Abstract
Simple mismatch negativity (MMN) to infrequent pitch deviants is impaired in individuals with long-term schizophrenia (Sz). The complex MMN elicited by pattern deviance often manifests later after deviant-onset than simple MMN, and can ascertain deficits in abstracting relationships between stimuli. Sz exhibit reduced complex MMN, but so far this has only been measured when deviance detection relies on a grouping rule. We measured MMN to deviants in pitch-based rules to see if MMN is also abnormal in Sz under these conditions. Three experiments were conducted. Twenty-seven Sz and 28 healthy matched controls (HC) participated in Experiments 1 and 2, and 24 Sz and 26 HC participated in Experiment 3. Experiment 1 was a standard pitch MMN task, and Sz showed the expected MMN reduction (~115 ms) to the simple pitch-deviant compared to HC. Experiment 2 comprised standard groups of six tones that ascended in pitch, and deviant groups where the last tone descended in pitch. Complex MMN was late (~510 ms) and significantly blunted in Sz. Experiment 3 comprised standard groups of 12 tones (six tones ascending in pitch followed by six tones descending in pitch, like a scale), and deviant groups containing two repetitions of six ascending tones (the scale restarted midstream). Complex MMN was also late (~460 ms) and significantly blunted in Sz. These results identify a late pitch pattern deviance-related MMN that is deficient in schizophrenia. This suggests specific deficits in later more complex deviance detection in schizophrenia for abstract patterns.
Keywords: deviance detection, long-term schizophrenia, EEG, auditory pattern
Graphical Abstract
The complex MMN elicited by pattern deviance often manifests later after deviant-onset than simple MMN, and can ascertain deficits in abstracting relationships between stimuli. Sz exhibit reduced complex MMN, but so far this has only been measured when deviance detection relies on a grouping rule. We report reduced late complex MMN to deviants in pitch-based rules suggesting specific deficits in later more complex deviance detection in schizophrenia for abstract patterns.
Introduction
Schizophrenia is associated with auditory abnormalities beyond the presence of auditory hallucinations. For example, individuals with schizophrenia show deficits in language processing (Strelnikov et al., 2010; DeLisi et al., 2001), acoustic segregation (Ramage et al., 2012; Weintraub et al., 2012), pitch discrimination (McLachlan et al., 2013), and tone-pair matching (Rabinowicz et al., 2000). Individuals with schizophrenia also show reduced evoked responses (e.g., smaller N1 responses; Shelley et al., 1999; Salisbury et al., 2009) that correlate with the perceptual deficits (Javitt et al., 2000). These deficits in basic auditory processing are associated with deficits in more complex processing in schizophrenia, such as identifying non-verbal vocal cues of emotion (Kahshan et al., 2015) and deficits in global functioning (Light et al., 2007). Together this indicates that the auditory deficits are linked to impairments in daily functioning.
One consistent measure of abnormal auditory processing in schizophrenia is mismatch negativity (MMN). MMN is a pre-attentive negative event-related potential (ERP) typically elicited in response to a rare deviant stimulus. For instance, rare 1.5 kHz tones presented among frequent 1 kHz tones generate MMN, and the amplitude of the MMN elicited by simple physical parameter deviance (hereafter simple MMN) is smaller in individuals with long-term schizophrenia compared to healthy controls (Shelley et al., 1999; Javitt et al., 2000; Shelley et al., 1991; Javitt et al., 1998; Michie et al., 2000; Salisbury et al., 2002; Umbricht & Krjles, 2005). The original description of simple MMN suggested that it reflected sensory memory (Näätänen, 1990), and more recent studies have described MMN as part of a computational model of error-detection (Winkler, 2007; Winkler et al., 2009). Others describe MMN as being a delayed and enlarged N1 due to stimulus-specific adaptation (May & Tiitinen, 2007; 2010) – the repeated standard tone causes adaptation to the specific pitch reflected in smaller N1, so that when a deviant-pitch tone is presented, another non-adapted area of tonotopic auditory cortex becomes active and produces a larger N1 response. However, N1 and MMN appear to originate from different parts of the brain and so are not the same components (Korzyukov et al., 1999). Ascertaining which mechanism drives the MMN signal would help to identify the specific deficits indexed by MMN reduction in schizophrenia. However, the simple infrequent physical parameter deviance protocol for measuring MMN makes it difficult to ascertain whether the smaller MMN in schizophrenia is due to deficits in stimulus-specific adaptation or deviance detection.
Several protocols have been developed that do not rely on simple infrequent physical parameter deviance, but instead rely on deviance from a sequential pattern of sounds. In fact, the deviant may be more frequent than other tones. Thus, the MMN elicited by deviance from complex auditory sequence patterns (hereafter complex MMN) cannot be explained by a non-adapted N1 effect. Most complex MMN studies in healthy individuals have focused on responses to deviant tone pairs in which the second tone descended in pitch relative to the first tone, compared to standard tone pairs that ascended in pitch (Gumenyuk et al., 2003; Paavilainen et al., 1998; 1999; van Zuijen et al., 2006; Korzyukov et al., 2003; Tervaniemi et al., 1999), suggesting that deviance-detection of abstract pitch-based features is reflected in the MMN. Several other studies have reported MMN from a variety of other complex pattern deviants: a repeated tone amongst a pattern of alternating tones (Alain et al., 1994), missing tones (Salisbury, 2012), and rules that help predict the duration and/or pitch of the next tone (Paavilainen et al., 2007). Unlike simple physical parameter deviance, complex pattern deviants appear to evoke multiple MMNs that represent different computational processes. Korpilahti and colleagues (2001) reported MMN between 150–200ms after a deviant complex tones and a second MMN between 350–450ms. Zachau and colleagues (2005) reported an early MMN at 146ms after deviant descending pairs of tones, and a second MMN around 340ms. Recasens and colleagues (2014) measured MMN to a deviant additional tone and detected an early MMN around 150–200ms and a later MMN around 250ms, each arising from separate generator locations. The appearance of multiple MMNs may represent deviance detection at different levels of the auditory hierarchy (Escara et al., 2014; Grimm et al., 2012), with the early MMN being more sensory-related (more in line with May & Tiitinen, 2007; 2010), and the late MMN being more cognitive-related (Näätänen, 1990; Winkler 2007; Winkler et al., 2009). Complex pattern deviants may then be a useful tool to uncover where in the auditory hierarchy individuals with schizophrenia show a deficit.
Only a few studies have investigated complex MMN in schizophrenia and results are inconsistent. Alain and colleagues (1998) reported reduced MMN to simple pitch deviants in schizophrenia. They showed similar MMN amplitudes (90–130 ms after deviant-onset) to the occasional repeat-deviant in both schizophrenia and controls, although referencing the MMN to the nose electrode revealed a marginally significant reduction in schizophrenia. Todd and colleagues (2014) also found smaller MMN in schizophrenia (peaks were picked between 200–250 ms after deviant-onset), but found that the schizophrenia group was able to modulate the amplitude of MMN according to how predictable the deviant was, similar to controls. The authors concluded that the individuals with schizophrenia were able to use rules to detect deviants. On the other hand, pairs of tones that descended in pitch produced a MMN when compared to expected pairs of tones that increased in pitch (Salisbury et al., 2017). A missing tone amongst standard groups of six tones produced a smaller MMN in individuals with long-term schizophrenia (150–200 ms after the expected onset of the tone; Salisbury et al., 2016) and in recent-onset schizophrenia patients (100–250 ms; Rudolph et al., 2015). We measured MMN to an extra tone that violated a quantity/number grouping rule in individuals with long-term schizophrenia (Haigh et al., 2016), and found no significant differences between groups in the early MMN, but found significant reductions in the late MMN in schizophrenia (~350 ms after the onset of the extra tone), suggesting specific deficits in later deviance processing. This was the first study to show specific deficits in the late MMN in schizophrenia, and it is possible that the late MMN may be more sensitive to deficits in complex deviance detection. To assess whether a late MMN can be elicited from other rule-based violations (not just grouping rules), and whether we could replicate the blunted late complex MMN in schizophrenia, we measured complex MMN to patterns that violated a complex pitch-based rule.
