Supplemental Digital Content is Available in the Text.
Key Words: atrial fibrillation, horse, dofetilide, ranolazine, combination therapy
Abstract
Background:
Antiarrhythmic compounds against atrial fibrillation (AF) often have reduced efficacy and may display cardiac and/or noncardiac toxicity. Efficacy can be improved by combining 2 compounds with distinct mechanisms, and it may be possible to use lower doses of each compound, thereby reducing the likelihood of adverse side effects. The purpose of this study was to investigate whether the effective doses of dofetilide and ranolazine can be reduced if the drugs are combined.
Methods:
Dofetilide, ranolazine, and a combination of these were administered in 4 incremental dosing regimens to horses with acutely pacing-induced AF. Time to cardioversion, atrial effective refractory period, and AF vulnerability and duration were assessed.
Results:
Of 8 horses, 6 cardioverted to sinus rhythm after infusion with a combination of 0.889 μg/kg dofetilide and 0.104 mg/kg ranolazine. Two horses cardioverted with 0.104 mg/kg ranolazine alone, and 3 cardioverted with 0.889 μg/kg dofetilide alone. The combination therapy decreased AF vulnerability (P < 0.05) and AF duration (P < 0.05). No change in atrial effective refractory period was detected with any of the drugs.
Conclusions:
The combination of dofetilide and ranolazine showed increased antiarrhythmic effects on acutely induced AF in horses, affecting time to cardioversion, AF vulnerability, and AF duration.
INTRODUCTION
Atrial fibrillation (AF) is the most common clinical arrhythmia in both humans and horses.1 However, current treatment options for restoring sinus rhythm are not optimal because available antiarrhythmic compounds possess cardiovascular and/or noncardiovascular toxicity, and the efficacy is limited.2 In recent years, ranolazine has been investigated for its antiarrhythmic potential both alone and in combination.3–5 Ranolazine has previously been reported to suppress QT-related arrhythmias,6 but a recent study reported an increased risk of torsades de pointes ventricular tachycardia (TdP) associated with its use.7 Ranolazine blocks a number of ion currents, most notably the late sodium current (INa,late) and in higher doses, the peak sodium current (INa,peak) and the rapid activating delayed rectifier potassium current (IKr) also.8,9
Dofetilide is a potent IKr blocker and is used as an antiarrhythmic agent in the treatment of AF.2 In the DIAMOND study,10 dofetilide was found to be effective for both cardioversion and maintenance of sinus rhythm in patients with congestive heart failure and AF. However, use of dofetilide may result in TdP because of a prolongation of the QT interval.11 This QT-prolonging effect is dose-dependent,2 and by combining the compound with another antiarrhythmic compound, it may be possible to reduce the dose of individual compounds, thus minimizing their adverse effects. Furthermore, the combination of more than one drug is hypothesized to increase the efficacy against AF, and some studies have already addressed this using ranolazine.4,12,13
The horse has been advocated as a promising animal model of AF because of the similarity to human physiology, including the cardiac ion channel distribution14 and occurrence of AF.1 Furthermore, AF can be easily induced experimentally in horses. Our research group has developed a model of acutely induced AF in horses, which allows us to examine the antiarrhythmic effects of different compounds.14,15
The present study was designed to test the hypothesis that combining dofetilide with ranolazine will make it possible to reduce the doses of both drugs to subefficacious levels and still reach the same antiarrhythmic potential as when either compound is used alone in higher doses.
METHODS
The study was approved by the local ethical committee at the Department of Veterinary Clinical Sciences, University of Copenhagen and The Danish Animal Experiments Inspectorate (license number 2012-15-2934-00198) and was performed in accordance with the European Commission Directive 86/609/EEC.
Twelve Standardbred horses were included [body weight (BW) 502 ± 37 kg, age 8.3 ± 3.5 years], of which 3 mares were enrolled in a safety study, and 6 mares and 3 geldings in an electrophysiology study (see Material Table S1, Supplemental Digital Content 1, http://links.lww.com/JCVP/A283). Before the study, the horses underwent a clinical examination and had a 24-hour electrocardiography (ECG) recording (Televet, Engel Engineering Services GmbH, Heusenstamm, Germany), as well as a routine echocardiographic examination (2-D, M-mode and colour-flow Doppler; Vivid I ultrasound machine with a 1.5 mHz phased array probe, GE Healthcare, Horten, Norway). Only horses with no signs of cardiovascular disease were included. All horses were included in 4 procedures [saline (control), dofetilide, ranolazine, and the combination of dofetilide and ranolazine], with 7 days recovery period in between. All procedures were performed on nonsedated horses restrained in a stock with access to hay and water. The horses were trained to stand quietly for longer periods before the studies, and therefore, no anesthesia was needed.
Drugs
Dofetilide (Cat. No. 3757, Tocris Bioscience, R&D systems, Abingdon, United Kingdom) was dissolved in 50% Poly (ethylene glycol) average Mn 400 (202398 Aldrich, Sigma-Aldrich, MO) and 50% saline (NaCl 9 mg/mL, B. Braun, Melsungen, Germany) to a solution of 0.04 mg/mL. Ranolazine dihydrochloride (Cat. No. 3118, Tocris Bioscience, R&D systems, Abingdon, United Kingdom) was dissolved in saline to a solution of 14 mg/mL. The ranolazine solution was pH-calibrated using 1M NaOH to a pH of just above 5.
