCI (ASIA Impairment Scale [AIS] A) were compared against those with incomplete SCI (AIS B, C, or D), the incidence did not significantly differ (P=0.2); however, the expert documentation differed significantly (P=0.007), where participants with complete SCI had a higher percent of participants with documentation (Fig. 5 and Table S3, Supplemental Digital Content 5, http://links.lww.com/CJP/A451). Among the participants with incomplete injuries, 35% lacked documentation compared with 18% of the participants with complete injuries. Across the cohorts, the time from injury to the earliest positive diagnosis or onset was significantly earlier for incomplete injuries (P=0.003) (see Table 2 for a summary). Figure 4 shows the cumulative distribution of NP onset between incomplete and complete injuries. Among all the participants who were diagnosed with NP during the acute phase, 80% of those with incomplete and 57% of those with complete injuries were diagnosed within 7 days. No such difference in onset was seen when participants with cervical injury were compared with those with thoracic or lumbar injury for all injury severities (AIS A to D) (P=0.5).
FIGURE 5.
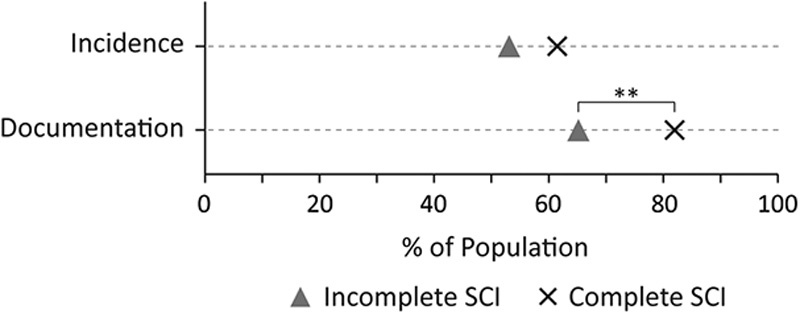
The dot plot comparisons of incomplete and complete SCIs based on either the incidence or the assessment documentation of neuropathic pain. SCI indicates spinal cord injury. **P<0.01.
TABLE 2.
Onset of NP by Injury Severity
NP Protocol Adherence
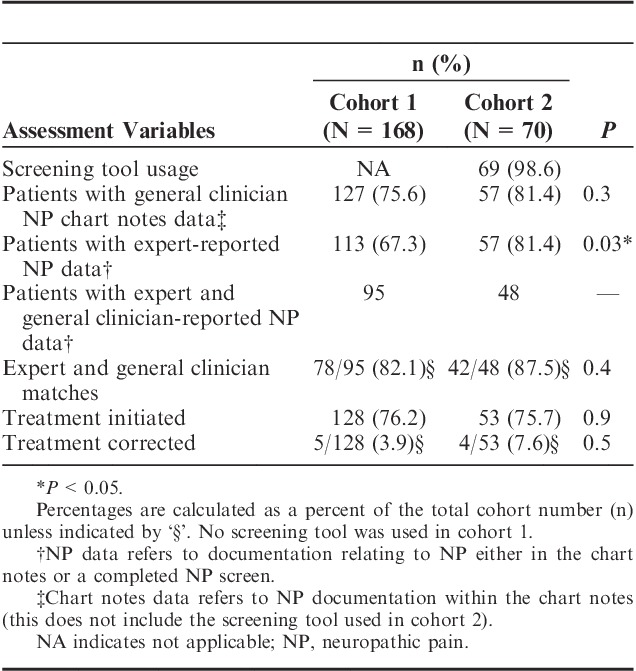
The comparisons of cohorts for NP screening, diagnosis, and treatment are summarized in Table 3. The screening portion of VANPTG implemented in cohort 2 had a high adherence of 99% (n=69). There was no difference among the cohorts in the documentation of NP in chart notes by general clinicians (P=0.3)—these data were collected by chart review only (excluding use of screening tool). There was a significant difference in the percentage of participants assessed by a pain expert following VANPTG. Among the cohorts, the diagnostic accuracy of the general clinicians (P=0.4), the number of participants who received treatment for NP (P=0.9), and treatment correction (P=0.5) also did not change significantly.
