Abstract
Acute respiratory distress syndrome (ARDS) is a diffuse lung injury that leads to a severe acute respiratory failure. Traditional diagnostic criteria for pulmonary hypertension (PH), in this situation, may be unreliable due to the effects of positive pressure ventilation and vasoactive agents. The aim of this study is to describe the hemodynamic characteristics of PH secondary to ARDS, in relation with respiratory parameters. We assessed the hemodynamic, respiratory function, and ventilator parameters in a cohort of 38 individuals with ARDS-associated PH defined by mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg. Individual characteristics: PaO2/FiO2 = 110 ± 60 mmHg, alveolar-arterial oxygen gradient (A-aO2) = 549 ± 148.9 mmHg, positive end-expiratory pressure (PEEP) = 8.7 ± 3.5 cmH2O, pulmonary static compliance (Cstat) = 30 ± 12.1 L*cmH2O-1, mPAP = 35.4 ±6.6 mmHg, pulmonary artery wedge pressure (PAWP) = 15.6 ± 5.5 mmHg, cardiac index (CI) = 3.4 ± 1.2 L/min/m2, pulmonary vascular resistance (PVR) = 3.3 ± 1.6 Wood units (WU), right atrial pressure (RAP) = 13.4 ± 5.4 mmHg, diastolic pulmonary gradient (DPG) = 12.6 ± 6.5 mmHg, and trans-pulmonary gradient (TPG) = 19.7 ± 7.7 mmHg. The composite marker—DPG >7 mmHg and PVR > 3 WU—is associated with lower CI (P = 0.016), higher mPAP (P = 0.003), and lower pulmonary static compliance (P = 0.028). We confirmed a poor prognosis of ARDS associated with PH, with a 50% survival rate after 17 days. We observed that the survival rate at 28 days was better in the case of improvement in the PaO2/FiO2 ratio in the first 24 h (log rank P = 0.003). ARDS associated with PH is a severe condition with a very poor survival rate. The composite marker DPG > 7 mmHg and PVR > 3 WU seemed to better describe the hemodynamic and respiratory dysfunction. The improvement in PaO2/FiO2 ratio in the first 24 h defined a better survival in our cohort of patients.
Keywords: ARDS, acute respiratory distress syndromes and acute lung injury, cardiac index, pulmonary hypertension, pulmonary vascular resistance
Introduction
Acute respiratory distress syndrome (ARDS) is a frequent and very severe form of acute respiratory failure. ARDS is defined clinically by the acute onset of hypoxemia associated with bilateral pulmonary infiltrates, which are not explained by elevated cardiac filling pressure.1 There are a multitude of causes which can produce an acute lung injury and this process results in a functional pulmonary shunt with an increase of perfusion in pulmonary areas less ventilated, resulting in severe hypoxemia non-responsive to oxygen therapy. In the era of lung protective ventilation (ARDSNet),2 the management of ARDS remains difficult with an unacceptable mortality rate, in the range of 32–41%.3,4 The fragile balance of pulmonary circulation can be altered by multiples conditions and therefore ARDS is a recognized cause of pulmonary hypertension (PH) and right ventricle failure, that could be classified as group 3 PH due to lung disease and/or hypoxia.5–7 The process of pulmonary vascular alteration is multifactorial and includes factors such as hypoxia, hypercapnia, vascular compression by edema or pulmonary fibrosis, mediator-induced vasoconstriction and remodeling, in-situ thrombosis and pulmonary embolism, increased alveolar pressure, transpulmonary pressure due to reduced pulmonary compliance, and increased intrathoracic pressure related to positive end-expiratory pressure (PEEP).6–10
The lung protective ventilatory strategy (plateau pressure < 30 cmH2O) showed better outcomes with decreased mortality for the ARDS patients.2 Nevertheless, right ventricular dysfunction remains a common complication in this condition, as described in some recent studies.11,12
The diastolic pulmonary gradient (DPG) was calculated as the difference between diastolic pulmonary arterial pressure (dPAP) and PAWP. The dPAP represents a substitute of left atrial pressure; it is less flow dependent than systolic pulmonary arterial pressure (sPAP), and it remains relatively unaffected by diuretic treatment. Recently, there was described that elevated DPG was associated with more advanced pulmonary vascular remodeling in PH due to left heart disease.13
The treatment of ARDS is a complex one, consisting of ventilatory assistance, vasoactive agents, and in some cases vasodilator treatment by NO inhalation and mechanical ventilation in prone position. Therefore, the hemodynamic measurements in this sentence could be influenced by the differences in overall fluid load.
