Abstract
Background:
One of the prevailing otologic infections in our country is chronic suppurative otitis media, especially the tubotympanic type for which various treatment protocols are followed. Usually, oral and topical antibiotics (mainly quinolones) are given alone or in combination. There is a lack of consensus as to whether topical drops alone are effective or a combined oral and systemic therapy should be prescribed. In our study, we have attempted to observe the efficacy of empirical therapy with combined ciprofloxacin versus topical drops only in patients with tubotympanic chronic suppurative otitis media for control of infection.
Methodology:
A total of 100 patients visiting the outpatient ENT department at our tertiary care hospital with clinically diagnosed chronic suppurative otitis media (tubotympanic type) were enrolled in our study. The study was reviewed and accepted by the ethical review committee. A detailed proforma was filled for all patients. All patients after aural toilet were subjected randomly to one of the 2 treatment methods, ie, topical ciprofloxacin ear drops plus an oral placebo or combined oral and topical ciprofloxacin. These patients were reviewed after 1 week of treatment.
Results:
It was observed that 48 of 50 (96%) patients responded to treatment in the group receiving topical ciprofloxacin, whereas 49 of 50 (98%) patients responded in the group receiving combined therapy. This difference was not significant. Moreover, age, sex, and duration of discharge did not have any effect on treatment. There were minimal side effects in both groups, which were also not significant and disappeared after discontinuation of treatment.
Conclusions:
The results of this study show that topical ciprofloxacin drops were as effective as combined oral and topical ciprofloxacin and that the addition of oral drug did not have any beneficial effect and added only to the cost of treatment.
Keywords: Chronic suppurative otitis media (CSOM), empirical antibiotics, otorrhea, Pseudomonas aeruginosa
Introduction
Experts harbor conflicting views regarding the true definition of chronic suppurative otitis media (CSOM).1 The World Health Organization (WHO) defines CSOM as “a stage of ear disease in which there is chronic infection of the middle ear cleft, a non-intact tympanic membrane (i.e. perforated eardrum) and discharge (otorrhea), for at least the preceding two weeks.” The 2 main groups include tubotympanic or safe type with a perforation in the pars tensa without cholesteatoma and atticoantral type when the perforation is in the attic or if there is presence of cholesteatoma.2,3
Chronic suppurative otitis media is frequently seen in patients presenting to the outpatient departments at various hospitals and is a common cause of preventable hearing impairment worldwide, particularly in the low- and middle-income countries. Risk factors include young age, overcrowding, inadequate housing, poor hygiene, lack of breastfeeding, poor nutrition, eustachian tube dysfunction, and inadequate or unavailable health care. Poverty is a major risk factor in developing countries.3 Pseudomonas aeruginosa is the most common pathogen isolated.4 Drug sensitivity patterns show that ciprofloxacin (quinolones) is active against most of the isolates, followed by amikacin, gentamicin, and other penicillins and cephalosporins.5,6
Medical management aims to stop the discharge, to heal small perforations in the tympanic membrane, to improve hearing, and to prevent infections and potentially life-threatening complications. Treatment options include the following: dry mopping, topical antiseptics or antibiotics, sometimes combined with steroids and systemic antibiotics.2 A number of topical antibiotics have been used. However, concerns exist regarding their ability to penetrate the middle ear and mastoid cavities as well as their activity against the causative bacteria. There also remains controversy and uncertainty about the possible ototoxic effect, in particular, topical aminoglycoside. For this reason, systemic treatments have been recommended and used alone or in combination with topical antibiotics. Because systemic aminoglycosides cause well-documented ototoxic and nephrotoxic side effects, systemic quinolones are often prescribed in these cases because of high incidence of gram-negative organisms such as Bacillus, Proteus, and P aeruginosa.7 However, systemic quinolones are contraindicated in pregnancy and in children.8 Also, they may cause arthralgia and gastrointestinal upset. Introduction of topical quinolones has gained interest in the medical management of CSOM because of their clinical efficacy and lack of side effects. Topical treatment with quinolones may be as effective as systemic treatment, in terms of efficacy and also safety, thereby avoiding the need for systemic therapy.9 This notion is especially relevant for low socioeconomic setup, where most of the patients cannot afford combination therapies.
