Background
Achieving the ambitious global HIV treatment goals will require a marked expansion of antiretroviral therapy (ART) coverage and close attention to HIV service quality [1]. In response, a growing body of evidence supports the use of differentiated ART services (DARTS) for subgroups of HIV-positive individuals [2–14]. Although differentiated services can support diverse patient groups, most current DARTS initiatives target stable adult patients, that is, those demonstrating ART adherence and viral suppression or viral suppression alone [15]. Both community-based and facility-based DARTS can reduce the burdens associated with frequent and lengthy clinic visits for both patients and health providers. Ultimately, these models aim to enhance retention, ART adherence, viral suppression, and quality of life [16].
The implementation of such novel approaches involves fundamental changes to delivery systems. This can make monitoring services more challenging, requiring new approaches to ensure that data are available to inform the care of individuals engaged in DARTS and enable programmatic evaluation of diverse DARTS models.
Important lessons have been learned regarding HIV program monitoring and evaluation (M&E). These include the critical importance of parsimony and pragmatism, focusing on information that is feasible to collect and essential for patient and program management [17]. These principles are particularly important as existing M&E systems evolve in response to increasingly diverse DARTS models.
This article highlights common gaps in M&E systems with respect to DARTS. We outline elements of an integrated, streamlined approach that provides robust information to guide patient and program management, while minimizing the burden of data collection and aggregation. Lastly, we note that additional information is needed for assessment and strategic planning at the health system level; this may require additional data collection and/or special studies.
Patient-level data: documenting eligibility for and engagement in differentiated antiretroviral therapy services
An increasing number of countries endorse the use of DARTS, such as facility-based adherence clubs, community-based antiretroviral groups, fast-track visits, outreach services, and/or community drug pickups [18]. In order to take DARTS to scale, it is critically important that providers are able to identify which patients are eligible for DARTS, given current local guidelines; whether they are enrolled in DARTS; and in which model they are participating. As eligibility may change over time, this information will need to be updated at each visit. Additionally, providers need to know at a glance whether and when patients have received their ART medication and required laboratory assessments, as well as their adherence levels and psychosocial support needs.
At present, standardized, structured approaches to document this information are scarce. Existing tools were not designed to capture patient-level information relevant to DARTS and, thus, are limited in guiding providers through the key steps in a DARTS patient visit. Modification of the ART medical record form will be required to capture these unique elements. Using the ART medical record, which is ubiquitous across countries, as the primary repository of DARTS information is essential to avoid the fragmentation that occurs whenever some information is documented at the clinic and some only in separate forms to be completed in the community, the pharmacy, or the laboratory.
In addition to a modified ART record, supplementary tools are needed to collect information regarding membership in patient DARTS groups [19], and to capture essential information from encounters that occur outside the facility (e.g. community ART groups), such as new symptoms, especially those that might indicate tuberculosis, adherence with ART and pregnancy status, as well as other information that may necessitate timely follow-up and referral for appropriate complementary services. These data should be regularly communicated with staff at the health facility wherever the patient receives clinical care, to trigger appropriate action such as recall to the health facility; they should also be transcribed into each patient's ART record.
Program-level data: understanding differentiated antiretroviral therapy services coverage and performance
At the program level, information for monitoring and evaluating DARTS models is also important, particularly early in their implementation and scale-up. Examples of relevant programmatic questions include the proportion of health facilities offering DARTS, the proportion of eligible patients enrolled in DARTS, and the proportion of patients enrolled in specific DARTS models who are retained and achieved durable viral suppression (Fig. 1). Some of this information may also be useful at district, provincial, or regional levels, for example, to compare coverage of DARTS and patient outcomes within and between localities; (though, cross-country comparisons may be limited by varying eligibility criteria for DARTS).
Fig. 1.