The purpose of this study was to examine complex MMN to deviant tones in sequences of stimuli defined by pitch patterns to determine if a later MMN could be isolated from an earlier MMN, and whether the late complex MMN was impaired in schizophrenia. Three experiments were conducted: a simple pitch-deviant task, and two complex pitch- pattern sequence tasks. The simple MMN task served to establish the well-replicated finding that simple MMN is significantly reduced in this sample of individuals with schizophrenia. Two different complex MMN tasks were used to test whether having a more salient deviant (a larger difference in pitch between what was expected and what was presented) would increase the separation in complex MMN amplitude between schizophrenia and controls. If simple and complex MMNs are abnormal in schizophrenia, then deficits in extracting pitch-based abstract relationships or deviance detection that are independent of abnormalities in early sensory processing.
Experiment 1
Methods
Participants
Twenty-seven participants with schizophrenia (Sz) were compared with 28 healthy control (HC) participants who were matched for age, gender, Wechsler Abbreviated Scale of Intelligence (WASI) IQ, and parental socioeconomic status. Twenty-three Sz had a diagnosis of schizophrenia (undifferentiated=9; paranoid=8; residual=5; disorganized=1), and four had schizoaffective disorder (depressed=4).
All participants were recruited from Western Psychiatric Institute and Clinic (WPIC) inpatient and outpatient services. All Sz had at least 5 years length of illness, or were hospitalized at least three times for psychosis.
All subjects had normal hearing as assessed by audiometry, at least nine years of schooling, and an IQ over 85. None of the participants had a history of concussion or head injury with sequelae, history of alcohol or drug addiction, detox in the last five years, or neurological comorbidity. The 4-factor Hollingshead Scale was used to measure socioeconomic status (SES) in participants and in their parents. As expected, Sz had lower SES than HC, consistent with social and occupational impairment as a disease consequence (see Table 1 for demographic measures). All participants provided informed consent and were paid for participation. All procedures were approved by the University of Pittsburgh IRB, and were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Table 1.
Demographic and diagnostic information for the Sz and HC groups for the simple pitch MMN and the ascending-pitch MMN (Experiments 1 & 2), with t/chi-square statistics and p-values for group comparisons. Medication is listed in Chlorpromazine (CPZ) equivalents. Dosages primarily from Andreasen et al. (2010), and remaining dosages from Gardner et al. (2010).
| SZ | HC | ||||
|---|---|---|---|---|---|
| Age | 36.07 | ±8.22 | 32.40 | ±10.54 | t(53)=1.40, p=.166 |
| Gender | M 18/F 9 | M 18/F 10 | x2(53)=0.03, p=.853 | ||
| % Right handed | 89% | 100% | x2(53)=2.15, p=.142 | ||
| SES | 29.85 | ±13.37 | 42.25 | ±11.28 | t(53)=3.72, p<.001 |
| Parental SES | 35.80 | ±13.53 | 41.00 | ±10.13 | t(53)=1.62, p=.111 |
| IQ | 102.78 | ±14.06 | 107.00 | ±11.29 | t(53)=1.23, p=.224 |
| MCCB (MATRICS) | 25.81 | ±4.36 | 28.36 | ±3.93 | t(53)=2.26, p=.028 |
| Medication (CPZ mg/day) | 566.98 | ±320.11 | |||
| Length of illness (years) | 13.72 | ±6.85 | |||
| PANSS total | 69.81 | ±13.73 | |||
| PANSS positive | 14.96 | ±4.94 | |||
| PANSS negative | 19.69 | ±6.21 | |||
| SAPS (global items) | 3.59 | ±2.79 | |||
| SAPS (symptom items) | 8.59 | ±9.77 | |||
| SANS (global items) | 12.19 | ±3.86 | |||
| SANS (symptom items) | 37.19 | ±12.15 | |||
| UPSA communication | 7.04 | ±1.71 | |||
| UPSA financial | 8.23 | ±1.77 | |||
Diagnostic Assessments
Diagnosis was based on the Structured Clinical Interview for DSM-IV (SCID-P). Symptoms were rated using the Positive and Negative Symptom Scale (PANSS), Scale for Assessment of Positive Symptoms (SAPS), and Scale for Assessment of Negative Symptoms (SANS). All tests were conducted by an expert diagnostician.
Neuropsychological Tests
All participants completed the MATRICS Cognitive Consensus Battery and the Wechsler Abbreviated Scale of Intelligence (WASI). Social functioning was assessed with the brief UCSD Performance-based Skills Assessment (UPSA-B). See Table 1 for neuropsychological scores.
Procedure
Stimuli were generated with Tone Generator (NCH Software), and presented in Presentation (Neurobehavioral Systems, Inc.). Binaural auditory stimuli were presented at 80 dB using Etymotic 3A insert earphones, with loudness confirmed with a sound meter. Participants watched a silent nature video whilst tones were played over earphones. They were asked to concentrate on the movie and ignore the tones.
Stimuli
Standard 1kHz tones lasting 50ms in duration with 5ms rise/fall times were presented. Pitch-deviants (1.2kHz, 50ms, 5ms rise/fall) were presented 10% of the time (150 presentations). At least two standard tones preceded every deviant tone. Tones had a stimulus-onset asynchrony (SOA) of 330ms.
EEG Recording
EEG was recorded from a custom 72 channel Active2 high impedance system (BioSemi), comprising 70 scalp sites including the mastoids, 1 nose reference electrode, and 1 electrode below the right eye. The EEG amplifier bandpass was DC to 104Hz (24 dB/octave rolloff) digitized at 512Hz, referenced to a common mode sense site (near PO1). Processing was done off-line with BESA 6 (BESA GMBH) and BrainVision Analyzer2 (Brain Products GMBH). First, using BESA, EEG was filtered between 0.5–20Hz; the relatively high low cutoff was to remove DC drifts and skin potentials, the high cutoff was to remove muscle and other high frequency artifact. Data were visually examined and bad channels were interpolated. Independent components analysis (ICA) was used to remove one vertical and one horizontal EOG component. Eye corrected data were then analyzed in BrainVision Analyzer2 and rereferenced to averaged mastoids (to help visualize complex MMN, which originates outside of superior temporal plane in scalp topography maps).
Data Analysis
Epochs (350ms) were extracted from the EEG based on stimulus triggers, including a 50ms prestimulus baseline. All epochs were baseline corrected, and linear DC detrended between baseline (−50–0ms) and the last 50ms of the epoch was removed to ensure that the data were not skewed by skin potentials and steady-state drift. Epochs were subsequently rejected if any site contained activity ±50μV.
MMN waveforms were calculated by subtracting the ERP in response to the standard tone from the deviant tone ERP. The window for calculating the MMN amplitudes depended on the maximal response for both groups in the grand averages. For the MMN to the simple pitch-deviants, the average voltage was calculated between 105–125ms. Groups did not significantly differ in the number of epochs included in the averages (HC: mean 127.4, std ±50.8; Sz: mean 114.9, std ±46.1; t(53)=0.96, p=.344).