A study about the drug sensitivity in 164 patients of CSOM revealed that Staphylococcus aureus showed highest sensitivity to gentamicin (82.5%), whereas P aeruginosa was 100% sensitive to ceftazidime. The overall sensitivity to standard CSOM antibiotic is on higher side.10 In a study on 124 people of Malawi rural areas, empirical otologic drops of ofloxacin were administered right after collection of cultures. It showed 33 failures for complete resolution of CSOM. Culture results showed that most of the persistent bacteria are either enterococci or water bacteria. Typical CSOM-causing organisms had higher sensitivity toward ofloxacin.11 A prospective randomized trial was conducted on 110 patients with active CSOM. Group A was prescribed antibiotics after culture and sensitivity, whereas group B had an empirical therapy of 2 weeks without microbiology done. The results showed no significant (P = .2) difference between 2 groups with respect to resolution and recurrence or persistence of ear discharge.12
In light of this evidential background, we designed a prospective study without proceeding to culture which is both expensive and unaffordable for low-income population. Comparison of efficacy of empirical treatment with topical ciprofloxacin alone versus combined oral and topical ciprofloxacin was the primary objective of our research.
Materials and Methods
Study setting
This study was constructed as a double-blinded, prospective, randomized trial conducted during a 15-month period at the ENT outpatient department of Jinnah Medical College Hospital (JMCH), which provides subsidized health care to patients, most of them belong to low socioeconomic class.
Study design and protocol
The sample size was calculated using the WHO sample size calculator, the confidence interval was 95%, and the desire of power was 0.8; with 2-tailed hypothesis, it was found to be 98 patients (49 in each group). A total of 100 consecutive patients attending the outpatient department and being diagnosed with CSOM of tubotympanic type were selected and randomly divided into 2 groups of 50 each. Group “A” patients received only topical ciprofloxacin drops plus an oral placebo, whereas Group “B” patients received both oral and topical ciprofloxacin for 1 week. The patients were blinded and did not know what drug they were given, whereas the physicians were blinded and given the drugs in the form of codes, ie, codes A and B, randomly organized in sealed envelopes to maintain the randomization. There were 100 sealed nonlabeled envelopes (50 for each group): half containing prescriptions for topical ciprofloxacin and oral placebo and the other half with prescription for both oral and topical ciprofloxacin. Patients were randomly allocated to either of these envelopes. Only on opening the envelope, the treatment was started. Topical ciprofloxacin drops in both the groups were given every 8 hourly with 3 to 4 drops each time for 7 days. In Group “A,” oral placebo was given every 12 hours for the same duration. In Group “B,” oral ciprofloxacin was given at a dose of 200 mg every 12 hours also for 7 days. Aural hygiene and water prevention were advised to patients. Both the patients and the physicians were blinded to the treatment medications. Patients were reassessed in ENT OPD after 7 days, and the ear was examined with respect to discharge, perforation, and resolution/worsening of symptoms.
Exclusion criteria
Those patients who had already received treatment within 2 weeks for the same complaint or who had taken antibiotics for other complaints, eg, upper respiratory tract infections, were excluded. Patients having attic perforation or cholesteatoma/granulations on examination were excluded. Patients having ear pathology besides CSOM (eg, otitis externa) were also excluded from the study. Patients with anatomical abnormalities of external or middle ear on examination were not included in the study, as were those who reported allergy to study drugs, were pregnant, or had comorbidities such as diabetes or immune suppression.
Ethical review
The Ethical Review Board of JMCH approved the study. The respondents were informed of their right to refuse at any time of the study. Confidentiality and anonymity of the data were maintained at all times. The protocol was designed according to the guidelines laid down by the Helsinki Declaration.13
Study questionnaire
The study instrument comprised 3 sections. The first section was concerned with the demographics of the subjects and included variables such as age and sex. The second section included questions pertaining to CSOM such as duration of discharge and whether the discharge resolved after 7 days. The last section aimed to assess the adverse events arising out of the treatment regimen advised.
Analysis of data
Data from the questionnaire were entered in SPSS (Statistical Package for the Social Sciences) version 17 for analysis and the results were compared.