Safety Study
The safety study is described in detail in the Supplemental Digital Content 1 (see Table S1, http://links.lww.com/JCVP/A283).
Electrophysiology
An overview of the experimental protocol is presented in Figure 1A. Two multipolar steerable nonfixative electrodes (Inquiry Steerable Diagnostic Catheter, 6Fr/110 cm; St. Jude Medical, MN) were used for each horse; 1 for atrial pacing and 1 for obtaining atrial electrograms, as previously described.15 The electrodes were placed through 2 introducer sheaths (One Piece/Tuohy-Borst Catheter Introducer with Integral Hemostasis Valve, 8F, Argon Medical Devices, Holte, Denmark) in 1 of the jugular veins and advanced to the right atrium using the deflections on the electrogram to determine positioning. The recording electrode was placed so that the deflection on the atrial electrogram appeared in conjunction with the P wave on 2 simultaneously recorded surface ECGs. The pacing electrode was positioned to have consistent atrial capture at 60 beats per minute (bpm), with a threshold of excitation lower than 1.4 mA. Intra-atrial recordings and surface ECGs were monitored and stored for later analysis using Labchart 7 software (ADInstruments, Oxford, United Kingdom).
FIGURE 1.
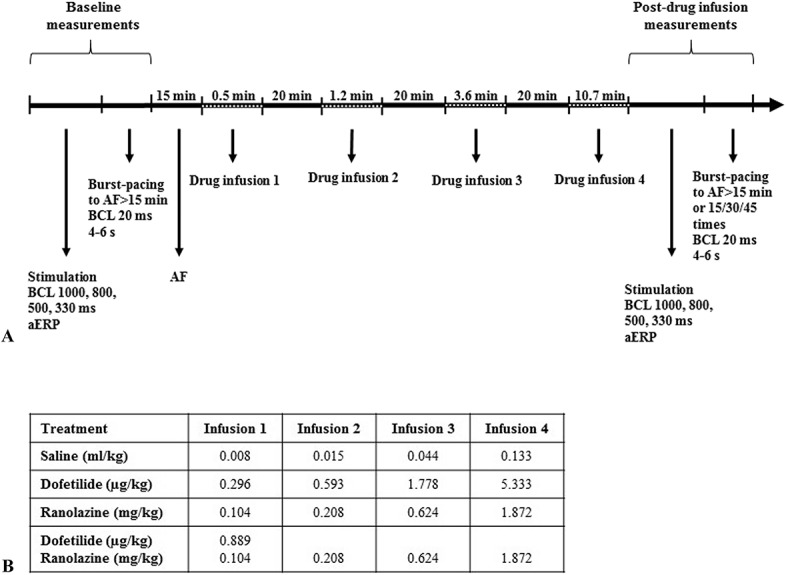
Experimental protocol. A, Outline of the experimental protocol. B, Schematic overview of the doses used at the different drug infusions.
One horse (ID11) was excluded from the study, as it did not complete the combination procedure because of the development of supraventricular tachycardia during an AF episode before drug administration.
Atrial Refractory Period
Atrial effective refractory periods (aERPs) were determined to be the longest S1-S2 stimuli interval failing to capture with 9 basic stimuli (S1) followed by an extra stimulus (S2) with 10-millisecond increments (pulse width 2 milliseconds, current 3 mA). Measurements were performed 3 times and averaged at atrial pacing rates of 60, 75, 120, and 182 bpm before and after drug administration. Each S1-S2 interval was applied a minimum of 3 times at each pacing rate, and aERP was determined as the S1-S2 interval before the one at which complete or intermittent atrial capture occurred.
AF Induction, AF Vulnerability, and AF Duration
AF was induced after aERP measurements using a current of 10 mA and burst-pacing at 50 Hz for 4–6 seconds. Drug treatment was initiated once the horse developed an AF episode longer than 15 minutes. After drug administration and cardioversion, aERP was measured and an attempt was made to reintroduce AF. After treatment, burst-pacing was performed until AF lasted for more than 15 minutes, or a maximum of 15, 30, or 45 times in cases where the induction of AF before drug administration required ≤15, ≤30, and ≤45 burst-pacings, respectively. The number of burst-pacings required until AF that lasted for more than 15 minutes was counted to evaluate AF vulnerability. The mean duration of AF episodes after burst-pacings (maximum 15 minutes) was termed AF duration.
Drug Treatment
The order of saline, dofetilide, and ranolazine procedures was randomized, whereas all horses received the combination treatment in their last procedure. Dofetilide was administered intravenously at a dose of 8.0 μg/kg BW, in accordance with recommendations in humans.16 Ranolazine was administered intravenously at a dose of 2.4 mg/kg BW, in accordance with studies in pigs,17 as these studies have demonstrated antiarrhythmic effects using an intravenous route of administration, whereas studies in humans use oral administration. Saline was administered at 0.2 mL/kg BW to mimic the volume of the drugs infused. All solutions were administered at a rate of 0.0125 mL/kg BW/min according to the recommendations for dofetilide administration.16,18 The doses were administered in 4 steps—each with a 3-fold increase in the total dose (Fig. 1B). Each administration step was given with 20-minute intervals. For the combination procedure, ranolazine was administered in 4 steps, using the same dose as when administered alone. Dofetilide was given with the first dose of ranolazine at a dose of 0.889 μg/kg, as this corresponded to the last noncardioverting dose of dofetilide when used alone. All horses were given all 4 doses of each drug regardless of cardioversion time, to allow for the comparison of electrophysiology parameters.