Statistics
Group demographics and neuropsychological scores were compared using t-tests and chi-squared tests where appropriate. MMN analyses utilized repeated-measures ANOVA, with group (Sz, HC) as the between subjects factor, and electrode chain (F or FC) and site (left, central, or right) as within subjects factors. The Huynh-Feldt epsilon was used to correct for multiple comparisons of site. Current source density (CSD) topography of complex MMN was calculated to indicate a possible dipole source. Interpolation was done using spherical splines, with the order of splines set to 4, the maximum degree of Legendre polynomials set to 10, and a default lambda of 1e-5. Two-tailed Spearman’s correlations were used as a preliminary and exploratory analysis of the relationships between complex MMN at FCz (where it was largest) and demographic, clinical, and neuropsychological items. Values are reported as Mean ±SD. Significance was attained at p <.05.
Results
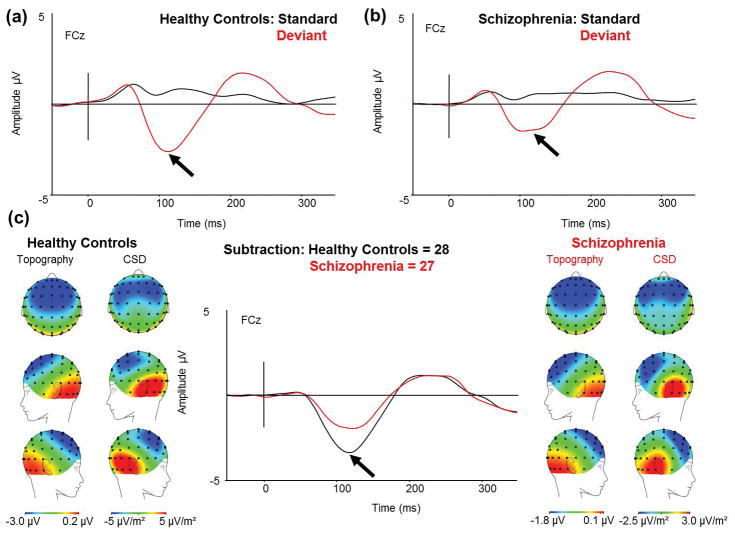
The simple pitch deviant tone produced a negative deflection in HC (Fig 1a) and in Sz (Fig 1b) not seen to the standard tone. The MMN was significantly larger in HC (mean −3.22μV, std ±0.34μV) than in Sz (mean −1.93μV, std ±0.35μV; F(1,53)=6.95, p=.011; Cohen’s d =3.74) (Fig 1c). The scalp topography and CSD maps showed the expected MMN distribution suggesting sources in temporal lobe (Fig 1c).
Fig 1.
(a) ERP waveforms in HC in response to the pitch-deviant (red) and the standard tone (black) for FCz electrode site. (b) ERP waveforms in Sz in response to the pitch-deviant (red) and the standard tone (black) for FCz electrode. (c) The MMN response (standard waveform subtracted from deviant waveform) to the pitch-deviant in HC and Sz. (From Left to Right) Scalp topography and current source density (CSD) maps of the MMN to the pitch-deviant in HC; simple MMN waveform to the pitch-deviant in HC (black) and Sz (red) as indicated by the arrow for FCz electrode; scalp topography and CSD maps to the pitch-deviant in Sz. Scales of topography and CSD maps were adjusted to accurately reflect distribution in each group unconfounded by the main effect.
Correlations with Simple MMN Amplitudes
Pitch MMN at FCz did not significantly correlate with any neuropsychiatric measures for HC or Sz. In Sz, those with worse pitch MMN were less symptomatic on PANSS measures of passive/apathetic social withdrawal (ρ(23)=-.44, p=.024) and measures of conceptual disorganization (ρ(26)=-.50, p=.008).
Experiment 1 Discussion
The results from Experiment 1 replicate previous findings that Sz exhibit smaller pitch MMN than HC (Umbricht & Krjles, 2005). The correlations, however, are the opposite of what we would expect: healthier MMN in those who are more symptomatic, and were only significant in two individual scores (one negative symptom and one positive). It is unclear whether the simple pitch MMN deficits are due to deficits in low-level sensory abnormalities or specific deficits in deviance detection.
A deviant in a complex pattern can distinguish between these two possibilities; deviance detection in a complex pattern has to rely on the ability to detect rule-based patterns and cannot depend on a single stimulus parameter to detect deviance. Experiment 2 tested whether Sz show deficits in complex deviance detection by measuring MMN to a standard pattern of six tones that ascended in pitch, and an infrequent deviant group of six tones where the last tone descended in pitch. The deviant descending tone was a repeat of a previously presented tone and so did not differ from the standard group of tones in its stimulus parameters. Therefore, deviance detection would have to have relied on detecting the abstract pitch-based rule.
Experiment 2
Methods
All methods were the same as above with the following differences.
Participants
The same participants from Experiment 1 participated in Experiment 2 (Table 1).
Stimuli
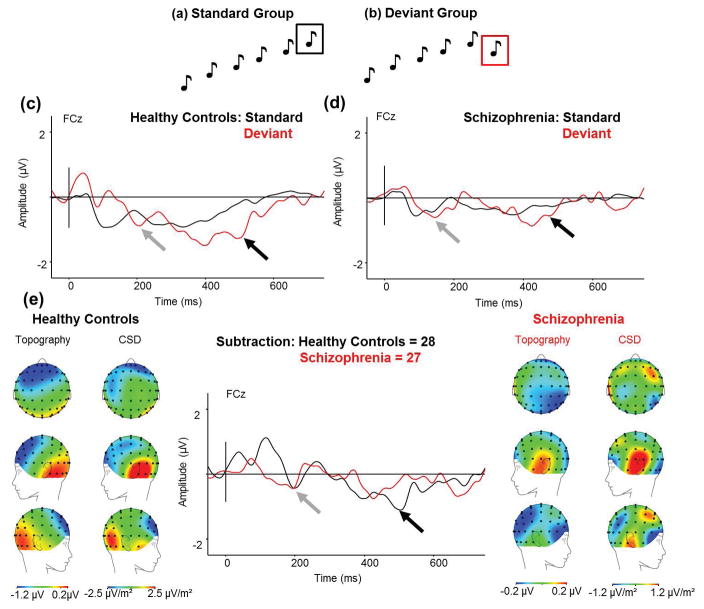
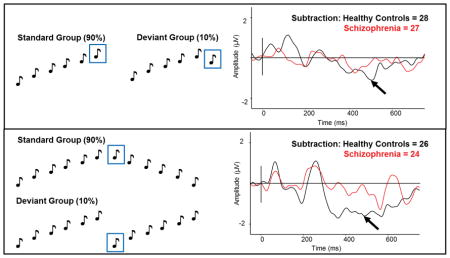
Stimuli were the same as those described by Coffman and colleagues (2016). Temporal proximity and rhythm were used to form discrete groups of tones, with a SOA within groups of 330ms and an inter-trial interval of 800 ms. The standard pattern consisted of six tones ascending in pitch (5ms rise/fall): 1.5kHz, 2kHz, 2.5kHz, 3kHz, 3.5kHz, 4kHz. The deviant pattern contained the same first five tones as the standard pattern, but the last tone descended in pitch: 1.5 kHz, 2 kHz, 2.5 kHz, 3 kHz, 3.5 kHz, 3 kHz (Fig 2a&b), and were presented 50 times. Deviant groups never immediately followed one another and were presented 10% of the time.