TABLE 3.
Comparison of Assessment for NP by Cohort
NP Management Practice
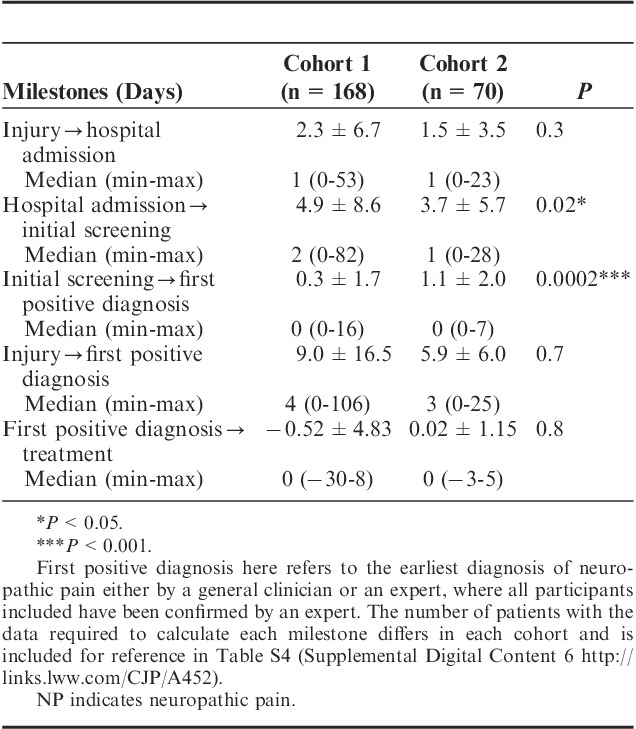
A comparison of cohorts for the milestones of NP management is summarized in Table 4. There was no change in the time from injury to the hospital admission among the cohorts (P=0.3). The initial screening occurred within a week following admission to the hospital and was significantly less following implementation of the VANPTG; the mean time was 3.7±5.7 days (P=0.02) from hospital admission to the initial NP assessment. The mean time was 1.1 days (SD=2.0) from the initial screening to the positive diagnosis of NP, which was significantly longer in cohort 2; however, the time from injury to first positive diagnosis was not significantly different following implementation.
TABLE 4.
Comparison of Cohorts for Mean Time to Care Milestones for Assessing and Managing NP
DISCUSSION
This study determined the NP incidence and early onset in an acute SCI population and evaluated the effect of VANPTG on pain assessment and diagnosis compared with previous practice. The average incidence of NP during the entire study period was 56% and onset was on average, 8 days following injury, with ∼71% diagnosed in the first week. The implementation of VANPTG significantly increased the percent of patients assessed with NP documentation and significantly decreased the average number of days from hospital admission to initial screening, without impacting treatment or diagnosis.
NP Incidence, Documentation, and Onset
To date, the incidence of NP in the SCI population is primarily reported in the rehabilitation or community setting.4,20–24 The prospective studies have focused on discrete time points to screen for NP, contrasting the current study that occurs in an acute setting where patients are regularly assessed for pain. We have examined all NP together and have not separated by location (above level or below level), so direct comparisons are difficult. Siddall et al3,4 reported that at 2 weeks following injury 38% and 14% of their population had at-level and below-level NP, respectively, and at 5 years this increased to 41% and 34%. At 3 months, Finnerup et al,6,7 had >30% of their population with NP, which increased to 49% at 3.5 years. According to these studies, our 56% incidence is at the higher end of the range. Although our study was retrospective and conducted as a quality improvement initiative, the data were collected from current clinical practice and reflects a high incidence during an early acute timeframe that has not been analyzed previously.
Our incidence is higher than Street et al5 who reported an incidence of 15% in a similar traumatic SCI population. 5 The difference is likely due to the reporting source. We used NP diagnosis by a pain expert as the definition of NP in our study, and this incidence was similar across cohorts, regardless of the protocol. Street et al used surgeon reports during their weekly rounds to determine the status of NP. The rounds may not have captured cases where NP was effectively managed. As our measurement of incidence occurs continuously, immediately following admission, and includes a team of pain experts for the diagnosis, we believe it is an accurate estimate of the incidence in an acute traumatic SCI population.