We hypothesized that DPG and pulmonary vascular resistance (PVR) can better define the vascular disease in PH associated to ARDS. Knowing the interdependence between the pulmonary and vascular system, we assessed the correlations between the ventilatory parameters and pulmonary hemodynamics.
Material and methods
Study design
We examined data of 38 consecutives individuals with ARDS complicated with PH defined by a mPAP > 25 mmHg, measured by right heart catheterization (RHC). We used a retrospective analysis of medical data from individuals recruited between March 2005 and May 2015.
This study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of the French learned society for respiratory medicine “Société de Pneumologie de Langue Française” (CEPR no. CEPRO 2017-006).
All the patients investigated in the present study met the Berlin definition criteria for ARDS:1,14
respiratory failure within one week, new or worsening respiratory symptoms;
bilateral opacities consistent with pulmonary edema must be present on a chest radiograph or computed tomographic (CT) scan. These opacities must not be fully explained by pleural effusions, lobar collapse, lung collapse, or pulmonary nodules;
the patient’s respiratory failure must not be fully explained by cardiac failure or fluid overload. An objective assessment (e.g. echocardiography) to exclude hydrostatic pulmonary edema is required if no risk factors for ARDS are present;
a moderate to severe impairment of oxygenation must be present, as defined by the ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2). The severity of the hypoxemia defines the severity of the ARDS: mild ARDS – the PaO2/FiO2 is >200 mmHg, but ≤300 mmHg, on ventilator settings that include PEEP or continuous positive airway pressure (CPAP) ≥5 cm H2O; moderate ARDS – the PaO2/FiO2 is >100 mmHg, but ≤200 mmHg, on ventilator settings that include PEEP ≥5 cm H2O; severe ARDS – the PaO2/FiO2 is ≤100 mmHg on ventilators setting that include PEEP ≥5 cm H2O.
The ventilation therapy used was in volume-assist control mode with target tidal volume of 6–8 mL/Kg (predicted body weight). For the patients with persistent severe hypoxemia (PaO2/FiO2 < 100 mmHg) despite an optimal ventilation therapy (a high level of PEEP and maximal pressure plateau at 30 cmH2O), prone-positioning and/or inhaled NO were used.
The RHC was performed 24 h after admission to the intensive care unit (ICU). We used two validated measures of pulmonary vascular dysfunction: DPG and PVR. The DPG, defined as the mean dPAP – PAWP, is used to better define the pulmonary vascular dysfunction in the case of post-capillary PH; a value of 7 mmHg or greater has been defined as an elevated DPG.13 The PVR is a measure of vascular resistance in the pulmonary bed defined by (mPAP – PAWP) / cardiac output. The cardiac output (CO) was measured by thermodilution method.
Investigations
The following parameters were recorded immediately after the insertion of the Swan–Ganz catheter:
ventilator parameters: tidal volume (Vt), PEEP, plateau pressure;
pulmonary characteristics: static pulmonary compliance (Cstat);
oxygenation parameters: PaO2/FiO2, PaCO2, pH, A-aO2. The A-aO2 was determined by the equation: (FiO2 * 760 – 0.8 * PaCO2) – PaO2;
hemodynamic variables: sPAP, dPAP, mPAP, PAWP, cardiac index (CI), PVR, right atrial pressure (RAP), DPG, and trans-pulmonary gradient (TPG);
laboratory test: procalcitonin, lactic acid.
