Abstract
Purpose of review
Cerebral impairment and acute kidney injury (AKI) are independent predictors of mortality in both adults and children with severe falciparum malaria. In this review, we present recent advances in understanding the pathophysiology, clinical features, and management of these complications of severe malaria, and discuss future areas of research.
Recent findings
Cerebral malaria and AKI are serious and well recognized complications of severe malaria. Common pathophysiological pathways include impaired microcirculation, due to sequestration of parasitized erythrocytes, systemic inflammatory responses, and endothelial activation. Recent MRI studies show significant brain swelling in both adults and children with evidence of posterior reversible encephalopathy syndrome-like syndrome although targeted interventions including mannitol and dexamethasone are not beneficial. Recent work shows association of cell-free hemoglobin oxidation stress involved in the pathophysiology of AKI in both adults and children. Paracetamol protected renal function likely by inhibiting cell-free-mediated oxidative stress. It is unclear if heme-mediated endothelial activation or oxidative stress is involved in cerebral malaria.
Summary
The direct causes of cerebral and kidney dysfunction remain incompletely understood. Optimal treatment involves prompt diagnosis and effective antimalarial treatment with artesunate. Renal replacement therapy reduces mortality in AKI but delayed diagnosis is an issue.
Keywords: cerebral malaria, malaria-associated acute kidney injury, pathophysiology, treatment
INTRODUCTION
Severe malaria incidence is approximately two million cases with nearly 430,000 deaths annually [1]. It is a medical emergency characterized by multisystem disease with different clinical manifestations between adults and children. However, recent studies show that cerebral involvement, kidney dysfunction, and acidosis are independent predictors of mortality in both adults and children (Fig. 1) [2,3]. This is supported by a meta-analysis of children with severe malaria that found prognostic indicators with the strongest association with death to be acute kidney injury (AKI) (odds ratio 5.96, 95% confidence interval, CI: 2.9–12.11) and coma (4.83, 95% CI: 3.11–7.5) [4▪▪].
FIGURE 1.
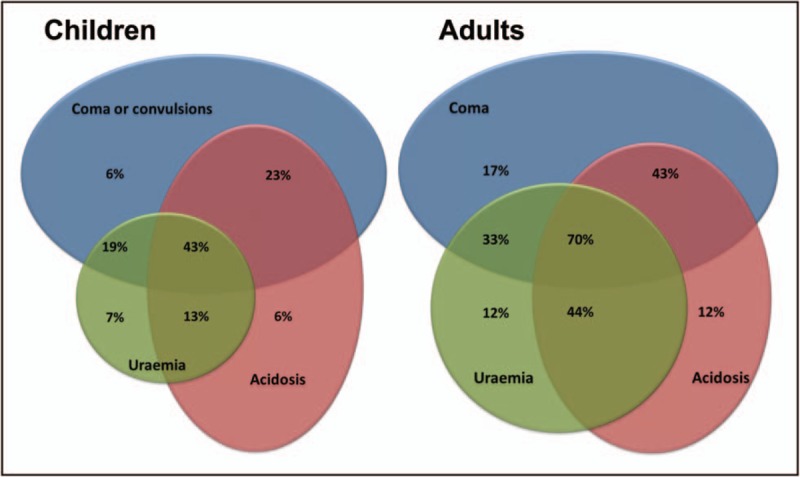
Venn diagrams of mortality of adults and children associated with prognostic manifestations of severe malaria [2,3]. Surface areas represent relative prevalence in severe malaria. Uremia defined as blood urea nitrogen more than 20 mg/dl in children and more than 48 mg/dl in adults. Acidosis defined as base excess less than −8 mmol/l in children and less than −3 mmol/l in adults. Coma score defined as Blantyre Coma Score less than 3 in children and Glasgow Coma Score less than 11 in adults. Reprinted from Tropical Medicine and International Health 19, Supplement 1, World Health Organization, Severe Malaria. Page 16. Copyright (2014).
Cerebral malaria is a clinical syndrome of impaired consciousness associated with malaria in the absence of hypoglycemia, convulsions, drugs, and nonmalarial causes characterized by unrousable coma defined by a Glasgow Coma Score less than11 (adults) [5] or Blantyre Coma Score less than 3 (children) [6–8]. Two large intervention trials in Asian adults and African children with severe malaria found that 54% of adult and 34% of pediatric patients had cerebral malaria [9,10]. AKI in severe falciparum malaria is caused by acute tubular necrosis and defined as a creatinine more than 265 μmol/l or urea more than 20 mmol/l [6]. In adults with severe malaria, AKI develops in up to 40% of patients, whereas in children, the incidence is historically reported at approximately 10% [9,10]. As the WHO definition does not define AKI adequately for pediatric malaria, the reported incidence of AKI in children is likely underestimated. The recent Kidney Disease: Improving Global Outcomes (KDIGO) classification standardizes AKI for clinical practice and research [11]. In adult severe malaria, 58% had AKI as defined by KDIGO, of whom 40% died, accounting for 71% of overall mortality [12▪]. Among children with severe malaria 46% had AKI as defined by KDIGO, of whom 12–24% died with increasingly severe AKI [13▪]. In two large multicenter studies, approximately 25% of children with severe malaria had increased blood urea nitrogen, accounting for roughly 50% of total deaths [10,14]. These studies imply that AKI complicating pediatric severe malaria has been previously underdiagnosed [13▪].
Notably, the majority of patients surviving these complications have complete recovery after appropriate treatment. The direct cause of coma and AKI complicating severe malaria are incompletely understood but likely share common pathophysiological mechanisms. This review will highlight recent developments in our understanding of the pathophysiologic and pathologic processes associated with cerebral malaria and malaria-associated AKI in addition to the clinical presentation, diagnosis and treatments of these complications.
