Abstract
Resolving the mechanisms that switch competition to cooperation is key to understand biological organization1. This is particularly relevant for intrasexual competition, which often leads to males harming females2. Recent theory proposes that kin selection may modulate female harm by relaxing competition among relatives3–5. We experimentally manipulated the relatedness of groups of male Drosophila melanogaster competing over females to demonstrate that, as expected, within group relatedness inhibits male competition and female harm. Females exposed to three brothers unrelated to the female had higher lifetime reproductive success and slower reproductive ageing compared to females exposed to triplets of males unrelated to each other. Triplets of brothers also fought less with each other, courted females less intensively and lived longer than triplets of unrelated males. However, associations among brothers may be vulnerable to invasion by minorities of unrelated males: when two brothers were matched with an unrelated male, the latter sired on average twice as many offspring as either brother. These results demonstrate that relatedness can profoundly affect fitness through its modulation of intrasexual competition, as flies plastically adjust sexual behaviour in a way consistent with kin selection theory.
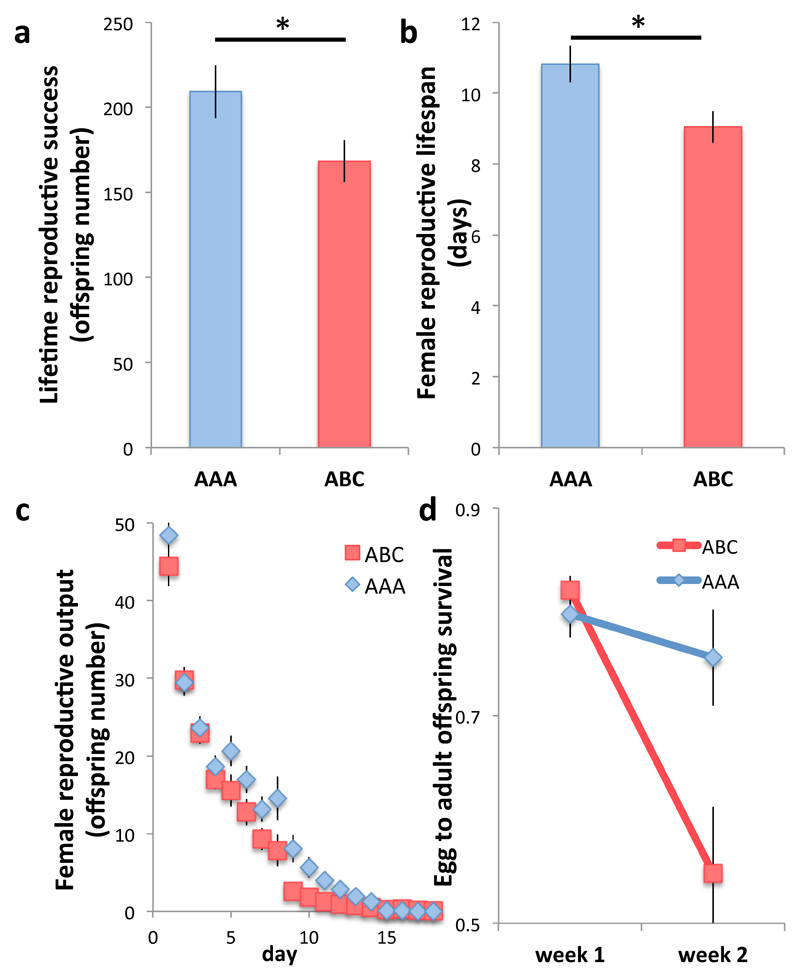
We first tested the effect of relatedness of males within a group on female fitness, by quantifying different aspects of fitness and life-history (experiment 1) in females exposed to male triplets. Males were unrelated to the female and either full-sib brothers of each other (AAA) or unrelated to each other (ABC), and were replaced weekly until female death. Consistent with expectations3–5, we found that females exposed to AAA-males had significantly higher lifetime reproductive success than females exposed to ABC-males (Fig. 1a). This was due to the fact that while total female lifespan did not differ on average between treatments (F1, 119 = 1.66, P = 0.2), females exposed to AAA-males had significantly longer reproductive lifespan (from eclosion to last egg-laying day6, Fig. 1b), and female reproductive lifespan was positively correlated with female lifetime reproductive success (F1, 117 = 484.59, P < 0.001). Two non-mutually exclusive mechanisms might cause this. First, high-fecundity females might die faster when exposed to ABC-males, leading to an average higher productivity of AAA-replicates (‘selective death’). Second, individual females might suffer a steeper rate of age-dependent decline in reproductive output when exposed to ABC- rather than AAA-males (‘reproductive ageing’). We found no evidence