Fig 2.
(a) Standard groups of six tones ascending in pitch (the waveform to the black boxed tone is used as the standard response in the analysis), and (b) deviant groups with five tones ascending in pitch with the sixth tone descending in pitch (the waveform to the ref boxed tone is used as the deviant response in the analysis). (c) ERP waveforms in HC in response to the ascending-pitch deviant (red) and the standard tone (black) for FCz electrode site. (d) ERP waveforms in Sz in response to the ascending-pitch deviant (red) and the standard tone (black) for FCz electrode. (e) The MMN response (standard waveform subtracted from the deviant waveform) to the ascending-pitch deviant in HC and Sz from the onset of the deviant to show maximal MMN amplitude. (From Left to Right) Scalp topography and current source density (CSD) maps of the MMN to the ascending-pitch deviant in HC; simple MMN waveform to the ascending-pitch deviant in HC (black) and Sz (red) as indicated by the arrow for FCz electrode; Scalp topography and CSD maps to the ascending-pitch deviant in Sz. Scales of topography and CSD maps were adjusted to accurately reflect distribution in each group unconfounded by the main effect. Zero denotes the onset of the last tone in the group. Early MMN indicated by grey arrow, mid MMN indicated by mid-grey arrow, and late MMN indicated by black arrow.
Data Analysis
Epochs of the last tone in the standard group and of the last tone in the deviant group were extracted (−50 before to 700ms after stimulus onset). All epochs were baseline corrected, and DC detrended between baseline (−50–0ms) and the last 50ms of the epoch to ensure that the data were not skewed by skin potentials and steady-state drift. Epochs were subsequently rejected if any site contained activity ±50μV. Complex MMN waveforms were calculated by subtracting the ERP waveform in response to the last tone in the standard group of tones from the deviant stimulus waveform. The window for calculating the MMN amplitudes depended on the maximal response for both groups in the grand averages. For the MMN to ascending-pitch deviant, two MMNs were detected. The average response was calculated between 190–210ms after stimulus-onset, and 500–520ms. Groups did not significantly differ in the number of epochs included in averages (HC: mean 46.1, std ±3.2; Sz: mean 45.0, std ±8.6; t(53)=0.62, p=.541).
Results
The deviant descending pitch tone produced two negative deflections not seen in the standard waveform in HC (Fig 2c). The second negativity (500–520ms) was not as pronounced in Sz (Fig 2d). The early complex MMN was around the same latency as the simple MMN, and was not significantly reduced in Sz (HC: mean −0.43μV, std ±0.34μV; SZ: mean −0.37μV, std ±0.35μV; F(1,53)=0.01, p=.907). The late MMN was significantly blunted in Sz (HC: mean −1.13μV, std ±0.35μV; SZ: mean −0.11μV, std ±0.35μV; F(1,53)=4.26, p=.044; Cohen’s d = 2.91; Fig 2e). Scalp topography in HC showed negativity over frontocentral areas. CSD maps indicated temporal source activity (Fig 2e).
Correlations with Ascending-Pitch MMN Amplitudes
HC who produced larger ascending-pitch MMN performed better on the Brief Visuospatial Memory Task in the MATRICS battery (ρ(26)=-.49, p=.012). For Sz, the ascending-pitch MMN did not show any significant correlations with any of the symptom or neuropsychiatric measures.
Experiment 2 Discussion
The significant reduction in late MMN in Sz suggests deficits in pure deviance detection that cannot be based on detecting changes in simple stimulus parameters. The timing of the late MMN also suggests that the complex pattern deviants were processed later in the auditory hierarchy, and may involve cognitive processing to help detect the complex deviant. The late MMN in HC correlated with a measure of visuospatial memory, potentially suggesting memory abilities help improve deviance detection in complex patterns.
To test whether the difference in complex MMN between HC and Sz increases with a more salient deviant, we measured complex MMN in a different pitch-based paradigm. Experiment 3 used standard groups of 12 tones, with an initial six tones that ascended in pitch (as in Experiment 2) followed by six tones that descended in pitch (creating a scale-like pattern). The deviant group comprised two repetitions of six tones that ascended in pitch. Therefore, the difference between the expected pitch and the presented pitch is much larger in Experiment 3 than in Experiment 2. If late MMN tracks deviance detection, then a more salient deviant should increase the size of the late MMN. Replication of the smaller late MMN in Sz would also demonstrate a deficit in pure deviance detection in pitch-based rules that is not dependent on the type of complex pattern used.
Experiment 3
Methods
Methods were the same as described above with the following differences:
Participants
Twenty-four Sz were compared to 26 HC and also participated in Experiments 1 and 2. Twenty Sz had a diagnosis of schizophrenia (undifferentiated=8; paranoid=5; residual=6; disorganized=1), and four had schizoaffective disorder (depressed=4). As expected, Sz had lower SES than HC, consistent with social and occupational impairment as a disease consequence (see Table 2 for demographic measures).
Table 2.
Demographic and diagnostic information for the Sz and HC groups for the scales-pitch MMN (Experiment 3), with t/chi-square statistics and p-values for group comparisons. Medication is listed in Chlorpromazine (CPZ) equivalents. Dosages primarily from Andreasen et al. (2010), and remaining dosages from Gardner et al. (2010).
| SZ | HC | ||||
|---|---|---|---|---|---|
| Age | 35.48 | ±8.33 | 31.99 | ±10.38 | t(48)=1.28, p=.205 |
| Gender | M 17/F 7 | M 16/F 10 | x2(48)=0.48, p=.488 | ||
| % Right handed | 92% | 100% | x2(48)=2.26, p=.133 | ||
| SES | 29.08 | ±11.68 | 42.27 | ±11.30 | t(48)=4.06, p<.001 |
| Parental SES | 35.33 | ±12.38 | 40.21 | ±10.05 | t(48)=1.54, p=.131 |
| IQ | 102.04 | ±14.70 | 106.81 | ±11.32 | t(48)=1.29, p=.203 |
| MATRICS | 25.67 | ±4.40 | 28.50 | ±4.02 | t(48)=2.38, p=.021 |
| Medication (CPZ mg/day) | 560.81 | ±325.63 | |||
| Length of illness (days) | 5173.92 | ±2446.23 | |||
| PANSS total | 70.29 | ±14.19 | |||
| PANSS positive | 14.96 | ±5.15 | |||
| PANSS negative | 20.08 | ±6.31 | |||
| SAPS (global items) | 3.58 | ±2.95 | |||
| SAPS (symptom items) | 8.79 | ±10.34 | |||
| SANS (global items) | 12.38 | ±3.79 | |||
| SANS (symptom items) | 38.33 | ±12.23 | |||
| UPSA communication | 6.88 | ±1.68 | |||
| UPSA financial | 8.04 | ±1.71 | |||
Stimuli
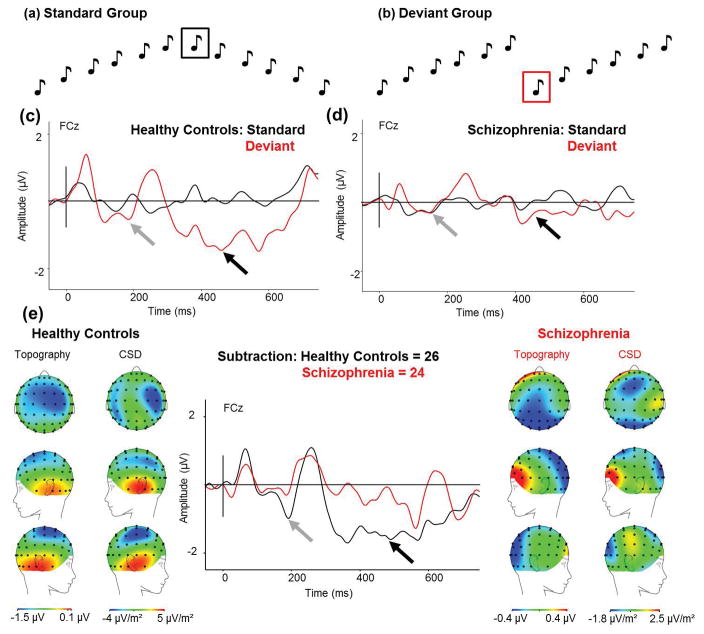
The standard pattern consisted of twelve tones: six tones with ascending pitch and six tones with descending pitch (5ms rise/fall): 1.5kHz, 2kHz, 2.5kHz, 3kHz, 3.5kHz, 4kHz, 4kHz, 3.5kHz, 3kHz, 2.5kHz, 2kHz, 1.5kHz. The deviant pattern contained the first six tones from the standard pattern repeated twice: 1.5kHz, 2kHz, 2.5kHz, 3kHz, 3.5kHz, 4kHz, 1.5kHz, 2kHz, 2.5kHz, 3kHz, 3.5kHz, 4kHz (Fig 3a&b), and were presented 50 times. Deviant groups never immediately followed one another and were presented 10% of the time.
