Abstract
The pulmonary vasculature plays an important role in many lung pathologies, such as pulmonary arterial hypertension, primary graft dysfunction of lung transplant, and acute respiratory distress syndrome. Therapy for these diseases is quite limited, largely due to dose-limiting side effects of numerous drugs that have been trialed or approved. High doses of drugs targeting the pulmonary vasculature are needed due to the lack of specific affinity of therapeutic compounds to the vasculature. To overcome this problem, the field of targeted drug delivery aims to target drugs to the pulmonary endothelial cells, especially those in pathological regions. The field uses a variety of drug delivery systems (DDSs), ranging from nano-scale drug carriers, such as liposomes, to methods of conjugating drugs to affinity moieites, such as antibodies. These DDSs can deliver small molecule drugs, protein therapeutics, and imaging agents. Here we review targeted drug delivery to the pulmonary endothelium for the treatment of pulmonary diseases. Cautionary notes are made of the risk–benefit ratio and safety—parameters one should keep in mind when developing a translational therapeutic.
Keywords: ARDS, acute respiratory distress syndromes and acute lung injury, drug delivery, endothelium, inflammation, vascular targeting
The pulmonary vasculature is an important target for therapeutic interventions. Pulmonary endothelial cells are implicated in numerous pulmonary diseases, including pulmonary arterial hypertension (PAH), primary graft dysfunction (PGD) of lung transplant, and acute respiratory distress syndrome (ARDS). Targeting drugs to the pulmonary vasculature may be beneficial for the treatment of these and other conditions as it offers a more precise spatiotemporal control of the pharmacological effect.
In addition to the pulmonary endothelium’s important role in numerous diseases, it also has unique features that make it a practical target for drug delivery systems (DDSs) via the intravenous (IV) route. First, the pulmonary endothelium represents ∼25% of the total vascular surface in the body, thus providing an enormouse surface area for binding.1 Second, the pulmonary vasculature receives the entire first pass of IV-administered drug. Third, it collects the entire cardiac output and does so at lower shear rates than arteries; hydrodynamic conditions aid the binding of targeted drug delivery vectors.1,2
The absence of affinity of most drugs and DDSs to endothelial cells can be overcome by “vascular targeting,” or conjugation of DDS with ligands that bind to the endothelium. This experimental strategy enables delivery to, into, or across endothelial cells.16–21. In this review, we will discuss how such vascular-targeted DDSs have been used to deliver drugs to the pulmonary endothelium for the treatment of animal models that mimic multiple important human lung diseases.
Endothelial determinants for targeting drugs to the pulmonary vasculature
“Endothelilal target determinants” are features of DDSs that anchor a drug or drug carrier to the endothelium in the area of interest and may also provide sub-cellular addressing. The vast majority of published endothelial target determinants are usually affinity moieities, such monoclonal antibodies, that bind to epitopes on the endothelium. The list of endothelial determinants tentatively useful for vascular drug targeting is growing.3,4 Techniques such as selective proteomics of the endothelial plasmalemma5,6 and in vivo phage display7 introduce new targets, as they recognize binding sites available from the circulation.8 Table 1 briefly lists the promising and most commonly investigated candidates for vascular drug targeting.9–12
Table 1.
Target determinants for endothelial drug delivery.
| Target determinant | Sub-cellular localization | Effect of pathology on target availability | Potential utility as target for drug delivery | References |
|---|---|---|---|---|
| PECAM-1 | Cell–cell junctions in endothelial layer | Not usually affected | Prophylactic and therapeutic delivery to endothelium in lungs and other organs | 9,13 |
| ICAM-1 | Tetraspanin microdomains at apical membrane | Upregulated in inflammation | Prophylactic and therapeutic delivery to vasculature in lungs and other organs, imaging of vascular pathology | 10,14–17 |
| VCAM-1 | Tetraspanin microdomains at apical membrane | Upregulated in inflammation | Selective delivery to and imaging of inflamed endothelium in some organs | 18–20 |
| TM | Cell surface, single pass type I membrane protein | TM level can be suppressed in various pathological states | Cannot be used as a target | 21–24 |
| E-selectin | Cell surface, single pass type I membrane protein | Upregulated in inflammation | Selective delivery to and imaging of inflamed endothelium in some organs | 25–27 |
| P-selectin | Intracellular granules | Released upon inflammation | Selective delivery to and imaging of inflamed endothelium in some organs | 28,29 |
| Integrins αvβ3, αvβ5, α5β1 | Cell surface | αvβ3 is upregulated in response to vascular damage, αvβ5 is upregulated by VEGF, TGF-a | Selective delivery to and imaging of tumor vasculature | 30 |
| ACE | Apical domains in plasmalemma | Suppressed in vascular pathology | Selective delivery to the pulmonary microvasculature | 31–35 |
| APP | Caveolae | Unknown | Delivery and imaging of caveolar pathways and trans-endothelial delivery | 36–38 |
| PV1 (Plvap) | Caveolae and fenestrae | Upregulated by VEGF | Delivery to caveolar pathways | 38,39 |
PECAM-1, platelet-endothelial cell adhesion molecule 1; ICAM-1, intercellular cell adhesion molecule 1; VCAM-1, vascular cell adhesion molecule; TM, thrombomodulin; ACE, angiotensin-converting enzyme; APP, aminopeptidase P; PV1/Plvap, plasmalemma vesicle associated protein.
Some target determinants useful for vascular targeting are expressed on the endothelium throughout the vasculature. Adhesion molecules platelet-endothelial cell adhesion molecule 1 (PECAM-1) and intercellular adhesion molecule 1 (ICAM-1) are not only expressed on endothelial cells, but they are also constitutively present in other cells accessible to blood (platelets and leukocytes). However, compared to all of these competitor cells, surface levels of PECAM-1 and ICAM-1 are the highest in endothelial cells. While PECAM-1 is mainly located in the inter-endothelial borders,14,40,41 ICAM-1 tends to localize in lipid rafts in the luminal membrane either as a monomer or as an oligomer form.42 Adhesion molecules are involved in cellular recognition, adhesion, and migration of cells like leukocytes.43 Notably, inhibition of such functions may be considered favorable during the treatment of some types of inflammation.44–48
The pan-endothelial targets (e.g. PECAM-1) discussed above can be valuable for drug delivery to treat systemic maladies (e.g. sepsis). However, considering the high percentage of total body endothelial surface localized in pulmonary tissue (∼ 25%), and that drugs dosed intravenously first pass through the pulmonary circulation,1 agents with endothelial affinity primarily accumulate in the lungs after IV administration. This why endothelial target determinants improve lung concentrations of DDSs more than any other organ, even if though such pan-endothelial target determinants are consistently distributed throughout all endothelial cells in the body.49,50
Pathological states, such as inflammatory conditions, also affect the expression levels of some cell adhesion molecules (CAMs) such as ICAM-1 by inducing its synthesis, leading to an increase in its surface expression of approximately twofold in some inflamed types of endothelium51 and other cell types.52 Vascular cell adhesion molecule-1 (VCAM-1), P-selectin, and E-selectin, which support early phases of leukocyte adhesion, are sometimes referred to as “inducible” adhesion molecules.53 Stimulants such as cytokines, oxidants, and abnormal flow trigger P-selectin molecules to translocate from their baseline intracellular position to the cell surface within 10–30 min,54 and prompt de novo synthesis and surface expression of E-selectin27 and VCAM-1.14 DDSs bearing antibodies targeted to these markers can therefore selectively bind to activated endothelium,20,55–59 where in some cases the endothelial determinants such as E-selectin and VCAM-1 are localized more in the arteries and skin microvasculature than in the pulmonary vasculature.57 This preferred specificity for activated endothelium is especially appealing for visualization of activated endothelium in inflamed areas via delivery of conjugated radioisotopes60 or ultrasound contrast agents61,62 for imaging applications.
Additional pulmonary specificity can be found in the case of angiotensin-converting enzyme (ACE), which is expressed at the endothelial luminal surface in the pulmonary capillaries at a higher level than in other organs.23,63–67 ACE antibody-conjugated preparations have been found to be promising in directing biotherapeutics and genetic cargos to the pulmonary endothelium in animal studies.31,68–75 Lung visualization using labeled anti-ACE selectively targeting the lungs after IV injection has been reported in rats, mice, cats, primates, and even humans.64,76–78 These studies demonstrate the translational potential of ACE-targeted DDSs.79
Caveolar epitopes represent another class of endothelial determinant. Caveolae are found in many different types of cells, and are particularly copious in the pulmonary endothelium.33,34 Caveolae are cholesterol-rich domains of cell membrane, decorated on the cytosolic side by the caveolin and cavin family proteins.35 Caveolae and caveolae-derived endosomes play important roles in subcellular activities such as molecular transport and cell signaling.80 Extensive studies have been done in applying antibodies and other affinity ligands to target therapeutics to caveolae36,37,39 or to caveolar endosomes in order to cross endothelial cells.38 Caveolar determinants such as aminopeptidase P (APP)36,39 and plasmalemma vesicle associated protein (PV1 or Plvap) actively accumulate in lungs after IV administration. However, considering the narrow diameter of the caveolar opening (∼ 50 nm), some larger nanocarriers may be unsuitable for caveolar delivery.
Intra- and trans-cellular addressing of drugs within the pulmonary vasculature
In some cases, delivery of drug to the endothelial surface is enough for a therapeutic goal to be reached. However, very often effective therapy may be achieved only when drugs are delivered to specific sub-cellular addresses.81
The endothelium exerts active non-specific fluid phase uptake via macropinocytosis.82 The vesicles generated in this process transport compounds, including drugs, present in the blood at high concentrations, but the low efficacy and non-specific nature of this pathway does not meet the requirements for drug targeting. In contrast, uptake of ligands that bind to internalizing receptors is more effective, specific, and controlled than passive fluid phase uptake.
Like the majority of cells, endothelial cells internalize ligands by phagocytosis or receptor-mediated endocytosis, in either clathrin-dependent83 or clathrin-independent84 pathways such as caveolar endocytosis.82,85,86 Recent studies on the link between signaling and internalization have demonstrated a vast endocytic network used by cells for endocytosis, sorting, and trafficking of ligand-receptor complexes.87 Ligand-dependent types of endocytosis have high selectivity to certain cellular receptors and are able to deliver cargoes to distinct destinations.
Target determinants are localized in different domains and clusters in the plasmalemma (Table 1).88 Thus, PECAM-1 and vascular endothelial cadherin (VE-cadherin) are predominantly localized at the cell–cell border,89,90 while VCAM-1 and ICAM-1 are found in tetraspanin microdomains, specialized types of membrane rafts on apical surface.18,42,91 GP85 presents in the plasmalemma over a thin organelle-free part of the endothelial cell separating alveoli from blood,92,93 whereas APP and Plvap are both found in caveolae.39 Target localization regulates both the accessibility of target to circulating drug carriers and their fate after anchoring and uptake.94 For example, endothelial cells internalize selectins via clathrin-mediated endocytosis,95–97 which favors intracellular delivery into endothelial cells of E-selectin-targeted liposomes,26 drugs,26,98 and genetic materials.99
Endothelial cells do not internalize unconjugated antibodies to PECAM-1 or ICAM-1, but rapidly internalize multivalent anti-ICAM-1 and anti-PECAM-1 carriers via a non-canonical type of endocytosis.81 Internalized carriers arrive in lysosomes several hours after uptake, which is slow compared to many classical endocytic pathways that deliver their ligands to lysosomes within 10–20 min.100,101 It has been recognized for a long time that conversion of monovalent or bivalent ligands into multivalent carriers that can engage numerous copies of the receptors enhances internalization.81 An extensive clustering of receptors eliciting strong endocytic signaling, as well as dissociation of pre-existing clusters and “unnatural” signaling, among other factors, may be involved in the enhanced uptake of multivalent carriers. Although high carrier avidity is viewed as favorable for intracellular delivery, excessively tight binding may be detrimental for the subsequent dissociation of the carrier from the receptors during intracellular trafficking.
