Abstract
Gastric neuroendocrine tumors (GNETs) are rare lesions characterized by enterochromaffin-like cells of the stomach. Optimal management of GNETs has not yet been definitively determined. Endoscopic resection is approximately recommended for small GNETs associated with hypergastrinemia. However, endoscopic resection might present risk of perforation or positive vertical margin because neuroendocrine tumors occur in the deep mucosa, with some invading the submucosa. In this case, a patient with type A chronic atrophic gastritis had a small subepithelial lesion in a deep submucosal layer, and we diagnosed it as GNET using endoscopic ultrasound-guided fine-needle aspiration biopsy using a forward-viewing and curved linear-array echoendoscope. Moreover, our results show that laparoscopic and endoscopic cooperative surgery with regional lymph node dissection is a safe and feasible procedure for GNETs, especially those that cross to the muscularis propria. We suggest this approach as one therapeutic option for GNETs because it safely minimizes resection and is less invasive.
Keywords: Gastric neuroendocrine tumor (GNET), subepithelial lesion (SEL), endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA), forward-viewing and curved linear-array echoendoscope (FVCLA-EUS), laparoscopic and endoscopic cooperative surgery (LECS)
Introduction
Gastric neuroendocrine tumors (GNETs) are classified into 3 distinct subgroups: type I to III.1 Type I GNETs arise in patients with chronic atrophic gastritis including autoimmune gastritis. The treatment strategy for GNETs is decided by the presence of associated conditions (Rindi classification), the number and size of the tumors, the depth of invasion, the distribution of proliferation markers (Ki-67 index), and the presence of metastasis. However, optimal management for GNETs, especially small GNETs, has not yet been definitively determined because the clinical outcomes of the different treatment approaches remain unclear. Endoscopic resection is recommended as the treatment for type I GNETs smaller than 10 mm and limited to mucosa or submucosa.2 However, endoscopic resection might entail risks of perforation or positive vertical margin because neuroendocrine tumors (NETs) arise from neuroendocrine cells present in the deep layers of mucosa and they frequently grow expansively into submucosal layers. Laparoscopic and endoscopic cooperative surgery (LECS) is applied to epithelial neoplasms, which are difficult to treat with endoscopic submucosal dissection (ESD). We herein report a very small subepithelial lesion (SEL) in a deep layer of submucosa associated with type A chronic atrophic gastritis, which was diagnosed as type I GNET by endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA) using a forward-viewing and curved linear-array echoendoscope (FVCLA-EUS)3 and was treated by LECS with regional lymph node dissection.
Case Report
An 80-year-old man was referred to our hospital for pancytopenia, including anemia. Esophagogastroduodenoscopy (EGD) showed type A chronic atrophic gastritis and small SEL on the greater curvature of the gastric body (Figure 1A and B). Endoscopic ultrasonography (miniprobe, 20 MHz) revealed a homogeneous, hypoechoic lesion located mainly within the submucosal layer closer to the muscularis propria. The size of the SEL was 6 mm (Figure 1C). Laboratory tests showed hyperchromic macrocytic anemia and vitamin B12 deficiency. Fasting serum gastrin levels were elevated: >3000 pg/mL (normal levels: 13-115 pg/mL). Serum antibodies to gastric parietal cells were not elevated (Table 1). We subsequently performed a deep endoscopic biopsy for the SEL, but we were unable to reach a histologic diagnosis. Therefore, we performed EUS-FNA using FVCLA-EUS with an attached cap (XGIF-UCT160 J-AL5; Olympus Corp., Tokyo, Japan; Figure 2A). An adequate histologic sample was obtained, and immunohistologic examination showed that chromogranin A was positive (Figure 2B and C). We diagnosed the SEL as a small (<10 mm) GNET in a deep layer of submucosa that was associated with type A chronic gastritis.
Figure 1.