Fig 3.
(a) Standard groups of six tones ascending in pitch followed by six tone descending in pitch (the waveform to the black boxed tone is used as the standard response in the analysis), and (b) deviant groups of tones with the six ascending tones repeating twice (the waveform to the red boxed tone is used as the deviant response in the analysis). (c) ERP waveforms in HC in response to the scales-pitch-deviant (red) and the standard tone (black) for FCz electrode site. (d) ERP waveforms in Sz in response to the scales-pitchdeviant (red) and the standard tone (black) for FCz electrode. (e) The MMN response (standard waveform subtracted from the deviant waveform) to the scales-pitch-deviant in HC and Sz from the onset of the deviant to show maximal MMN amplitude. (From Left to Right) Scalp topography and current source density (CSD) maps of the MMN to the scales-pitch-deviant in HC; simple MMN waveform to the scales-pitch-deviant in HC (black) and Sz (red) as indicated by the arrow for FCz electrode; scalp topography and CSD maps to the scales-pitch-deviant in Sz. Scales of topography and CSD maps were adjusted to accurately reflect distribution in each group unconfounded by the main effect. Zero denotes the onset of seventh tone in the group. Early MMN indicated by grey arrow, mid MMN indicated by mid-grey arrow, and late MMN indicated by black arrow.
Data Analysis
The same analyses were conducted as Experiment 2, except for the following differences.
Epochs of the seventh tone from the standard group and from the deviant group were extracted (−50 before stimulus-onset to 700ms after stimulus-onset). For the MMN to the scale-pitch pattern deviant, two MMNs were detected. The average response was calculated between 180–200ms and 430–480ms after stimulus-onset (the second MMN was much longer than the others and so a 50ms peak was measured; results were the same with a 20ms peak). Groups significantly differed in the number of epochs included in averages (HC: mean 55.4, std ±16.5; Sz: mean 47.1, std ±9.6; t(48)=2.15,p=.037).
Results
The deviant tone in the middle of the group (restarting the scale) produced two negative deflections not seen in the standard waveform in HC (Fig 3c). Again, the late MMN was not as pronounced in Sz (Fig 3d). The early MMN was not significantly different between HC and Sz (HC: mean −0.62μV, std ±0.42μV; SZ: mean −0.07μV, std ±0.44μV; F(1,48)=1.30, p=.261); however, the second MMN was significantly smaller in Sz (HC: mean −1.24μV, std ±0.31μV, Sz: mean −0.28μV, std ±0.31μV; F(1,48)=5.20, p=.027; Cohen’s d = 3.10; Fig 3e). Scalp topography showed frontocentral negativity for both MMNs in HC. CSD maps indicated bilateral temporal source activity (Fig 3e).
Correlations with Scale-Pitch MMN Amplitudes
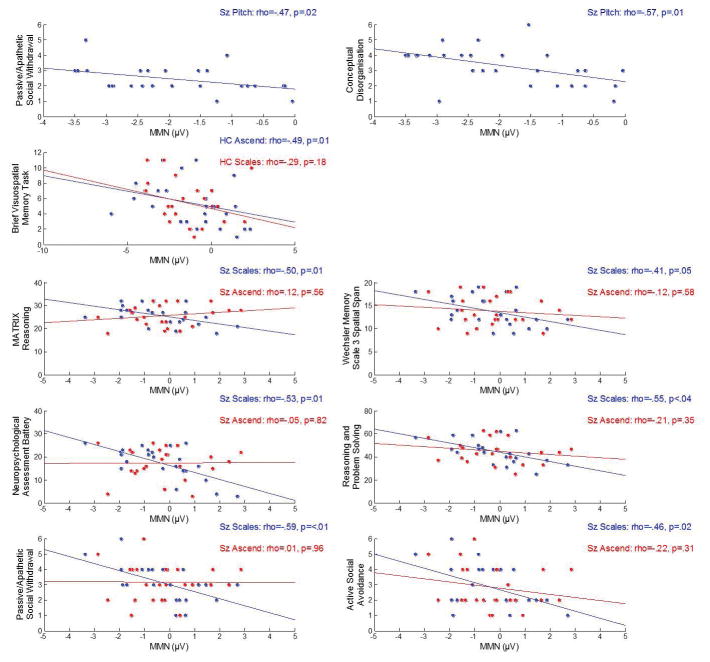
HC showed no significant correlations between scale-pitch MMN and any of the neuropsychological test scores. For Sz, the late scale-pitch MMN showed significant negative correlations with overall MATRIX reasoning scores (ρ(22)=-.50, p=.013), Wechsler Memory Scale 3 Spatial Span (ρ(22)=-.41, p=.048), Neuropsychological Assessment Battery (ρ(22)=-.53, p=.008), and the composite t-score for the Reasoning and Problem Solving subscale of the MATRICS (ρ(22)=-.55, p=.004). In addition, two PANSS scores correlated with scale-pitch late MMN; passive/apathetic social withdrawal scores (ρ(22)=-.59, p=.002), and active social avoidance (ρ(22)=-.46, p=.024; Fig 4). Together this suggests that larger late MMN related to better performance on neuropsychological tests, but that these individuals were more symptomatic.
Fig 4.
Correlations between MMN and neuropsychological or symptom scores. Correlations shown in blue were significant (p<.05). Correlations in red were not significant (p>.05) and demonstrate how reliable the correlations were with other MMN signals. For HC, only scores in the Brief Visuospatial Memory Task were significant. All other correlations were for the Sz group.
Experiment 3 Discussion
Once again, HC exhibited a late complex MMN that was significantly blunted in Sz. This demonstrates that the late MMN is not dependent on the type of complex pattern or on the type of deviant used to elicit MMN. The correlations in Sz are again surprising. Those who performed better on neuropsychological tests exhibited larger late MMNs, but were more symptomatic. The correlation in HC between visuospatial memory and late MMN detected in Experiment 2 was not replicated in Experiment 3.