ECG Analysis
ECGs were obtained throughout each procedure and for 24 hours after drug infusion using a Holter unit (Televet).15 QT intervals and QRS durations were manually analyzed on lead II. Most measurements were made at ventricular rates of 30–50 bpm (resting heart rate for horses is 28–40 bpm); however, the rates have been up to a maximum of 105 bpm. The QT measurements were corrected using a piecewise linear regression model specifically developed for horses. This regression model shows the lowest prediction errors at almost all heart rates when compared with other models.19 Because of technical problems, ECGs were not available for analysis for 2 dofetilide and 2 ranolazine procedures.
Data Analysis
All data are presented as mean ± standard error of measurement. Analyses were performed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA) or R 3.3.1 software (The R Foundation for Statistical Computing, Vienna, Austria), with P ≤ 0.05 considered significant. Changes in QRS duration and corrected QT intervals (QTc) in the animals of the safety study were analyzed by 1-way repeated analysis of variance followed by a Dunnett's multiple comparisons test using a time point of 5 minutes before drug injection, T(-5), as a reference. The QRS duration and QTc from the electrophysiology study were analyzed using a linear mixed model, and aERP data were analyzed by 2-way repeated-measures analysis of variance, followed by the Bonferroni post-test for pairwise comparisons. Changes in excitation threshold were analyzed using Wilcoxon signed-rank tests. AF vulnerability and AF duration were analyzed by Mann–Whitney U tests. Time to cardioversion was analyzed by 1-way repeated analysis of variance, followed by Dunnett's multiple comparisons test.
RESULTS
Safety of Dofetilide and Ranolazine
The safety of dofetilide (8.0 μg/kg BW) and ranolazine (2.4 mg/kg BW) for use in horses was first assessed by administering the compounds in 2 steps to healthy horses without AF, and monitoring them closely.
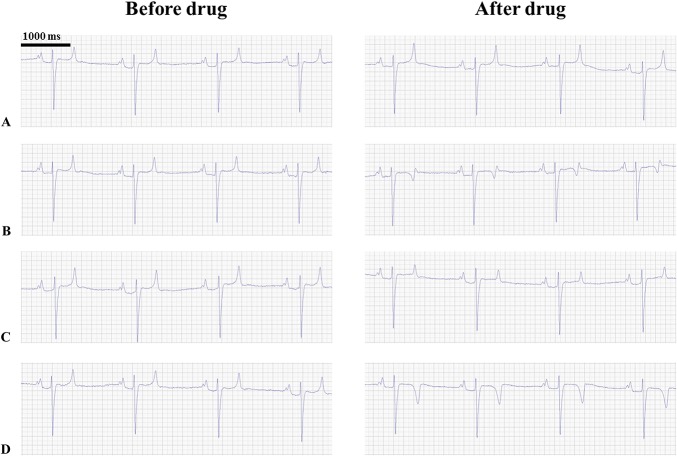
None of the horses in the study experienced adverse clinical reactions or an elevation of the cardiac biomarkers cardiac Troponin I and Creatinine Kinase fraction MB as a result of the drugs either alone or in combination (see Material Figure 1, Supplemental Digital Content 1, http://links.lww.com/JCVP/A283), and blood pressure remained unchanged after drug administration in the safety study (see Material Figure 1, Supplemental Digital Content 1, http://links.lww.com/JCVP/A283). The QRS duration and QTc in the safety study were prolonged by all treatments (Figs. 2 and 3). The T-wave morphology changed after dofetilide administration both alone and in combination in all 3 safety procedures (Figs. 4B, D). When the T wave was initially positive, it changed to a biphasic or negative deflection, and when it was initially biphasic, it became negative. This phenomenon occurred despite a steady heart rate, and was not observed after administration of saline or ranolazine alone (Figs. 4A, C).
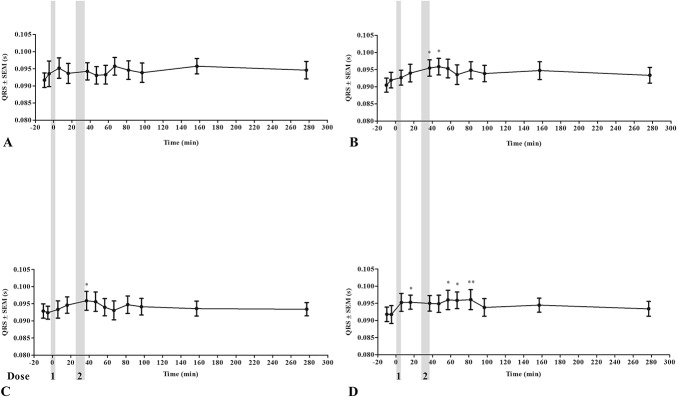
FIGURE 2.
QRS duration during drug treatment. QRS duration during the 4 safety procedures: saline/control (A), dofetilide (B), ranolazine (C), and a combination of dofetilide and ranolazine (D). The gray areas represent dose administrations. *P < 0.05; **P < 0.01.
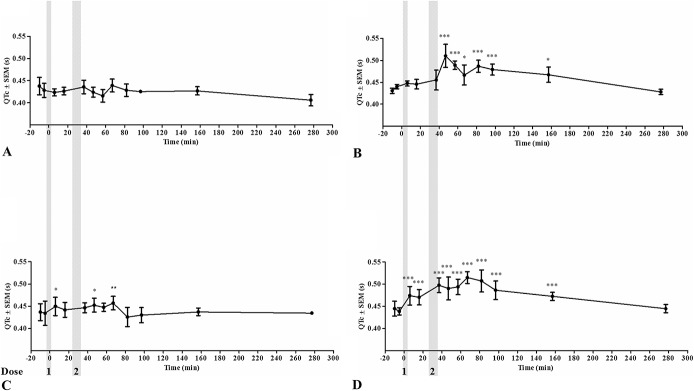
FIGURE 3.