The introduction of new treatment guidelines did not significantly impact the incidence of NP in this patient population, but did significantly increase the documented assessment of NP, irrespective of the profession recording the clinical data. The entire population of the second cohort had NP documentation by the general clinician, with 99% screened using the VANPTG, showing the guidelines’ value and adherence. The higher incidence reported by the general clinician compared with the pain experts was expected due to the methodology of the guidelines that promote an initial screen by a general clinician with less stringent criteria, lacking a physical examination, compared with the pain expert consultation. To correct for any over diagnosis, all participants screened as positive by the general clinician are confirmed by a pain expert and treated accordingly. The VANPTG ensured all participants were assessed, had NP documentation and streamlined the pain expert consultation process.
There is limited evidence regarding the onset of NP following SCI, particularly early in the acute phase (or <1 month following injury). Siddall and colleagues found that the majority of their participants with NP first report at 2 weeks post-injury (53% of those with at-level NP and 41% of below-level NP).3 Interestingly, several groups have found different progression of at-level versus below-level NP, where at-level pain has an earlier onset.3,4,6,7 In the present study, the majority of our participants also reported NP at 2 weeks, 87% of those with NP were first diagnosed by 14 days and 71% by 7 days. Average onset was 8 days (SD=14) following injury across our study period, which indicates that NP emerges earlier than any of the current literature screens for NP.
When the incidence, documentation, and onset of NP were compared by completeness of injuries, the incidence did not differ significantly (P=0.2), whereas the percent of participants with NP documentation and the onset of NP did differ between the complete and incomplete populations in the study. The percent of the participants with NP documentation was found to be significantly higher in the complete SCI population (P=0.007) compared with the incomplete population and the onset earlier for the incomplete SCI population (P=0.003) compared with the complete population. This demonstrates that participants with incomplete SCI are less likely to be assessed for NP. Those that lack NP documentation could represent the less severely injured proportion of the incomplete injuries that have a shorter length of acute stay. Of clinical interest, the onset of NP was significantly earlier for incomplete compared with complete injuries (6.2 vs. 10.9 d), suggesting the importance of early NP assessment and documentation of incomplete injuries. Two prospective longitudinal studies did not find differences in the prevalence of NP based on the completeness of injury.4,7 However, one of these studies did find that incomplete injuries had a higher prevalence of allodynia in the first 6 months following injury.3 The impact that the severity of injury has on the incidence of NP in this population will require further study with a prospective design to reduce the number of participants that lack documentation of an NP assessment.
NP Management Practice
The relatively high incidence of NP in the traumatic SCI population likely reinforced the importance of early assessment, treatment, and expert consultation in the ASU, even before the new treatment guidelines. The implementation of the VANPTG protocol was effective with almost all participants being screened and all participants having NP documentation. As a result of implementing the VANPTG, there was a significant 1 day decrease (4.9 d in cohort 1 to 3.7 d in cohort 2) in the time to initial screening following admission to VGH. However, there were no significant differences in the overall time from injury to obtaining a positive diagnosis (P=0.7) or in the time from positive diagnosis to receiving treatment (P=0.8). Taken together with the consistently high cohort incidence, these results suggest that the VANPTG had only a minor impact on management practice and diagnosis of NP in the ASU.
The negative time from positive diagnosis to treatment demonstrates that the ASU team is comfortable initiating treatment while confirming the diagnosis and management with the pain expert. The extent (84%) to which the general clinician diagnosis of NP agrees with the expert diagnosis suggests that in a setting with a high incidence and access to pain experts, general clinicians are proficient in recognizing and treating NP. Having access to a pain expert on the ASU was in place before 2004 and may explain why no significant changes were seen in treatment. In the recently published SCI Acute and Rehabilitation Standards by Accreditation Canada,25,26 there is a requirement for the program to evaluate and manage pain. Future studies should evaluate the VANPTG program at a center that does not have a specialized spine unit to analyze the impact on NP treatment.