Together, this demonstrates that Sz are deficient in their ability to detect a deviant in a pattern that violates an abstract pitch-based rule.
General Discussion
Sz exhibited a blunted MMN to the simple pitch deviant (Experiment 1; Fig 1), to the late complex ascending-pitch deviant (Experiment 2; Fig 2), and to the late complex scale-pitch deviant (Experiment 3; Fig 3) compared to HC. The peak amplitude of all three MMNs was maximal over frontocentral areas and exhibited CSDs consistent with bilateral sources from temporal lobe, although source modelling was not performed. The complex MMN tasks generated both an early (~125 ms) and a late MMN (~500ms), while the simple pitch MMN task only generated an early MMN (~125 ms), consistent with previous studies (Korpilahti et al., 2000; Zachau et al., 2005; Recasens et al., 2014; Haigh et al., 2016; Korpilahti et al., 2016). The results shown here demonstrate that while the early MMN responses on complex pattern tasks appear to be within normal limits in Sz, the later (and in HC, the most prominent) MMN is deficient in Sz.
This study replicates previous findings that the late complex MMN is significantly reduced in Sz (Haigh et al., 2016). However, they used complex patterns of five identical tones with infrequent deviant groups of six tones. The detection of the deviant sixth tone relied on the ability to form a numerical quantity grouping rule. The current study demonstrates that the late MMN also appears when employing a pitch-based rule to help detect deviant tones, and that Sz still show a deficit in generating the late MMN. Interestingly, the effect size for the difference between HC and Sz in the size of the late MMN was slightly larger to the complex scale-pitch deviant than the ascending-pitch deviant, suggesting that the more salient deviant may be better at distinguishing between the two groups. However, the amplitude of the late MMN in HC was only nominally larger to the scales-pitch deviant. What is evident is that the late scales-pitch MMN elicited a later/more sustained MMN (see Fig 3e). Measuring the behavioral correlates of deviant detection may be able to address how the magnitude of the deviant stimulus impacts the late MMN, and whether more salient pattern deviants can further increase the difference between groups.
Previous studies that have examined auditory ERPs to groups of tones found a sustained ERP, termed the auditory sustained potential (ASP), that lasted the duration of the auditory pattern and increased in amplitude over the first couple of presentations. The ASP was interpreted to reflect auditory segmentation. Sz showed a smaller ASP amplitude, suggesting deficits in the ability to segment groups of auditory stimuli (Coffman et al., 2016). It is also worth noting that the late complex MMN is not related to the ability to suppress frequently presented stimuli (Coffman et al., 2017). It is therefore possible that the blunted late complex MMN in Sz may be due to deficits in being able to establish auditory rules to help segment the acoustic environment.
The timing of the late MMN in the current study (~500 ms after deviant-onset) differs from the late MMN reported to a deviant in group-based patterns: ~350 ms after deviant-onset (Haigh et al., 2016) and ~250 ms after deviant-onset (Recasens et al., 2014). Other pitch-based pattern deviant also report late MMNs with earlier timing than the current study: late MMN to a sine-wave deviant appeared ~400 ms (Korpilahti et al., 2001), and a paired-tones deviant appeared ~340 ms after deviant-onset (Zachau et al., 2005). One possibility for this discrepancy is that the ascending-pitch and the scale-pitch patterns are more complex than the grouping-based or the other pitch-based patterns reported previously. If so, then perhaps the auditory perceptual systems take longer to detect the deviant. Future studies manipulating the complexity of the pattern deviant may be able to test this to see how the timing of late MMN adjusts with complexity.
The source of the MMN appears to differ depending on the complexity of the deviant. Alho (1995) found that the source of a MMNm (MEG version) to a deviant tone was more posterior from the source of the MMNm to a chord or pattern. Similarly, Recasens et al. (2014) showed different sources for their early and late MMN, where the late MMN moved away from auditory cortex. In the current study, it is difficult to ascertain whether the source of the late MMNs differ from the simple MMN, but the CSD maps in HC do look different with regards to the orientation of the source. It is possible that communication in the network that generates the late MMN is deficient in Sz, and that this network exists outside of auditory cortex.
The early complex MMN was not significantly reduced in Sz, similar to previous findings (Haigh et al., 2016), raising the question as to why simple MMN is significantly reduced compared to HC, but not the early complex MMN, despite them being at the same time intervals post stimulus onset. One major difference is that the simple MMN is larger in amplitude than the early complex MMN. The larger amplitude may indicate temporal overlap of multiple MMN components in responses to the simple pitch deviant and separation of these components in the complex MMN response. There is evidence of a biphasic simple MMN in HC to syllable stimuli (Jacobsen et al., 2013), which may support the theory of multiple MMN components. Again, systematic manipulation of deviance complexity could help ascertain if multiple MMNs appear simultaneously and have an additive effect on simple MMN amplitude.
Scales-pitch late MMN significantly correlated with several measures of working memory and with PANSS measures of social withdrawal and avoidance. However, the direction of the correlations suggests that Sz with a larger late MMN were the most symptomatic, which is the opposite of what was expected. These correlations were not significant with the late ascending-pitch MMN. This may be due to the fact that the late MMNs were so small in Sz. If the late MMNs in both experiments are essentially noise, this would not produce meaningful correlations. These findings are preliminary and exploratory, and further investigation on the relationship between late MMN and general functioning will be able to check these findings. In addition, while there was no significant correlation with medication dosage, it is difficult to ascertain the effect of medication on the complex MMN.
There is a growing literature investigating how sensory abnormalities contribute to general functioning (Javitt & Sweet, 2015). For instance, difficulties in detecting irregular changes in pitch could lead to difficulties detecting non-verbal cues of emotion, leading to abnormal social interactions, which are characteristic of schizophrenia. Correlations between difficulty discriminating between tones and deficits in identifying non-verbal vocal cues of emotion have been reported (Jahshan et al., 2015), and reduced MMN to deviant phonemes correlated with poor verbal memory in schizophrenia (Kawakubo et al., 2006). These results are purely correlational and so the causality or the directionality cannot be established; however, they emphasize that understanding the roles that sensory abnormalities play in more complex processing may help to understand Sz pathology.
Conclusion
Deviants from complex patterns evoke a late MMN that is significantly blunted in individuals with long-term schizophrenia. This finding was consistent across two pitch-based sequence paradigms. The late MMN may reflect deviance detection later in the auditory hierarchy, which is abnormal in Sz.
Acknowledgments
This work was supported by the National Institute of Mental Health at the National Institutes of Health (R01 MH094328) to DFS. The authors would like to thank Christian Andreaggi for his work in analyzing the data, and the clinical staff at Western Psychiatric Institute and Clinic for recruitment and assessment of participants.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Authors’ Contribution: Sarah M Haigh analyzed and interpreted the data, and wrote the paper. Mario De Matteis analyzed and interpreted the data, and read final version of the paper. Brian A Coffman helped interpret the data, and read the final version of the paper. Timothy K Murphy designed the study, collected and helped analyze the data, and read the final version of the paper. Christiana D Butera collected and helped analyze the data, and read the final version of the paper. Kayla L Ward collected and helped analyze the data, and read the final version of the paper. Justin R Leiter-Mcbeth conducted some of the analyses, and read the final version of the paper. Dean F Salisbury designed the study, helped interpret the results, and helped write the paper.
Data Accessibility: Data will be available through the National Institute of Mental Health after publication of the results.