Box 1.
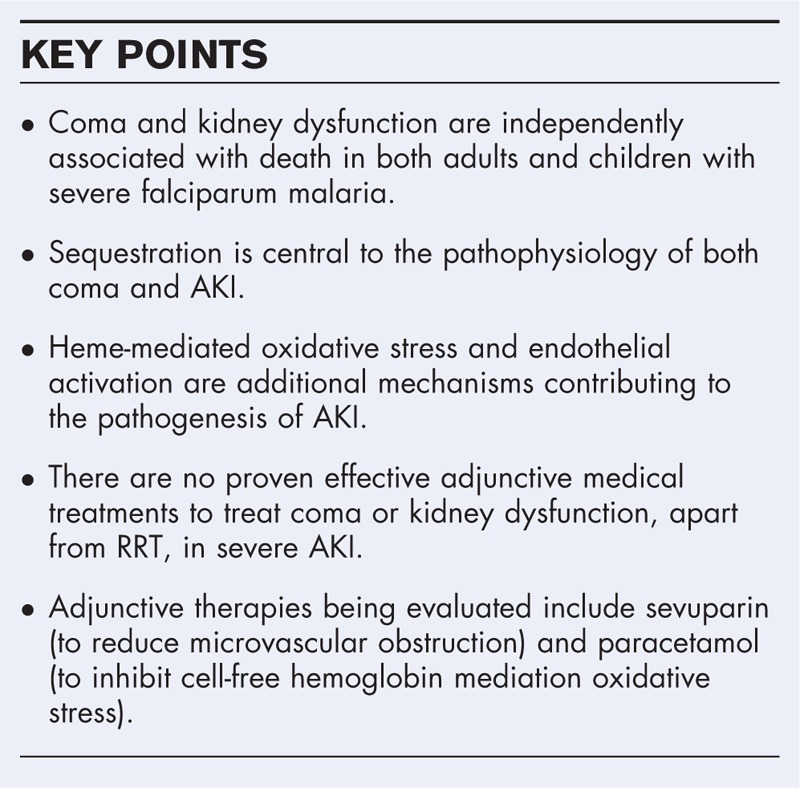
no caption available
PATHOGENESIS
Severe malaria is predominantly caused by Plasmodium falciparum because of its ability to induce infected red blood cell (RBC) cytoadherence to the vascular endothelium and consequent end-organ dysfunction. Other plasmodium species can cause severe disease and AKI [15], although their ability to cause coma is debated [6].
Microvascular obstruction
Parasites developing within the infected RBC transport P. falciparum erythrocyte membrane protein 1 (PfEMP1) to the RBC membrane functioning as a key ligand for cytoadherence [16]. PfEMP1 is expressed on RBC protrusions, or ‘knobs’, that confer points of attachment to the endothelium. PfEMP1 is strain specific, encoded by a highly variable var gene family, which provides antigenic variation for immune evasion and differential endothelial receptor binding. CD36 is an endothelial receptor constitutively expressed on most vascular beds [17]. The key endothelial receptor in the brain is intercellular adhesion molecule-1 [18]. Recent studies have identified endothelial protein C receptor as an important receptor in the brain that binds to a specific PfEMP1 domain (CIDRα1) [19▪,20–22]. Cytoadhesion results in sequestration of parasitized RBCs in the capillaries and postcapillary venules causing heterogeneous blockage of the microcirculation and tissue hypoxia [23]. In addition to flow obstruction by sequestered parasitized RBCs, microcirculatory flow is thought to be further compromised by increased rigidity of both infected and uninfected RBCs and clumping of infected RBCs (platelet-mediated autoagglutination) and uninfected RBCs adhering to infected RBCs (rosette formation) [24].
Direct visualization of microvascular obstruction is observed in the retina of adults and children with cerebral malaria, termed ‘malaria retinopathy’ [25,26]. Cerebral blood flow is not decreased in adults [27,28]. Intracranial pressure is often increased in children, but less so in adult patients [29,30]. Indirect assessment of sequestration via estimated total parasite biomass, measured by P. falciparum histidine rich protein 2, was shown to contribute to AKI in adults with severe malaria [31]. Autopsy studies of adults and retinopathy-positive children dying from cerebral malaria show prominent sequestration in the brain microvasculature compared to adults with fatal noncerebral malaria and retinopathy-negative children [32,33]. Postmortem studies report sequestration of parasitized RBCs in renal glomerular and peritubular capillaries in adults and children [32,34].
Endothelial activation
Microvascular obstruction-induced tissue hypoxia is compounded by microvascular dysfunction [35,36] and increased oxygen demand [36,37]. In adults with cerebral malaria, there is endothelial and astroglial activation in the brain [18], with variable inflammatory responses [38] and mild functional change to the blood–brain barrier [39,40]. In children with strictly defined retinopathy-positive cerebral malaria, breakdown of the endothelial barrier is observed particularly in areas of sequestration [32]. Patterns of histopathological change within the brain in cerebral malaria vary between adults and children, with less inflammatory cellular infiltrates and edema in adult cases [41,42]. A recent pediatric autopsy study found that HIV coinfection influences histopathology, increasing the degree of platelet and monocyte infiltration around damaged microvasculature [43].
In a recent MRI study of similarly defined children, 35% had evidence of brain swelling most commonly in fatal cases implicating brainstem herniation as the cause of death [44]. Another recent serial MRI study in India including adults and children found that 50% of patients had evidence of brain swelling with posterior vasogenic edema and vascular congestion in the basal nuclei [45▪]. All patients had rapid clinical improvement and radiological reversibility with hallmarks suggestive of posterior reversible encephalopathy syndrome. The exact cause of the brain swelling is yet unclear.