The rate of endothelial uptake of drugs and nanocarriers directed to PECAM-1 and ICAM-1 is modulated by the functional status of endothelium,102 parameters of flow and endothelial adaptation to shear stress,102,103 as well as the size of the nanocarriers.104,105 Furthermore, endothelial internalization of PECAM-1-targeted carriers depends on the selection of anchoring epitopes on the PECAM-1 molecule.106 Indeed, ligands binding to distinct epitopes of the same anchoring molecules may enter cells differently. This makes selection of ligands facilitating cellular uptake an important goal in drug delivery research.107,108 Using a phage-display library, a series of peptides binding to diverse VCAM-1 epitopes was identified, some of which have shown enhanced uptake.57 Labeled VCAM-1-binding peptides improved imaging of vascular inflammation in animal models.20,57 Phage display and other high-throughput methods may facilitate selection of internalizable antibodies and their fragments.109–111
Endothelial cells more effectively internalize polyvalent conjugates targeted to ICAM-1 than to PECAM-1,13 although small (i.e. < 100 nm) PECAM-1-targeted carriers enter the endothelium fairly effectively.104 Size is generally important in the vascular targeting paradigm. For example, the specificity of pulmonary targeting was shown to be dependent on the size of the carriers; exceeding a sub-micron limit leads to enhanced non-specific mechanical retention in the lungs.112 Therefore, PECAM-1 and ICAM-1 represent highly unusual endothelial targets providing either surface anchoring or internalization, with the choice controlled by the parameters of the carrier design, i.e. valence of binding.105 This feature permits targeting of drugs that either need to be retained on the cell surface such as monovalent anti-thrombotic fusion proteins or delivered inside the cell in the form of drug-loaded multivalent nanocarriers.113
Further, clustering of ICAM-1 leads to activation of sphingomyelinase and subsequent cleavage of sphingomyelin, stimulating endocytosis of anti-ICAM-1-coated carriers in the size range of < 100 to > 1000 nm in diameter.114 This mechanism does not operate in sphingomyelinase-deficient cells and animals; however, carriers coated with both anti-ICAM-1 and the enzyme, devised to deliver enzyme replacement therapy in Neumann–Pick syndrome, do compensate for the genetic defect and are internalized by cells.115 Furthermore, co-immobilization of sphingomyelinase with ligands anchoring carriers to transferrin receptor permitted internalization of carriers larger than those normally permitted by clathrin vesicles (< 200 nm).116
Examples of PECAM-1 and ICAM-1 illustrate the case when endothelial cells internalize nanocarriers coated with normally non-internalizable molecules. In the opposite scenario, coating a nanocarrier with internalizable molecules does not necessarily result in internalization. For example, the uptake of a large, micron-size carrier may exceed the cellular endocytic capacity; formation of such a large vacuole would require prohibitively extensive mobilization of the cell membrane and cytoskeleton. Further, coupling ligands to carriers may impede their interaction with receptors and epitopes localized in invaginations and other domains of the plasmalemma inaccessible to the particles of such size. For example, conjugation of ligands of caveolar epitopes to carriers larger than the diameter of the neck of caveolae (>40–70 nm) abolishes endothelial targeting and transfer.117
Binding to endothelium may also lead to transport across the cells. Some ligands of receptors involved in endocytosis via clathrin-coated pits, such as transferrin receptor,118 and caveolae,119–122 such as aminopeptidase P39 and annexin A1,38 are capable of crossing the endothelial barrier. These pathways provide an opportunity for trans-endothelial transport of nanocarriers with size suitable for these endocytic vesicles. For example, antibodies to aminopeptidase P undergo fast (within seconds to 1 min) caveolae-dependent transport across the endothelium and stay there for days.39 However, whether this phenomenon can provide efficient tool for tissue delivery of drugs remains to be investigated. In addition, the potential side effects of engaging caveolar determinants must be better understood in order to define the biomedical utility of this transcellular pathway.123–125 For example, many caveolae localized proteins are critical players in cell cignaling and an interefence with their functioning may cause unwanted consequences. In addition, disease conditions, including inflammation, may affect this pathway.82,117,126–129 It has been shown that anti-ICAM-1 coated nanocarriers (∼100 nm diameter spheres) can be transported across the cellular monolayer without cell damage or disruption of intercellular junctions.130 Similar to caveolar delivery, ICAM-dependent tissue drug delivery requires further studies.
Effect of pathological factors on vascular targeting
In order to obtain selective delivery to pathological tissues, targeting agents (e.g. monoclonal antibodies and their derivatives) are often utilized that bind to markers that are upregulated in diseased or injured tissues. Typically, targets are upregulated through mechanisms such as synthesis of new protein molecules, mobilization of sequestered intracellular stores, or unmasking of target epitopes.
Delivery of drugs may occur in two settings, prophylactic or therapeutic. Markers that are constitutively expressed in the lung are most suitable for prophylactic delivery (e.g. PECAM-1, ACE, and APP), while molecules that either remain expressed (PECAM-1)113 or are induced following injury (e.g. VCAM-1 and certain selectins) may find utility in therapeutic interventions and diagnostic imaging.20,57,60,62,99 However, in some cases, targets molecules are shed from the endothelial surface following injury (e.g. ACE or thrombomodulin),22,32,67,131 suggesting that they may not be optimal markers for therapeutic delivery to sites of acute inflammation.
Beyond simple consideration of target expression, pathological changes in tissue physiology may lead to alterations in targeting to the site of injury. Local alterations in physiology may occur either at focal points within a tissue or on a whole-tissue level (less common clinically). One example of a spatially heterogeneous condition is ARDS, which classically appears in patches of the tissue when visualized on a computed tomography (CT) scan.
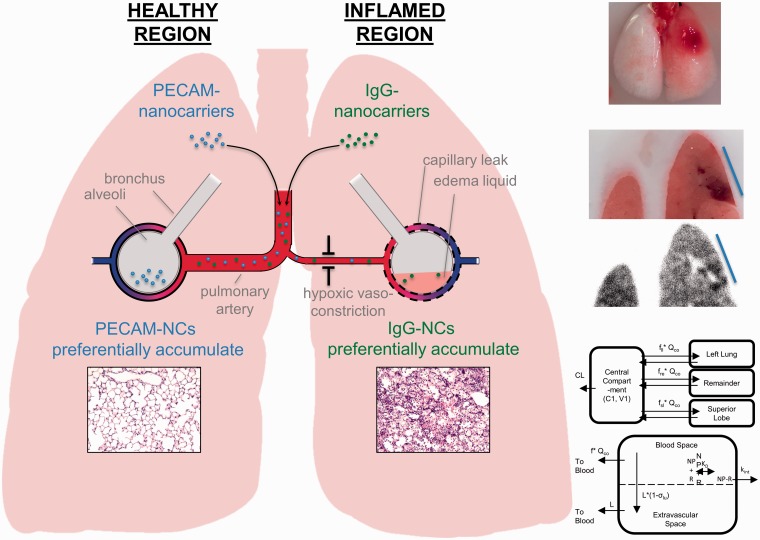
Recently, our group has developed a mouse model of ARDS wherein injury is induced in a single lobe of the lung, more closely recapitulating the human condition than classical ARDS models that display homogeneous injury throughout the tissue.132 The impact of injury on sub-tissue distribution of nanocarriers targeted to cell adhesion molecules was measured in order to elucidate mechanisms that could control drug disposition in this injury model. Several mechanisms were evaluated in this model, including hypoxic vasoconstriction (measured by changes in local blood flow), edema (measured by protein leak and tissue weight), and target protein expression (measured by western blot). The injured lobe was observed to have decreased blood flow and increased edema relative to the healthy lobes.
Interestingly, it was found that different targeting strategies had different distribution patterns within the lung and that these could be explained by the various tested mechanisms. For example, untargeted particles preferentially accumulated in the injured lobe, likely due to enhanced vascular leak, while particles targeted to PECAM-1 (no change in expression following injury) were found to accumulate in the healthy lobe, due to the reduced blood flow to the injured lobe. These mechanisms are depicted in Fig. 1. However, ICAM-1 targeted nanocarriers selectively accumulated in the injured lobe, due to a large upregulation of ICAM-1 expression at the injured site. A pharmacokinetic model was developed that considered all of these mechanisms, which was able to make a priori predictions of the sub-tissue distribution of these carriers, based solely on changes in physiology following lung injury.
Fig. 1.
Impact of pathology on nanocarrier delivery to the inflamed lung. The left panel depicts mechanisms controlling sub-tissue delivery of PECAM-targeted (hypoxic vasoconstriction) and IgG-coated (capillary leak) nanocarriers in the unilateral ARDS mouse model. The top right depicts the localized injury induced in this model and the bottom right shows a semi-physiologic pharmacokinetic model describing sub-tissue nanocarrier disposition in this animal model, which described disposition using a one-compartment model linked to a physiolgic lung model. Within the lung space, nanocarriers were allowed to discribute based on physiologically relevant values to the injury model.
The above example described how in ARDS / acute lung injury (ALI), pathological changes can markedly change how targeted DDSs distribute within the lungs. Numerous other lung pathologies are very likely to also change DDS distribution and efficacy. For example, in PAH, the plexiform lesions of the small arterioles greatly disrupt blood flow, which is very likely to change where DDSs deposit in the pulmonary vascular tree, as compared to normal lungs. Similarly, in pulmonary embolism (PE), the occlusion of the pulmonary arteries by the clot causes numerous vasodilatory changes in the lungs, which may make it harder for DDSs to reach the offending clot. These and numerous other examples illustrate the paramount importance of examining the distribution of DDSs within the lungs of animal models of disease early. Indeed, determining what fraction of a DDS reaches the site of pathology in the lung should be the very first step in development of a DDS for pulmonary diseases.
Targeting antioxidants to the pulmonary endothelium
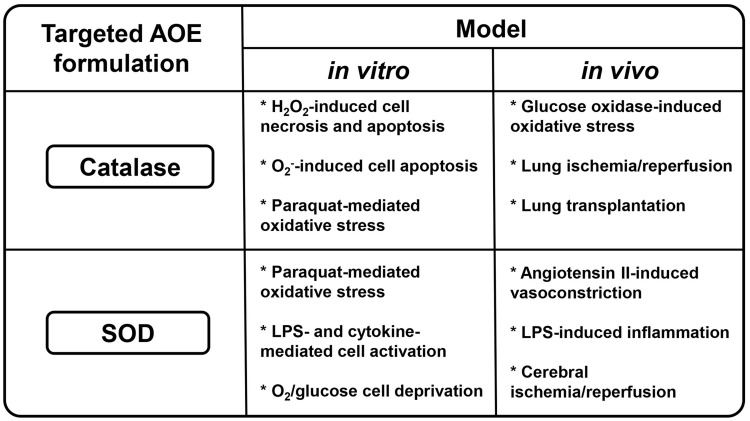
The disappointing lack of results over the last several decades of antioxidant research, including large-scale clinical trials testing effects of antioxidants and antioxidant enzymes (AOE) catalase and superoxide dismutase (SOD), can be interpreted as a drug delivery problem.133–135 Indeed, data have shown that when antioxidant pharmacokinetics (PK) are improved via chemical modifications using polyethylene glycol (PEG)136 or PEG-based pluronic137 or by loading into PEGylated carriers (liposomes138 or polymeric carriers139), the enhanced bioavailability of modified catalase, SOD, or other antioxidants moderately reduced systemic oxidative stress in animals.140,141 Further, SOD and catalase conjugated with either cationic membrane-permeating peptides142 or heparin-binding peptides enhanced cellular binding and uptake, resulting in modest improved protective effects in animal models of inflammation143 (Fig. 2). However, none of these non-targeted delivery approaches provided a decisive improvement of antioxidant interventions in controlled animal studies.