References
- Alain C, Hargrave R, Woods DL. Processing of auditory stimuli during visual attention in patients with schizophrenia. Biological Psychiatry. 1998;44(11):1151–1159. doi: 10.1016/s0006-3223(97)00478-2. doi: http://dx.doi.org/10.1016/S0006-3223(97)00478-2. [DOI] [PubMed] [Google Scholar]
- Alain C, Woods DL, Ogawa KH. Brain indices of automatic pattern processing. NeuroReport. 1994;6(1):140–144. doi: 10.1097/00001756-199412300-00036. [DOI] [PubMed] [Google Scholar]
- Alho K. Cerebral Generators of Mismatch Negativity (MMN) and Its Magnetic Counterpart (MMNm) Elicited by Sound Changes. Ear and Hearing. 1995;16(1):38–51. doi: 10.1097/00003446-199502000-00004. [DOI] [PubMed] [Google Scholar]
- Althen H, Grimm S, Escera C. Simple and complex acoustic regularities are encoded at different levels of the auditory hierarchy. European Journal of Neuroscience. 2013;38(10):3448–3455. doi: 10.1111/ejn.12346. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic Dose Equivalents and Dose-Years: A Standardized Method for Comparing Exposure to Different Drugs. Biological Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. doi: http://dx.doi.org/10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman BA, Haigh SM, Murphy TK, Salisbury DF. Event-related potentials demonstrate deficits in acoustic segmentation in schizophrenia. Schizophrenia Research. 2016;173(1–2):109–115. doi: 10.1016/j.schres.2016.03.012. doi: http://dx.doi.org/10.1016/j.schres.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman BA, Haigh SM, Murphy TK, Salisbury DF. Impairment in Mismatch Negativity but not Repetition Suppression in Schizophrenia. Brain Topogr. 2017;30:521–530. doi: 10.1007/s10548-017-0571-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi LE. Speech disorder in schizophrenia: review of the literature and exploration of its relation to the uniquely human capacity for language. Schizophrenia bulletin. 2001;27(3):481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- Escera C, Malmierca MS. The auditory novelty system: an attempt to integrate human and animal research. Psychophysiology. 2014;51(2):111–123. doi: 10.1111/psyp.12156. [DOI] [PubMed] [Google Scholar]
- Frost JD, Winkler I, Provost A, Todd J. Surprising sequential effects on MMN. Biological Psychology. 2016;116:47–56. doi: 10.1016/j.biopsycho.2015.10.005. doi: http://dx.doi.org/10.1016/j.biopsycho.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International Consensus Study of Antipsychotic Dosing. American Journal of Psychiatry. 2010;167(6):686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Grimm S, Escera C. Auditory deviance detection revisited: Evidence for a hierarchical novelty system. International Journal of Psychophysiology. 2012;85(1):88–92. doi: 10.1016/j.ijpsycho.2011.05.012. doi: http://dx.doi.org/10.1016/j.ijpsycho.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Gumenyuk V, Korzyukov O, Alho K, Winkler I, Paavilainen P, Näätänen R. Electric brain responses indicate preattentive processing of abstract acoustic regularities in children. Neuroreport. 2003;14(11):1411–1415. doi: 10.1097/00001756-200308060-00001. [DOI] [PubMed] [Google Scholar]
- Haigh SM, Coffman BA, Murphy TK, Butera CD, Salisbury DF. Abnormal auditory pattern perception in schizophrenia. Schizophrenia Research. 2016;176(2–3):473–479. doi: 10.1016/j.schres.2016.07.007. doi: http://dx.doi.org/10.1016/j.schres.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horacek M, Kärgel C, Scherbaum N, Müller BW. The effect of deviance predictability on mismatch negativity in schizophrenia patients. Neuroscience Letters. 2016;617:76–81. doi: 10.1016/j.neulet.2016.02.010. doi: http://dx.doi.org/10.1016/j.neulet.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Jacobsen TK, Steinberg J, Truckenbrodt H, Jacobsen T. Mismatch Negativity (MMN) to successive deviants within one hierarchically structured auditory object. International Journal of Psychophysiology. 2013;87(1):1–7. doi: 10.1016/j.ijpsycho.2012.09.012. doi: http://dx.doi.org/10.1016/j.ijpsycho.2012.09.012. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, Green MF. Relationship between auditory processing and affective prosody in schizophrenia. Schizophrenia Research. 2015;143(2):348–353. doi: 10.1016/j.schres.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Grochowski S, Shelley AM, Ritter W. Impaired mismatch negativity (MMN) generation in schizophrenia as a function of stimulus deviance, probability, and interstimulus/interdeviant interval. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1998;108(2):143–153. doi: 10.1016/s0168-5597(97)00073-7. doi: http://dx.doi.org/10.1016/S0168-5597(97)00073-7. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley AM, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clinical Neurophysiology. 2000;111(10):1733–1737. doi: 10.1016/s1388-2457(00)00377-1. doi: http://dx.doi.org/10.1016/S1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Sweet RA. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci. 2015;16(9):535–550. doi: 10.1038/nrn4002. http://dx.doi.org/10.1038/nrn4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, Kudo N, Rogers MA, Nakagome K, Itoh K, Kato N. Phonetic mismatch negativity predicts verbal memory deficits in schizophrenia. NeuroReport. 2006;17(10):1043–1046. doi: 10.1097/01.wnr.0000221828.10846.ba. [DOI] [PubMed] [Google Scholar]
- Korpilahti P, Krause CM, Holopainen I, Lang AH. Early and Late Mismatch Negativity Elicited by Words and Speech-Like Stimuli in Children. Brain and Language. 2001;76(3):332–339. doi: 10.1006/brln.2000.2426. doi: http://dx.doi.org/10.1006/brln.2000.2426. [DOI] [PubMed] [Google Scholar]
- Korpilahti P, Lang H, Aaltonen O. Is there a late-latency mismatch negativity (MMN) component? Electroencephalography and Clinical Neurophysiology. 2016;95(4):P96. doi: 10.1016/0013-4694(95)90016-G. [DOI] [PubMed] [Google Scholar]
- Korzyukov OA, Winkler I, Gumenyuk VI, Alho K. Processing abstract auditory features in the human auditory cortex. NeuroImage. 2003;20(4):2245–2258. doi: 10.1016/j.neuroimage.2003.08.014. doi: http://dx.doi.org/10.1016/j.neuroimage.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Korzyukov O, Alho K, Kujala A, Gumenyuk V, Ilmoniemi RJ, Virtanen J, et al. Electromagnetic responses of the human auditory cortex generated by sensory-memory based processing of tone-frequency changes. Neuroscience Letters. 1999;276(3):169–172. doi: 10.1016/s0304-3940(99)00807-1. doi: http://dx.doi.org/10.1016/S0304-3940(99)00807-1. [DOI] [PubMed] [Google Scholar]
- Lecaignard F, Bertrand O, Gimenez G, Mattout J, Caclin A. Implicit learning of predictable sound sequences modulates human brain responses at different levels of the auditory hierarchy. Frontiers in Human Neuroscience. 2015;9:505. doi: 10.3389/fnhum.2015.00505. doi: http://doi.org/10.3389/fnhum.2015.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL. Preattentive Sensory Processing as Indexed by the MMN and P3a Brain Responses is Associated with Cognitive and Psychosocial Functioning in Healthy Adults. Journal of Cognitive Neuroscience. 2007;19(10):1624–1632. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJC, Tiitinen H. The role of adaptation-based memory in auditory cortex. International Congress Series. 2007;1300(0):53–56. doi: http://dx.doi.org/10.1016/j.ics.2007.01.051. [Google Scholar]