Statistical analysis
We used SPSS Statistics version 20 for all statistical analyses. Continuous variables are described as mean ± SD. Significant differences at baseline, between the subgroups (mild to moderate ARDS vs. severe ARDS) were determined using the Mann–Whitney U test. Correlation coefficients are calculated using Spearman’s test. Survival rate was evaluated using the Kaplan–Meier method, and the survival rates are compared using the long-rank test. A P value < 0.05 was considered statistically significant. All parameters with a P value < 0.20 entered into the multivariate Cox proportional hazards analysis.
Results
The study population included 38 patients with PH associated to ARDS, all diagnosed by RHC. The baseline characteristics of the patients are presented in Table 1; the mean age was 63 ± 12.7 years, with a male predominance of 71.1%. The main cause of ARDS was pulmonary sepsis (76% of cases), followed by aspiration pneumonia (16% of cases) and non-pulmonary sepsis (8%).
Table 1.
Baseline characteristics.
| Patients (n = 38) | Min | Max | Mean ± SD |
|---|---|---|---|
| Age (years) | 31 | 86 | 63 ± 12.7 |
| BMI (kg/m2) | 15.1 | 56.8 | 30 ± 9.5 |
| PaO2/FiO2 (mmHg) | 36 | 286 | 110 ± 60 |
| Vt (mL/kg) | 5.5 | 11.1 | 7.5 ± 1.3 |
| Pplat (mmHg) | 14 | 36 | 25 ± 7.3 |
| PEEP (mmHg) | 5 | 15 | 8.7 ± 3.5 |
| Cstat (mL/mmHg | 13 | 48 | 30 ± 12.1 |
| PCT | 0.17 | 22.8 | 5 ± 8.2 |
| mPAP (mmHg) | 25 | 47 | 35.4 ± 6.7 |
| PAWP (mmHg) | 8 | 28 | 15.6 ± 5.5 |
| RAP (mmHg) | 6 | 26 | 13.4 ± 5.4 |
| CI (L/min/m2) | 1.2 | 7.1 | 3.4 ± 1.2 |
| PVR (Wood Units) | 0.9 | 6.9 | 3.3 ± 1.6 |
| DPG (mmHg) | 3 | 25 | 12.6 ± 6.5 |
Fourteen patients (36.8%) received a vasodilator treatment by inhaled NO and only four patients (10.5%) were ventilated in prone position. A grand majority of the patients (34 individuals) received vasoactive treatment by norepinephrine.
The pulmonary features at baseline were: PaO2/FiO2 = 110 ± 60 mmHg; A-aO2 = 549 ± 148.9 mmHg; PEEP = 8.7 ±3.5 cmH2O; Vt = 7.5 ± 1.3 L/min; inspiratory plateau pressure = 25 ± 7.3 cmH2O; Cstat = 30 ± 12.1 mL/mmHg. Fourteen patients were under inhaled NO and four patients were placed in prone position.
The hemodynamic profile was: mPAP = 35.4 ±6.6 mmHg; PAWP = 15.6 ± 5.5 mmHg; CI = 3.4 ± 1.2 L/min/m2; PVR = 3.3 ± 1.6 WU; RAP = 13.4 ± 5.4 mmHg; DPG = 12.6 ± 6.5 mmHg; and TPG = 19.7 ± 7.7 mmHg.