Results
A total of 100 patients were enrolled in this study. Of these, 67 were men with the mean age of 34.12 years and 33 were women with mean age of 31.68 years, giving a male to female ratio of 1:0.49. The ages of the subjects ranged from 18 to 50 years, with a mean age of 33.2 ± 8.7 years. The mean duration of discharge was 55.2 days (SD + 33.3) with a minimum of 14 days and maximum of 140 days. The left ear was affected in 55 patients, whereas the right ear was affected in 45 patients, with none having bilateral pathology.
After a week of therapy, both the groups were compared with respect to resolution of discharge and adverse effects. Of 100 patients enrolled in this study, 97 had complete resolution of discharge, whereas 3 failed to show complete resolution. Of 50 patients, 48 (96%) in group “A” taking topical ciprofloxacin showed resolution of discharge, whereas 49 of 50 patients (98%) in group “B” had resolution of discharge. There was no statistical difference between the 2 groups with respect to effectiveness of treatment. There were minimal adverse effects in both the groups.
In group “A,” only 2 failed to show any resolution of discharge. On further examination, one of the patients had fungal overgrowth which accounted for persistent otorrhea. A culture swab was taken from the second patient’s ear whose discharge failed to resolve after 1 week of topical ciprofloxacin. In group “B,” there was only 1 failure. Graphical representation of these findings is given in Figure 1.
Figure 1.

Group-wise resolution of discharge.
The χ2 test was used to compare the 2 groups in terms of sex distribution and resolution of discharge. It was observed that there was no significant difference between 2 groups in terms of sex (P = .2) and resolution of discharge (P = 1.0). The Student t test was used to compare the 2 treatment groups with respect to age and duration of discharge. No significant difference was found in age (P = .9) and duration of discharge (P = .88) between 2 groups. Tabular representation of categorical variables was given in Table 1, whereas Table 2 represents continuous variables.
Table 1.
Categorical variables and their associations.
| Group A | Group B | P value | ||
|---|---|---|---|---|
| Gender | Male | 30 | 37 | .2 |
| Female | 20 | 13 | ||
| Resolution of discharge | Yes | 48 | 49 | 1.0 |
| No | 02 | 01 |
Table 2.
Continuous variables and their associations.
| Group A | Group B | P value | |
|---|---|---|---|
| Mean age, y | 33.28 | 33.24 | .98 |
| Duration of discharge, d | 55.72 | 54.75 | .88 |
A total of 15 patients of 100 complained of adverse effects to the drugs prescribed. Of these, 4 patients were in Group “A” taking topical ciprofloxacin ear drops, whereas 11 patients were in Group “B” taking combined oral and topical ciprofloxacin (this finding is graphically represented in Figure 2). In group “A,” 3 patients complained of mild earache, whereas only 1 patient developed fungal overgrowth. In Group “B,” there were 11 patients having side effects of which 8 patients complained of transient gastrointestinal disturbance, 2 patients had mild arthralgia, and 1 patient had vertigo.
Figure 2.
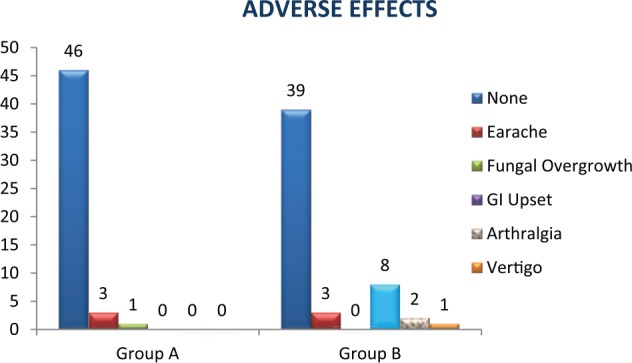
Comparison of adverse effects. GI indicates gastrointestinal.