of ‘selective death’: across both treatments (AAA and ABC) females characterized by a relatively low (rather than high) initial oviposition rate died significantly faster than high-fecundity females (F1, 117 = 11.038, P = 0.0012; treatment*oviposition rate interaction, F1, 117 = 0.224, P = 0.64), which does not support the prediction that high-fecundity females die faster in ABC- compared to AAA-trials. In contrast, we found robust support for ‘reproductive ageing’: the rate of offspring production declined with age significantly faster for females exposed to ABC-males than for females exposed to AAA-males (Fig. 1c). This was partly due to the fact that offspring egg-to-adult viability declined significantly faster as females aged in the ABC- then the AAA-treatment (Fig. 1d). We explored the generality of these results by estimating rate-sensitive female fitness costs under different intrinsic rates of population growth6, and confirmed that exposure to ABC-males resulted in relative fitness costs, both for individual females and entire female cohorts, that were particularly pronounced in contracting or stable populations (SI). Experiment 1 therefore indicates that relatedness within male groups promotes female lifetime reproductive success largely by delaying reproductive ageing.
Figure 1. The effect of male-male relatedness on female fitness.
(a) Female lifetime reproductive success was higher in the high-male relatedness (AAA) than in the low-male relatedness treatment (ABC, F1, 119 = 4.11, P = 0.045). This difference was highly significant when we included female reproductive lifespan and its interaction with treatment as factors in the analysis (F1,117 = 20.83, P < 0.001). (b) Female reproductive lifespan was longer (ABC, F1,119 = 6.55, P = 0.012) and the probability to cease reproducing at any given time was lower (χ22 = 3.95, P = 0.047; nAAA = 63, nABC = 62) in the high-male relatedness (AAA) than in the low-male relatedness treatment (ABC). (c) Female reproductive rates declined more sharply in individual females exposed to ABC- rather than to AAA-male (average number of offspring produced by AAA- and ABC-females over successive days of their life: treatment, χ21 = 4.11, P = 0.043; day, χ21 = 1570.8, P < 0.001; treatment*day interaction, χ21 = 7.55, P = 0.006). (d) Offspring viability (egg to adult survival) declined more sharply over time in females exposed to ABC- rather than AAA-males (treatment*week interaction: χ21 = 9.23, P = 0.002, estimated difference in viability drop AAA-ABC, mean±se: estimate = -0.231±0.075). Vertical bars = se, horizontal bar with asterisk = significant comparisons; nAAA = 61, nABC = 60, unless stated otherwise.