Studies of patients with severe malaria having AKI show reduced renal cortical blood flow [46], increased kidney size [47], and endothelial changes in both glomerular and peritubular capillaries on histopathology [34]. Cell-free hemoglobin and lipid peroxidation markers are strongly associated with AKI and renal replacement therapy (RRT) requirement in adults with severe malaria [12▪]. In children with severe malaria, an elevated heme-to-hemopexin ratio was associated with hemoglobinuria, stage 3 AKI, and 6-month mortality [48▪].
Cytokines
There is an imbalance of proinflammatory and anti-inflammatory responses in severe malaria [49]. The role of cytokines and chemokines in cerebral malaria has been recently reviewed [50]. However, many of these studies are in the murine experimental cerebral malaria model, the relevance of which has been questioned [51]. Conflicting evidence has emerged from human studies as to the association between cerebral malaria and levels of numerous cytokines such as tumor necrosis factor α (TNFα) [49,52–56]. Although cytokines and/or chemokines are clearly involved in the pathogenesis of malarial fever and may be associated with disease severity and/or cerebral malaria, it is not established that they are a cause of coma.
The role of cytokines and chemokines in the pathophysiology of AKI in severe malaria was recently highlighted. Plasma-soluble urokinase-type plasminogen activator receptor, a marker of immune activation, was independently associated with AKI and RRT requirement [31]. Previously, it was shown that TNFα, but not inteleukin (IL)6 or IL6:IL10 ratio, was associated with AKI suggesting that TNFα may induce localized renal tubular cell injury [57].
CLINICAL FEATURES
The clinical presentation of cerebral malaria is diffuse symmetrical encephalopathy with fever and absent or few focal neurological signs. In children, coma can rapidly develop after fever onset (mean, 2 days) [7]. In adults, coma is typically gradual with increasing drowsiness, confusion, obtundation, and high fevers (mean duration, 5 days). Convulsions are present in approximately 15% of adults and 80% of children with severe malaria [9,10] and frequently herald development of coma. Patients may recover full consciousness after a convulsion, thus transient postictal coma must be excluded [6]. Multiple convulsions are common and up to 50% of comatose children have subclinical seizures or status epilepticus. Ocular funduscopic findings include vessel color change, macular and extramacular whitening, and white-centered retinal hemorrhages [58] (Fig. 2).
FIGURE 2.
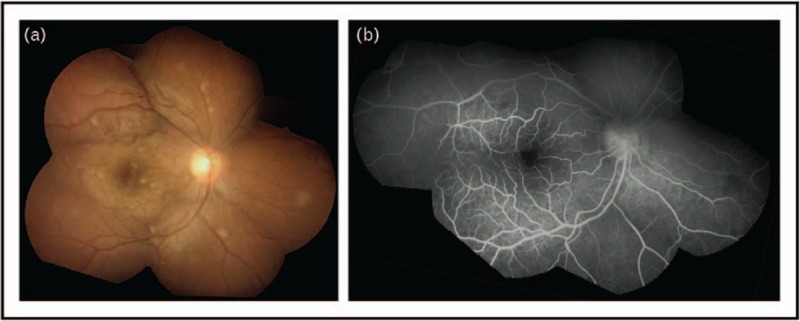
Malaria retinopathy in a Bangladeshi child with cerebral malaria. (A) Composite fundus photograph. (B) Fluorescein angiogram of same fundus image. Images show widespread retinal whitening and patchy hypoperfusion with a white-centered hemorrhage. Typical malarial retinopathy can include four findings: first, macular (perifoveal) and peripheral retinal whitening, second, retinal vessel whitening/discoloration, third, white-centered hemorrhages, and fourth, papilledema. The former two features (first and second) are specific for malaria and the latter two features (third and fourth) are also found in nonmalarial conditions. Reproduced with permission from BMJ Publishing Group Ltd [97].
Among survivors, the median time to coma recovery is roughly 24 h in children and 48 h in adults [9,10]. Retinal abnormalities resolve with no residual visual deficit. Neurologic sequelae occur in less than 1% of adults but up to 12% of children in the quinine-therapy era, including hemiplegia, cortical blindness, aphasia, and cerebellar ataxia [59]. Studies suggest that neurologic deficits may reflect slow neurological recovery [10,60]. Postmalaria neurological syndrome is self-limiting [61]; however, longer term neurological sequelae, including cognitive deficits and epilepsy, are reported among children [62,63▪].
The majority of malaria patients have risk factors for developing AKI, including volume depletion, hypoalbuminemia, male sex, previous AKI, concomitant bacterial sepsis, blackwater fever (BWF), or comorbidity, such as, diabetes. Severe intravascular hemolysis and hemoglobinuria in severe malaria, with or without AKI, is known as BWF. Although oliguria clinically indicates decreased function with a prerenal component, up to 80% of patients with malaria have nonoliguric AKI [64–66]. Thus, the clinical diagnosis will capture established anuric AKI but will underdiagnose moderate AKI and delay diagnosis. AKI complicating severe malaria can be categorized into four groups:
-
(1)
Few severity criteria with prerenal AKI that resolves with fluids.
-
(2)
Several severity criteria including AKI that resolves without RRT.
-
(3)
Progressive AKI that resolves with antimalarial treatment and RRT.
-
(4)
Multiorgan dysfunction, often with anuric AKI and cerebral malaria, who die prior to or during RRT with hemodynamic shock and/or respiratory failure.