Discussion
This study was conducted to compare the efficacy of topical ciprofloxacin ear drops versus combined topical and oral ciprofloxacin in the medical management of tubotympanic-type CSOM. A vacant area appears in our country regarding the trend of chronic middle ear infections, the treatment prescribed, and the outcomes to various forms of treatment. Various physicians and otolaryngologists differ in their approach to the medical treatment for CSOM, and the usual trend is to prescribe oral antibiotics in addition to quinolone drops. Till date, no prevalence study has been conducted locally to observe the burden of CSOM. There has been a paucity of randomized controlled trials in our setup to observe the efficacy of ciprofloxacin drops. One trial was reported in 2003, where the authors compared the effects of topical quinolones with topical aminoglycosides with encouraging results but aside from that a comparative study is lacking.14 With a major chunk of population below the poverty line, any additional treatment becomes costly and adding oral antibiotics without evidence only adds to the cost and adverse effects; hence, this study is another attempt in our local setup to prove the efficacy of topical ciprofloxacin in tubotympanic CSOM. It is a plea to the otolaryngologists and physicians who regarding the poor hygienic conditions in our part of the world commonly prescribe systemic antibiotics; to take a step forward and commensurate a new trend of prescription next time, they examine a patient with tubotympanic CSOM.
Since the advent of quinolones in late 1980s, there had been a gradual shift toward prescribing these medications for middle ear infection. This is due to their broader antimicrobial coverage and the lack of ototoxic side effects. Both topical and systemic formulations are available, but there has been a lack of consensus as to whether topical, oral, or both forms of antibiotics are effective for CSOM. Researchers have studied the effects of single and combined therapy and have come up with varying results. A study by Mittal et al concluded that topical antibiotics and aural toilets constitute the first line of treatment for CSOM. It was also observed that intravenous antibiotics show increased side effect profile and significant potential to produce antibiotic resistance.15
A number of studies have compared the modalities of controlling infection for CSOM. A study on the efficacy of ofloxacin showed that clinical success of oral and topical ofloxacin varies from 75% to 90%. In this evidence-based review, Manolidis et al reviewed various ear infections including otitis media which were treated with ciprofloxacin and aminoglycosides. He observed that fluoroquinolones have better efficacy as compared with aminoglycosides. It was concluded that ofloxacin 0.3% otic solution has comparable or better efficacy than most of the conventional antibacterial therapies for middle ear infections.11
In 1990, Esposito studied the effectiveness of oral versus topical ciprofloxacin.16 In this randomized trial, 3 groups were assigned for oral, topical, and combined therapy with 20 patients in each group. This study favored the use of topical drops only, with a success rate of 85% and the non-necessity of oral treatment, but there was no mention about the safety profile and adverse effects of ciprofloxacin in this study. Similarly, in our study, there were 2 arms for topical and combined treatment but the number of patients is more (50 in each group). Also, the success rate is higher (97%). We also observed that the addition of oral ciprofloxacin had no significant effect in treating CSOM and only added to the systemic side effects.
Perhaps, the most important evidence for the use of topical ciprofloxacin comes from the Cochrane Database Reviews. In 2000, Acuin et al from Philippines published a review paper in the Cochrane Database to assess the effects of different treatments for CSOM.17 They reviewed 24 randomized trials including 1660 people. Topical quinolones were found to be more effective than nonquinolones in 5 trials but combining topical and systemic antibiotics was not more effective than topical antibiotic drops alone. He further went on to prepare the document and guidelines for prevention of blindness and deafness for the WHO in 2004.18 Within this document, it is also stated that topical drops alone are effective for noncomplicated chronic otitis media.
In 2004, Acuin et al conducted another extensive review on the burden and management options of CSOM. In this Cochrane Review, it was evident that topical ciprofloxacin (86%) is more effective as compared with oral ciprofloxacin (60%) in terms of bacteriologic cure and clinical efficacy. It was also observed that topical quinolones have better efficacy than topical nonquinolones.19 Acuin et al published an update of the previous reviews in 2007. This review addressed 48 different studies, discussing the management plan in the patients of CSOM with an emphasis on the usage of topical quinolones.20
A prospective, randomized multicenter clinical trial was conducted in 2000 which compared topical ciprofloxacin 0.2% solution with the combination of polymyxin B, neomycin, and hydrocortisone suspension. It was observed that topical ciprofloxacin solution as a single dose is more effective and showed better tolerance rate in the patients of CSOM.21 de Miguel in 1999 published a randomized trial of 125 patients where he selected 4 different treatment groups to study the effectiveness of ciprofloxacin in CSOM.22 These were oral ciprofloxacin alone, topical ciprofloxacin alone, oral and topical ciprofloxacin, and topical polymyxin/neomycin drops, respectively. He concluded that topical ciprofloxacin remained the most effective form of treatment. This study had 4 groups and had the advantage of comparing topical antibiotics with topical ciprofloxacin. Recent literature clearly indicates the superiority of topical ciprofloxacin over other topical antibiotics; therefore, we omitted the addition of an extra part of oral or topical nonquinolone antibiotic.