Fig. 2.
Demonstrated functional activities of targeted antioxidant enzymes catalase and superoxide dismutase in vitro and in vivo. LPS, lipopolysaccharide.
In contrast, AOE conjugated with antibodies to ACE,70 ICAM-1,31 and PECAM-1144 but not control IgG specifically bound to the endothelium,13,145,146 and accumulated in the lungs after IV injection in rats, mice, pigs, and dogs.13,31,70 In models of acute oxidative stress, the conjugates protected the perfused lungs in these animals much more effectively than their untargeted counterparts in these species.146–148 Most notably, catalase targeting to pulmonary endothelium via PECAM-1 or ACE was very protective in models of lung transplantation in rats,72,148 mice,149 and pigs.150
The benefits of targeted AOE provide specificity to their corresponding reactive oxygen species (ROS) substrates (SOD and catalase degrade superoxide radical and hydrogen peroxide, respectively), while low molecular weight antioxidants mostly lack this specificity. For example, a model of pulmonary ischemia/reperfusion injury showed that targeted catalase, but not SOD, was protective, implicating hydrogen peroxide H2O2 as the chief damaging ROS.149 In contrast, targeted SOD inhibited vasoconstriction induced by angiotensin II in mice, confirming the key role of superoxide O2.- produced by endothelial NADPH-oxidase (NOX) in depleting the vascular pool of NO, whereas catalase not only failed to attenuate the vasoconstriction effect, but showed some tendency to aggravate it.149 Furthermore, targeted SOD, but not catalase, inhibited pathological endothelial activation induced by cytokines.13 PECAM-1-targeted SOD attenuated leukocyte adhesion in cerebral vasculature and reduced the infarction zone in a model of brain ischemia-reperfusion injury.151
The recent data on anti-inflammatory effects of SOD13,151 demonstrate the importance of intracellular SOD delivery. Because ROS are small, promiscuous species that act without specificity on numerous biological targets, the value of localization within target cells of antioxidants to their therapeutic effects could be underestimated. The range of action of superoxide is on the scale of nanometers, if not angstroms, from their influx site, due to high reactivity and limited membrane diffusion of superoxide.152 Recent studies show that sub-cellular addressing of SOD is indeed important; PECAM-1-targeted SOD formulations accumulating in endosomes quenched superoxide anion produced in the vesicular lumen by NOX, thereby intercepting this organelle-specific pathological pathway.13,48
The diversity of antioxidant formulations targeted to the pulmonary vasculature includes liposomes and polymeric nanocarriers, in addition to antibody-drug conjugates (Fig. 2). Catalase was found to be protective against oxidative stress in vitro145,147,153 as well as in in vivo models of oxidative stress, lung ischemia/reperfusion, and transplantation.147,149,150 Delivery of SOD was protective in cellular models of oxidative stress and in case of pro-inflammatory endothelila cell activation.13,154 In animal models, SOD may attenuate inflammation, angiotensin II-induced hypertension, and cerebral ischemia/reperfusion.13,151
Liposomes are an established means to deliver antioxidants, protecting them from clearance and deactivation in vivo, and facilitating access to injured or inflamed cells and tissues.155 Hydrophobic antioxidants can be incorporated within the hydrophobic bilayer (e.g. vitamin E, flavonoids, among many others),156 while water-soluble antioxidants such as ascorbate, glutathione, and AOE can be encapsulated in the inner aqueous space of liposomes157 or polymersomes.158 Cell culture and some animal studies imply that liposomal antioxidants have better pharmacokinetics (PK) and efficacy than free drugs, especially in systemic subtle oxidative stress.159–161 Varying methodology and content of liposomal SOD formulations allows for modulation of the loading efficacy, localization within the liposomal compartments, and enzymatic activity of resultant SOD/liposomes.162 These studies provided the context for targeting antioxidant liposomes to the pulmonary vasculature.
PECAM-1-targeted liposomes loaded with MJ33, a NOX inhibitor, accumulated in the endothelial cells and inhibited ROS production in cell culture. They provided more potent protective effects than non-targeted counterparts against oxidative stress and inflammation in perfused mouse lungs ex vivo, and in LPS-induced inflammation in mice.163 Similar results were obtained with a SOD/catalase mimetic EUK-134. Targeted liposomes loaded with EUK-134 bound to endothelial cells in culture and in pulmonary vasculature in mice and provided anti-inflammatory effects in a mouse model of endotoxin-induced lung injury, whereas untargeted IgG/EUK liposomes provided neither delivery of cargo to endothelium nor protection.164
Furthermore, targeting of AOE encapsulated into a porous milieu permeable to ROS, but impermeable to proteases, has been designed using polymeric nanocarriers based on PEG-PLGA copolymers, allowing access to H2O2 (but not to superoxide), and protecting catalase from proteases.165 Additionally, porous vesicular polymersomes encapsulating SOD provided access of the enzyme to superoxide, while preventing SOD degradation, and proving effective in a brain injury model.158 Enzyme PEGylation may also increase protection against proteases, while modulating the molar ratio and size of PEG and PLGA chains in the copolymer controls the shape of nanocarriers, allowing geometries ranging from spherical to filamentous.166
PECAM-1-targeted PEG-PLGA nanocarriers loaded with catalase provide effective endothelial delivery in vitro and in vivo, and prolonged antioxidant protection of the endothelium.167 Encapsulation of either catalase or SOD formed by controlled precipitation of magnetic nanoparticles (MNPs) using calcium and oleate provides composite nanocarriers (200–300 nm diameter) containing active catalase or SOD accessible to either H2O2 or superoxide, and protected from proteases.168 Additionally, in vivo PECAM-1-directed non-polymeric MNPs loaded with SOD or catalase accumulated specifically in the pulmonary endothelium, and catalase-loaded endothelial-targeted particles alleviated pulmonary edema and leukocyte infiltration in a mouse model of endotoxin-induced lung injury, whereas SOD-loaded targeted carriers mitigated LPS-induced lung inflammation.169
The last two decades have demonstrated significant progress in the formulation of delivery systems carrying antioxidant enzymes, and their applications, to pathologies critically susceptible to oxidative stress including organ transplantation, ischemia-reperfusion, and inflammation. Going forward, testing new target molecules, as well as new delivery systems and the optimization of current platforms, will provide important steps in the translation of targeted antioxidants into the clinical domain, thereby improving management of disease conditions involving this pathological mechanism.
Endothelial targeting of antithrombotic agents
While typically associated with diseases of the systemic circulation, such as myocardial infarction and ischemic stroke, thrombosis and thromboembolism in the pulmonary vasculature are not only a source of direct morbidity and mortality (e.g. in patients with PE), but prominent features of the pathophysiology of nearly all of the lung diseases discussed in this review.170,171 In a landmark study in 1983, Tomashefski et al. used a variety of pre- and post-mortem techniques to examine the pulmonary vessels of 22 patients who died of ARDS, noting thromboemboli in all but one patient and microvascular thrombosis in 19 of 22. Microscopic evidence of acute endothelial injury, even in patients who had survived the early hemorrhagic and exudative phase of the syndrome, suggested local coagulation as a critical and ongoing pathophysiologic event.172 This process has since been shown to result from both an increase in procoagulant forces (predominantly tissue factor) and failure of endogenous anticoagulant systems.24,173,174 Similarly, histopathologic studies of PAH have shown a high incidence of thrombotic lesions, even in the subset of patients with arteriopathy and plexiform lesions.175,176 As with ARDS, increased expression of tissue factor and loss of key antithrombotic molecules, like thrombomodulin, have been identified as contributors to local coagulation and disease progression.28,177,178
Given the suspected pathophysiologic role of coagulation and, specifically, dysfunction of endogenous antithrombotic mechanisms in lung disease, it is no surprise that a number of efforts have been made to boost these protective pathways pharmacologically. Rather than directly target pulmonary endothelial function, however, most therapeutic programs have focused on systemic infusion of endothelial-derived agents. Among the drugs studied in lung diseases are several small molecules with vasodilatory and antiplatelet activity, such as nitric oxide and prostacyclin (epoprostenol), and biotherapeutics from the three major endogenous anticoagulant pathways, including activated protein C (APC), tissue factor pathway inhibitor, and antithrombin.2,179–183 While some of these therapeutics have found clinical application (e.g. epoprostenol in PAH), the majority of the clinical results have been disappointing and there is increasing consensus that systemic infusion simply may be inadequate to reproduce the activity of highly regulated and localized endothelial systems.
As an alternative, a number of groups have used targeted drug delivery to direct antithrombotic agents to endothelial cells.184 In some cases, the intention has been localization of therapeutic activity to increase efficacy or diminish the risk of bleeding toxicity, whereas in others, the goal has been to alter the phenotype of the endothelium itself, restoring endogenous anticoagulant and anti-adhesive mechanisms which may have been compromised with the progression of disease.185 While theoretically attractive, this approach has required new technology to circumvent a significant practical challenge, the rapid internalization of multivalent antibody targeted NPs or protein therapeutics by vascular endothelial cells. To promote retention on the endothelial surface and sustained interaction with the bloodstream, protein cargo has been genetically fused to monovalent affinity ligands, such as single chain variable fragments (scFv), which generally have a lower rate of endocytosis following binding to cell adhesion molecules and other surface determinants.132,186
Our group has pursued delivery of two major classes of antithrombotic proteins to the vascular endothelium. The first is tissue plasminogen activators (tPA), clinically approved for treatment of ischemic stroke and myocardial infarction and recently studied as a means of improving outcome in massive and sub-massive PE.28 A fusion protein of a single chain fibrinolytic enzyme and a PECAM-1 targeted scFv was found to accumulate in the cerebral vasculature and, when used prophylactically, enhanced reperfusion and reduced BBB disruption following cerebral embolization of fibrin clots.186 The same therapeutic was also effective in the pulmonary vasculature, demonstrating approximately 2–3 times more fibrinolysis than free enzyme in the setting of venous thromboembolism.179 However, the apparent need for prophylactic administration limits the practical utility of endothelial-targeted fibrinolytic agents and, while it is possible that vascular targeting may also result in improved efficacy or diminished toxicity after embolic insult, this hypothesis has not been rigorously tested.