Study Limitations
It is important to consider the limitations when interpreting the results of this study. As this study used retrospective data collected from hospital charts, only data recorded as part of a routine clinical practice were available. As clinicians generally document problems that require attention, the absence of NP would not be as meticulously recorded as the presence of NP, especially in cohort 1, which did not have an effective documentation tool to prompt clinicians to record the absence of NP symptoms. If the absence of NP was not recorded in the medical chart, the assessment was not likely documented leading to a falsely reported longer time to the initial screening. Some clinicians may not have documented the presence of NP but initiated the treatment based on clinical judgment, which could have led to the treatment initiation percentage being higher than the NP diagnosis percentage. Moreover, our gold standard of diagnosis by the pain experts has not been independently validated. A future prospective design with an independently validated VANPTG would improve the quality of data on NP incidence and onset.
The VANPTG was developed as a hospital-wide initiative and was not targeted to patients sustaining a traumatic SCI. Since its development, there have been advances in the standardized classification SCI pain with the publication of the International Spinal Cord Injury Pain (ISCIP) Classification.27 A prospective study will include more details specific to SCI pain, including the ISCIP classification and other domains of pain (location, intensity, and extent).
Although the present study did not address side effects of the treatments for NP, the side effects to these pharmaceuticals are an important part of treatment and should be included in future prospective research. In addition, we were only able to report on the number of comorbidities and not specific comorbidities that may influence the development of NP. For the scope of this paper, as a retrospective chart review, these details were not included; however, it should be noted that few participants were taken off medication (or had treatment corrected) suggesting that side effects were not an issue for the treatment of this patient population.
CONCLUSIONS
Results from this study support the perception that the incidence of NP in the acute traumatic SCI population is higher (56%) and the onset is earlier (8±14 d) than previously reported. The finding that incomplete injuries have an earlier onset is novel and should be included in SCI pain management education, and to inform future research as little is known about NP immediately following injury. The implementation of a hospital-wide NP protocol improved assessment documentation and timely care in a specialized SCI center by increasing the NP documentation and decreasing the time to initiate screening but did not impact the overall time from injury to treatment. However, given that the ASU was vigilant in the management of NP, lessons learned from this program may inform other SCI programs.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.
Footnotes
Funding for this project was provided by the Rick Hansen Institute Vancouver, BC, Canada, Health Canada Ottawa, ON, Canada, and the Western Economic Diversification Canada Vancouver, BC, Canada. V.K.N., S.E.P., N.P.T., and T.S. receive a salary from the Rick Hansen Institute. The remaining authors declare no conflict of interest.
REFERENCES
- 1.Rekand T, Hagen E, Gronning M. Chronic pain following spinal cord injury. Tidsskr Nor Legeforen. 2013;8:974–979. [DOI] [PubMed] [Google Scholar]
- 2.Levi R, Hultling C, Nash M, et al. The Stockholm Spinal Cord Injury Study: 1. Medical problems in a regional SCI population. Paraplegia. 1995;33:308–315. [DOI] [PubMed] [Google Scholar]
- 3.Siddall PJ, Taylor DA, McClelland JM, et al. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. 1999;81:187–197. [DOI] [PubMed] [Google Scholar]
- 4.Siddall PJ, Mcclelland JM, Rutkowski SB, et al. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. [DOI] [PubMed] [Google Scholar]
- 5.Street JT, Thorogood NP, Cheung A, et al. Use of the Spine Adverse Events Severity System (SAVES) in patients with traumatic spinal cord injury. A comparison with institutional ICD-10 coding for the identification of acute care adverse events. Spinal Cord. 2013;51:472–476. [DOI] [PubMed] [Google Scholar]