(A, B) Esophagogastroduodenoscopy showing a 6-mm diameter submucosal tumor on the greater curvature of the gastric body with no mucosal change. (C) Endoscopic ultrasound demonstrating a homogeneous, hypoechoic lesion located mainly within the submucosal layer closer to the muscularis propria.
Table 1.
Laboratory data.
| Blood cell count | |
|---|---|
| WBC | 3340/mL |
| RBC | 142 × 104/mL |
| Hb | 6.4 g/dL |
| Hct | 18.2% |
| MCV | 128.2 fL |
| Plt | 5.2 × 104/mL |
| Blood chemistry | |
| AST | 29 IU/L |
| ALT | 15 IU/L |
| LDH | 803 IU/L |
| ALP | 168 IU/L |
| γ-GTP | 8 IU/L |
| T-bil | 1.8 mg/dL |
| D-bil | 0.6 mg/dL |
| Fe | 178 μg/dL |
| UIBC | 21 U/mL |
| Ferritin | 166 ng/mL |
| Vitamin B12 | <50 pg/mL |
| Folic acid | 12.0 ng/mL |
| Gastrin | >3000 pg/mL |
| PG1 | 7.0 ng/mL |
| PG2 | 9.7 ng/mL |
| PG1/2 | 0.7 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GTP, γ-guanosine triphosphate; Hb, hemoglobin; Hct, hematocrit; LDH, lactate dehydrogenase; MCV, mean corpuscular volume; PG, prostaglandin; Plt, platelet; RBC, red blood cell; UIBC, unsaturated iron–binding capacity; WBC, white blood cell.
Antiparietal cell antibody: negative.
Anti-Helicobacter pylori IgG antibody: <3 U/mL.
Figure 2.

(A) Image of endoscopic ultrasound-guided fine-needle aspiration procedure with a forward-viewing and curved linear-array echoendoscope. A long arrow shows the needle, and a short arrow shows the subepithelial lesion. (B) Microscopic examination revealed small, nearly homogeneous neoplastic cells, with round or oval nuclei and rich cytoplasm (HE, ×400). (C) Immunohistologic examination showed that chromogranin A was positive (chromogranin A, ×1,000).
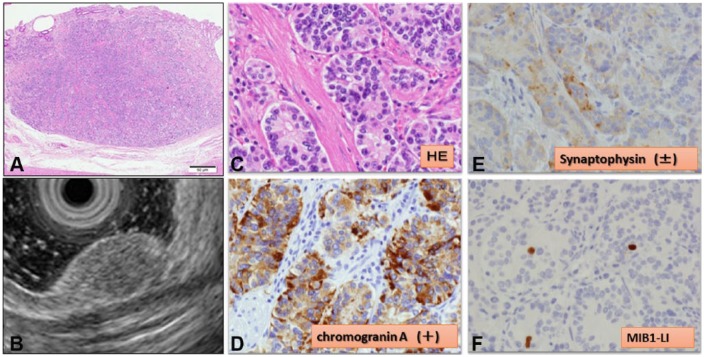
Although we considered ESD as a minimally invasive treatment, there was a potential risk of perforation and positive vertical margin. Therefore, we chose to perform a full-thickness, local excision using LECS (Figure 3). After inducing general anesthesia, we secured the laparoscopic view. Although we were unable to detect the tumor laparoscopically from the serous membrane side, the gastric tumor was confirmed by EGD. The lesion was marked endoscopically. Laparoscopically, we incised the greater omentum and approached into the omental bursa cavity. We ligated the right gastroepiploic artery and vein and dissected the nearby lymph nodes designated as No 4d in the tumor area. Subsequently, the sections frozen intraoperatively revealed that those lymph nodes were negative for cancer. A full-thickness layer excision was performed along the marking point using a Dual knife and insulation-tip knife 2 (Olympus Corp., Tokyo, Japan). After an en bloc–resected specimen was removed via the abdominal cavity using an “Endobag,” we sutured the postexcisional defect in the gastric posterior wall under laparoscopic view. After completing the full-thickness closure, the endoscope was inserted into the stomach to confirm that it passed the suture site easily and that no air leakage had occurred despite insufflation of the stomach. The surgical procedure was uneventful. The total operation time was 182 minutes. Pathologic examination revealed small, nearly homogeneous neoplastic cells with round or oval nuclei and rich cytoplasm (Figure 4C). Immunostaining for chromogranin (Figure 4D) and synaptophysin (Figure 4E) was positive. The mitotic count was <2% (Figure 4F). These findings led to the final diagnosis of a GNET G1. The tumor had invaded into a deep layer of submucosa (Figure 4A), but the horizontal and vertical margins were free of tumor cells. The patient recovered uneventfully and was discharged on postoperative day 14.