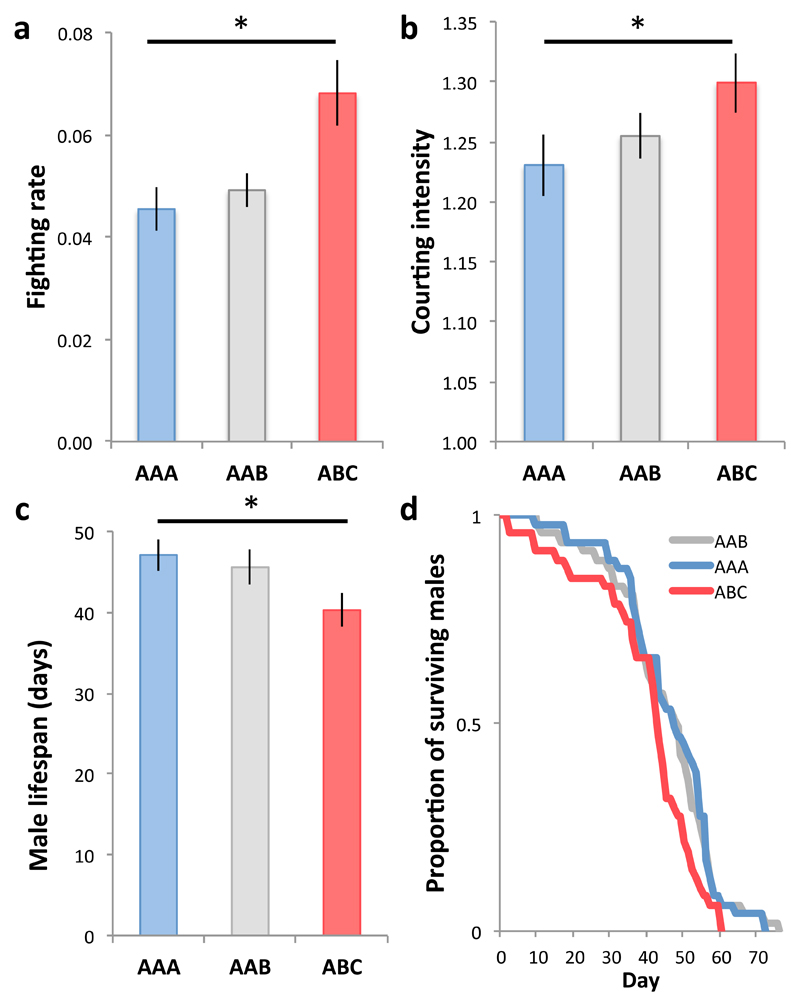
We then investigated the signature of within-group relatedness on male competition. Relatedness can influence the way males compete over access to mating opportunities (pre-copulatory competition) and/or the way their ejaculates compete over fertilization (post-copulatory competition)4. For example, when females mate in a patch, then disperse to a new patch where they mate again, pre-copulatory competition occurs locally and post-copulatory competition, globally. We tested the effect of male relatedness within a group on male pre-copulatory competition (experiment 2), by measuring how males respond to changes in within-group male relatedness. We assembled male triplets that comprised three full-sib brothers (AAA treatment), two full-sib brothers and an unrelated male (AAB), or three males unrelated to each other (ABC), exposed each triplet to a single female unrelated to the males, and maintained vials without replacement. We detected no difference in mating rates across treatments (χ22 = 0.071, P = 0.965; mating rate [number of matings per 100 scans] estimate±se: AAA = 0.70 ± 0.158, AAB = 0.76 ± 0.214, ABC = 0.83 ± 0.260). However, consistent with expectations, fighting was more common in triplets of unrelated males (ABC) than in AAA- and AAB-triplets (Fig 2a). ABC-males also courted the female more intensely than AAA-triplets (Fig. 2b). We further confirmed the effect of within-group male relatedness on male behavior using the first axis of a Principal Component Analysis summarizing different aspects of male fighting and courting (SI). Within-group relatedness was also associated with variation in male longevity. First, AAA-males lived on average longer than ABC-males (Fig. 2c). Second, survival analysis by means of a Cox proportional hazards model detected significant overall treatment effects in male mortality risk across treatments (Fig. 2d). While this experiment was not designed to test treatment effects on female fitness because males were allowed to co-age with females, we found non-significant trends for females exposed to ABC-males to suffer shorter reproductive lifespan and lower lifetime reproductive success, in line with the findings of experiment 1 (Table S1). We next tested whether within-group relatedness also influences the intensity of male post-copulatory competition. For example, competing with relatives may inhibit male ejaculate investment in accessory gland products (Acps) such as Sex Peptide, which in D. melanogaster inhibits female re-mating, hence delaying sperm competition, and boosts female egg-laying rates7, 8, but can also induce contribute to female harm and reproductive ageing under certain conditions9, 10. We tested this idea (experiment 3) by monitoring mating duration with the first male, latency to re-mate with a new male, and egg-laying rates in females, which were first mated to a male from the AAA-treatment, a male from the ABC-treatment or a control male kept in isolation. We found no difference in the mating duration, remating latency or egg-laying rate of the females first mated to AAA- vs. ABC-males (SI). These results suggest that within-group relatedness is associated with longer male lifespan and relaxes the key aspects of pre- (rather than post-) copulatory competition in this species: courtship and fighting.