- 6.Finnerup NB, Norrbrink C, Trok K, et al. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J Pain. 2014;15:40–48. [DOI] [PubMed] [Google Scholar]
- 7.Finnerup NB, Jensen MP, Norrbrink C, et al. A prospective study of pain and psychological functioning following traumatic spinal cord injury. Spinal Cord. 2016;54:816–821. [DOI] [PubMed] [Google Scholar]
- 8.Cragg JJ, Haefeli J, Jutzeler CR, et al. Effects of pain and pain management on motor recovery of spinal cord-injured patients: a longitudinal study. Neurorehabil Neural Repair. 2016;30:753–761. [DOI] [PubMed] [Google Scholar]
- 9.Mann R, Schaefer C, Sadosky A, et al. Burden of spinal cord injury-related neuropathic pain in the United States: retrospective chart review and cross-sectional survey. Spinal Cord. 2013;51:564–570. [DOI] [PubMed] [Google Scholar]
- 10.Sadler A, Wilson J, Colvin L. Acute and chronic neuropathic pain in the hospital setting use of screening tools. Clin J Pain. 2012;29:507–511. [DOI] [PubMed] [Google Scholar]
- 11.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new Neuropathic Pain Diagnostic Questionnaire (DN4). Pain. 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- 12.Bennett M. The LANSS pain scale: the Leeds Assessment of Neuropathic Symptoms and Signs. Pain. 2001;92:147–157. [DOI] [PubMed] [Google Scholar]
- 13.Attal N. Neuropathic pain: mechanisms, therapeutic approach, and interpretation of clinical trials. Continuum. 2012;18:161–175. [DOI] [PubMed] [Google Scholar]
- 14.Hagen EM, Rekand T. Management of neuropathic pain associated with spinal cord injury. Pain Ther. 2015;4:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moulin D, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenehan B, Street J, Kwon BK, et al. The epidemiology of traumatic spinal cord injury in British Columbia, Canada. Spine (Phila Pa 1976). 2012;37:321–329. [DOI] [PubMed] [Google Scholar]
- 17.Santos A, Gurling J, Dvorak MF, et al. Modeling the patient journey from injury to community reintegration for persons with acute traumatic spinal cord injury in a Canadian centre. PLoS One. 2013;8:e72552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treede RD, Jensen TS, Campbell JN, et al. Redefinition of neuropathic pain and a grading system for clinical use: consensus statement on clinical and research diagnostic criteria. Neurology. 2008;70:1630–1635. [DOI] [PubMed] [Google Scholar]
- 19.Noonan V, Kwon B, Soril L, et al. The Rick Hansen Spinal Cord Injury Registry (RHSEIR): a national patient-registry. Spinal Cord. 2012;50:22–27. [DOI] [PubMed] [Google Scholar]
- 20.Chiu W-T, Lin H-C, Lam C, et al. Epidemiology of traumatic spinal cord injury: comparisons between developed and developing countries. Asia Pac J Public Health. 2010;22:9–18. [DOI] [PubMed] [Google Scholar]
- 21.Vall J, Mauricio C, Costa DC, et al. Neuropathic pain characteristics in patients from Curitiba (Brazil) with spinal cord injury. Arq Neuropsiquiatr. 2011;69:64–68. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Rohilla RK, Siwach R, et al. Health-related problems and effect of specific interventions in spinal cord injury: an outcome study in Northern India. Eur J Phys Rehabil Med. 2010;46:47–53. [PubMed] [Google Scholar]
- 23.Finnerup NB, Johannesen IL, Sindrup SH, et al. Pain and dysesthesia in patients with spinal cord injury: a postal survey. Spinal Cord. 2001;39:256–262. [DOI] [PubMed] [Google Scholar]
- 24.New PW, Lim TC, Hill ST, et al. A survey of pain during rehabilitation after acute spinal cord injury. Spinal Cord. 1997;35:658–664. [DOI] [PubMed] [Google Scholar]
- 25.Accreditation Canada. Qmentum Program: Spinal Cord Injury Acute Services. Ottawa: Accreditation Canada; 2015. [Google Scholar]
- 26.Accreditation Canada. Qmentum Program: Spinal Cord Injury Rehabilitation Services. Ottawa: Accreditation Canada; 2015. [Google Scholar]
- 27.Bryce T, Biering-Sørensen F, Finnerup N, et al. International Spinal Cord Injury Pain Classification: part I. Background and description. March 6-7, 2009. Spinal Cord. 2012;50:413–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.clinicalpain.com.