DIAGNOSIS
Any comatose patient with a history of fever and/or travel to malaria-endemic regions must be considered to have cerebral malaria until proven otherwise. In children, febrile convulsions should be distinguished from cerebral malaria, wherein coma will persist beyond 1 h after anticonvulsive treatment is administered. Absence of fever does not rule out malaria. Antimalarial treatment should not be delayed in severely ill patients if diagnostics are unavailable or delayed.
Parasitological diagnosis is by microscopy of stained thin and thick blood smears. A rapid diagnostic test for parasite antigens can be performed if microscopy is unavailable. Patients may have a low circulating parasitemia because of sequestration, thus a low parasitemia is not reassuring [67]. In high-transmission settings, children with partial immunity tolerate higher parasitemia without severe symptoms and may be asymptomatic at low parasitemia. A parasitemia of more than 1 000 000/μl in African children with cerebral malaria is associated with fatal outcomes [7].
Funduscopy for malaria retinopathy improves specificity for diagnosis of cerebral malaria and is prognostic in patients with severe malaria [26,68,69] (Fig. 2). Alternative causes of coma must be ruled out including hypoglycemia, and bacterial, fungal, or viral meningoencephalitis. Lumbar puncture does not increase mortality in stable, comatose children with suspected cerebral malaria even when MRI brain swelling or papilledema is present [70▪].
There is no robust prognostic risk model or biomarker that can predict AKI or RRT requirement [71,72▪]. All patients with malaria should be considered at risk of developing AKI. To improve outcomes, early diagnosis and management is critical. AKI diagnosis requires quantification of creatinine (or urea) or observing low urine volume (<0.5 ml/kg/h) for 6 h (Fig. 3) [6,11]. Urine output is difficult to accurately assess in malaria endemic countries and may delay diagnosis. As the WHO creatinine threshold is not applicable to children, the diagnosis of AKI must be considered using all available patient data. The KDIGO AKI definition of a creatinine rise at least 1.5 times baseline is the current standard, and baseline creatinine can be back-calculated using the Modified Diet in Renal Disease (>19 years) or Swartz equation (≤18 years) [11,73].
FIGURE 3.
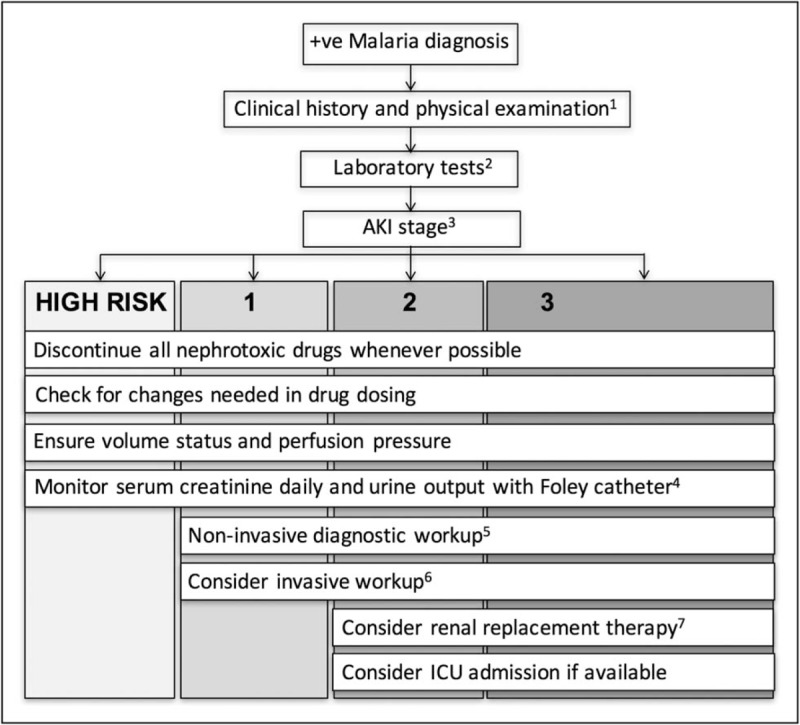
General management of AKI in malaria based on KDIGO guidelines. 1History including preadmission medications, comorbidities, and physical examination focusing on volume status and signs of concomitant sepsis. 2Laboratory tests including serum creatinine, urea, electrolytes, and full blood count. 3Stage using KDIGO staging criteria. If baseline creatinine is unknown, estimate using back-calculation of MDRD equation (>19 years) or Swartz equations (≤18 years). Urinary bladder catheterization to monitor initial urine output if unconscious. If ambulating, urine should be collected to monitor output. Patients should be managed according to AKI stage, as stage correlates with increased morbidity and mortality. 4Daily creatinine and urine output to monitor change in AKI stage severity and guide management. 5Additional investigations to assess AKI etiology: urine analysis, sediment microscopy, creatinine, and sodium; renal ultrasound to assess kidney size, presence of pyelonephritis, and inferior vena cava filling as a gauge of volume status; AKI biomarkers if applicable. Nephrotoxic drugs, that is, aminoglycosides, should be avoided whenever possible. Discontinuation of nephrotoxic drugs may assist with determining AKI etiology. 6If resources permit, monitor hemodynamic variables. Static central venous pressure is of limited value but recommended target is 0 to +5 cmH2O. Arterial pulse pressure as a dynamic variable may be more useful to gauge response to fluid administration. 7Early referral to center with RRT, particularly if one indication for RRT is present. Patients with multiorgan dysfunction are recommended to receive urgent dialysis within 2 h [6]. Patients should be evaluated 3 months after AKI resolution to monitor resolution of kidney function and/or development of chronic kidney disease. AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes; MDRD, modification of diet in renal disease [11]; RRT, renal replacement therapy. Reprinted from Kidney International Supplements 2, Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group, KDIGO Clinical Practice Guideline for Acute Kidney Injury. Page 25. Copyright (2012) with permission from Elsevier.