Corrected QT intervals (QTc) during drug treatment. QTc during the 4 safety procedures: saline/control (A), dofetilide (B), ranolazine (C), and a combination of dofetilide and ranolazine (D). The gray areas represent dose administrations. *P < 0.05; **P < 0.01; ***P < 0.001.
FIGURE 4.
Changes in T-wave morphology following drug treatment. ECGs (lead II) from the same horse before and after administration of drugs: saline (A), dofetilide (B), ranolazine (C), and a combination of dofetilide and ranolazine (D). Note the changes in the T-wave morphology after dofetilide and combination treatment (B, D).
Electrophysiology
The time to spontaneous cardioversion after the first drug injection was compared with the time to spontaneous cardioversion after saline injection in the time-matched control procedures (Fig. 5). The mean time to cardioversion in minutes was 128.3 ± 29.9, 67.0 ± 22.7, 50.8 ± 10.2, and 24.5 ± 12.6 in the control, dofetilide, ranolazine, and combination procedure groups, respectively (see Material Table S2, Supplemental Digital Content 1, http://links.lww.com/JCVP/A283). Both ranolazine (P < 0.05) and the combination treatment (P < 0.05) significantly shortened the time to cardioversion. The combination of 0.889 μg/kg dofetilide and 0.104 mg/kg ranolazine cardioverted 6 of 8 horses (dose 1 combination, Fig. 5), whereas the same doses of the drugs administered individually were only able to cardiovert 3 (dose 2 dofetilide, Fig. 5) and 2 horses (dose 1 ranolazine, Fig. 5), respectively. Infusion of 8.0 μg/kg dofetilide did not result in cardioversion rates that were different from those in the control group.
FIGURE 5.
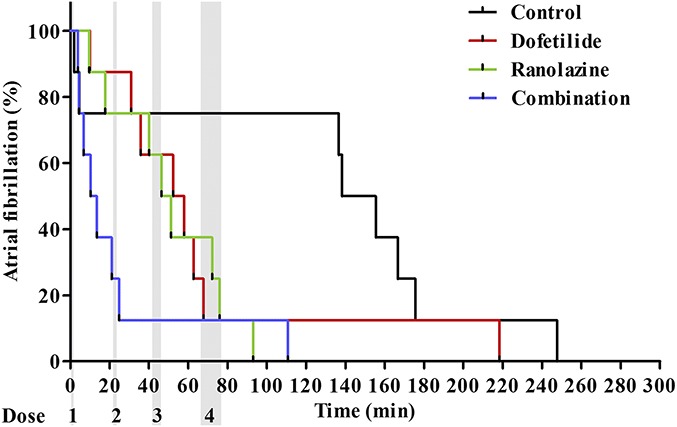
Time from start of drug administration to cardioversion. The percentage of horses remaining in AF as a function of time (in minutes). Time 0 is the start of the first drug administration. The gray areas represent the 4 dose administrations. In all groups n = 8.
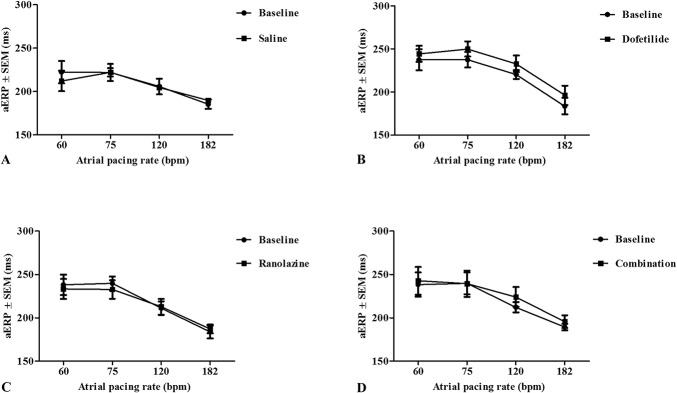
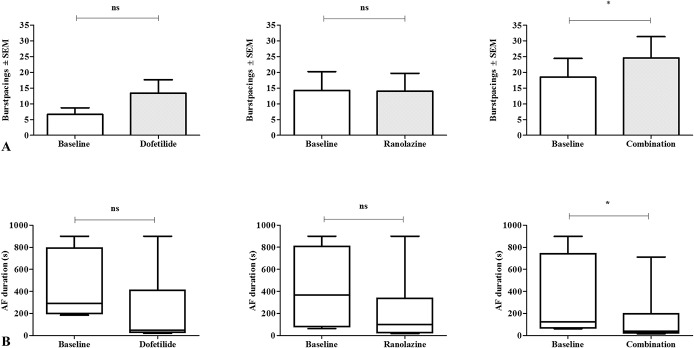
The threshold of excitation, aERP, AF vulnerability, and AF duration were studied before treatment and after the horses had received the full dose of each drug. It was not possible to perform the investigations after drug infusion in 6 saline, 1 dofetilide, and 1 combination procedure, because the horses were not in sinus rhythm. The threshold of excitation was in the range of 0.3–1.3 mA before drug treatment, and after drug treatment 0.3–1.1 mA (P > 0.05 for all procedures). In the control animals, aERP varied with pacing frequency from ∼240 milliseconds at 60 bpm to ∼180 milliseconds at 182 bpm (Fig. 6A). None of the treatments significantly prolonged aERP (Fig. 6). The combination treatment decreased AF vulnerability (P < 0.05) and AF duration (P < 0.05), whereas the drugs used individually had no effect on these parameters (Figs. 7 and 8).