The other major application for which scFv targeted fusion proteins have been explored is as a means of augmenting the endothelial protein C (PC) pathway.181 The key component of this endogenous enzymatic system is the endothelial membrane protein, thrombomodulin (TM), which binds thrombin, changes its enzymatic specificity, and inhibits both coagulation and vascular barrier leak.180 As mentioned above, the pleiotropic effects of TM, its high level of expression in the pulmonary microvasculature, and its suppression in the presence of disease have led to significant interest in its role in the pathogenesis of lung diseases.24,28,187 Loss of endothelial TM and a second protein, the endothelial protein C receptor (EPCR), which binds protein C and positions it optimally for cleavage by the thrombin–TM complex, results in an imbalance between thrombin and APC, a protease previously approved for the treatment of severe sepsis and studied in a clinical trial of ARDS.188 While this study was terminated due to apparent lack of efficacy, its authors noted that a low dose was used due to concerns for bleeding risk, the drug’s major toxicity and reason for ultimate withdrawal from the market. As an alternative with lower risk of spontaneous hemorrhage, our group fused TM with the same PECAM-1 specific scFv used for delivery of fibrinolytics and found significant protection in murine models of pulmonary inflammation, hyperoxia, and ischemia/reperfusion injury.179
This preliminary success motivated further investigation of underlying mechanisms and led to the discovery that scFv/TM fusion protein bound to PECAM-1 does not interact with EPCR in the same way as endogenous TM.181 The result has been the development of two distinct strategies for augmenting the endothelial protein C pathway, one in which scFv/TM is targeted to ICAM-1, which improves enzymatic partnering with endogenous EPCR, and the other in which two recombinant fusion proteins, scFv/TM and scFv/EPCR, are delivered to adjacent epitopes of PECAM-1, allowing synergistic interaction on the endothelial membrane.181,189 In addition, fusion proteins which incorporate human TM and human specific or species cross-reactive scFv have now been developed.186,190 These agents have allowed confirmation of the antithrombotic effects of scFv/TM in humanized systems, including a recently described microfluidic model of tissue factor-driven, inflammatory thrombosis. These studies not only support the translational prospects of targeted TM, but demonstrate the potential utility of microfluidic models of the endothelial–blood interface and alveolar–capillary unit as tools for testing drug delivery systems in healthy and injured lung vasculature.186,191
Diseases to treat by targeting the pulmonary vasculature
As mentioned above, the pulmonary vasculature plays a significant role in many, if not all, lung diseases. However, only a subset of pulmonary diseases warrants the development of a DDS that targets the pulmonary endothelium. Just a few parameters can be used to evaluate the utility of endothelium-targeted DDSs in a particular disease.185
First, endothelium-targeted DDSs are not optimal for delivery to the airways. Many lung diseases, most notably asthma, are primarily diseases of the airways. Inhaled delivery to those airways is already sufficient and therefore endothelium-targeted DDSs are not needed for diseases of the airways.
Second, the role of the endothelium in the pathophysiology should be well understood. For example, there is certainly evidence that the endothelium plays a role in the development of COPD, but the mechanisms are uncertain. However, a caveat to this guideline is that even if the endothelium’s role in the pathophysiology is not well understood, endothelium-targeted DDSs could still help by turning the endothelium into a drug depot that elutes to the rest of the parenchyma. Many small molecule drugs diffuse well out of cells, so if the DDS delivers drugs to the endothelium, those drugs could then act on nearby other cell types.
Third, the disease must have a severity and acuity that are compatible with a medication that is IV-delivered. All known endothelium-targeting DDSs require IV injection. IV injections are not practical for most outpatient diseases (e.g. chronic cough), unless the disease is very severe (e.g. PAH). However, for some diseases, it is conceivable that patients would comply with a monthly visit to an infusion center, as is done for many protein therapeutics for rheumatological diseases. Additionally, in select severe diseases like PAH, long-term central lines (e.g. a Hickman catheter) are currently used by some patients and could be used for endothelium-targeted DDSs. But the diseases most amenable to obligate-IV medications are ICU diseases, such as ARDS and PE. In such ICU diseases, the patients almost always already have an IV catheter in place, often in a central vein, making IV infusions facile.
Using these three criteria, we can come up with a focused list of goal diseases to treat with endothelium-targeted DDSs (Table 2). Each of these diseases has seen some research using endothelium-targeted DDSs, but ARDS has had many more studies and further progress than the rest.192 Therefore, below we provide an overview of endothelium-targeted DDSs directed towards ARDS (Table 3), which can serve as an archetype of future targeted drug development for other pulmonary diseases.
Table 2.
Lung diseases and target regions for therapeutic interventions.
| Disease | Target region of lung | Examples of targeted formulations in animal studies |
|---|---|---|
| ARDS | Alveoli, preferably only “flooded alveoli” (filled with edema liquid and leukocytes) | Explained in detail in Table 3 |
| Primary graft dysfunction (PGD) | Alveoli | Antibody-catalase conjugates targeted to PECAM and ACE in rodents and pig lung transplant models 71,148,149 |
| Pulmonary arterial hypertension (PAH) | Small pulmonary arterioles (< 2 mm), possibly with preference for plexiform lesions | Co-administration of CAR (a peptide assumed to locate specifically to PAH-affected vessels but not normal ones) and small molecule vasodilators into rats (e.g. fasudil)193,194 Covalently conjugated octa-arginine (R8) peptide-liposomes loaded with the chemotherapeutic paclitaxel studied in rats195 Adenoviruses decorated with bispecific antibodies containing anti-ACE carrying several genetic cargoes in rat69,196 |
| Pulmonary embolism | Clots that impede flow | Anti-PECAM scFv/low molecular weight single chain urokinase (lmw-scuPA) fusion protein in mice179 Anti-PECAM scFv/thrombomodulin fusion protein in mice22 |
| Idiopathic pulmonary fibrosis | Unknown. Possibly delivery to the endothelium can serve as a drug depot for surrounding alveolar cells | Anti-surfactant protein A (anti-SPA)-coated liposomes loaded with dexamethasone in bleomycin-induced model of lung fibrosis in rats197 |
Table 3.
Brief list of targeted formulations used in animal models of ARDS.
| Targeted delivery system | Cargo | Target cell/tissue | Animal model of ARDS (species/ inflammatory stimulant) | Major findings | References |
|---|---|---|---|---|---|
| IgG-coated immunoliposomes | Dexamethasone | Putatively FcγR-expressing leukocytes such as macrophages and neutrophils | Mouse/mechanical ventilation | Intravenously-injected dex-loaded liposomes improved decreased pulmonary inflammatory markers (cytokines and neutrophil infiltrate). This ameliorating effect was augmented by conjugating IgG to the surface of the liposomes, which that paper’s authors speculated may have increased liposome uptake in macrophages and neutrophils | 198 |
| Anti-PECAM coated liposomes | MJ33 (indirect inhibitor of NADPH oxidase) | Vascular endothelium | Mouse/intratracheally administered lipopolysaccharide (LPS) | MJ33 in anti-PECAM targeted liposomes could reduce the lung permeability and pulmonary VCAM expression, much more effectively than free MJ33. This report is one of the early demonstrations on using a targeted nanomedicine to improve ARDS phenotypes in therapeutic (after disease induction) rather than just prophylactic (before disease induction) condition | 163 |
| Anti-PECAM conjugate of SOD and catalase | SOD or catalase | Vascular endothelium | Mouse/intravenously injected LPS | PECAM-targeted SOD, but not catalase, decreased pulmonary VCAM expression as ARDS marker. Moreover, it added to the protective effect of NO donors | 13,151 |
| Anti-PECAM coated instant supramolecular co-precipitated nanoparticles (also called as Protective Antioxidant Carrier for Endothelial Targeting [PACkET]) | Combination of SOD and catalase | Vascular endothelium | Mouse/intratracheally injected LPS | As a modular DDS, catalase-PACKET decreased bronchoalveolar (BAL) protein content and leukocyte cell population by almost 50%, while SOD-PACKET attenuated inflammatory markers (serum and on endothelium) by almost 70% | 169 |
| Fusion protein consists of TM and scFv fragment of anti-PECAM antibody | TM | Vascular endothelium | Mouse/intratracheal injection of LPS, followed by exposure to hyperoxia | The prepared fusion protein alleviated inflammatory markers, neutrophil infiltration, and lung permeability to a greater extent than un-targeted soluble TM | 22 |
| Liposomes coated with anti-ACE antibodies | siRNA against non-muscle myosin light chain kinase (nmMLCK; a regulator of endothelial contraction) | Vascular endothelium | Mouse/mechanical ventilation or intratracheal injection of LPS | Prophylactic treatment with this siRNA-containing nanomedicine markedly reduce BAL protein content and WBC infiltration by almost 50% in both ventilator-induced and IT-LPS-induced lung injury ARDS models | 202 |
TM, thrombomodulin.
To illustrate the utility of endothelium-targeted DDSs in ARDS, it is helpful to first examine the unmet need in ARDS. ARDS afflicts ∼ 190,000 Americans each year, has a mortality rate of ∼ 40%, and no FDA-approved drugs to treat it. Nearly two dozen drugs have failed in ARDS clinical trials. Why have there been so many failures in ARDS? From a pharmacology perspective, three reasons stand out: (1) ARDS patients are fragile, with multi-system organ dysfunction, so do not tolerate off-target side effects; (2) the inhaled route of drug delivery is challenging because of the layer of liquid filling the alveoli; and (3) the disease is heterogeneous within a population, so targeting a single pathway is unlikely to work on a mixed population.
Endothelium-targeted DDSs seem well suited to these pharmacological challenges. By targeting the drug solely to the flooded alveoli, DDSs could avoid off-target side effects. Additionally, these DDSs avoid the inhaled route, as they are obligate-IV formulations. Finally, the DDSs are capable of carrying multiple cargo drugs, which could act on multiple different signaling pathways simultaneously. A DDS designed to meet these specifications should greatly improve the chances of a successful clinical trial. Several endothelium-targeted DDSs have been designed to meet these specifications. They all involve using three main features: carrier that contains the therapeutic; a lung-targeting moiety (almost always an antibody or fragment thereof); and, of course, the cargo drugs. We summarize each of these three components below.
Multiple carriers have been used to target therapeutics to the lungs of animal models of ARDS. The most common have been liposomes.163,197,198 Liposomes are ∼ 100 nm-diameter lipid bilayers. Liposomes been used in patients as drug carriers since the 1990s, which means that they have a long history of safety and the industrial manufacturing processes have been well worked out. While liposomes have the advantage of longer and broader use, several other drug carriers have been used in ARDS studies, including enzyme–protein conjugates13,151 and polymer NPs.199 Another very notable carrier design is that of a fusion protein. In a fusion protein, a single chain variable fragment (scFv) of an antibody is genetically fused to a therapeutic protein. One such fusion protein, with an scFv targeting PECAM-1 fused to the anti-thrombotic/anti-inflammatory protein thrombomodulin, showed an ability to prophylactically prevent ARDS phenotypes in a mouse model of ARDS.22 In choosing a carrier for targeting ARDS, it is best to match the carrier to the cargo therapeutic, while also considering carrier’s safety and manufacturability.
The vast majority of studies have used as their targeting moiety antibodies that bind to endothelial luminal proteins, such as PECAM-1, ICAM-1, APP, and others listed in the sections above. One study,197 however, used anti-surfactant protein A (anti-SPA) antibodies, which is only expressed on the alveolar epithelium. Unfortunately, that study did not assess the fraction of the DDS that reached the lungs, so it is difficult to compare its targeting capabilities to those of endothelial targets. Indeed, a first step in determining which affinity moiety to employ is a quantitative assessment of the fraction of the DDS that reaches the lungs, preferably the most injured alveoli.