TREATMENT
The two key pillars of severe malaria treatment are prompt antimalarial treatment and supportive management. Adjunctive therapies targeted at the underlying pathophysiology are unproven.
Antimalarial treatment
Two landmark trials in patients with severe malaria definitively showed that intravenous artesunate reduced mortality by 35 and 23% in adults and children, respectively, compared to quinine [9,10]. Intravenous artesunate is now the first-line treatment for severe malaria as recommended by the WHO. Artemether and quinine are the second-line therapies [74]. The mechanism of improved survival over quinine is the rapid cidal activity of artesunate on young ring forms, preventing parasite maturation and sequestration [75]. Once the patient is able to take oral medication, and after a minimum of 24 h of artesunate, an oral artemisinin-based combination therapy can be initiated to complete the treatment.
Supportive treatment
Despite the best available artemisinin therapy for malaria, mortality remains unacceptably high and supportive management is key to reducing this. Comatose patients require endotracheal intubation with mechanical ventilation for airway protection. Rapid sequence intubation should be performed to prevent transient hypercapnia and increased intracranial pressure. Routine care should be implemented including regular turning, lateral positioning (‘recovery position’), and catheterization. Nasogastric tube insertion and suctioning may protect against aspiration, however, enteral feeding in nonintubated patients should be delayed (>60 h) because of increased risk of aspiration pneumonia [76].
The majority of children with cerebral malaria experience convulsions. Glucose replacement to ensure euglycemia and fever control with paracetamol are important. Prophylactic anticonvulsant therapy is not recommended. A randomized controlled trial (RCT) of phenobarbital in pediatric cerebral malaria showed increased mortality, likely caused by respiratory depression [77].
Fluid management and nephrotoxic drug avoidance are cornerstones for management of malaria-associated AKI. Cautious fluid management is important, as patients with AKI are not necessarily hypovolemic and are at high risk of developing pulmonary edema [78–81]. Rapid infusions may exacerbate intracranial hypertension and precipitate cerebral herniation. The large multicenter Fluid Expansion as Supportive Therapy study of African children with severe febrile illness showed a relative risk for death of 1.59 (95% CI: 1.10–2.31) with fluid bolus therapy among those with malaria [14]. The WHO recommends individualized restrictive fluid management, keeping the patient slightly dry, using slow infusion of isotonic crystalloids [74]. Patients with BWF require creatinine and hemoglobin monitoring as resulting severe anemia requires whole blood transfusion.
Treatment of malaria-associated AKI with RRT reduces mortality from 75 to 26% [64]. In general, RRT is urgently indicated when biochemical disturbances and volume overload refractory to conventional therapy are present. The additional thresholds included in the WHO malaria guidelines are based on findings that anuria and elevated or rapidly rising creatinine are sensitive indicators for RRT [6,64]. As AKI in malaria rapidly progresses and is often compounded by multiorgan dysfunction, early RRT is recommended. Although intermittent hemodialysis and continuous venovenous hemofiltration have been shown to be superior to peritoneal dialysis in adults with severe malaria [82], in the absence of hemodialysis, life-saving peritoneal dialysis should be initiated if this is the only modality available [83]. RRT has also been shown to be effective in the management of malaria-associated AKI in pediatric patients [84].
Many adjunctive therapies have been suggested, mainly driven by studies in murine experimental cerebral malaria. However, none has proven benefit in humans. Evidence for exchange transfusion and the more recently employed RBC exchange transfusion remains limited [85▪,86,87]. Mannitol [88,89], steroids [90,91], and monoclonal antibodies to TNF [54,56] are not recommended as treatments in cerebral malaria as studies show no benefits and potential harm. Furosemide and mannitol are ineffective in preventing and treating AKI and BWF, respectively, and may be harmful [92,93]. On the basis of the ability of paracetamol to inhibit hemoprotein-mediated AKI [94], a recent RCT of paracetamol in Bangladeshi adults with severe malaria found that acetaminophen improved kidney function and reduced the development of AKI, particularly in patients with high cell-free hemoglobin levels at enrollment [95▪,96]. Larger studies of paracetamol in adults and children with malaria are currently ongoing to further assess this renoprotective effect.
CONCLUSION
Cerebral malaria and AKI complicating severe malaria are prognostic for mortality in both adults and children. Microvascular obstruction and endothelial dysfunction are common mechanisms for both of these complications. Future study of adjunctive therapies should target reducing sequestration, improving endovascular function, and reducing hemoglobin-mediated oxidative stress.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by the Wellcome Trust of Great Britain.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.World. Health Orgranization. World Malaria Report. 2016 September 2, 2017. Available from: http://www.who.int/malaria/publications/world-malaria-report-2016/en/ [Google Scholar]
- 2.Dondorp AM, Lee SJ, Faiz MA, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis 2008; 47:151–157. [DOI] [PubMed] [Google Scholar]
- 3.von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in african children: findings from a large randomized trial. Clin Infect Dis 2012; 54:1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪▪.Sypniewska P, Duda JF, Locatelli I, et al. Clinical and laboratory predictors of death in African children with features of severe malaria: a systematic review and meta-analysis. BMC Med 2017; 15:147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meta-analysis of 30 publications showing that AKI (n = 4757) and coma (n = 16 573) are prognostic factors with the strongest association with death in pediatric severe malaria.