FIGURE 6.
Right aERP after drug treatment. The aERP was measured at different pacing rates before and after the infusion of drugs: saline (A), dofetilide (B), ranolazine (C), and the combination of dofetilide and ranolazine (D). A, Only 2 horses cardioverted within the time of the experiment, therefore, n = 8 at the baseline and n = 2 in the saline group. B, One horse did not cardiovert within the time of the experiment and 1 horse had unstable placement of the electrode: n = 6. C, n = 8. D, One horse did not cardiovert within the time of the experiment: n = 7.
FIGURE 7.
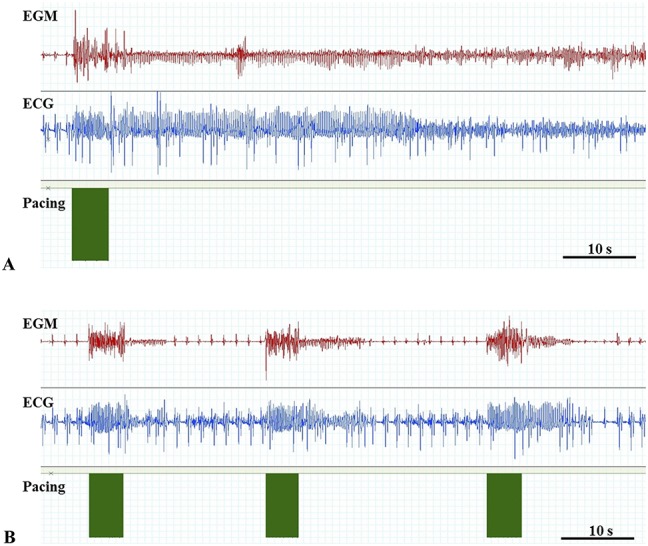
Induction of AF. Electrogram (EGM) from the right atrium, ECG, and pacing from the same horse before (A) and after (B) administration of the combination therapy with dofetilide and ranolazine. In both images tachypacing is performed leaving no visible gap between each stimuli. Note the short AF episodes (AF duration) after AF induction after combination therapy (B).
FIGURE 8.
Atrial fibrillation (AF) vulnerability and mean AF duration after drug treatment. A, AF vulnerability presented as the mean number of burst-pacings required to induce an AF episode of ≥15 minutes. B, AF duration is presented in box plots and whiskers represent minimum to maximum values. In the dofetilide procedures n = 6, in the ranolazine procedures n = 7 and in the combination procedures n = 7. * P < 0.05; ns = nonsignificant.
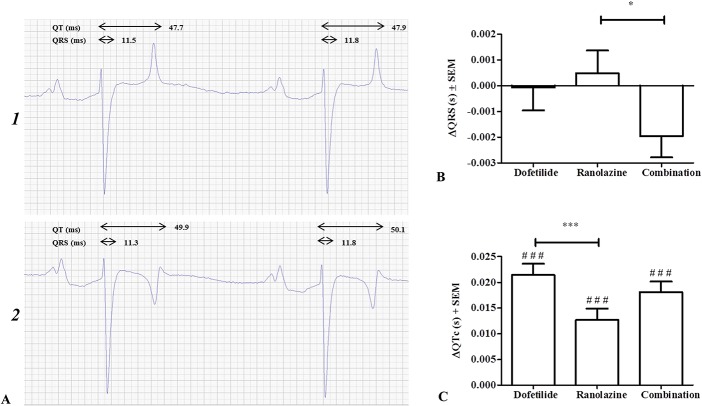
With respect to ventricular electrophysiology, none of the treatments altered the QRS duration when compared with the control group, yet this was significantly prolonged in the ranolazine group when compared with the combination group (ΔQRSran = 0.00049 ± 0.001 seconds, ΔQRScombi = −0.0020 ± 0.001 seconds, P < 0.05; Fig. 9B). Dofetilide, ranolazine, and the combination treatment all significantly increased QTc when compared with the control group (ΔQTcdof = 0.021 ± 0.002 seconds, ΔQTcran = 0.013 ± 0.002 seconds, ΔQTccombi = 0.018 ± 0.002 seconds, P < 0.001 for all 3 comparisons; Fig. 9C). No significant differences were found when comparing either drug used individually to the combination, but dofetilide prolonged the QTc more than ranolazine (P < 0.001). The maximum QTc reached was 0.43 ± 0.01 seconds in the control procedure, 0.48 ± 0.01 seconds after dofetilide treatment, 0.44 ± 0.01 seconds after ranolazine treatment, and 0.45 ± 0.01 seconds after combination treatment. No ventricular arrhythmias were detected during or after any of the drug treatments.
FIGURE 9.
Ventricular effects of drug treatments. A, ECG from the same horse before (1) and after (2) dofetilide administration with illustration of QRS duration and QT interval. Heart rate is 36 bpm in both ECGs. B, QRS duration and (C) corrected QT intervals (QTc) after drug treatment presented as the mean prolongation ± SEM compared with the control group receiving saline. #Represents significance levels of the comparison between the different drug treatments and the control group. *P < 0.05; ***P < 0.001; ###P < 0.001.