There are many possible cargo drugs for ARDS. For example, the list of >20 drugs that failed in clinical trials, mostly due to off-target side effects, are a reasonable place to start. Additionally, dozens of other drugs have shown efficacy in animal models, but often at doses that are unrealistic in humans. Among the drugs that have shown promise already in endothelium-targeted DDSs in ARDS model are: corticosteroids;197,198 small molecule antioxidants such as N-acetylcysteine, α-tocopherol, MJ33, and EUK-134;163,164,200,201; anti-oxidant enzymes such as catalase and superoxide dismutase;13,151,153 and thrombomodulin.22 Very intriguingly, nucleic-acid based cargos have also shown promise. For example, siRNA directed against non-muscle myosin light chain kinase was loaded into liposomes coated with anti-ACE antibodies, and when given prophylactically, reduced ARDS-like phenotypes in mice.202 This proof-of-principle suggests that other genetic cargoes (plasmid DNA, siRNA, shRNA, miRNA, mRNA, etc) might also open a large number of pathways to attack with endothelium-targeted DDSs.
While many of the DDSs described above show promise in ARDS models, much work is to be done. First, most of the studies used the drugs as prophylaxis, not treatment, which is probably unrealistic for ARDS. Second, it is important to use multiple animal models that display severe pathology, not just ones with mild pathology. Finally, it is important to consider toxicology early, as a DDS may shift toxicity from one organ to another. And notably, the above paradigm, as described for ARDS, can be used for the other pulmonary diseases of Table 2.
Cautionary notes: remember Hippocrates
There is no such thing as a perfect targeted therapeutic, especially when facing one of the most sophisticated, complex, sensitive, and important cell types in the body. The aim of vascular targeting in the diseases mentioned here is to improve lung health, rather than the simpler task of targeted drug delivery in cancer, which must only destroy invading cancerous cells. Thus, the standards of safety are different in dealing with cardiovascular, pulmonary, neurological or metabolic complications than for tumor-targeting applications.203 Therapeutics developed to improve these systemic pathologies are usually more benign than anti-cancer agents; thus, pan-endothelial delivery of antioxidant, anti-inflammatory, or anti-thrombotic agents throughout the vasculature seems to be a suitable option for translation. In addition, physiology plays a major role in this situation, as the target for the vast majority of endothelial targeted nanomedicine targets is the lung, which truly favors the first pass phenomenon.
One of the major concerns in every type of targeting strategy is how non-target cells expressing the ligand of interest could affect or even change the intended therapeutic intervention. As mentioned earlier, most endothelial determinants are ubiquitously expressed throughout the endothelial vasculature of different organs. Moreover, some targets such as CAMs are expressed on other cell types such as epithelial cells or and leukocytes, although mostly at lower protein concentrations.
Another major concern in targeting vascular determinants, especially in systemic disorders, is that the no-harm rule should be carefully followed in terms of targeted cells. Unlike the “find and kill” mission of cancer nanomedicine, in which, an “incidental” toxic effect on the target tumor cell is often cheered, here, endothelial disturbance must be minimized. Moreover, engagement of target determinants by a multivalent carrier presenting antibodies against that target may induce their shedding, internalization, and/or dysfunction. In all the scenarios, there is a chance to induce aggravation of oxidative stress, inflammation, and thrombosis, which should be avoided.
For example, there are several reports on using antibodies against the endothelial glycoprotein thrombomodulin to prepare liposomal formulations targeted to the pulmonary endothelium in vivo.204,205 However, thrombomodulin affects thrombin, and the inhibition of its protective function by engaging it with antibody-functionalized NPs increases the risk of thrombosis and inflammation.180,206 This concern basically makes it impossible to use this endothelial determinant as a suitable target for endothelial-directed DDSs.
Another example is targeting DDSs to ACE and APP, peptidases that cleave mediators including bradykinin.207 Engagment of DDSs with these peptidases may have consequences such as vascular edema and severe hypotension due to elevation of bradykinin and other signaling peptides. It should be noted that inhibition of angiotensin II production by ACE may be valuable in hypertension and inflammation. Therefore, it is always important to evaluate the impact of using each determinant for nanocarrier targeting in each clinical context.
A common caution with decorating NPs with antibodies is that their unique characteristics may create the chance of activation of complement and other host defense systems in the bloodstream. Complement components and immune cells interact with the Fc fragment of antibodies, leading to rapid clearance from the circulation and immune reaction. As an alternative to antibodies, antibody derivatives called single-chain variable fragments (scFv) can be used as targeting moieties for DDs. scFv retain most of the positive features of antibodies while eliminating Fc-mediated immune responses and clearance mechanisms. However, it should be noted that selective tissue uptake obtained with whole antibodies is usually higher than the one achieved by antibody fragments.
Lastly, we should be aware that proven biocompatibility for each individual components of a DDS does not necessarily translate into a safe DDS.208 Loading a relatively benign agent into a relatively safe carrier decorated by harmless ligands may generate a combination with pro-inflammatory or adjuvant characteristics. These and other aforementioned cautionary notes are critical in pushing forward the clinical endothelial nanomedicine for pulmonary, cardiovascular, cerebrovascular, and other diseases in need of therapy.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research was supported by National Heart, Lung, and Blood Institute Grants 1RO1 HL125462-02 (VRM), 1RO1 HL128398-02 (VRM) and RO1 HL126874-01A1 (VRM).
2017 Grover Conference Series
This review article is part of the 2017 Grover Conference Series. The American Thoracic Society and the conference organizing committee gratefully acknowledge the educational grants provided for the support of this conference by Actelion Pharmaceuticals US, Inc., Gilead Sciences, Inc., and United Therapeutics Corporation. Additionally, the American Thoracic Society is grateful for the support of the Grover Conference by the American Heart Association, the Cardiovascular Medical Research and Education Fund, and the National Institutes of Health.
References
- 1.Scherpereel A, Rome JJ, Wiewrodt R, et al. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J Pharmacol Exp Ther 2002; 300: 777–786. [DOI] [PubMed] [Google Scholar]
- 2.Danielyan K, Ding BS, Gottstein C, et al. Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J Pharmacol Exp Ther 2007; 321: 947–952. [DOI] [PubMed] [Google Scholar]
- 3.Giordano RJ, Cardó-Vila M, Lahdenranta J, et al. Biopanning and rapid analysis of selective interactive ligands. Nat Med 2001; 7: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 4.Kinniry P, Pick J, Stephens S, et al. KL4-surfactant prevents hyperoxic and LPS-induced lung injury in mice. Pediatr Pulmonol 2006; 41: 916–928. [DOI] [PubMed] [Google Scholar]
- 5.Durr E, Yu J, Krasinska KM, et al. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol 2004; 22: 985–992. [DOI] [PubMed] [Google Scholar]
- 6.Oh P, Li Y, Yu J, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature 2004; 429: 629–635. [DOI] [PubMed] [Google Scholar]
- 7.Rajotte D, Arap W, Hagedorn M, et al. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J Clin Invest 1998; 102: 430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajitou A, Trepel M, Lilley CE, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell 2006; 125: 385–398. [DOI] [PubMed] [Google Scholar]
- 9.Christofidou-Solomidou M, Muzykantov VR. Antioxidant strategies in respiratory medicine. Treat Respir Med 2006; 5: 47–78. [DOI] [PubMed] [Google Scholar]
- 10.Muzykantov VR. Targeting of superoxide dismutase and catalase to vascular endothelium. J Control Release 2001; 71: 1–21. [DOI] [PubMed] [Google Scholar]
- 11.Pasqualini R, Arap W, McDonald DM. Probing the structural and molecular diversity of tumor vasculature. Trends Mol Med 2002; 8: 563–571. [DOI] [PubMed] [Google Scholar]
- 12.Pasqualini R, McDonald DM, Arap W. Vascular targeting and antigen presentation. Nat Immunol 2001; 2: 567–568. [DOI] [PubMed] [Google Scholar]
- 13.Shuvaev VV, Han J, Yu KJ, et al. PECAM-targeted delivery of SOD inhibits endothelial inflammatory response. FASEB J 2011; 25: 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albelda SM. Endothelial and epithelial cell adhesion molecules. Am J Respir Cell Mol Biol 1991; 4: 195–203. [DOI] [PubMed] [Google Scholar]
- 15.Chittasupho C, Xie SX, Baoum A, et al. ICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cells. Eur J Pharm Sci 2009; 37: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo P, Huang J, Wang L, et al. ICAM-1 as a molecular target for triple negative breast cancer. Proc Natl Acad Sci U S A 2014; 111: 14710–14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopkins AM, Baird AW, Nusrat A. ICAM-1: targeted docking for exogenous as well as endogenous ligands. Adv Drug Deliv Rev 2004; 56: 763–778. [DOI] [PubMed] [Google Scholar]
- 18.Cook-Mills JM, Marchese M, Abdala-Valencia H. VCAM-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal 2011; 15: 1607–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar AG, Dai XY, Kozak CA, et al. Murine VCAM-1. Molecular cloning, mapping, and analysis of a truncated form. J Immunol 1994; 153: 4088–4098. [PubMed] [Google Scholar]
- 20.Tsourkas A, Shinde-Patil VR, Kelly KA, et al. In vivo imaging of activated endothelium using an anti-VCAM-1 magneto-optical probe. Bioconjug Chem 2005; 16: 576–581. [DOI] [PubMed] [Google Scholar]
- 21.Carnemolla R, Greineder CF, Chacko AM, et al. Platelet endothelial cell adhesion molecule targeted oxidant-resistant mutant thrombomodulin fusion protein with enhanced potency in vitro and in vivo. J Pharmacol Exp Ther 2013; 347: 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding B-S, Hong N, Christofidou-Solomidou M, et al. Anchoring fusion thrombomodulin to the endothelial lumen protects against injury-induced lung thrombosis and inflammation. Am J Respir Crit Care Med 2009; 180: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford VA, Stringer C, Kennel SJ. Thrombomodulin is preferentially expressed in Balb/c lung microvessels. J Biol Chem 1992; 267: 5446–5450. [PubMed] [Google Scholar]
- 24.Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol 2003; 285: L514–521. [DOI] [PubMed] [Google Scholar]
- 25.Haraldsen G, Kvale D, Lien B, et al. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in human microvascular endothelial cells. J Immunol 1996; 156: 2558–2565. [PubMed] [Google Scholar]
- 26.Kessner S, Krause A, Rothe U, et al. Investigation of the cellular uptake of E-Selectin-targeted immunoliposomes by activated human endothelial cells. Biochim Biophys Acta 2001; 1514: 177–190. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto TK, Rothlein R. Integrins, ICAMs, and selectins: role and regulation of adhesion molecules in neutrophil recruitment to inflammatory sites. Adv Pharmacol 1994; 25: 117–169. [DOI] [PubMed] [Google Scholar]