- 5.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Severe malaria. Trop Med Int Health 2014; 19 Suppl 1:7–131. [DOI] [PubMed] [Google Scholar]
- 7.Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med 1989; 71:441–459. [PubMed] [Google Scholar]
- 8.Newton CR, Chokwe T, Schellenberg JA, et al. Coma scales for children with severe falciparum malaria. Trans R Soc Trop Med Hyg 1997; 91:161–165. [DOI] [PubMed] [Google Scholar]
- 9.Dondorp A, Nosten F, Stepniewska K, et al. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005; 366:717–725. [DOI] [PubMed] [Google Scholar]
- 10.Dondorp AM, Fanello CI, Hendriksen IC, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 2010; 376:1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2:1–138. [Google Scholar]
- 12▪.Plewes K, Kingston HWF, Ghose A, et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis 2017; 17:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed observational pathophysiological study suggesting that cell-free hemoglobin and lipid peroxidation are associated with AKI and RRT requirement in Bangladeshi adults with severe malaria.
- 13▪.Conroy AL, Hawkes M, Elphinstone RE, et al. Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis 2016; 3:ofw046. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed observational study of 180 Ugandan children with severe malaria found that 46% had AKI as defined by KDIGO, of whom 12–24% died with increasingly severe AKI and 50% evolved or developed AKI after admission.
- 14.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011; 364:2483–2495. [DOI] [PubMed] [Google Scholar]
- 15.Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv Parasitol 2012; 80:151–201. [DOI] [PubMed] [Google Scholar]
- 16.Magowan C, Wollish W, Anderson L, Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med 1988; 168:1307–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke B, Coppel R, Wahlgren M. Falciparum malaria: sticking up, standing out and out-standing. Parasitol Today 2000; 16:416–420. [DOI] [PubMed] [Google Scholar]
- 18.Turner GD, Morrison H, Jones M, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol 1994; 145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 19▪.Bernabeu M, Danziger SA, Avril M, et al. Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc Natl Acad Sci USA 2016; 113:E3270–E3279. [DOI] [PMC free article] [PubMed] [Google Scholar]; Translational study showing that parasites isolated from adults with severe malaria exhibit specific var genes/PfEMP1 transcripts associated with parasite biomass and activated protein C/endothelial protein C receptor pathway disruption.
- 20.Gillrie MR, Avril M, Brazier AJ, et al. Diverse functional outcomes of Plasmodium falciparum ligation of EPCR: potential implications for malarial pathogenesis. Cell Microbiol 2015; 17:1883–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moxon CA, Wassmer SC, Milner DA, Jr, et al. Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children. Blood 2013; 122:842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner L, Lavstsen T, Berger SS, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013; 498:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silamut K, Phu NH, Whitty C, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol 1999; 155:395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop 2004; 89:309–317. [DOI] [PubMed] [Google Scholar]
- 25.Beare NA, Taylor TE, Harding SP, et al. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg 2006; 75:790–797. [PMC free article] [PubMed] [Google Scholar]
- 26.Maude RJ, Beare NA, Abu Sayeed A, et al. The spectrum of retinopathy in adults with Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 2009; 103:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clavier N, Rahimy C, Falanga P, et al. No evidence for cerebral hypoperfusion during cerebral malaria. Crit Care Med 1999; 27:628–632. [DOI] [PubMed] [Google Scholar]
- 28.Warrell DA, White NJ, Veall N, et al. Cerebral anaerobic glycolysis and reduced cerebral oxygen transport in human cerebral malaria. Lancet 1988; 2:534–538. [DOI] [PubMed] [Google Scholar]
- 29.Newton CR, Kirkham FJ, Winstanley PA, et al. Intracranial pressure in African children with cerebral malaria. Lancet 1991; 337:573–576. [DOI] [PubMed] [Google Scholar]
- 30.Waller D, Crawley J, Nosten F, et al. Intracranial pressure in childhood cerebral malaria. Trans R Soc Trop Med Hyg 1991; 85:362–364. [DOI] [PubMed] [Google Scholar]
- 31.Plewes K, Royakkers AA, Hanson J, et al. Correlation of biomarkers for parasite burden and immune activation with acute kidney injury in severe falciparum malaria. Malar J 2014; 13:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milner DA, Jr, Whitten RO, Kamiza S, et al. The systemic pathology of cerebral malaria in African children. Front Cell Infect Microbiol 2014; 4:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pongponratn E, Turner GD, Day NP, et al. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg 2003; 69:345–359. [PubMed] [Google Scholar]
- 34.Nguansangiam S, Day NP, Hien TT, et al. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health 2007; 12:1037–1050. [DOI] [PubMed] [Google Scholar]
- 35.Yeo TW, Lampah DA, Gitawati R, et al. Recovery of endothelial function in severe falciparum malaria: relationship with improvement in plasma L-arginine and blood lactate concentrations. J Infect Dis 2008; 198:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeo TW, Lampah DA, Kenangalem E, et al. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis 2013; 207:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Day NPJ, Phu NH, Bethell DP, et al. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet 1996; 348:219–223. [DOI] [PubMed] [Google Scholar]
- 38.MacPherson GG, Warrell MJ, White NJ, et al. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol 1985; 119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 39.Davis TM, Suputtamongkol Y, Spencer JL, et al. Measures of capillary permeability in acute falciparum malaria: relation to severity of infection and treatment. Clin Infect Dis 1992; 15:256–266. [DOI] [PubMed] [Google Scholar]
- 40.Warrell DA, Looareesuwan S, Phillips RE, et al. Function of the blood-cerebrospinal fluid barrier in human cerebral malaria: rejection of the permeability hypothesis. Am J Trop Med Hyg 1986; 35:882–889. [DOI] [PubMed] [Google Scholar]
- 41.Medana IM, Day NP, Sachanonta N, et al. Coma in fatal adult human malaria is not caused by cerebral oedema. Malar J 2011; 10:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponsford MJ, Medana IM, Prapansilp P, et al. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis 2012; 205:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hochman SE, Madaline TF, Wassmer SC, et al. Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. MBio 2015; 6:e01390–e01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seydel KB, Kampondeni SD, Valim C, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med 2015; 372:1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45▪.Mohanty S, Benjamin LA, Majhi M, et al. Magnetic resonance imaging of cerebral malaria patients reveals distinct pathogenetic processes in different parts of the brain. mSphere 2017; 2:e00193–e00217. [DOI] [PMC free article] [PubMed] [Google Scholar]; Detailed serial MRI study of adults and children with severe malaria in India showing vasogenic and cytotoxic edema with clinical and radiological reversibliity at 48–72 h.