DISCUSSION
This study is the first to examine the effects of combining dofetilide and ranolazine for the termination of AF. The combination protocol demonstrated antiarrhythmic potential by cardioverting horses in acutely induced AF and decreasing AF vulnerability and duration after drug administration.
Combination Therapy in the Treatment of AF
One case report has previously found the combination of dofetilide and ranolazine to be effective in maintaining sinus rhythm in a patient with a history of paroxysmal AF.20 Several studies have combined ranolazine with amiodarone or dronedarone, finding the combination to be superior to either drug used individually.4,12,13,21,22 Dofetilide has also been proposed for use in combination therapy for the termination of AF.23 Previous studies combining antiarrhythmic compounds in the treatment of AF have shown synergistic effects.12,13,23,24 In this study, we demonstrated an increased antiarrhytmic effect for the combination of dofetilide and ranolazine relative to monotherapy with either drug. We demonstrated that the combination of 0.889 μg/kg dofetilide and 0.104 mg/kg ranolazine cardioverted 6 of 8 horses, whereas the individual use of these drugs at the same doses were only able to cardiovert 3 and 2 of the 8 horses, respectively. Furthermore, a decrease in AF vulnerability and duration only occurred after treatment with the combination of drugs. This protective mechanism against reinduction of AF corresponds well with previous findings of a combination strategy that was able to decrease the AF burden in patients with paroxysmal AF.4,20
Ranolazine as Monotherapy in AF
Ranolazine has shown the ability to terminate AF and atrial flutter in a canine sterile pericarditis model,25 and the drug was able to cardiovert induced AF to atrial flutter in anesthetised pigs.17 In humans, a “pill-in-pocket” approach, using a single oral dose of ranolazine to cardiovert AF of acute onset (between 3 and 48 hours of AF) was successful in 72% of cases.5 However, it is worth noting that no control group was included in this study, and spontaneous cardioversion is highly likely to have occurred in some of the patients.5 Other studies have found ranolazine useful for maintenance of sinus rhythm,26,27 whereas the AF burden was not decreased in the HARMONY trial after ranolazine administration alone.4 In this study, 2.4 mg/kg of ranolazine produced a significant antiarrhythmic effect, which is in contrast to the individual use of dofetilide up to a dose of 8.0 μg/kg. However, no effects on AF vulnerability or AF duration were found with the administration of either drug alone, indicating the lack of a protective effect on the initiation of new AF episodes.
Atrial Electrophysiology and Underlying Mechanisms
Both dofetilide and ranolazine have been reported to cause an increase in aERP.3,17,18,21 In this study, ranolazine resulted in aERP values similar to the baseline, and the 10–15-millisecond prolongation observed in aERP after dofetilide (Fig. 6B) did not reach statistical significance. As dofetilide alone did not display significant antiarrhythmic properties, it may be the case that the IKr antiarrhythmic target is not expressed in a sufficient level in horse atria to lead to cardioversion. The chosen maximum dose was based on the recommendations for safe and efficacious dosing in humans,16 which can be argued not to apply in horses. The expression of the gene encoding for the IKr (KCNH2) in the equine atria has been shown,14,28 but a higher expression of KCNH2 was found in the right ventricle compared with the right atrial appendage,14 in contrast to the uniform expression level in human atrial and ventricular myocardium.29 The functional role of IKr has only been demonstrated in equine ventricular tissue,30 and therefore, further studies are warranted. Despite an increased antiarrhythmic effect seen with the combination of drugs no changes in aERP were seen. It is unlikely that dofetilide acted in another way than IKr block and changes in repolarization. The possible explanations for the lack of effect on aERP are therefore; 1) dofetilide acted by blocking IKr and prolonging the action potential duration, but the prolongations were not sufficiently large to achieve statistical significance; 2) dofetilide acted through IKr block, but the effect was mediated by changes in action potential morphology that enhanced ranolazine-induced INa block but did not significantly prolong the action potential duration.31 In a study by Aguilar et al,31 it was shown that potassium channel block potentiates the AF-selective antiarrhythmic effects of optimized sodium-channel blockade. The mechanisms behind this synergism are rate dependent, and the effect is therefore largest in the atria during AF.
Effect of Drugs on Ventricular Electrophysiology
It has been shown that dofetilide can cause TdP in humans.2 No adverse cardiovascular or noncardiovascular reactions were encountered in the horses receiving ranolazine or dofetilide in this study. Both drugs displayed QTc-prolonging effects, in accordance with studies in other species.6,9,23 Dofetilide prolonged the QTc more than ranolazine, presumably because dofetilide mainly blocks IKr, and this prolongs the entire repolarisation phase of the action potential. Conversely, ranolazine blocks INa,late with great potency in ventricular myocytes, thereby shortening the early repolarization.8,32 None of the compounds prolonged the QRS interval, indicating that the ventricular effects were on the repolarization of the action potential. Human studies have shown similar QRS results after dofetilide administration, but a slight prolongation of the QRS duration after ranolazine administration.32 It is worth noting that in the safety part of this study, some prolongation of the QRS interval was seen, but these results were based on only 3 horses (Fig. 2). Little is known about the T-wave morphology in horses, but it was evident that dofetilide changed the morphology in this study (Figs. 4B, D). Infusion of ranolazine also seems to change the morphology, as the T wave appears to have smaller amplitude (Fig. 4C). An IKr block induced by dofetilide or ranolazine is known to affect T-wave morphology in humans by causing concentration-dependent T-wave flatness, asymmetry, and notching.33 The changes in T-wave morphology observed in this study may be unique to horses and should be studied further to determine causes and consequences.