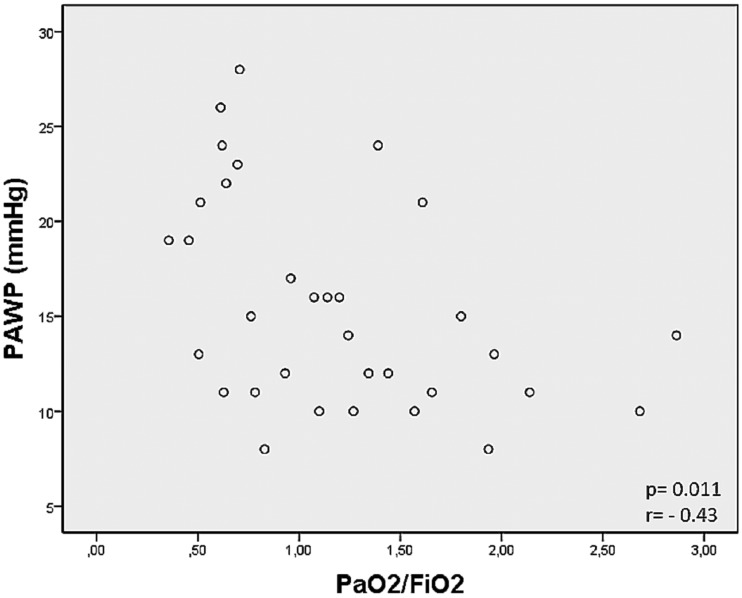
We found that PAWP was correlated with A-aO2 (P = 0.01, r = 0.44) and PaO2/FiO2 (P = 0.011, r = –0.43) (Fig. 1). The PEEP was correlated with hemodynamic status: PVR (P = 0.002, r = –0.68); DPG (P < 0.001, r =−0.538); TPG (P = 0.001, r = –0.577); and PAWP (P = 0.026, r = 0.40). Also, the pulmonary compliance was correlated with mPAP (P = 0.043, r = –0.68), PVR (P = 0.052, r = –0.66), and PaO2/FiO2 (P = 0.038, r = 0.69).
Fig. 1.
Correlation test between PAWP and PaO2/FiO2 ratio.
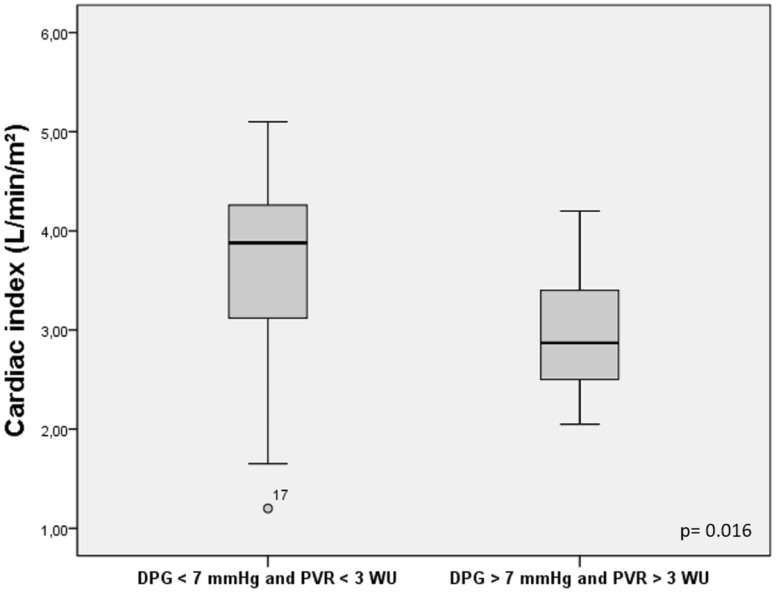
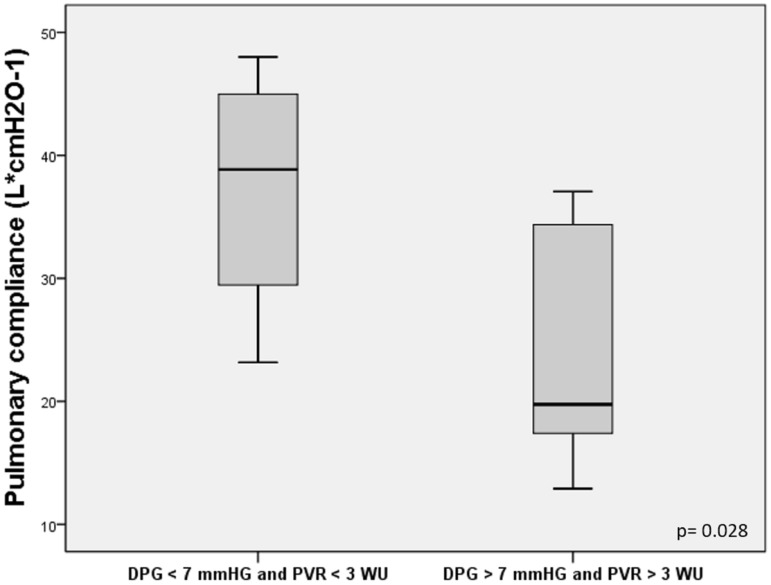
We compared the patients with DPG > 7 mmHg and PVR > 3 WU vs. DPG < 7 mmHg and PVR < 3 WU, measured at baseline. We found that the individuals with DPG > 7 mmHg and PVR > 3 WU had lower CI (2.9 vs. 3.6 L/min/m2, P = 0.016) (Fig. 2) and lower pulmonary compliance (26.3 vs. 45.6 L*cmH2O–1, P = 0.028) (Fig. 3).