Figure 2. The effect of male-male relatedness on males.
(a) Triplets of unrelated males (ABC) had a significantly higher frequency of male-male fighting than triplets of brothers (i.e. AAA, proportion of focal scans in which male-male fighting was observed, χ22 = 14.46, P < 0.001; Tukey, ABC-AAA, z = 3.73, P < 0.001, ABC-AAB, z = 2.92, P = 0.01, nAAA=47, nAAB=47, nABC=45). (b) Compared to triplets of brothers (AAA), triplets of unrelated males (ABC) were characterised by higher courting intensity (i.e. number of courting males when courting was observed, χ22 = 5.01, P = 0.081; Tukey ABC-AAA: z = 2.38, p = 0.045; nAAA=47, nAAB=47, nABC=45). (c) Male longevity was significantly lower in unrelated triplets (ABC) than among full-sib brothers (AAA, F2, 128 = 3.77, P = 0.026; Estimated differential lifespan for ABC, mean±se: -5.62±2.63, t = -2.139, P = 0.034; nAAA=43, nAAB=44, nABC=45). (d) We found significant differences in male mortality risk across treatments (χ22 = 10.47, P = 0.005), and post-hoc direct comparisons between the treatments indicated that this effect was due to males in unrelated triplets (ABC) being more likely to die than in AAA- (χ22 = 9.55, P = 0.002) and AAB-triplets (χ22 = 6.66, P = 0.010; nAAA = nAAB = nABC = 47). Vertical bars = se, horizontal bar with asterisk = significant post-hoc comparisons.
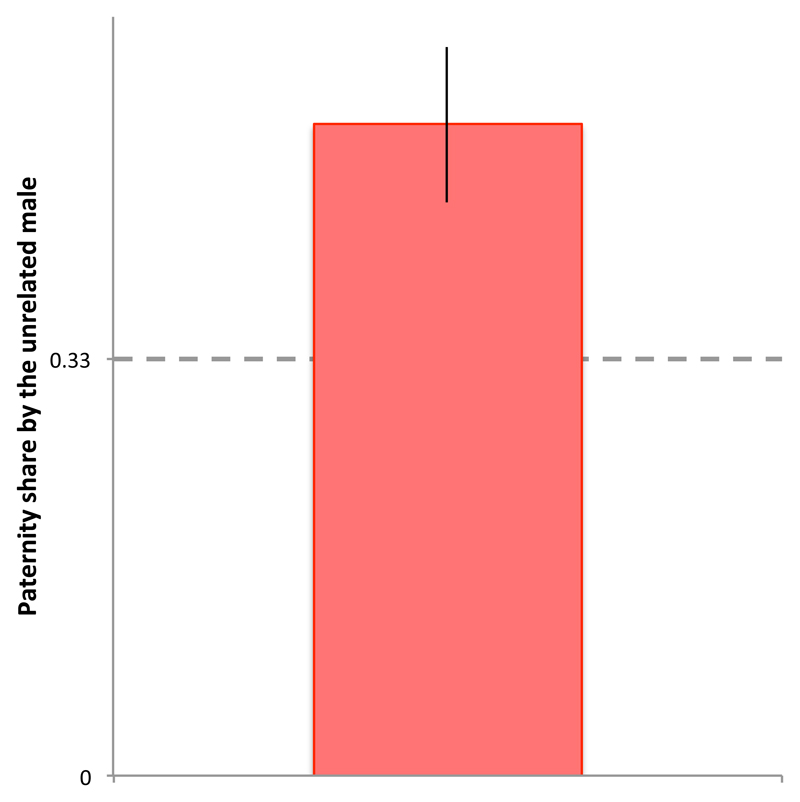
To study how groups of relatives interact with unrelated competitors, we assembled (experiment 4) triplets comprising two brothers and one male unrelated to them (i.e. AAB), replicated across three different genetic stocks (wild-type -wt-, and two homozygous recessive mutants -sepia (sep)11 and sparkling (spa)12- each backcrossed into the wild-type Dahomey population9, 13, 14) and exposed to a single female double homozygous recessive for both sep and spa. This design enabled us to test whether males behaved differentially towards related (A) or unrelated (B) competitors, and to assign offspring paternity to A- or B-males in each trial. We found no evidence of differential behavioural interactions: an A-male was just as likely to fight with his brother than with the unrelated B-male (mean±se proportion of all fights that were direct to the B-male = 0.51±0.07; effect of relatedness: z = 0.20, P = 0.84). Similarly, the unrelated of the three males (B) did not court (0.34±0.03, difference from expected 0.33: z = 0.20, P = 0.84) or mate with the female more frequently than each of the two brothers (0.38±0.07, difference from expected 0.33: z = 0.63, P = 0.53). However, the unrelated B-male sired on average twice as many offspring as either A-male (Fig. 3), suggesting that a minority of selfish unrelated competitors may be able to invade and persist in groups of male relatives.
Figure 3. Unrelated males outcompete brothers.
Proportion of offspring sired by the unrelated male (B) in male triplets in which two brothers were matched with an unrelated male (AAB, n =54). The B-male sired on average half of the offspring produced by the female, leaving the two brothers to share the other half. This distribution of paternity deviated significantly from an equalitarian distribution of paternity across the three males (i.e. 033, dashed line, z = 3.99, P < 0.001), and was independent of male stock (i.e. sep, spa). Vertical bars = se.