Figure 3.

Laparoscopic and endoscopic cooperative surgery procedure. (A, B) A circumferential incision was made around the tumor by an endoscopic submucosal dissection technique using an insulation-tip knife 2. (C, D) The postexcisional hole in the stomach was sutured under laparoscopic view and clipped endoscopically.
Figure 4.
(A, B) The depth of the tumor invasion was into the submucosal layer closer to the muscularis propria: comparison with endoscopic ultrasound findings (HE, ×100). (C) Pathologic examination revealed small, nearly homogeneous neoplastic cells with round or oval nuclei and rich cytoplasm (HE, ×1,000). (D) Chromogranin A expression was positive in tumor cells (chromogranin A, ×1,000). (E) Synaptophysin expression was positive in tumor cells. (F) MIB1-LI 0.8% (5/632) (MIB1-LI, ×1,000).
Discussion
In this case, no typical endoscopic findings of NETs were observed, such as a hemispherical reddish polyp with or without a central depression. Moreover, we were unable to reach a diagnosis even by a deeper endoscopic biopsy. Endoscopic ultrasound revealed that the lack of mucosal endoscopic features and inconclusive biopsy specimen was due to the tumor residing in a deep layer of the submucosa. In such a case, we considered that, according to SELs, EUS-FNA is an important modality for diagnosis. Akahoshi et al4 demonstrated that the diagnostic rates for gastrointestinal stromal tumors (GISTs) smaller than 20 mm, 20 to 40 mm, and 40 mm or larger were, respectively, 71%, 86%, and 100%. Although EUS-FNA could be conducted easily in large SELs, it was technically difficult to perform in small SELs. Our earlier study3 highlighted the diagnostic capability of EUS-FNA using a FVCLA-EUS with a cap for small SELs: although the mean diameter of the SELs was 10.6 mm and the specimen obtainment rate was 87.5%. Among SELs, NETs in particular might have a potential for metastasis even when they are of a small size. Soga et al5 reported that even NETs limited to the submucosa had a metastasis rate of 7.9% at <10 mm and 21.4% at 10 to 20 mm. Therefore, obtaining the histologic diagnosis before progression using EUS-FNA is important even for a small SEL from which a histologic specimen cannot be obtained using conventional biopsy methods, particularly for suspected NET.