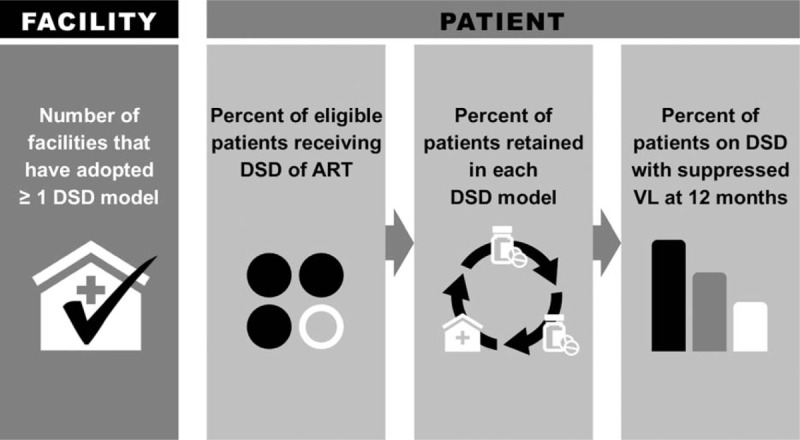
Indicators for differentiated antiretroviral therapy.
Unfortunately, existing widely used ART M&E systems are not yet able to measure the performance of DARTS in this way [20,21]. To describe DARTS coverage, uptake, and outcomes, M&E systems will need to collect and summarize the types of data described above. Depending on local resources and priorities, such data may be disaggregated by type of DARTS model, age, sex, and key population group.
In some contexts, tracking and manually calculating a new set of indicators using paper registers may not be feasible or advisable. However, countries with patient-level electronic ART databases may add data elements to capture DARTS eligibility and engagement for every patient at every visit. In such scenarios, it is feasible to aggregate patient data in an automated fashion and easily describe DARTS model coverage, and outcomes.
Health system-level
Some questions regarding the impact of DARTS at the health system level (e.g. patient/provider satisfaction, wait times, efficiency, and cost) can best be addressed via periodic data collection and/or special studies. Country programs may choose to convene annual/semiannual data review meetings, wherein facility-level or district-level staff present detailed information on a sample of patient records, as a complement to routinely reported information. Data collection and discussions could be focused on areas of high interest, such as specific quality improvement interventions. This could be supplemented with periodic assessments of provider–patient load, wait times, satisfaction, and measures to inform cost-effectiveness calculations.
Conclusion
As differentiated ART is implemented broadly, country programs, funders, and program managers will seek information on the coverage and performance of such models. Refinement of existing M&E systems is, therefore, required. Efforts to ‘differentiate’ M&E systems must be parsimonious and pragmatic; enabling data-driven learning to optimize services and outcomes.
Acknowledgements
The authors wish to acknowledge the contributions by the following individuals: Peter Ehrenkranz, Tiffany Harris, Maria Lahuerta, Ruby Fayorsey, and Fatima Tsiouris.
Funding: The authors acknowledge funding support from the Bill and Melinda Gates Foundation.
Authors’ contributions: W.R., M.R., M.S., and W.E.S. conceptualized the article. W.R., M.R., and A.S. wrote the initial draft and A.S. developed Figure 1. All authors reviewed and approved the final draft and approved of the decision to submit the manuscript for publication.
Conflicts of interest
There are no conflicts of interest.