Fig. 2.
Comparison of cardiac index for patients with DPG > 7 mmHg and PVR > 3 WU vs. DPG < 7 mmHg and PVR < 3 WU.
Fig. 3.
Comparison of pulmonary compliance for patients with DPG > 7 mmHg and PVR > 3 WU vs. DPG < 7 mmHg and PVR < 3 WU.
We compared, at baseline, the group of patients with severe ARDS vs. mild to moderate ARDS. We found that the patients with severe ARDS had higher PAWP (17.9 vs. 13.5 mmHg, P = 0.018) and a trend of higher RAP (15.3 vs. 11.5 mmHg, P = 0.069).
All the 38 individuals with ARDS and PH included in our analysis were treated by protective mechanical ventilation (plateau pressure < 30 cmH2O), treatment support, and inotropic agents.
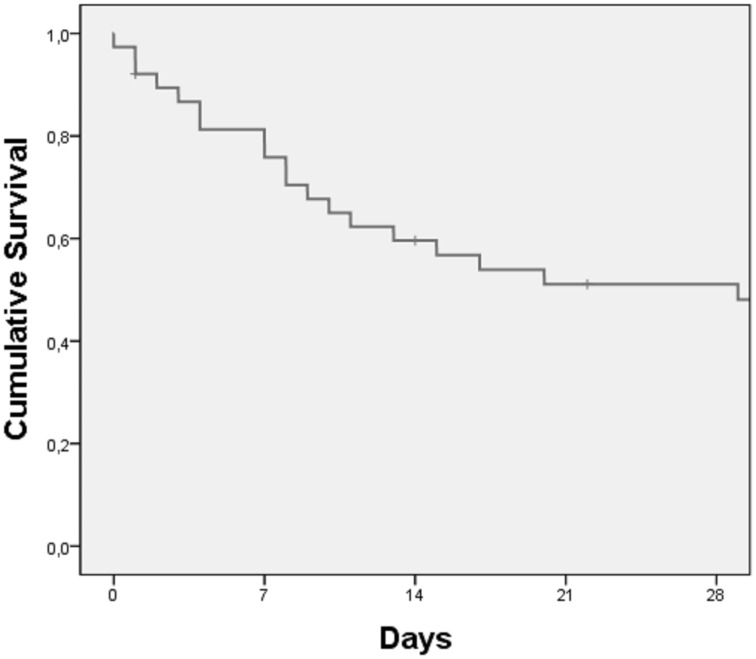
In our group of ARDS associated to PH, 23 individuals (60.5%) died (including all causes of death) during the first month of hospitalization in ICU. The median survival was 29 ± 10.7 days for a median follow-up time of 18.5 days (95% confidence interval = 7.9–50.1) (Fig. 4).
Fig. 4.
Cumulative survival at 28 days.
