Abstract
Disulfiram was the first pharmacotherapy approved to treat alcohol use disorder (AUD) in the 1950s. Disulfiram alters ethanol pharmacokinetics (PK) and causes uncomfortable reactions (e.g.: headache, tachycardia, nausea, flushing and hypotension) when alcohol is consumed. Subsequently, a better understanding of the neurobiological pathways involved in AUD led to the development of other medications (e.g.: naltrexone and acamprosate) to treat AUD. These neurobiological-based medications act on AUD-related phenotypes including craving, stress, and/or withdrawal. The original approach to treat AUD, by altering ethanol PK has been much less investigated. Recent research on ethanol PK has shed light on the mechanisms of action underlying AUD and how some medications that alter ethanol PK may be helpful in treating AUD. This review summarizes and discusses the complex PK of ethanol, and proposes that altering ethanol PK via novel pharmacological approaches may be a viable approach to treat AUD.
Keywords: pharmacokinetics (PK), alcohol use disorder (AUD), ethanol, biobehavioral mechanisms, human laboratory studies
Introduction
Alcohol use disorder (AUD) is a complex medical disease with dramatic consequences to our society in terms of mortality and morbidity (Rehm et al., 2010). Research efforts have focused on different strategies to treat AUD (Edwards et al., 2011); one such strategy has been to develop medications to treat AUD. Pharmacological interventions to treat AUD may act by reversing the acute effects of ethanol (amethysic treatment), mitigating craving (urge to drink), and reducing stress and/or withdrawal (negative symptoms). Disulfiram was the first drug approved in the US for the treatment of AUD, and today it is the only medication used clinically whose mechanism of action is based on altering ethanol pharmacokinetics (PK).
This review describes the complexity of drug-ethanol interactions and discusses medications used to treat AUD that share PK pathways with ethanol. Critically, this review highlights possible pharmacological interventions for AUD that target ethanol-drug interactions without causing the overwhelming disulfiram-like adverse reactions to ethanol. This approach opens the possibility that exploring medications that alter ethanol PK may modulate biobehavioral effects of ethanol and improve drinking outcomes.
Overview of drug-ethanol interactions
Drug-drug interactions contribute significantly to adverse drug reactions (Pirmohamed et al., 2004). They occur when the expected effects of a medication are enhanced or diminished by the administration of another medication. Drug-drug interactions can result in therapeutic failure (e.g.: lower seizure threshold for phenytoin) and/or adverse events due to potentiation of their pharmacological effects (e.g.: bleeding for warfarin).
Ethanol can also influence the effect of a drug by increasing the risk and/or exacerbating side-effects caused by the drug when used alone (e.g.: hepatotoxicity for acetaminophen; gastrointestinal ulcers and/or bleeding for nonsteroidal anti-inflammatory drugs).
In most cases, PK interactions between ethanol and other drugs are unintended and should be avoided (Breslow et al., 2015). On the other hand, there may be beneficial drug-drug interactions that could be used in clinical practice. For example, the co-formulated lopinavir/ritonavir led to the development of a gold standard combination antiretroviral therapy (Sham et al., 1998), since ritonavir inhibits CYP metabolism to improve the bioavailability of protease inhibitors. Although this strategy has never been evaluated or applied clinically to the treatment of AUD patients, targeting ethanol PK might hold promise for the development of future medications in the AUD field.
Medication development for AUD by using ethanol-drug interaction
Historically, AUD treatments have targeted complete abstinence from ethanol. Although disulfiram was developed as a pharmacological intervention that would facilitate abstinence; it is an approach that uses a therapeutically intended ethanol-drug interaction to deter individuals from consuming ethanol. More recently, it has been suggested that while alcohol abstinence remains the ideal therapeutic outcome to achieve, a reduction in alcohol drinking below harmful levels may be a beneficial goal for AUD individuals. An analysis that evaluated two large clinical trials demonstrated the utility of adopting the percent subjects with no heavy drinking days (PSNHDDs) as a clinically informative end point measure (Falk et al., 2010). The development of medications that target reduction of alcohol consumption, rather than complete abstinence, represent a novel pharmacological approach to facilitate reduction of alcohol drinking below harmful levels. As such, altering ethanol PK parameters, without producing overwhelming adverse reactions like disulfiram may represent a novel target to treat AUD.