A recent epidemiological study found an increased risk of TdP related to the use of ranolazine in a population of patients with prolonged QTc.7 By contrast, previous studies have reported that ranolazine reduces ventricular arrhythmias by suppressing early afterdepolarizations, despite the QTc-prolonging effect.6,9 In a canine model, TdP was induced using dofetilide, but when ranolazine was added, the number of TdP episodes was significantly decreased.6 No ventricular arrhythmogenic events were noted in this study, despite the observed QTc prolongation.
Study Limitations
The size of the study population poses an important limitation, which became apparent when 2 horses cardioverted very early in the control procedures. The decision to use 15 minutes of AF as the threshold for the start of treatment was based on previous studies,15,34 yet it could be argued that this was too short a time in this study.
Knowledge of the pharmacodynamics of dofetilide and ranolazine in horses is currently lacking. More information on this would be valuable to compare the findings from this study to those in other species. In humans, the elimination half-life of dofetilide is around 10 hours,35 and for ranolazine, is around 1.5 hours.36 As a consequence, the dosing regimen of this study (with 20 minutes in between treatments) is not considered to be problematic in terms of comparisons between dofetilide used alone and in combination. However, ranolazine plasma concentrations may not have reached the levels that could be expected if the total dose was given at once.
The level of electrophysiological analysis is too limited to define the exact mechanisms of interaction between dofetilide and ranolazine. The mechanisms suggested in the study therefore remain speculative. Furthermore, the mechanism of AF in this model is unknown and extrapolation of results should be performed with caution.
CONCLUSIONS
The combination of dofetilide and ranolazine showed clear antiarrhythmic effects—terminating acutely induced AF in horses, protecting from the reinduction of AF and shortening AF episodes induced after treatment. Ranolazine as a monotherapy was also found to terminate AF, but it did not display other antiarrhythmic effects. The use of the drugs individually and in combination prolonged the QTc, which may indicate the possibility of proarrhythmic events in the ventricles, although no such events were encountered.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge all staff members at The Large Animal Teaching Hospital, Department of Veterinary Clinical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen who were involved in the project. The authors also wish to thank GE Healthcare Denmark, for lending us equipment for blood pressure monitoring.
Footnotes
Supported by The Danish Council for Independent Research (DFF-1331-00313B) and The Danish Horse Levy Foundation.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcvp.org).
REFERENCES
- 1.Reef VB, Bonagura J, Buhl R, et al. Recommendations for management of equine athletes with cardiovascular abnormalities. J Vet Intern Med. 2014;28:749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125:381–389. [DOI] [PubMed] [Google Scholar]
- 3.Burashnikov A, Di Diego JM, Zygmunt AC, et al. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiffel JA, Camm AJ, Belardinelli L, et al. The HARMONY trial: combined ranolazine and dronedarone in the management of paroxysmal atrial fibrillation: mechanistic and therapeutic synergism. Circ Arrhythm Electrophysiol. 2015;8:1048–1056. [DOI] [PubMed] [Google Scholar]
- 5.Murdock DK, Kersten M, Kaliebe J, et al. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a review of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol J. 2009;9:260–267. [PMC free article] [PubMed] [Google Scholar]
- 6.Antoons G, Oros A, Beekman JD, et al. Late na(+) current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. J Am Coll Cardiol. 2010;55:801–809. [DOI] [PubMed] [Google Scholar]
- 7.Romero J, Baldinger SH, Goodman-Meza D, et al. Drug-induced torsades de pointes in an underserved urban population. Methadone: is there therapeutic equipoise? J Interv Card Electrophysiol. 2016;45:37–45. [DOI] [PubMed] [Google Scholar]
- 8.Antzelevitch C, Burashnikov A, Sicouri S, et al. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm 2011;8:1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antzelevitch C, Belardinelli L, Wu L, et al. Electrophysiologic properties and antiarrhythmic actions of a novel antianginal agent. J Cardiovasc Pharmacol Ther. 2004;9(suppl 1):S65–S83. [DOI] [PubMed] [Google Scholar]
- 10.Torp-Pedersen C, Møller M, Bloch-Thomsen PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–865. [DOI] [PubMed] [Google Scholar]
- 11.Abraham JM, Saliba WI, Vekstein C, et al. Safety of oral dofetilide for rhythm control of atrial fibrillation and atrial flutter. Circ Arrhythm Electrophysiol. 2015;8:772–776. [DOI] [PubMed] [Google Scholar]