The patients who survived showed a lower RAP and acid lactate level (10.9 ± 4.8 vs. 16.4 ± 5.6 mmHg, P = 0.015 and 1.86 ± 0.87 vs. 4.59 ± 4.6 mmol/L, respectively, P = 0.027) with a higher PaO2/FiO2 (1.72 ± 0.76 vs. 1.06 ± 0.58 mmHg, P = 0.009) and a normal pH value (7.38 ± 0.06 vs. 7.30 ± 0.11, P = 0.009).
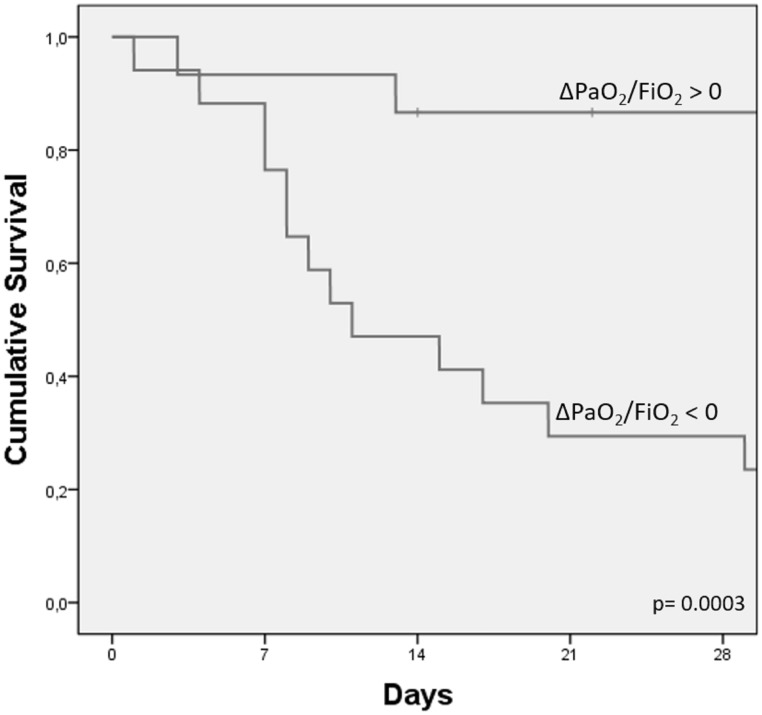
The results of the univariate analysis on the survival rate are presented in Table 2. We observed that the survival rate 28 days after ICU admission was better in the case of improvement in the PaO2/FiO2 ratio after 24 h (Δ PaO2/FiO2 > 0) (Log Rank P = 0.003) (Fig. 5) and A-aO2 < 432 mmHg (Log Rank P = 0.001), measured at baseline. After multivariate analysis only Δ PaO2/FiO2 > 0 showed a significant improvement of the 28-day survival (P = 0.007) (Table 3).
Table 2.
Univariate analysis of factors associated with mortality.
| Variable | P value |
|---|---|
| Δ PaO2/FiO2 < 0 | 0.003 |
| A-aO2 > 432 mmHg | 0.001 |
| pH < 7.29 | 0.149 |
| Lactate acid > 1.8 mmol/L | 0.175 |
| DPG > 7 mmHg and PVR > 3 WU | 0.21 |
| POD > 15 mmHg | 0.31 |
| PEEP > 10 cmH2O | 0.67 |
| Inhaled NO | 0.65 |
| Prone positioning | 0.54 |
Fig. 5.
The cumulative survival by evolution of hypoxemia in the first 24 h after ICU admission (measured by PaO2/FiO2 ratio).
Table 3.
Multivariate analysis of factors associated with mortality.
| Variable | HR | 95% CI | P value |
|---|---|---|---|
| Δ PaO2/FiO2 < 0 | 0.109 | 0.022–0.543 | 0.007 |
| A-aO2 > 432 mmHg | 1.239 | 0.284–5.412 | 0.776 |
| pH < 7.29 | 0.284 | 0.065–1.245 | 0.095 |
| Lactate acid > 1.8 mmol/L | 0.911 | 0.213–3.906 | 0.901 |
Discussion
The main finding of our study was that the marker DPG >7 mmHg and PVR >3 WU seemed to better define a worsen hemodynamic and pulmonary status; DPG >7 mmHg and PVR >3 WU was associated with lower CI and lower pulmonary compliance suggesting a more severe illness and thus identifying patients with worse outcomes. We thought that the DPG could better define the vascular dysfunction because the dPAP is less or not influenced by the cardiac flow.13 Furthermore, the occurrence of severe ARDS was associated with higher RAP, a sign of right heart failure.