- 46.Sitprija V, Vongsthongsri M, Poshyachinda V, Arthachinta S. Renal failure in malaria: a pathophysiologic study. Nephron 1977; 18:277–287. [DOI] [PubMed] [Google Scholar]
- 47.Atalabi OM, Orimadegun AE, Adekanmi AJ, Akinyinka OO. Ultrasonographic renal sizes, cortical thickness and volume in Nigerian children with acute falciparum malaria. Malar J 2013; 12:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48▪.Elphinstone RE, Conroy AL, Hawkes M, et al. Alterations in systemic extracellular heme and hemopexin are associated with adverse clinical outcomes in ugandan children with severe malaria. J Infect Dis 2016; 214:1268–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]; Observation study of Ugandan children with severe malaria (n = 179) showing that an elevated heme-to-hemopexin ratio was associated with jaundice, hemoglobinuria, stage 3 AKI, and 6-month mortality.
- 49.Day NP, Hien TT, Schollaardt T, et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis 1999; 180:1288–1297. [DOI] [PubMed] [Google Scholar]
- 50.Dunst J, Kamena F, Matuschewski K. Cytokines and chemokines in cerebral malaria pathogenesis. Front Cell Infect Microbiol 2017; 7:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craig AG, Grau GE, Janse C, et al. The role of animal models for research on severe malaria. PLoS Pathog 2012; 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grau GE, Taylor TE, Molyneux ME, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 1989; 320:1586–1591. [DOI] [PubMed] [Google Scholar]
- 53.Kwiatkowski D, Hill AV, Sambou I, et al. TNF concentration in fatal cerebral, nonfatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 1990; 336:1201–1204. [DOI] [PubMed] [Google Scholar]
- 54.Kwiatkowski D, Molyneux ME, Stephens S, et al. Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med 1993; 86:91–98. [PubMed] [Google Scholar]
- 55.Shabani E, Ouma BJ, Idro R, et al. Elevated cerebrospinal fluid tumour necrosis factor is associated with acute and long-term neurocognitive impairment in cerebral malaria. Parasite Immunol 2017; 39:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Hensbroek MB, Palmer A, Onyiorah E, et al. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis 1996; 174:1091–1097. [DOI] [PubMed] [Google Scholar]
- 57.Lieberthal W, Koh JS, Levine JS. Necrosis and apoptosis in acute renal failure. Semin Nephrol 1998; 18:505–518. [PubMed] [Google Scholar]
- 58.Lewallen S, Taylor TE, Molyneux ME, et al. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology 1993; 100:857–861. [DOI] [PubMed] [Google Scholar]
- 59.Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet 1990; 336:1039–1043. [DOI] [PubMed] [Google Scholar]
- 60.van Hensbroek MB, Palmer A, Jaffar S, et al. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr 1997; 131:125–129. [DOI] [PubMed] [Google Scholar]
- 61.Nguyen TH, Day NP, Ly VC, et al. Postmalaria neurological syndrome. Lancet 1996; 348:917–921. [DOI] [PubMed] [Google Scholar]
- 62.Birbeck GL, Molyneux ME, Kaplan PW, et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol 2010; 9:1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63▪.Brim R, Mboma S, Semrud-Clikeman M, et al. Cognitive outcomes and psychiatric symptoms of retinopathy-positive cerebral malaria: cohort description and baseline results. Am J Trop Med Hyg 2017; 97:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]; First cohort study assessing MRI brain imaging with long-term outcomes in children with retinopathy-positive cerebral malaria (n = 221) showing that children less than 5 years had delays in motor, language, and social development at 1-month follow-up visits.
- 64.Tran TMT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis 1992; 15:874–880. [DOI] [PubMed] [Google Scholar]
- 65.Aloni MN, Nsibu CN, Meeko-Mimaniye M, et al. Acute renal failure in Congolese children: a tertiary institution experience. Acta Paediatr 2012; 101:e514–e518. [DOI] [PubMed] [Google Scholar]
- 66.Esezobor CI, Ladapo TA, Osinaike B, Lesi FE. Paediatric acute kidney injury in a tertiary hospital in Nigeria: prevalence, causes and mortality rate. PLoS One 2012; 7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White NJ, Krishna S. Treatment of malaria: some considerations and limitations of the current methods of assessment. Trans R Soc Trop Med Hyg 1989; 83:767–777. [DOI] [PubMed] [Google Scholar]
- 68.Beare NA, Southern C, Chalira C, et al. Prognostic significance and course of retinopathy in children with severe malaria. Arch Ophthalmol 2004; 122:1141–1147. [DOI] [PubMed] [Google Scholar]
- 69.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 2004; 10:143–145. [DOI] [PubMed] [Google Scholar]
- 70▪.Moxon CA, Zhao L, Li C, et al. Safety of lumbar puncture in comatose children with clinical features of cerebral malaria. Neurology 2016; 87:2355–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]; Retrospective study of 1075 children with cerebral malaria in Malawi found that performing lumbar punctures in clinically stable children (n = 866) did not increase mortality even if brain swelling on MRI or papilledema was present.