A similar study was conducted by Ramos and colleagues in 2003.23 Five treatment groups were assigned to patients with chronically discharging ears. They concluded that topical treatment with ciprofloxacin in chronic middle ear infection revealed improved results as compared with oral administration. This study had 300 patients and compared various strengths of topical ciprofloxacin versus oral quinolone and combined forms. This study had a bias of including patients with cholesteatoma, as well, which may have led to a less favorable treatment response, although they added fluocinolone. Our study focused on patients with tubotympanic CSOM to avoid this bias.
In 2006, Carolyn et al studied 9 randomized trials including 833 participants. This review article which was published in the Cochrane Database; the authors deduced that topical quinolones can resolve aural discharge better than systemic antibiotics and they were more effective than nonquinolone topical antibiotics or antiseptics, although none of these trials reported any long-term results regarding adverse effects. There was clearly no benefit detected of adding systemic antibiotics to topical quinolones.9
In all the above-mentioned trials, there is a trend of short treatment duration and a short follow-up. None of these trials were designed to observe the long-term effects of either short or continuous courses of quinolones. The same trend is seen in our study and we have focused only on the immediate effect of a short course of quinolone antibiotics and have not studied the long-term effectiveness or adverse outcomes in prolonged use of either oral or topical ciprofloxacin.
But the fact remains that the definitive management of tubotympanic CSOM is surgical treatment by tympanoplasty once the ear is free of discharge. In our opinion, there is no need for long-term use of any antibiotic for tubotympanic CSOM. The main aim of such treatment strategies is to prepare the patient for surgery by eradicating the infection. The other problem arising in long-term studies is that if the tympanic membrane is not grafted, there remains a breach in normal physiologic barrier; that is, the communication between the external and middle ear remains open. Due to the breach, it will be difficult to assess whether there is persistence or recurrence of infection in the middle ear cavity. Same is true for determining the side effects in terms of hearing loss in prolonged use. We also have concentrated only on single aspect of improvement regarding treatment, ie, resolution of discharge. We did not opt for calculating the hearing outcome in these patients after treatment, which has been mentioned in a few studies, but as mentioned earlier, hearing improvement depends on surgical part of treatment rather than medical, so it is out of scope of our study.
Conclusions
It was concluded that empirical treatment with topical ciprofloxacin drops alone were as effective as combined oral and topical ciprofloxacin and that the inclusion of oral drug did not have any additional beneficial effect but the cost-effectiveness is a major concern in low socioeconomic population. The frequency of adverse effects with the oral ciprofloxacin was high as compared with the topical drops. On the basis of our results, we can deduce that topical ciprofloxacin alone suffice for the treatment of tubotympanic CSOM. It is a costly option for the treatment of the disease. The idea now is to create awareness among otolaryngologists, physicians, and general practitioners to negate their false beliefs and to promote a cost-effective way of treatment for tubotympanic CSOM.