Recently, Bull et al. reported that elevated PVR and TPG were independently associated with increased mortality in ARDS, in a large trial with protocol-defined management strategies and using lung-protective ventilation.11 We think that hemodynamic status plays a key role in the case of ARDS complicated by right heart failure.
The hemodynamic profile is characterized by high mPAP (35.4 ± 6.6 mmHg) with borderline PAWP (15.6 ±5.5 mmHg), probably explained by fluid resuscitation and vasopressor treatment. The CI was normal (3.4 ± 1.2 L/min/m2) with slightly elevated PVR (3.3 ±1.6 WU). This PH feature was similar compared with other studies.11,15,16
Compared to normal lungs, injured lungs in ARDS need ventilatory support with application of PEEP and inspiratory plateau pressure. In our study, all the patients received a lung protective ventilation with a mean inspiratory plateau pressure at 25 cmH2O and total PEEP at 8.7 cmH2O. Surprisingly, in our study, PEEP was negatively correlated with PVR, which can suggest a protective role of PEEP by improving the oxygenation level. Probably, with the application of high PEEP the number of open alveoli will increase, followed by a fall in PVR due to the Whittenberger-U shaped relationship between PVR and lung volume. At the same time, the pulmonary compliance was negatively correlated to mPAP, which proves the relationship between the respiratory and hemodynamic system and therefore the principle treatment for group 3 PH is the cure of underlying lung disease.17,18
We found that the composite marker (DPG > 7 mmHg and PVR > 3 WU) could predict the hemodynamic and pulmonary impairment for the ARDS patients, complicated by PH. Bull et al. showed that the pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. TPG and PVR were independent risk factors for 60-day mortality.11
ARDS remains a major cause of death, even in the era of protective lung ventilation, and the mortality is unacceptably high.3,11 A recent study showed a higher 28-day mortality rate in the group with cor pulmonale associated with ARDS (60% vs. 36%).12 Similar to these findings, we found in our cohort a high mortality during ICU hospitalization (60.5%) with a median survival of 29 ± 10.7 days. The survivors showed a lower RAP and acid lactate level with a normal pH and better oxygenation. On the other hand, the level of mPAP or PVR were not correlated with changes in terms of survival. That can suggest that the response of right heart function to this hypoxic and pulmonary aggression is more important than the vascular reactive mechanism (elevation of mPAP and PVR).
We found that the patients with severe ARDS had higher PAWP (17.9 vs. 13.5 mmHg, P = 0.018). Probably, this post-capillary component of PH could be explained by a degree of diastolic heart failure and decreased cardiac compliance which could furthermore worsen the hypoxemia.
We observed that improvement in the PaO2/FiO2 ratio 24 h after admission was correlated with better survival which could explain that right heart failure is just a consequence of the respiratory failure in this situation.
Whether pharmacological treatment of pulmonary vascular dysfunction (which is possible in patients with ARDS, but without sustained proves in terms of benefit on mortality)19 or prone positioning (which improves pulmonary ventilation, with reduced air pressure and decreased RV overload)20 may modify the mortality outcomes in patients with PH associated to ARDS warrants further investigation. The treatment by inhaled NO, prone positioning, or vasoactive therapy did not change the survival rates in our cohort, but only a minority of these patients followed this therapeutic strategy.
Study limitations
Our study has some limitations. It retrospectively analyzed a highly selected population of patients in a single university hospital. Our cohort represents only a minority of ARDS patients because of the lack of interest in using RHC, an invasive procedure, in this situation. Therefore, no estimate of incidence or prevalence of PH in ARDS can result from this study.