References
- 1.El-Sadr WM, Rabkin M, DeCock KM. Population health and individualized care in the global AIDS response: synergy or conflict?. AIDS 2016; 30:2145–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2016. [PubMed] [Google Scholar]
- 3.Mutasa-Apollo T, Ford N, Wiens M, Socias ME, Negussie E, Wu P, et al. Effect of frequency of clinic visits and medication pick-up antiretroviral therapy outcomes: a systematic review and meta-analysis. J Int AIDS Soc 2017; 20 Suppl 4:21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bemelmans M, Baert S, Goemaere E, Wilkinson L, Vandendyck M, van Cutsem G, et al. Community-supported models of care for people on HIV treatment in sub-Saharan Africa. Trop Med Int Heal 2014; 19:968–977. [DOI] [PubMed] [Google Scholar]
- 5.Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community-based antiretroviral therapy programs can overcome barriers to retention of patients and decongest health services in sub-Saharan Africa: a systematic review. Int Health 2013; 5:169–179. [DOI] [PubMed] [Google Scholar]
- 6.Grimsrud A, Lesosky M, Kalombo C, Bekker L-G, Myer L. Community-based adherence clubs for the management of stable antiretroviral therapy patients in Cape Town, South Africa: a cohort study. J Acquir Immune Defic Syndr 2016; 71:e16–e23. [DOI] [PubMed] [Google Scholar]
- 7.Koole O, Tsui S, Wabwire-Mangen F, Kwesigabo G, Menten J, Mulenga M, et al. Retention and risk factors for attrition among adults in antiretroviral treatment programmes in Tanzania, Uganda and Zambia. Trop Med Int Heal 2014; 19:1397–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luque-Fernandez MA, Van Cutsem G, Goemaere E, Hilderbrand K, Schomaker M, Mantangana N, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One 2013; 8:e56088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ssonko C, Gonzalez L, Mesic A, da Fonseca MS, Achar J, Safar N, et al. Delivering HIV care in challenging operating environments: the MSF experience towards differentiated models of care for settings with multiple basic healthcare needs. J Int AIDS Soc 2017; 20 Suppl 4:21654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsondai P, Wilkinson L, Grimsrud A, Mdlalo P, Trivino A, Boulle A. High rates of retention and viral suppression in the scale-up of antiretroviral therapy adherence clubs in Cape Town, South Africa. J Int AIDS Soc 2017; 20 Suppl 4:21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogt F, Kalenga L, Lukela J, Salumu F, Diallo I, Nico E, et al. Decentralizing ART supply for stable HIV patients to community-based distribution centres: Programme outcomes from an urban context in Kinshasa, DRC. J Acquir Immune Defic Syndr 2017; 74:326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilkinson LS. ART adherence clubs: a long-term retention strategy for clinically stable patients receiving antiretroviral therapy. South Afr J HIV Med 2013; 14:48–50. [Google Scholar]
- 13.Woodd SL, Grosskurth H, Levin J, Amuron B, Namara G, Birunghi J, et al. Home-based versus clinic-based care for patients starting antiretroviral therapy with low CD4+ cell counts: findings from a cluster-randomized trial. AIDS 2014; 28:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wools-Kaloustian KK, Sidle JE, Selke HM, Vedanthan R, Kemboi EK, Boit LJ, et al. A model for extending antiretroviral care beyond the rural health centre. J Int AIDS Soc 2009; 12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldrop G, Doherty M, Vitoria M, Ford N. Stable patients and patients with advanced disease: consensus definitions to support sustained scale up of antiretroviral therapy. Trop Med Int Heal 2016; 21:1124–1130. [DOI] [PubMed] [Google Scholar]
- 16.Duncombe C, Rosenblum S, Hellmann N, Holmes C, Wilkinson L, Biot M, et al. Reframing HIV care: putting people at the centre of antiretroviral delivery. Trop Med Int Heal 2015; 20:430–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organisation (WHO). Consolidated strategic information guidelines for HIV in the health sector. Geneva: WHO; 2015. [PubMed] [Google Scholar]
- 18.Swaziland Ministry of Health. Standard operating procedures for implementing community-centred models of ART service delivery (CommART) in Swaziland. June 2016. Swaziland: Ministry of Health; 2016. [Google Scholar]
- 19.Médecins Sans Frontières. CAG Register. 2013. [Google Scholar]
- 20.UNAIDS. Global Aids Monitoring 2017: indicators for monitoring the 2016 United Nations Political Declaration on HIV and AIDS. Geneva: UNAIDS; 2017. [Google Scholar]
- 21.PEPFAR. Monitoring, evaluation, and indicator reference guide October v.2.1. 2017. [Google Scholar]