Sexual selection favours males that outcompete each other over access to females or their ova to a point that often harms female fitness2, with pronounced repercussions for the population as a whole, reducing productivity and even leading to local extinctions15, 16, a process akin to the tragedy of the commons17. In structured populations however, where local rivals can be genetically related to each other, harming females impacts the inclusive fitness of a male by reducing the reproductive success of his male relatives, and kin selection should discourage female harm by relaxing competition among related males3–5. Our study provides experimental support for these expectations in D. melanogaster. A proximate explanation is that elevated rates of harassment and male-male fighting, induced by low within-group male relatedness, impose cumulative costs on females and accelerate their reproductive ageing13. By mating with genetically different (i.e. unrelated) males, females may also incur higher immunological costs18. We found little evidence that differential female harm is mediated by post-copulatory traits, suggesting that post-copulatory male competition may occur on a more global scale than pre-copulatory competition4. It would therefore appear that in the evolutionary past, the structure of natural D. melanogaster populations generated sufficient opportunity for the evolution of kin-selected sexual behaviours. Natural fly populations display limited dispersal and a tendency for local aggregations19, 20, and although the extent to which different lab-adapted populations have retained kin-biased sexual behaviour is unclear, evidence of differential sexual responses based on kinship have been shown in some fly lab populations including our own study population14.
Although insects have inspired a large body of literature documenting how relatedness among group members structures social interactions, this work has largely focused on the particular case of eusociality1, 21, 22. However, the influence of relatedness transcends eusociality and can modulate fundamental aspects of social behaviour more broadly. Sexual cooperation among related males has been observed in different animal societies23–27, however the fitness consequences for females have previously received little attention. While the possibility that sexual selection results in males harming females is well established2, we currently lack a framework to understand the high variability in female harm observed across and within taxa5. Our study indicates that variation in relatedness and conditional behavioural responses to kin are potentially key factors underpinning such diversity. Whilst the genetic make-up of social groups was proposed as a modulator of female harm28, 29, it was only recently that kin selection was explicitly applied to sexually-selected female harm3–5. This process is reminiscent of the way kin selection modulates virulence in pathogens30. In both female harm and virulence, selfishness leads to a tragedy of the commons, which is inhibited by the relatedness of local competitors5, 30. As in other cooperative systems1, we found that in groups of relatives unrelated rivals can have disproportionate success. This may be due to a number of mechanisms, including an imperfect kin recognition system1, e.g. males might respond to the average relatedness of the group because they are unable to recognize their relatedness to individual group members. Although it is difficult to extrapolate these experimental findings to the complexities of natural populations (e.g. variable patterns of relatedness among the offspring of polyandrous females), these results indicate that the benefits of relaxed competition among relatives may be dynamic, diminishing rapidly as populations become less viscous, a result consistent with our finding that the benefits of within-group male relatedness are higher in contracting populations. In conclusion, we present an experimental demonstration that genetic relatedness of social groups modulates the switch between sexual competition and cooperation. Future work should investigate the generality of these results and further resolve underpinning proximate mechanisms and evolutionary dynamics.