We could not describe the echocardiographic profile of our patients because of the lack of these data in our retrospective analysis. Therefore, we cannot show any data of follow-up for these cases of PH related to ARDS.
We included patients during a ten-year period; however, our mechanical ventilation strategy did not vary during the study period.
In conclusion, we found a strong correlation between the hemodynamic impairment, pulmonary characteristics, and ventilatory parameters in patients with PH secondary to ARDS. The composite marker of DPG > 7 mmHg and PVR > 3 WU seemed to better define a worsen hemodynamic and pulmonary status.
The rapid improvement of PaO2/FiO2 was associated with better survival. Similarly, the patients with higher RAP showed a worse prognostic, which can suggest that, the presence of right heart failure influences the survival in this situation.
Furthermore, specific treatment and measures aimed to improve pulmonary vascular and right heart dysfunction should be tested on ARDS patients with PH in the future.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ranieri VM, Rubenfeld GD, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012; 307(23): 2526–2533. [DOI] [PubMed] [Google Scholar]
- 2.Brower RG, Matthay MA, et al. Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342(18): 1301–1308. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353(16): 1685–1693. [DOI] [PubMed] [Google Scholar]
- 4.Phua J, Badia JR, Adhikari NKJ, et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am J Respir Crit Care Med 2009; 179(3): 220–227. [DOI] [PubMed] [Google Scholar]
- 5.Sibbald WJ, Driedger AA, Myers ML, et al. Biventricular function in the adult respiratory distress syndrome. Chest 1983; 84(2): 126–134. [DOI] [PubMed] [Google Scholar]
- 6.Vieillard-Baron A, Jardin F. Why protect the right ventricle in patients with acute respiratory distress syndrome? Curr Opin Crit Care 2003; 9(1): 15–21. [DOI] [PubMed] [Google Scholar]
- 7.Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med 1977; 296(9): 476–480. [DOI] [PubMed] [Google Scholar]
- 8.Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med 2001; 29(8): 1551–1555. [DOI] [PubMed] [Google Scholar]
- 9.Jardin F, Brun-Ney D, Cazaux P, et al. Relation between transpulmonary pressure and right ventricular isovolumetric pressure change during respiratory support. Cathet Cardiovasc Diagn 1989; 16(4): 215–220. [DOI] [PubMed] [Google Scholar]
- 10.Moloney ED, Evans TW. Pathophysiology and pharmacological treatment of pulmonary hypertension in acute respiratory distress syndrome. Eur Respir J 2003; 21(4): 720–727. [DOI] [PubMed] [Google Scholar]
- 11.Bull TM, Clark B, McFann K, et al. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2010; 182(9): 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013; 39(10): 1725–1733. [DOI] [PubMed] [Google Scholar]
- 13.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in ‘out-of-proportion’ pulmonary hypertension. Chest 2013; 143(3): 758–766. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38(10): 1573–1582. [DOI] [PubMed] [Google Scholar]
- 15.Osman D, Monnet X, Castelain V, et al. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med 2009; 35(1): 69–76. [DOI] [PubMed] [Google Scholar]
- 16.McNeil K, Dunning J, Morrell NW. The pulmonary physician in critical care. 13: the pulmonary circulation and right ventricular failure in the ITU. Thorax 2003; 58(2): 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeger W, Adir Y, Barberà JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62(25 Suppl): D109–116. [DOI] [PubMed] [Google Scholar]
- 18.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Espanola Cardiol Engl Ed 2016; 69(2): 177. [DOI] [PubMed] [Google Scholar]
- 19.Afshari A, Brok J, Møller AM, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev 20107, CD002787. [DOI] [PubMed] [Google Scholar]
- 20.Vieillard-Baron A, Charron C, Caille V, et al. Prone positioning unloads the right ventricle in severe ARDS. Chest 2007; 132(5): 1440–1446. [DOI] [PubMed] [Google Scholar]