- 71.Hanson J, Hasan MM, Royakkers AA, et al. Laboratory prediction of the requirement for renal replacement in acute falciparum malaria. Malar J 2011; 10:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72▪.van Wolfswinkel ME, Koopmans LC, Hesselink DA, et al. Neutrophil gelatinase-associated lipocalin (NGAL) predicts the occurrence of malaria-induced acute kidney injury. Malar J 2016; 15:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pilot study of imported malaria cohort showing that urine neutrophil gelatinase-associated lipocalin had strong positive predictive value, negative predictive value, and Area Under the curve of the Receiver Operating Characteristic curve for AKI. Although this study is limited by a small sample size, if this finding bears out in larger studies and this biomarker is developed as a point-of-care test, it could facilitate prompt AKI diagnosis in patients with severe malaria.
- 73.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 2017; 376:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.World Health Organization. World Health Organization Guidelines for the treatment of malaria. Third edition. Geneva: World Health Organization, 2015. Available from: http://www.who.int/malaria/publications/atoz/9789241549127/en/ [Google Scholar]
- 75.ter Kuile F, White NJ, Holloway P, et al. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol 1993; 76:85–95. [DOI] [PubMed] [Google Scholar]
- 76.Maude RJ, Hoque G, Hasan MU, et al. Timing of enteral feeding in cerebral malaria in resource-poor settings: a randomized trial. PLoS One 2011; 6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crawley J, Waruiru C, Mithwani S, et al. Effect of phenobarbital on seizure frequency and mortality in childhood cerebral malaria: a randomised, controlled intervention study. Lancet 2000; 355:701–706. [DOI] [PubMed] [Google Scholar]
- 78.Hanson J, Anstey NM, Bihari D, et al. The fluid management of adults with severe malaria. Crit Care 2014; 18:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanson J, Lam SW, Mohanty S, et al. Central venous catheter use in severe malaria: time to reconsider the World Health Organization guidelines? Malar J 2011; 10:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hanson JP, Lam SW, Mohanty S, et al. Fluid resuscitation of adults with severe falciparum malaria: effects on acid-base status, renal function, and extravascular lung water. Crit Care Med 2013; 41:972–981. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen HP, Hanson J, Bethell D, et al. A retrospective analysis of the haemodynamic and metabolic effects of fluid resuscitation in Vietnamese adults with severe falciparum malaria. PLoS One 2011; 6:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med 2002; 347:895–902. [DOI] [PubMed] [Google Scholar]
- 83.Mishra SK, Mahanta KC. Peritoneal dialysis in patients with malaria and acute kidney injury. Perit Dial Int 2012; 32:656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asinobi AO, Ademola AD, Alao MA. Haemodialysis for paediatric acute kidney injury in a low resource setting: experience from a tertiary hospital in South West Nigeria. Clin Kidney J 2016; 9:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85▪.Calvo-Cano A, Gomez-Junyent J, Lozano M, et al. The role of red blood cell exchange for severe imported malaria in the artesunate era: a retrospective cohort study in a referral centre. Malar J 2016; 15:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]; Retrospective study of imported malaria (n = 42) found four patients who underwent RBC exchange. The procedure required a median of 12 units of packed RBCs and did not shorten parasite elimination rate.
- 86.Gyr K, Speck B, Ritz R, et al. Cerebral tropical malaria with blackwater fever. A current diagnostic and therapeutic problem [in German]. Schweiz Med Wochenschr 1974; 104:1628–1630. [PubMed] [Google Scholar]
- 87.Tan KR, Wiegand RE, Arguin PM. Exchange transfusion for severe malaria: evidence base and literature review. Clin Infect Dis 2013; 57:923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohanty S, Mishra SK, Patnaik R, et al. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis 2011; 53:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Namutangula B, Ndeezi G, Byarugaba JS, Tumwine JK. Mannitol as adjunct therapy for childhood cerebral malaria in Uganda: a randomized clinical trial. Malar J 2007; 6:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffman SL, Rustama D, Punjabi NH, et al. High-dose dexamethasone in quinine-treated patients with cerebral malaria: a double-blind, placebo-controlled trial. J Infect Dis 1988; 158:325–331. [DOI] [PubMed] [Google Scholar]
- 91.Warrell DA, Looareesuwan S, Warrell MJ, et al. Dexamethasone proves deleterious in cerebral malaria. A double-blind trial in 100 comatose patients. N Engl J Med 1982; 306:313–319. [DOI] [PubMed] [Google Scholar]
- 92.Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010; 65:283–293. [DOI] [PubMed] [Google Scholar]
- 93.Ho KM, Sheridan DJ. Meta-analysis of frusemide to prevent or treat acute renal failure. Br Med J 2006; 333:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci USA 2010; 107:2699–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95▪. Plewes K. Intravascular haemolysis, oxidative stress and acute kidney injury in severe falciparum malaria [DPhil]. University of Oxford, (Nuffield Department of Medicine; Green Templeton College.) University of Oxford, 2016. [Google Scholar]; Phase 2 RCT of paracetamol in adults with severe and moderately severe malaria found that paracetamol improved kidney function and reduced odds of developing AKI, particularly in patients with elevated cell-free hemoglobin at enrollment.
- 96.Plewes K, Kingston HWWT, Ghose A, et al. editors. Paracetamol as adjunctive treatment in severe and moderately severe falciparum malaria: An open label, randomised controlled trial. In: American Society of Tropical Medicine and Hygiene 65th Annual Meeting. Philadelphia, Pennsylvania, 2015. [Google Scholar]
- 97.Maude RJ, Plewes K, Dimock J, Dondorp AM. Low-cost portable fluorescein angiography. Br J Ophthalmol 2011; 95:1213–1215. [DOI] [PubMed] [Google Scholar]