Methods Summary
Across experiments, male triplets were set-up by collecting recently hatched (virgin) males from controlled 24h pairings of one-week-old (virgin) pairs of flies. Families were brought up in the same vials. Triplets consisted of three full-sib males (AAA), two full-sib males and one unrelated male (AAB), or three unrelated males (ABC). Male triplets were set up between 48-72h before the beginning of a trial, which begun by introducing a 48/72h-old virgin female (unrelated to any of the males in the triplet) into a vial with a male triplet.
Supplementary Material
Supplementary Information is available in the online version of the paper.
Acknowledgements
We thank the following funding agencies: Marie Curie fellowship (PIEF-GA-2010-273010) to P.C., the Wellcome Trust VIP award and NERC fellowship to S.W., NERC research grant and the Leverhulme Trust to T.P. We thank C. Garroway, J. Perry, S. Michaelides for technical help, M. Bonsall, A. Buckling, G. McDonald, D. Noble, J. Perry, R. Snook, S. West for helpful discussions, and three anonymous referees for excellent comments on the manuscript.
Footnotes
Author contributions. Expt.1 was designed by P.C., S.W. and T.P., conducted by P.C. and F.A. and analysed by P.C. Expt. 2 was designed by P.C., C.K.W.T., S.W. and T.P., and conducted/analysed by P.C. Expt. 3 was designed and conducted by S.W. and P.C. and analysed by P.C. Expt. 4 was designed by C.K.W.T., T.P. and S.W., and conducted/analysed by C.K.W.T. The article was conceived and written by T.P. with input from P.C., C.K.W.T. and S.W.
References
- 1.Bourke AFG. Principles of Social Evolution. Oxford Univ. Press; Oxford: 2011. [Google Scholar]
- 2.Parker GA. Sexual conflict over mating and fertilization: an overview. Philos Trans Roy Soc B. 2006;361:235–259. doi: 10.1098/rstb.2005.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rankin DJ. Kin selection and the evolution of sexual conflict. J Evol Biol. 2010;24:71–81. doi: 10.1111/j.1420-9101.2010.02143.x. [DOI] [PubMed] [Google Scholar]
- 4.Wild G, Pizzari T, West SA. Sexual conflict in viscous populations: the effect of the timing of dispersal. Theor Population Biol. 2011;80:298–316. doi: 10.1016/j.tpb.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Pizzari T, Gardner A. The sociobiology of sex: inclusive fitness consequences of inter-sexual interactions. Phil Trans Roy Soc B. 2012;367:2314–2323. doi: 10.1098/rstb.2011.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edward DA, Fricke C, Gerrard DT, Chapman T. Quantifying the life-history response to male exposure in female Drosophila melanogaster. Evolution. 2010;65:564–573. doi: 10.1111/j.1558-5646.2010.01151.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman T, et al. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Fricke C, Bretman A, Chapman T. Female nutritional status determines the magnitude and sign of responses to a male ejaculate signal in Drosophila melanogaster. J Evol Biol. 2010;23:157–165. doi: 10.1111/j.1420-9101.2009.01882.x. [DOI] [PubMed] [Google Scholar]