- 12.Burashnikov A, Sicouri S, Di Diego JM, et al. Synergistic effect of the combination of ranolazine and dronedarone to suppress atrial fibrillation. J Am Coll Cardiol. 2010;56:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sicouri S, Burashnikov A, Belardinelli L, et al. Synergistic electrophysiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circ Arrhythm Electrophysiol. 2010;3:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haugaard MM, Hesselkilde EZ, Pehrson S, et al. Pharmacologic inhibition of small-conductance calcium-activated potassium (SK) channels by NS8593 reveals atrial antiarrhythmic potential in horses. Heart Rhythm. 2015;12:825–835. [DOI] [PubMed] [Google Scholar]
- 15.Haugaard MM, Pehrson S, Carstensen H, et al. Antiarrhythmic and electrophysiologic effects of flecainide on acutely induced atrial fibrillation in healthy horses. J Vet Intern Med. 2015;29:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindeboom JE, Kingma JH, Crijns HJ, et al. Efficacy and safety of intravenous dofetilide for rapid termination of atrial fibrillation and atrial flutter. Am J Cardiol. 2000;85:1031–1033. [DOI] [PubMed] [Google Scholar]
- 17.Kumar K, Nearing BD, Carvas M, et al. Ranolazine exerts potent effects on atrial electrical properties and abbreviates atrial fibrillation duration in the intact porcine heart. J Cardiovasc Electrophysiol. 2009;20:796–802. [DOI] [PubMed] [Google Scholar]
- 18.Sedgwick ML, Lip G, Rae AP, et al. Chemical cardioversion of atrial fibrillation with intravenous dofetilide. Int J Cardiol. 1995;49:159–166. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen PJ, Kanters JK, Buhl R, et al. Normal electrocardiographic QT interval in race-fit Standardbred horses at rest and its rate dependence during exercise. J Vet Cardiol. 2013;15:23–31. [DOI] [PubMed] [Google Scholar]
- 20.Shah SA, Koyama G, Da Costa D, et al. Combination use of ranolazine with dofetilide for the maintenance of atrial fibrillation. Int J Cardiol. 2014;172:e428–e429. [DOI] [PubMed] [Google Scholar]
- 21.Frommeyer G, Milberg P, Uphaus T, et al. Antiarrhythmic effect of ranolazine in combination with class-III drugs in an experimental whole heart model of atrial fibrillation. Cardiovasc Ther. 2013;31:e63–e71. [DOI] [PubMed] [Google Scholar]
- 22.Fragakis N, Koskinas KC, Katritsis DG, et al. Comparison of effectiveness of ranolazine plus amiodarone versus amiodarone alone for conversion of recent-onset atrial fibrillation. Am J Cardiol. 2012;110:673–677. [DOI] [PubMed] [Google Scholar]
- 23.Blaauw Y, Schotten U, van Hunnik A, et al. Cardioversion of persistent atrial fibrillation by a combination of atrial specific and non-specific class III drugs in the goat. Cardiovasc Res. 2007;75:89–98. [DOI] [PubMed] [Google Scholar]
- 24.Kirchhoff JE, Diness JG, Sheykhzade M, et al. Synergistic antiarrhythmic effect of combining inhibition of Ca(2)(+)-activated K(+) (SK) channels and voltage-gated Na(+) channels in an isolated heart model of atrial fibrillation. Heart Rhythm. 2015;12:409–418. [DOI] [PubMed] [Google Scholar]
- 25.Bhimani AA, Yasuda T, Sadrpour SA, et al. Ranolazine terminates atrial flutter and fibrillation in a canine model. Heart Rhythm. 2014;11:1592–1599. [DOI] [PubMed] [Google Scholar]
- 26.Hammond DA, Smotherman C, Jankowski CA, et al. Short-course of ranolazine prevents postoperative atrial fibrillation following coronary artery bypass grafting and valve surgeries. Clin Res Cardiol. 2015;104:410–417. [DOI] [PubMed] [Google Scholar]
- 27.Miles RH, Passman R, Murdock DK. Comparison of effectiveness and safety of ranolazine versus amiodarone for preventing atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2011;108:673–676. [DOI] [PubMed] [Google Scholar]
- 28.Finley MR, Li Y, Hua F, et al. Expression and coassociation of ERG1, KCNQ1, and KCNE1 potassium channel proteins in horse heart. Am J Physiol Heart Circ Physiol. 2002;283:H126–H138. [DOI] [PubMed] [Google Scholar]
- 29.Gaborit N, Le Bouter S, Szuts V, et al. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen PJ, Thomsen KB, Olander ER, et al. Molecular cloning and functional expression of the equine K+ channel KV11.1 (ether a Go-Go-related/KCNH2 gene) and the regulatory subunit KCNE2 from equine myocardium. PLoS One. 2015;10:e0138320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aguilar M, Xiong F, Qi XY, et al. potassium channel blockade enhances atrial fibrillation-selective antiarrhythmic effects of optimized state-dependent sodium channel blockade. Circulation. 2015;132:2203–2211. [DOI] [PubMed] [Google Scholar]
- 32.Johannesen L, Vicente J, Mason JW, et al. Differentiating drug-induced multichannel block on the electrocardiogram: randomized study of dofetilide, quinidine, ranolazine, and verapamil. Clin Pharmacol Ther. 2014;96:549–558. [DOI] [PubMed] [Google Scholar]
- 33.Vicente J, Johannesen L, Mason JW, et al. Comprehensive T wave morphology assessment in a randomized clinical study of dofetilide, quinidine, ranolazine, and verapamil. J Am Heart Assoc. 2015;4:e001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohmura H, Nukada T, Mizuno Y, et al. Safe and efficacious dosage of flecainide acetate for treating equine atrial fibrillation. J Vet Med Sci. 2000;62:711–715. [DOI] [PubMed] [Google Scholar]
- 35.Lenz TL, Hilleman DE. Dofetilide, a new class III antiarrhythmic agent. Pharmacotherapy. 2000;20:776–786. [DOI] [PubMed] [Google Scholar]
- 36.Jerling M. Clinical pharmacokinetics of ranolazine. Clin Pharmacokinet. 2006;45:469–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.