Footnotes
Author Contributions: MAO and SBB conceived the topic of the study and were involved in designing the study, collecting and analyzing data, and drafting the initial manuscript. All were involved in data collection and critical revision of the manuscript. All authors who have affiliation outside the Jinnah Medical College Hospital worked as honorary researchers and contributed to data collection. All authors have read and approved the final manuscript.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Verhoeff M, van der Veen EL, Rovers MM, Sanders EA, Schilder AG. Chronic suppurative otitis media: a review. Int J Pediatr Otorhinolaryngol. 2006;70:1–12. [DOI] [PubMed] [Google Scholar]
- 2. Gleeson M, Browning GG, Burton MJ, et al. Scott-Brown’s Otolaryngology, Otology. 7th ed. Vol. 2 Oxford, UK: Butterworth-Heinemann; 2004. [Google Scholar]
- 3. World Health Organization. Prevention of hearing impairment from chronic otitis media: report of a WHO/CIBA Foundation workshop. Paper presented at: Prevention of hearing impairment from chronic otitis media: report of a WHO/CIBA Foundation Workshop; November 19-21, 1996; London, UK. [Google Scholar]
- 4. Aslam MA, Ahmed Z, Azim R. Microbiology and drug sensitivity patterns of chronic suppurative otitis media. J Coll Physicians Surg Pak. 2004;14:459–461. [PubMed] [Google Scholar]
- 5. Iqbal S, Udaipurwala IH, Hasan A, Shafiq M, Mughal S. Chronic suppurative otitis media: disease pattern and drug sensitivity. J Surg Pak. 2006;11:17–19. [Google Scholar]
- 6. de Miguel MI, et al. Aetiology and therapeutic considerations in chronic otitis media. Analysis of a 5 year period. Acta Otorrinolaringol Esp. 2005;56:459–462. [DOI] [PubMed] [Google Scholar]
- 7. Davidson SS. Davidson’s Principles and Practice of Medicine. London, England: Churchill Livingstone; 2002. [Google Scholar]
- 8. Goodman LS. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. Vol. 1157 New York, NY: Pergamon Press; 1990. [Google Scholar]
- 9. Macfadyen C, Acuin J, Gamble C. Systemic antibiotics versus topical treatments for chronically discharging ears with underlying eardrum perforations. Cochrane Database Syst Rev. 2006;1:CD005608. [DOI] [PubMed] [Google Scholar]
- 10. Ahmad S. Antibiotics in chronic suppurative otitis media: a bacteriologic study. Egypt J Ear, Nose, Throat Allied Sci. 2013;14:191–194. [Google Scholar]
- 11. Manolidis S, Friedman R, Hannley M, et al. Comparative efficacy of aminoglycoside versus fluoroquinolone topical antibiotic drops. Otolaryngol Head Neck Surg. 2004;130:S83–S88. [DOI] [PubMed] [Google Scholar]
- 12. Khanna V, Chander J, Nagarkar NM, Dass A. Clinicomicrobiologic evaluation of active tubotympanic type chronic suppurative otitis media. J Otolaryngol. 2000;29:148–153. [PubMed] [Google Scholar]
- 13. WMA. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subject. Paper presented at: 52nd WMA General Assembly; October, 2008; Edinburgh, Scotland. [Google Scholar]
- 14. Kadar AA, Usman M, Tirmizi S. Topical quinolones versus topical amynoglycosides in the medical management of chronic suppurative otitis media: a comparative trial. J Surg Pakistan. 2003;8:6–9. [Google Scholar]
- 15. Mittal R, Lici CV, Gerring R, et al. Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J Med Microbiol. 2015;64:1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esposito S, D’Errico G, Montanaro C. Topical and oral treatment of chronic otitis media with ciprofloxacin: a preliminary study. Arch Otolaryngol. 1990;116:557–559. [DOI] [PubMed] [Google Scholar]
- 17. Acuin J, Smith A, Mackenzie I. Interventions for chronic suppurative otitis media. Cochrane Database Syst Rev. 2000;2:CD000473. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization Library Cataloguing-in-Publication Data. Chronic Suppurative Otitis Media: Burden of Illness and Management Options. Geneva, Switzerland: WHO; 2004. [Google Scholar]
- 19. World Health Organization. Chronic suppurative otitis media: burden of illness and management options; 2004. http://www.who.int/pbd/publications/Chronicsuppurativeotitis_media.pdf.
- 20. Acuin J. Chronic suppurative otitis media. BMJ Clin Evid. 2007;2007:0507. [PMC free article] [PubMed] [Google Scholar]
- 21. Miró N. Controlled multicenter study on chronic suppurative otitis media treated with topical applications of ciprofloxacin 0.2% solution in single-dose containers or combination of polymyxin B, neomycin, and hydrocortisone suspension. Otolaryngol Head Neck Surg. 2000;123:617–623. [DOI] [PubMed] [Google Scholar]
- 22. Fairbanks D. Antimicrobial therapy for chronic suppurative otitis media. Ann Otol Rhinol Laryngol. 1981;90:58–62. [DOI] [PubMed] [Google Scholar]
- 23. Ramos A, Ayudarte F, de Miguel I, Cuyás JM, Cenjor C. Use of topical ciprofloxacin in chronic suppurating otitis media. Acta Otorrinolaringol Esp. 2003;54:485–490. [DOI] [PubMed] [Google Scholar]