- 11.Anxolabéhère D. Heterosis overdominance and frequency-dependent selection in Drosophila melanogaster at the Sepia locus. Evolution. 1976;30:523–534. doi: 10.1111/j.1558-5646.1976.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 12.Fu W, Noll M. The Pax2 homolog sparkling is required for development of cone and pigment cells in the Drosophila eye. Genes Dev. 1997;11:2066–2078. doi: 10.1101/gad.11.16.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Partridge L, Fowler K. Non-mating costs of exposure to males in female Drosophila melanogaster. J Insect Physiol. 1990;36:419–425. [Google Scholar]
- 14.Tan CKW, et al. Sex-specific responses to sexual familiarity, and the role of olfaction in Drosophila. Proc Roy Soc Lond B. 2013;280 doi: 10.1098/rspb.2013.1691. 20131691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Galliard JF, et al. Sex ratio bias, male aggression, and population collapse in lizards. Proc Natl Acad Sci USA. 2005;102:18231–18236. doi: 10.1073/pnas.0505172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rankin DJ, Kokko H. Sex, death and tragedy. Trends Ecol Evol. 2006;21:225–226. doi: 10.1016/j.tree.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Rankin DJ, Dieckmann U, Kokko H. Sexual conflict and the tragedy of the commons. Am Nat. 2011;177:780–791. doi: 10.1086/659947. [DOI] [PubMed] [Google Scholar]
- 18.Fedorka KM, Zuk M. Sexual conflict and female immune suppression in the cricket, Allonemobious socius. J Evol Biol. 2005;18:1515–1522. doi: 10.1111/j.1420-9101.2005.00942.x. [DOI] [PubMed] [Google Scholar]
- 19.McInnis DO, Schaffer HE, Mettler LE. Field dispersal and population sizes of native Drosophila from North-Carolina. Am Nat. 1982;119:319–330. [Google Scholar]
- 20.Robinson SP, Kennington WJ, Simmons LW. Preference for related mates in the fruit fly, Drosophila melanogaster. Anim Behav. 2012;84:1169–1176. [Google Scholar]
- 21.Hamilton WD. Altruism and related phenomena, mainly in social insects. Ann Rev Ecol System. 1972;3:193–232. [Google Scholar]
- 22.Bourke AFG, Frank NR. Social Evolution in Ants. Princeton, Univ. Press; Princeton: 1995. [Google Scholar]
- 23.Packer C, Pusey AE. Cooperation and competition within coalitions of male lions – kin selection or game theory. Nature. 1982;296:740–742. [Google Scholar]
- 24.McDonald DB, Potts WK. Cooperative display and relatedness among males in a lek-mating bird. Science. 1994;266:1030–1032. doi: 10.1126/science.7973654. [DOI] [PubMed] [Google Scholar]
- 25.Petrie M, Krupa A, Burke T. Peacocks lek with relatives even in the absence of social and environmental cues. Nature. 1999;401:155–157. [Google Scholar]
- 26.Krakauer AH. Kin selection and cooperative courtship in wild turkeys. Nature. 2005;434:69–72. doi: 10.1038/nature03325. [DOI] [PubMed] [Google Scholar]
- 27.Den Boer S, Baer B, Boomsma JJ. Seminal fluid mediates ejaculate competition in social insects. Science. 2010;327:1506–1509. doi: 10.1126/science.1184709. [DOI] [PubMed] [Google Scholar]
- 28.Moore AJ, Pizzari T. Quantitative genetic models of sexual conflict based on interacting phenotypes. Am Nat. 2005;165:S88–S97. doi: 10.1086/429354. [DOI] [PubMed] [Google Scholar]
- 29.Eldakar OT, Dlugos M, Pepper JW, Wilson DS. Population structure mediates sexual conflict in water striders. Science. 2009;326:816. doi: 10.1126/science.1180183. [DOI] [PubMed] [Google Scholar]
- 30.Buckling A, Brockhurst MA. Kin selection and the evolution of virulence. Heredity. 2008;100:484–488. doi: 10.1038/sj.hdy.6801093. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.