- 28.Sakamaki F, Kyotani S, Nagaya N, et al. Increased plasma P-selectin and decreased thrombomodulin in pulmonary arterial hypertension were improved by continuous prostacyclin therapy. Circulation 2000; 102: 2720–2725. [DOI] [PubMed] [Google Scholar]
- 29.Shamay Y, Elkabets M, Li H, et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Science Transl Med 2016; 8: 345ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winter PM, Neubauer AM, Caruthers SD, et al. Endothelial ανβ3 integrin–targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol 2006; 26: 2103–2109. [DOI] [PubMed] [Google Scholar]
- 31.Atochina EN, Balyasnikova IV, Danilov SM, et al. Immunotargeting of catalase to ACE or ICAM-1 protects perfused rat lungs against oxidative stress. Am J Physiol 1998; 275: L806–817. [DOI] [PubMed] [Google Scholar]
- 32.Atochina EN, Muzykantov VR, Al-Mehdi AB, et al. Normoxic lung ischemia/reperfusion accelerates shedding of angiotensin converting enzyme from the pulmonary endothelium. Am J Respir Crit Care Med 1997; 156: 1114–1119. [DOI] [PubMed] [Google Scholar]
- 33.Bagam P, Singh DP, Inda ME, et al. Unraveling the role of membrane microdomains during microbial infections. Cell Biol Toxicol 2017; 33: 429–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Krieken R, Krepinsky JC. Caveolin-1 in the pathogenesis of diabetic nephropathy: potential therapeutic target? Curr Diab Rep 2017; 17: 19. [DOI] [PubMed] [Google Scholar]
- 35.Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 2010; 72: 463–493. [DOI] [PubMed] [Google Scholar]
- 36.Chrastina A, Valadon P, Massey KA, et al. Lung vascular targeting using antibody to aminopeptidase P: CT-SPECT imaging, biodistribution and pharmacokinetic analysis. J Vasc Res 2010; 47: 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massey KA, Schnitzer JE. Targeting and imaging signature caveolar molecules in lungs. Proc Am Thorac Soc 2009; 6: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh P, Testa JE, Borgstrom P, et al. In vivo proteomic imaging analysis of caveolae reveals pumping system to penetrate solid tumors. Nat Med 2014; 20: 1062–1068. [DOI] [PubMed] [Google Scholar]
- 39.Oh P, Borgstrom P, Witkiewicz H, et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol 2007; 25: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romer LH, McLean NV, Yan HC, et al. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol 1995; 154: 6582–6592. [PubMed] [Google Scholar]
- 41.Scalia R, Lefer AM. In vivo regulation of PECAM-1 activity during acute endothelial dysfunction in the rat mesenteric microvasculature. J Leukoc Biol 1998; 64: 163–169. [DOI] [PubMed] [Google Scholar]
- 42.Almenar-Queralt A, Duperray A, Miles LA, et al. Apical topography and modulation of ICAM-1 expression on activated endothelium. Am J Pathol 1995; 147: 1278–1288. [PMC free article] [PubMed] [Google Scholar]
- 43.Nakada MT, Amin K, Christofidou-Solomidou M, et al. Antibodies against the first Ig-like domain of human platelet endothelial cell adhesion molecule-1 (PECAM-1) that inhibit PECAM-1-dependent homophilic adhesion block in vivo neutrophil recruitment. J Immunol 2000; 164: 452–462. [DOI] [PubMed] [Google Scholar]
- 44.Chacko AM, Hood ED, Zern BJ, et al. Targeted Nanocarriers for Imaging and Therapy of Vascular Inflammation. Curr Opin Colloid Interface Sci 2011; 16: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muro S, Gajewski C, Koval M, et al. ICAM-1 recycling in endothelial cells: a novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood 2005; 105: 650–658. [DOI] [PubMed] [Google Scholar]
- 46.Murohara T, Delyani JA, Albelda SM, et al. Blockade of platelet endothelial cell adhesion molecule-1 protects against myocardial ischemia and reperfusion injury in cats. J Immunol 1996; 156: 3550–3557. [PubMed] [Google Scholar]
- 47.Pan H, Myerson JW, Hu L, et al. Programmable nanoparticle functionalization for in vivo targeting. FASEB J 2013; 27: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shuvaev VV, Ilies MA, Simone E, et al. Endothelial targeting of antibody-decorated polymeric filomicelles. ACS Nano 2011; 5: 6991–6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McIntosh DP, Tan XY, Oh P, et al. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc Natl Acad Sci U S A 2002; 99: 1996–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muzykantov VR. Biomedical aspects of targeted delivery of drugs to pulmonary endothelium. Expert Opin Drug Deliv 2005; 2: 909–926. [DOI] [PubMed] [Google Scholar]
- 51.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal 2008; 10: 1713–1765. [DOI] [PubMed] [Google Scholar]
- 52.Rothlein R, Wegner C. Role of intercellular adhesion molecule-1 in the inflammatory response. Kidney Int 1992; 41: 617–619. [DOI] [PubMed] [Google Scholar]
- 53.Springer TA. Adhesion receptors of the immune system. Nature 1990; 346: 425–434. [DOI] [PubMed] [Google Scholar]
- 54.Kumasaka T, Quinlan WM, Doyle NA, et al. Role of the intercellular adhesion molecule-1(ICAM-1) in endotoxin-induced pneumonia evaluated using ICAM-1 antisense oligonucleotides, anti-ICAM-1 monoclonal antibodies, and ICAM-1 mutant mice. J Clin Invest 1996; 97: 2362–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 1991; 251: 788–791. [DOI] [PubMed] [Google Scholar]
- 56.Huang X, Molema G, King S, et al. Tumor infarction in mice by antibody-directed targeting of tissue factor to tumor vasculature. Science 1997; 275: 547–550. [DOI] [PubMed] [Google Scholar]
- 57.Kelly KA, Allport JR, Tsourkas A, et al. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res 2005; 96: 327–336. [DOI] [PubMed] [Google Scholar]
- 58.Spragg DD, Alford DR, Greferath R, et al. Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc Natl Acad Sci U S A 1997; 94: 8795–8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taichman DB, Cybulsky MI, Djaffar I, et al. Tumor cell surface alpha 4 beta 1 integrin mediates adhesion to vascular endothelium: demonstration of an interaction with the N-terminal domains of INCAM-110/VCAM-1. Cell Regul 1991; 2: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keelan ET, Harrison AA, Chapman PT, et al. Imaging vascular endothelial activation: an approach using radiolabeled monoclonal antibodies against the endothelial cell adhesion molecule E-selectin. J Nucl Med 1994; 35: 276–281. [PubMed] [Google Scholar]
- 61.Lindner JR, Klibanov AL, Ley K. Targeting inflammation. In: Muzykantov VR, Torchilin VP. (eds). Biomedical Aspects of Drug Targeting, Boston, MA: Kluwer Academic Pub, 2003, pp. 149–172. [Google Scholar]
- 62.Lindner JR, Song J, Christiansen J, et al. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 2001; 104: 2107–2112. [DOI] [PubMed] [Google Scholar]
- 63.Danilov S, Atochina E, Hiemisch H, et al. Interaction of mAb to angiotensin-converting enzyme (ACE) with antigen in vitro and in vivo: antibody targeting to the lung induces ACE antigenic modulation. Int Immunol 1994; 6: 1153–1160. [DOI] [PubMed] [Google Scholar]
- 64.Danilov SM, Gavrilyuk VD, Franke FE, et al. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am J Physiol Lung Cell Mol Physiol 2001; 280: L1335–1347. [DOI] [PubMed] [Google Scholar]
- 65.Heitsch H, Brovkovych S, Malinski T, et al. Angiotensin-(1-7)-stimulated nitric oxide and superoxide release from endothelial cells. Hypertension 2001; 37: 72–76. [DOI] [PubMed] [Google Scholar]
- 66.Metzger R, Franke FE, Bohle RM, et al. Heterogeneous distribution of angiotensin I-converting enzyme (CD143) in the human and rat vascular systems: vessel, organ and species specificity. Microvasc Res 2011; 81: 206–215. [DOI] [PubMed] [Google Scholar]
- 67.Muzykantov VR, Puchnina EA, Atochina EN, et al. Endotoxin reduces specific pulmonary uptake of radiolabeled monoclonal antibody to angiotensin-converting enzyme. J Nucl Med 1991; 32: 453–460. [PubMed] [Google Scholar]
- 68.Miller WH, Brosnan MJ, Graham D, et al. Targeting endothelial cells with adenovirus expressing nitric oxide synthase prevents elevation of blood pressure in stroke-prone spontaneously hypertensive rats. Mol Ther 2005; 12: 321–327. [DOI] [PubMed] [Google Scholar]
- 69.Morecroft I, White K, Caruso P, et al. Gene therapy by targeted adenovirus-mediated knockdown of pulmonary endothelial Tph1 attenuates hypoxia-induced pulmonary hypertension. Mol Ther 2012; 20: 1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muzykantov VR, Atochina EN, Ischiropoulos H, et al. Immunotargeting of antioxidant enzyme to the pulmonary endothelium. Proc Natl Acad Sci U S A 1996; 93: 5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nowak K, Hanusch C, Nicksch K, et al. Pre-ischaemic conditioning of the pulmonary endothelium by immunotargeting of catalase via angiotensin-converting-enzyme antibodies. Eur J Cardiothorac Surg 2010; 37: 859–863. [DOI] [PubMed] [Google Scholar]
- 72.Nowak K, Weih S, Metzger R, et al. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia-reperfusion injury of the lung in vivo. Am J Physiol Lung Cell Mol Physiol 2007; 293: L162–169. [DOI] [PubMed] [Google Scholar]
- 73.Reynolds AM, Xia W, Holmes MD, et al. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2007; 292: L1182–1192. [DOI] [PubMed] [Google Scholar]
- 74.Reynolds PN, Nicklin SA, Kaliberova L, et al. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat Biotechnol 2001; 19: 838–842. [DOI] [PubMed] [Google Scholar]
- 75.Reynolds PN, Zinn KR, Gavrilyuk VD, et al. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol Ther 2000; 2: 562–578. [DOI] [PubMed] [Google Scholar]
- 76.Balyasnikova IV, Metzger R, Visintine DJ, et al. Selective rat lung endothelial targeting with a new set of monoclonal antibodies to angiotensin I-converting enzyme. Pulm Pharmacol Ther 2005; 18: 251–267. [DOI] [PubMed] [Google Scholar]
- 77.Balyasnikova IV, Sun ZL, Metzger R, et al. Monoclonal antibodies to native mouse angiotensin-converting enzyme (CD143): ACE expression quantification, lung endothelial cell targeting and gene delivery. Tissue Antigens 2006; 67: 10–29. [DOI] [PubMed] [Google Scholar]
- 78.Danilov SM, Martynov AV, Klibanov AL, et al. Radioimmunoimaging of lung vessels: an approach using indium-111-labeled monoclonal antibody to angiotensin-converting enzyme. J Nucl Med 1989; 30: 1686–1692. [PubMed] [Google Scholar]
- 79.Nowak K, Kolbel HC, Metzger RP, et al. Immunotargeting of the pulmonary endothelium via angiotensin-converting-enzyme in isolated ventilated and perfused human lung. Adv Exp Med Biol 2013; 756: 203–12. [DOI] [PubMed] [Google Scholar]
- 80.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 2013; 14: 98–112. [DOI] [PubMed] [Google Scholar]
- 81.Muro S. Challenges in design and characterization of ligand-targeted drug delivery systems. J Control Release 2012; 164: 125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Curr Vasc Pharmacol 2004; 2: 281–299. [DOI] [PubMed] [Google Scholar]
- 83.Popova NV, Deyev IE, Petrenko AG. Clathrin-mediated endocytosis and adaptor proteins. Acta Naturae 2013; 5: 62–73. [PMC free article] [PubMed] [Google Scholar]
- 84.Mayor S, Parton RG, Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol 2014; 6: a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev 1997; 77: 759–803. [DOI] [PubMed] [Google Scholar]
- 86.Caron E, Hall A. Phagocytosis, Oxford: Oxford University Press, 2001. [Google Scholar]
- 87.Di Fiore PP, von Zastrow M. Endocytosis, signaling, and beyond. Cold Spring Harb Perspect Biol 2014; 6: a016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 2007; 100: 158–173. [DOI] [PubMed] [Google Scholar]
- 89.Newman PJ. The biology of PECAM-1. J Clin Invest 1997; 99: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell 2013; 26: 441–454. [DOI] [PubMed] [Google Scholar]