The European Neuroendocrine Tumor Society (ENETS) recommends endoscopic treatment for G1 NETs ≤10 mm that do not extend beyond the submucosal layer and which do not demonstrate lymph node metastasis and recommends surgical treatment for tumors ≥20 mm. The treatment strategy for tumors of 10 to 20 mm remains controversial.2 Endoscopic mucosal resection (EMR) has been recommended for well-localized, type I GNETs. However, their complete removal by snare polypectomy or conventional EMR is difficult because GNETs frequently invade the submucosa. Therefore, ESD is useful for removing submucosal tumors including type I GNETs. Studies have demonstrated superior complete resection rates of GNETs using ESD compared with those achieved with EMR.6 However, endoscopic resection might risk perforation or positive margin because NETs arise from neuroendocrine cells residing in a deep layer of mucosa and they frequently grow expansively in submucosal layers. However, LECS has been described by Hiki et al7 as the treatment for GIST. GISTs grow expansively in the gastric wall and rarely metastasize to regional lymph nodes. Therefore, partial gastrectomy or laparoscopic wedge resection has been used for the surgical treatment of gastric GIST. In other cases, LECS can prevent excessive resection of the gastric wall by combination with an endoscopic procedure. A retrospective multicenter study described that LECS can be a standard treatment for SELs of the stomach.8 Now, the technique is beginning to be employed for epithelial neoplasm, which is difficult to treat with ESD because of ulcer coexistence or tumor location, among other reasons. Reportedly, ESD for lesions on the greater curvature of the stomach entails increased perforation risk. We performed LECS in this case because the lesion was in a deep layer of submucosa that crossed to the muscularis propria. Moreover, unlike ESD, LECS can dissect the regional lymph node laparoscopically. Goto et al9 reported a successful case of nonexposed endoscopic wall-inversion surgery, a method of LECS, with sentinel node navigation surgery for early gastric cancer with a risk of lymph node metastasis. We applied this technique to GNET because GNETs ≤10 mm and limited to the submucosa have been shown to metastasize with sufficiently high frequency (7.9%).5 A multicenter study in Japan10 described 4 of 71 patients with small type I GNET (<10 mm) who were complicated with capillary invasion (lymphatic invasion in 2, venous invasion in 2).
In conclusion, EUS-FNA using a FVCLA-EUS with a cap is useful for the diagnosis of very small SELs. Subsequent LECS with regional lymph node dissection may be regarded as a therapeutic option even for small GNETs.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RI, AI, and GS managed the patient; RI, AY, and MF performed EUS-FNA; RI, GS, and NS performed LECS; AS, NA, YY, TI, YA, TM, SY, and IO provided clinical advice; RI and AI collected the data and wrote the paper; AI revised the paper; TS and HH supervised the report; all authors approved the final manuscript for publication.
Disclosures and Ethics: The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that we have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1. Rindi G, Luinetti O, Cornaggia M, Capella G, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006. [DOI] [PubMed] [Google Scholar]
- 2. Klöppel G, Couvelard A, Perren A, et al. ; Mallorca Consensus Conference Participants; European Neuroendocrine Tumor Society. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: towards a standardized approach to the diagnosis of gastroenteropancreatic neuroendocrine tumors and their prognostic stratification. Neuroendocrinology. 2009;90:162–166. [DOI] [PubMed] [Google Scholar]
- 3. Yamabe A, Irisawa A, Bhutani MS, et al. Usefulness of endoscopic ultrasound-guided fine-needle aspiration with a forward-viewing and curved linear-array echoendoscope for small gastrointestinal subepithelial lesions. Endosc Int Open. 2015;3:E161–E164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soga J. Early-stage carcinoids of the gastrointestinal tract. An analysis of 1914 reported cases. Cancer. 2005;103:1587–1595. [DOI] [PubMed] [Google Scholar]
- 6. Sato Y, Takeuchi M, Hashimoto S, et al. Usefulness of endoscopic submucosal dissection for type I gastric carcinoid tumors compared with endoscopic mucosal resection. Hepatogastroenterology. 2013;60:1524–1529. [DOI] [PubMed] [Google Scholar]
- 7. Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729–1735. [DOI] [PubMed] [Google Scholar]
- 8. Matsuda T, Nunobe S, Kosuga T, et al. ; Society for the Study of Laparoscopy and Endoscopy Cooperative Surgery. Laparoscopic and luminal endoscopic cooperative surgery can be a standard treatment for submucosal tumors of the stomach: a retrospective multicenter study. Endoscopy. 2017;49:476–483. [DOI] [PubMed] [Google Scholar]
- 9. Goto O, Takeuchi H, Kawakubo H, et al. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:434–439. [DOI] [PubMed] [Google Scholar]
- 10. Sato Y, Imamura H, Kaizaki Y, et al. Management and clinical outcomes of type I gastric carcinoid patients: retrospective, multicenter study in Japan. Dig Endosc. 2014;26:377–384. [DOI] [PubMed] [Google Scholar]