- May PJC, Tiitinen H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology. 2010;47(1):66–122. doi: 10.1111/j.1469-8986.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- McLachlan NM, Phillips DS, Rossell SL, Wilson SJ. Auditory processing and hallucinations in schizophrenia. Schizophrenia Research. 2013;150(2–3):380–385. doi: 10.1016/j.schres.2013.08.039. doi: http://dx.doi.org/10.1016/j.schres.2013.08.039. [DOI] [PubMed] [Google Scholar]
- Michie PT, Budd TW, Todd J, Rock D, Wichmann H, Box J, Jablensky AV. Duration and frequency mismatch negativity in schizophrenia. Clinical Neurophysiology. 2000;111(6):1054–1065. doi: 10.1016/s1388-2457(00)00275-3. doi: http://dx.doi.org/10.1016/S1388-2457(00)00275-3. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Arajärvi P, Takegata R. Preattentive detection of nonsalient contingencies between auditory features. NeuroReport. 2007;18(2):159–163. doi: 10.1097/WNR.0b013e328010e2ac. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Jaramillo M, Näätänen R. Binaural information can converge in abstract memory traces. Psychophysiology. 1998;35(5):483–487. doi: 10.1017/S0048577298970895. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Jaramillo M, Näätänen R, Winkler I. Neuronal populations in the human brain extracting invariant relationships from acoustic variance. Neuroscience Letters. 1999;265(3):179–182. doi: 10.1016/s0304-3940(99)00237-2. doi: http://dx.doi.org/10.1016/S0304-3940(99)00237-2. [DOI] [PubMed] [Google Scholar]
- Rabinowicz E, Silipo G, Goldman R, Javitt D. Auditory sensory dysfunction in schizophrenia: Imprecision or distractibility? Archives of General Psychiatry. 2000;57(12):1149–1155. doi: 10.1001/archpsyc.57.12.1149. http://dx.doi.org/10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- Ramage EM, Weintraub DM, Allen DN, Snyder JS. Evidence for stimulus-general impairments on auditory stream segregation tasks in schizophrenia. Journal of Psychiatric Research. 2012;46(12):1540–1545. doi: 10.1016/j.jpsychires.2012.08.028. doi: http://dx.doi.org/10.1016/j.jpsychires.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recasens M, Grimm S, Wollbrink A, Pantev C, Escera C. Encoding of nested levels of acoustic regularity in hierarchically organized areas of the human auditory cortex. Human Brain Mapping. 2014;35(11):5701–5716. doi: 10.1002/hbm.22582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph ED, Ells EML, Campbell DJ, Abriel SC, Tibbo PG, Salisbury DF, Fisher DJ. Finding the missing-stimulus mismatch negativity (MMN) in early psychosis: Altered MMN to violations of an auditory gestalt. Schizophrenia Research. 2015;166(1–3):158–163. doi: 10.1016/j.schres.2015.05.028. doi: http://dx.doi.org/10.1016/j.schres.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF. Finding the missing stimulus mismatch negativity (MMN): Emitted MMN to violations of an auditory gestalt. Psychophysiology. 2012;49(4):544–548. doi: 10.1111/j.1469-8986.2011.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 Auditory Event-Related Potentials in First-Hospitalized and Chronic Schizophrenia. Schizophrenia Bulletin. 2009;36(5):991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, McCathern AG. Abnormal Complex Auditory Pattern Analysis in Schizophrenia Reflected in an Absent Missing Stimulus Mismatch Negativity. Brain Topography. 2016;29(6):867–874. doi: 10.1007/s10548-016-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury D, Shenton M, Griggs C, Bonner-Jackson A, McCarley R. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Archives of General Psychiatry. 2002;59(8):686–694. doi: 10.1001/archpsyc.59.8.686. http://dx.doi.org/10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, McCathern AG, Coffman BA, Murphy TK, Haigh SM. Mismatch negativity responses to paired-tone deviants in schizophrenia. Schizophr Res. 2017 doi: 10.1016/j.schres.2017.04.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley AM, Silipo G, Javitt DC. Diminished responsiveness of ERPs in schizophrenic subjects to changes in auditory stimulation parameters: implications for theories of cortical dysfunction. Schizophrenia Research. 1999;37(1):65–79. doi: 10.1016/s0920-9964(98)00138-8. doi: http://dx.doi.org/10.1016/S0920-9964(98)00138-8. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Ward PB, Catts SV, Michie PT, Andrews S, McConaghy N. Mismatch negativity: an index of a preattentive processing deficit in schizophrenia. Biological psychiatry. 1991;30(10):1059–1062. doi: 10.1016/0006-3223(91)90126-7. [DOI] [PubMed] [Google Scholar]
- Smith DM, Grant B, Fisher DJ, Borracci G, Labelle A, Knott VJ. Auditory verbal hallucinations in schizophrenia correlate with P50 gating. Clinical Neurophysiology. 2013;124(7):1329–1335. doi: 10.1016/j.clinph.2013.02.004. doi: http://dx.doi.org/10.1016/j.clinph.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Strelnikov K. Schizophrenia and language — Shall we look for a deficit of deviance detection? Psychiatry Research. 2010;178(2):225–229. doi: 10.1016/j.psychres.2010.04.025. doi: http://dx.doi.org/10.1016/j.psychres.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Tervaniemi M, Radil T, Radilova J, Kujala T, Näätänen R. Pre-Attentive Discriminability of Sound Order as a Function of Tone Duration and Interstimulus Interval: A Mismatch Negativity Study. Audiology and Neurotology. 1999;4(6):303–310. doi: 10.1159/000013854. [DOI] [PubMed] [Google Scholar]
- Todd J, Michie PT, Schall U, Ward PB, Catts SV. Mismatch negativity (MMN) reduction in schizophrenia—Impaired prediction-error generation, estimation or salience? International Journal of Psychophysiology. 2012;83(2):222–231. doi: 10.1016/j.ijpsycho.2011.10.003. doi: http://dx.doi.org/10.1016/j.ijpsycho.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Todd J, Whitson L, Smith E, Michie PT, Schall U, Ward PB. What’s intact and what’s not within the mismatch negativity system in schizophrenia. Psychophysiology. 2014;51(4):337–347. doi: 10.1111/psyp.12181. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia Research. 2005;76(1):1–23. doi: 10.1016/j.schres.2004.12.002. doi: http://dx.doi.org/10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- van Zuijen TL, Simoens VL, Paavilainen P, Näätänen R, Tervaniemi M. Implicit, Intuitive, and Explicit Knowledge of Abstract Regularities in a Sound Sequence: An Event-related Brain Potential Study. Journal of Cognitive Neuroscience. 2006;18(8):1292–1303. doi: 10.1162/jocn.2006.18.8.1292. [DOI] [PubMed] [Google Scholar]
- Weintraub DM, Ramage EM, Sutton G, Ringdahl E, Boren A, Pasinski AC, et al. Auditory stream segregation impairments in schizophrenia. Psychophysiology. 2012;49(10):1372–1383. doi: 10.1111/j.1469-8986.2012.01457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachau S, Rinker T, Körner B, Kohls G, Maas V, Hennighausen K, Schecker M. Extracting rules: early and late mismatch negativity to tone patterns. NeuroReport. 2005;16(18) doi: 10.1097/00001756-200512190-00009. [DOI] [PubMed] [Google Scholar]