- 91.Barreiro O, Yanez-Mo M, Serrador JM, et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 2002; 157: 1233–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ghitescu L, Jacobson BS, Crine P. A novel, 85 kDa endothelial antigen differentiates plasma membrane macrodomains in lung alveolar capillaries. Endothelium 1999; 6: 241–250. [DOI] [PubMed] [Google Scholar]
- 93.Murciano JC, Harshaw DW, Ghitescu L, et al. Vascular immunotargeting to endothelial surface in a specific macrodomain in alveolar capillaries. Am J Respir Crit Care Med 2001; 164: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 94.Swaminathan TN, Liu J, Balakrishnan U, et al. Dynamic factors controlling carrier anchoring on vascular cells. IUBMB Life 2011; 63: 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuijpers TW, Raleigh M, Kavanagh T, et al. Cytokine-activated endothelial cells internalize E-selectin into a lysosomal compartment of vesiculotubular shape. A tubulin-driven process. J Immunol 1994; 152: 5060–5069. [PubMed] [Google Scholar]
- 96.Straley KS, Green SA. Rapid transport of internalized P-selectin to late endosomes and the TGN: roles in regulating cell surface expression and recycling to secretory granules. J Cell Biol 2000; 151: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.von Asmuth EJ, Smeets EF, Ginsel LA, et al. Evidence for endocytosis of E-selectin in human endothelial cells. Eur J Immunol 1992; 22: 2519–2526. [DOI] [PubMed] [Google Scholar]
- 98.Kok RJ, Everts M, Asgeirsdottir SA, et al. Cellular handling of a dexamethasone-anti-E-selectin immunoconjugate by activated endothelial cells: comparison with free dexamethasone. Pharm Res 2002; 19: 1730–1735. [DOI] [PubMed] [Google Scholar]
- 99.Harari OA, Wickham TJ, Stocker CJ, et al. Targeting an adenoviral gene vector to cytokine-activated vascular endothelium via E-selectin. Gene Ther 1999; 6: 801–807. [DOI] [PubMed] [Google Scholar]
- 100.Muro S, Cui X, Gajewski C, et al. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. Am J Physiol Cell Physiol 2003; 285: C1339–1347. [DOI] [PubMed] [Google Scholar]
- 101.Muro S, Wiewrodt R, Thomas A, et al. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci 2003; 116: 1599–1609. [DOI] [PubMed] [Google Scholar]
- 102.Han J, Zern BJ, Shuvaev VV, et al. Acute and chronic shear stress differently regulate endothelial internalization of nanocarriers targeted to platelet-endothelial cell adhesion molecule-1. ACS Nano 2012; 6: 8824–8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han J, Shuvaev VV, Davies PF, et al. Flow shear stress differentially regulates endothelial uptake of nanocarriers targeted to distinct epitopes of PECAM-1. J Control Release 2015; 210: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shuvaev VV, Muro S, Arguiri E, et al. Size and targeting to PECAM vs ICAM control endothelial delivery, internalization and protective effect of multimolecular SOD conjugates. J Control Release 2016; 234: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serrano D, Manthe RL, Paul E, et al. How carrier size and valency modulate receptor-mediated signaling: understanding the link between binding and endocytosis of ICAM-1-targeted carriers. Biomacromolecules 2016; 17: 3127–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garnacho C, Albelda SM, Muzykantov VR, et al. Differential intra-endothelial delivery of polymer nanocarriers targeted to distinct PECAM-1 epitopes. J Control Release 2008; 130: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tagliabue E, Centis F, Campiglio M, et al. Selection of monoclonal antibodies which induce internalization and phosphorylation of p185HER2 and growth inhibition of cells with HER2/NEU gene amplification. Int J Cancer 1991; 47: 933–937. [DOI] [PubMed] [Google Scholar]
- 108.Feero WG, Li S, Rosenblatt JD, et al. Selection and use of ligands for receptor-mediated gene delivery to myogenic cells. Gene Ther 1997; 4: 664–674. [DOI] [PubMed] [Google Scholar]
- 109.Zhou Y, Zhao L, Marks JD. Selection and characterization of cell binding and internalizing phage antibodies. Arch Biochem Biophys 2012; 526: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Y, Zou H, Zhang S, et al. Internalizing cancer antibodies from phage libraries selected on tumor cells and yeast-displayed tumor antigens. J Mol Biol 2010; 404: 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heitner T, Moor A, Garrison JL, et al. Selection of cell binding and internalizing epidermal growth factor receptor antibodies from a phage display library. J Immunol Methods 2001; 248: 17–30. [DOI] [PubMed] [Google Scholar]
- 112.Shuvaev VV, Tliba S, Pick J, et al. Modulation of endothelial targeting by size of antibody-antioxidant enzyme conjugates. J Control Release 2011; 149: 236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Muro S, Muzykantov VR. Targeting of antioxidant and anti-thrombotic drugs to endothelial cell adhesion molecules. Curr Pharm Des 2005; 11: 2383–2401. [DOI] [PubMed] [Google Scholar]
- 114.Serrano D, Bhowmick T, Chadha R, et al. Intercellular adhesion molecule 1 engagement modulates sphingomyelinase and ceramide, supporting uptake of drug carriers by the vascular endothelium. Arterioscler Thromb Vasc Biol 2012; 32: 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garnacho C, Dhami R, Simone E, et al. Delivery of acid sphingomyelinase in normal and niemann-pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J Pharmacol Exp Ther 2008; 325: 400–408. [DOI] [PubMed] [Google Scholar]
- 116.Ansar M, Serrano D, Papademetriou I, et al. Biological functionalization of drug delivery carriers to bypass size restrictions of receptor-mediated endocytosis independently from receptor targeting. ACS Nano 2013; 7: 10597–10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer 2003; 3: 571–581. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Y, Schlachetzki F, Pardridge WM. Global non-viral gene transfer to the primate brain following intravenous administration. Mol Ther 2003; 7: 11–18. [DOI] [PubMed] [Google Scholar]
- 119.Vogel SM, Easington CR, Minshall RD, et al. Evidence of transcellular permeability pathway in microvessels. Microvasc Res 2001; 61: 87–101. [DOI] [PubMed] [Google Scholar]
- 120.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem 2001; 49: 419–432. [DOI] [PubMed] [Google Scholar]
- 121.Testa JE, Chrastina A, Oh P, et al. Immunotargeting and cloning of two CD34 variants exhibiting restricted expression in adult rat endothelia in vivo. Am J Physiol Lung Cell Mol Physiol 2009; 297: L251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Valadon P, Darsow B, Buss TN, et al. Designed auto-assembly of nanostreptabodies for rapid tissue-specific targeting in vivo. J Biol Chem 2010; 285: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stan RV, Ghitescu L, Jacobson BS, et al. Isolation, cloning, and localization of rat PV-1, a novel endothelial caveolar protein. J Cell Biol 1999; 145: 1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem 1995; 270: 14399–14404. [DOI] [PubMed] [Google Scholar]
- 125.Schnitzer JE. Caveolae: from basic trafficking mechanisms to targeting transcytosis for tissue-specific drug and gene delivery in vivo. Adv Drug Deliv Rev 2001; 49: 265–280. [DOI] [PubMed] [Google Scholar]
- 126.Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest 1996; 98: 1949–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mehta D, Bhattacharya J, Matthay MA, et al. Integrated control of lung fluid balance. Am J Physiol Lung Cell Mol Physiol 2004; 287: L1081–1090. [DOI] [PubMed] [Google Scholar]
- 128.Pardridge WM, Buciak J, Yang J, et al. Enhanced endocytosis in cultured human breast carcinoma cells and in vivo biodistribution in rats of a humanized monoclonal antibody after cationization of the protein. J Pharmacol Exp Ther 1998; 286: 548–554. [PubMed] [Google Scholar]
- 129.Predescu D, Vogel SM, Malik AB. Functional and morphological studies of protein transcytosis in continuous endothelia. Am J Physiol Lung Cell Mol Physiol 2004; 287: L895–901. [DOI] [PubMed] [Google Scholar]
- 130.Ghaffarian R, Bhowmick T, Muro S. Transport of nanocarriers across gastrointestinal epithelial cells by a new transcellular route induced by targeting ICAM-1. J Control Release 2012; 163: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Atochina EN, Hiemisch HH, Muzykantov VR, et al. Systemic administration of platelet-activating factor in rat reduces specific pulmonary uptake of circulating monoclonal antibody to angiotensin-converting enzyme. Lung 1992; 170: 349–358. [DOI] [PubMed] [Google Scholar]
- 132.Brenner JS, Bhamidipati K, Glassman PM, et al. Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions. Nanomedicine 2017; 13: 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Siekmeier R, Steffen C, Marz W. Role of oxidants and antioxidants in atherosclerosis: results of in vitro and in vivo investigations. J Cardiovasc Pharmacol Ther 2007; 12: 265–282. [DOI] [PubMed] [Google Scholar]
- 134.Thomson MJ, Puntmann V, Kaski JC. Atherosclerosis and oxidant stress: the end of the road for antioxidant vitamin treatment? Cardiovasc Drugs Ther 2007; 21: 195–210. [DOI] [PubMed] [Google Scholar]
- 135.Carvalho AN, Firuzi O, Gama MJ, et al. Oxidative stress and antioxidants in neurological diseases: is there still hope? Curr Drug Targets 2017; 18: 705–718. [DOI] [PubMed] [Google Scholar]
- 136.White CW, Jackson JH, Abuchowski A, et al. Polyethylene glycol-attached antioxidant enzymes decrease pulmonary oxygen toxicity in rats. J Appl Physiol (1985) 1989; 66: 584–590. [DOI] [PubMed] [Google Scholar]
- 137.Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release 2002; 82: 189–212. [DOI] [PubMed] [Google Scholar]
- 138.Corvo ML, Boerman OC, Oyen WJ, et al. Intravenous administration of superoxide dismutase entrapped in long circulating liposomes. II. In vivo fate in a rat model of adjuvant arthritis. Biochim Biophys Acta 1999; 1419: 325–334. [DOI] [PubMed] [Google Scholar]
- 139.Simone EA, Dziubla TD, Muzykantov VR. Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv 2008; 5: 1283–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Freeman BA, Turrens JF, Mirza Z, et al. Modulation of oxidant lung injury by using liposome-entrapped superoxide dismutase and catalase. Fed Proc 1985; 44: 2591–2595. [PubMed] [Google Scholar]
- 141.Koyama S, Kobayashi T, Kubo K, et al. Recombinant-human superoxide dismutase attenuates endotoxin-induced lung injury in awake sheep. Am Rev Respir Dis 1992; 145: 1404–1409. [DOI] [PubMed] [Google Scholar]
- 142.Jin LH, Bahn JH, Eum WS, et al. Transduction of human catalase mediated by an HIV-1 TAT protein basic domain and arginine-rich peptides into mammalian cells. Free Radic Biol Med 2001; 31: 1509–1519. [DOI] [PubMed] [Google Scholar]
- 143.Gao B, Flores SC, Leff JA, et al. Synthesis and anti-inflammatory activity of a chimeric recombinant superoxide dismutase: SOD2/3. Am J Physiol Lung Cell Mol Physiol 2003; 284: L917–925. [DOI] [PubMed] [Google Scholar]
- 144.Shuvaev VV, Dziubla T, Wiewrodt R, et al. Streptavidin-biotin crosslinking of therapeutic enzymes with carrier antibodies: nanoconjugates for protection against endothelial oxidative stress. Methods Mol Biol 2004; 283: 3–19. [DOI] [PubMed] [Google Scholar]
- 145.Shuvaev VV, Christofidou-Solomidou M, Scherpereel A, et al. Factors modulating the delivery and effect of enzymatic cargo conjugated with antibodies targeted to the pulmonary endothelium. J Control Release 2007; 118: 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Muzykantov VR, Christofidou-Solomidou M, Balyasnikova I, et al. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc Natl Acad Sci U S A 1999; 96: 2379–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Christofidou-Solomidou M, Scherpereel A, Wiewrodt R, et al. PECAM-directed delivery of catalase to endothelium protects against pulmonary vascular oxidative stress. Am J Physiol Lung Cell Mol Physiol 2003; 285: L283–292. [DOI] [PubMed] [Google Scholar]
- 148.Kozower BD, Christofidou-Solomidou M, Sweitzer TD, et al. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol 2003; 21: 392–398. [DOI] [PubMed] [Google Scholar]
- 149.Shuvaev VV, Christofidou-Solomidou M, Bhora F, et al. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J Pharmacol Exp Ther 2009; 331: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Preissler G, Loehe F, Huff IV, et al. Targeted endothelial delivery of nanosized catalase immunoconjugates protects lung grafts donated after cardiac death. Transplantation 2011; 92: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shuvaev VV, Han J, Tliba S, et al. Anti-inflammatory effect of targeted delivery of SOD to endothelium: mechanism, synergism with NO donors and protective effects in vitro and in vivo. PLoS ONE 2013; 8: e77002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 2009; 11: 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han J, Shuvaev VV, Muzykantov VR. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J Pharmacol Exp Ther 2011; 338: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shuvaev VV, Tliba S, Nakada M, et al. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther 2007; 323: 450–457. [DOI] [PubMed] [Google Scholar]
- 155.Tanswell AK, Freeman BA. Liposome-entrapped antioxidant enzymes prevent lethal O2 toxicity in the newborn rat. J Appl Physiol 1987; 63: 347–352. [DOI] [PubMed] [Google Scholar]
- 156.Stone WL, Smith M. Therapeutic uses of antioxidant liposomes. Mol Biotechnol 2004; 27: 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gonnet M, Lethuaut L, Boury F. New trends in encapsulation of liposoluble vitamins. J Control Release 2010; 146: 276–290. [DOI] [PubMed] [Google Scholar]
- 158.Kartha S, Yan L, Weisshaar CL, et al. Superoxide dismutase-loaded porous polymersomes as highly efficient antioxidants for treating neuropathic pain. Adv Healthc Mater 2017; 6. doi:10.1002/adhm.201700500. [DOI] [PMC free article] [PubMed]
- 159.Corvo LM, Jorge JCS, van’t Hof R, et al. Superoxide dismutase entrapped in long-circulating liposomes: formulation design and therapeutic activity in rat adjuvant arthritis. Biochim Biophys Acta 2002; 1564: 227–236. [DOI] [PubMed] [Google Scholar]
- 160.Gaspar MM, Boerman OC, Laverman P, et al. Enzymosomes with surface-exposed superoxide dismutase: in vivo behaviour and therapeutic activity in a model of adjuvant arthritis. J Control Release 2007; 117: 186–195. [DOI] [PubMed] [Google Scholar]
- 161.Gaspar MM, Martins MB, Corvo ML, et al. Design and characterization of enzymosomes with surface-exposed superoxide dismutase. Biochim Biophys Acta 2003; 1609: 211–217. [DOI] [PubMed] [Google Scholar]
- 162.Xu X, Costa A, Burgess DJ. Protein encapsulation in unilamellar liposomes: high encapsulation efficiency and a novel technique to assess lipid-protein interaction. Pharm Res 2012; 29: 1919–1931. [DOI] [PubMed] [Google Scholar]
- 163.Hood ED, Greineder CF, Dodia C, et al. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J Control Release 2012; 163: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Howard MD, Greineder CF, Hood ED, et al. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J Control Release 2014; 177: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dziubla TD, Karim A, Muzykantov VR. Polymer nanocarriers protecting active enzyme cargo against proteolysis. J Control Release 2005; 102: 427–439. [DOI] [PubMed] [Google Scholar]
- 166.Simone EA, Dziubla TD, Discher DE, et al. Filamentous polymer nanocarriers of tunable stiffness that encapsulate the therapeutic enzyme catalase. Biomacromolecules 2009; 10: 1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dziubla TD, Shuvaev VV, Hong NK, et al. Endothelial targeting of semi-permeable polymer nanocarriers for enzyme therapies. Biomaterials 2008; 29: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chorny M, Hood E, Levy RJ, et al. Endothelial delivery of antioxidant enzymes loaded into non-polymeric magnetic nanoparticles. J Control Release 2010; 146: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hood ED, Chorny M, Greineder CF, et al. Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials 2014; 35: 3708–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lannan KL, Phipps RP, White RJ. Thrombosis, platelets, microparticles and PAH: more than a clot. Drug Discov Today 2014; 19: 1230–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maniatis NA, Kotanidou A, Catravas JD, et al. Endothelial pathomechanisms in acute lung injury. Vascul Pharmacol 2008; 49: 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tomashefski JF, Jr, Davies P, Boggis C, et al. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol 1983; 112: 112–126. [PMC free article] [PubMed] [Google Scholar]
- 173.Carraway MS, Welty-Wolf KE, Miller DL, et al. Blockade of tissue factor: treatment for organ injury in established sepsis. Am J Respir Crit Care Med 2003; 167: 1200–1209. [DOI] [PubMed] [Google Scholar]
- 174.Creasey AA, Chang AC, Feigen L, et al. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest 1993; 91: 2850–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pietra GG, Edwards WD, Kay JM, et al. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 1989; 80: 1198–1206. [DOI] [PubMed] [Google Scholar]
- 176.Bjornsson J, Edwards WD. Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc 1985; 60: 16–25. [DOI] [PubMed] [Google Scholar]
- 177.White RJ, Meoli DF, Swarthout RF, et al. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007; 293: L583–590. [DOI] [PubMed] [Google Scholar]
- 178.Meoli DF, White RJ. Thrombin induces fibronectin-specific migration of pulmonary microvascular endothelial cells: requirement of calcium/calmodulin-dependent protein kinase II. Am J Physiol Lung Cell Mol Physiol 2009; 297: L706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ding B-S, Gottstein C, Grunow A, et al. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood 2005; 106: 4191–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Esmon C. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J 1995; 9: 946–955. [DOI] [PubMed] [Google Scholar]
- 181.Greineder CF, Howard MD, Carnemolla R, et al. Advanced drug delivery systems for antithrombotic agents. Blood 2013; 122: 1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Murciano JC, Medinilla S, Eslin D, et al. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol 2003; 21: 891–896. [DOI] [PubMed] [Google Scholar]
- 183.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, et al. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc Natl Acad Sci U S A 1996; 93: 10212–10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709. [DOI] [PubMed] [Google Scholar]
- 185.Brenner JS. Nanomedicine for the treatment of acute respiratory distress syndrome. The 2016 ATS Bear Cage Award-winning Proposal. Ann Am Thorac Soc 2017; 14: 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Greineder CF, Johnston IH, Villa CH, et al. CAM-1-targeted thrombomodulin prevents tissue-factor driven inflammatory thrombosis in a human endothelialized microfluidic model. Blood Adv 2017; 1: 1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Welsh CH, Hassell KL, Badesch DB, et al. Coagulation and fibrinolytic profiles in patients with severe pulmonary hypertension. Chest 1996; 110: 710–717. [DOI] [PubMed] [Google Scholar]
- 188.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med 2008; 178: 618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Greineder CF, Brenza JB, Carnemolla R, et al. Dual targeting of therapeutics to endothelial cells: collaborative enhancement of delivery and effect. FASEB J 2015; 29: 3483–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Greineder CF, Hood ED, Yao A, et al. Molecular engineering of high affinity single-chain antibody fragment for endothelial targeting of proteins and nanocarriers in rodents and humans. J Control Release 2016; 226: 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Seo J, Conegliano D, Farrell M, et al. A microengineered model of RBC transfusion-induced pulmonary vascular injury. Sci Rep 2017; 7: 3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Brenner JS, Greineder CF, Shuvaev VV, et al. Endothelial nanomedicine for the treatment of pulmonary disease. Expert Opin Drug Deliv 2015; 12: 239–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Toba M, Alzoubi A, O’Neill K, et al. A novel vascular homing peptide strategy to selectively enhance pulmonary drug efficacy in pulmonary arterial hypertension. Am J Pathol 2014; 184: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Urakami T, Järvinen TAH, Toba M, et al. Peptide-directed highly selective targeting of pulmonary arterial hypertension. Am J Pathol 2011; 178: 2489–2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yin Y, Wu X, Yang Z, et al. The potential efficacy of R8-modified paclitaxel-loaded liposomes on pulmonary arterial hypertension. Pharm Res 2013; 30: 2050–2062. [DOI] [PubMed] [Google Scholar]
- 196.Reynolds AM, Holmes MD, Danilov SM, et al. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J 2012; 39: 329–343. [DOI] [PubMed] [Google Scholar]
- 197.Chen X-Y, Wang S-M, Li N, et al. Creation of lung-targeted dexamethasone immunoliposome and its therapeutic effect on bleomycin-induced lung injury in rats. PLoS One 2013; 8: e58275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hegeman MA, Cobelens PM, Kamps J, et al. Liposome-encapsulated dexamethasone attenuates ventilator-induced lung inflammation. Br J Pharmacol 2011; 163: 1048–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hood E, Simone E, Wattamwar P, et al. Nanocarriers for vascular delivery of antioxidants. Nanomedicine (Lond) 2011; 6: 1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fan J, Shek PN, Suntres ZE, et al. Liposomal antioxidants provide prolonged protection against acute respiratory distress syndrome. Surgery 2000; 128: 332–338. [DOI] [PubMed] [Google Scholar]
- 201.Rocksén D, Ekstrand-Hammarström B, Johansson L, et al. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am J Respir Cell Mol Biol 2003; 28: 199–207. [DOI] [PubMed] [Google Scholar]
- 202.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol 2011; 44: 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Muzykantov VR. Targeted therapeutics and nanodevices for vascular drug delivery: quo vadis? IUBMB Life 2011; 63: 583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mori A, Kennel SJ, van Borssum Waalkes M, et al. Characterization of organ-specific immunoliposomes for delivery of 3',5'-O-dipalmitoyl-5-fluoro-2'-deoxyuridine in a mouse lung-metastasis model. Cancer Chemother Pharmacol 1995; 35: 447–456. [DOI] [PubMed] [Google Scholar]
- 205.Wilson A, Zhou W, Champion HC, et al. Targeted delivery of oligodeoxynucleotides to mouse lung endothelial cells in vitro and in vivo. Mol Ther 2005; 12: 510–518. [DOI] [PubMed] [Google Scholar]
- 206.Christofidou-Solomidou M, Kennel S, Scherpereel A, et al. Vascular immunotargeting of glucose oxidase to the endothelial antigens induces distinct forms of oxidant acute lung injury. Am J Pathol 2002; 160: 1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Skidgel RA. Bradykinin-degrading enzymes: structure, function, distribution, and potential roles in cardiovascular pharmacology. J Cardiovasc Pharmacol 1992; 20(Suppl 9): S4–9. [DOI] [PubMed] [Google Scholar]
- 208.Howard MD, Jay M, Dziubla TD, et al. PEGylation of nanocarrier drug delivery systems: state of the art. Journal of Biomedical Nanotechnology 2008; 4: 133–148. [Google Scholar]