Pharmacological approaches that target ethanol metabolism via ALDH inhibition
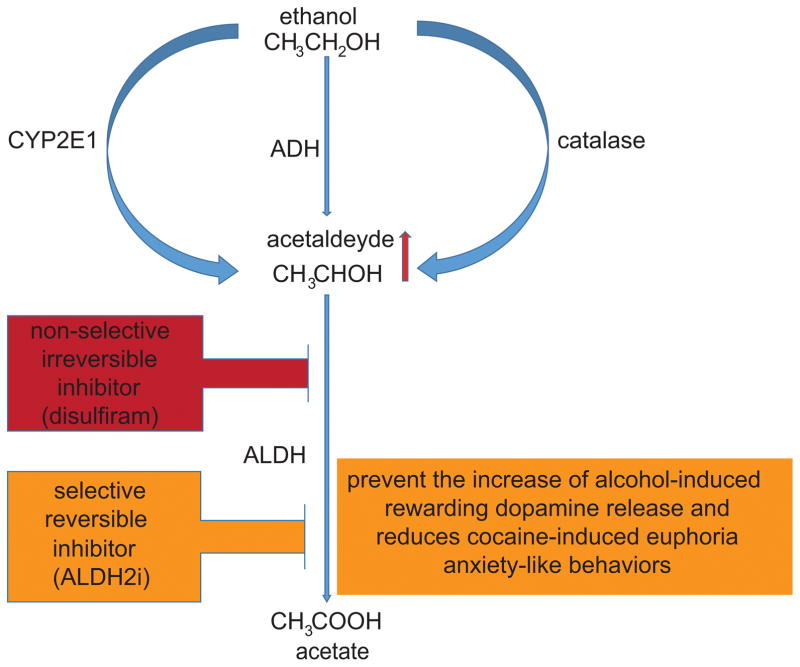
The use of a compound able to produce aversive effects after concurrent consumption of ethanol was first suggested by a rubber plant physician (Williams, 1937). Subsequently, it was proposed that an amethysic compound can be utilized as a pharmacotherapy for alcoholism (Hald and Jacobsen, 1948). The aversive approach was developed based on the idea that severe reaction to ethanol consumption would increase the patient’s motivation to remain abstinent. The effect of disulfiram is to inhibit acetaldehyde dehydrogenase (ALDH), causing acetaldehyde to accumulate after alcohol intake, resulting in an immediate feeling of nausea after alcohol intake than ALDH2. Initial research on active metabolites of disulfiram suggests that its metabolites may inhibit ALDH with fewer adverse effects than the parent disulfiram (Madan and Faiman, 1994). The metabolic products of disulfiram mediate the molecular mechanisms of ALDH by irreversible inhibition (covalent binding to the enzyme). However, glutathione is able to diminish this inhibition in less potent metabolites, while does not affect inhibition by the more potent ones (Mays et al., 1998). In addition, disulfiram is more potent for ALDH1A1 than ALDH2. Together, this observation raise the possibility that reversible inhibition via ALDH2 analogues (mediating specific, non-covalent interaction), may be a better pharmacological approach for AUD (Koppaka et al., 2012).
Furthermore, selective ALDH2 inhibitors (e.g.: ALDH2i) have been shown to be suitable for addiction since they prevent the increase of alcohol-induced rewarding dopamine release in the brain (Arolfo et al., 2009) and induce a decrease in norepinephrine release which results in reduced excitation of dopaminergic neurons and reduced cocaine-induced euphoria in rats (Yao et al., 2010). Finally, highly selective and reversible ALDH2 inhibitors exhibit anxiolytic properties in animal models of anxiety-like behavior (Overstreet et al., 2009), a critical treatment component since feelings of anxiety can be prevalent during ethanol withdrawal and relapse (Shen et al., 2012; Lukas et al., 2013).
Peripheral acetaldehyde accumulation is a deterrent for alcohol consumption while brain (rather than liver) ALDH functionality closely correlates with ethanol preference (Amir, 1978). The brain itself can generate acetaldehyde from ethanol through three pathways: catalase (60%), CYP2E1 (20%) and ADH (20%) (Figure 1), for review see (Hipolito et al., 2007). Alcohol preference may result from acetaldehyde produced in the ventral tegmental area (VTA), via catalase, which has shown to have rewarding properties (Karahanian et al., 2011). Since there is a putative contribution of brain acetaldehyde to ethanol tolerance and addiction (Deng and Deitrich, 2008), reducing acetaldehyde levels in the brain may have therapeutic benefit for AUD.
Figure 1. Pharmacological approaches that target ethanol metabolism via ALDH inhibition.
Non selective irreversible inhibition of ALDH (disulfiram) produces aversive side-effects. Selective ALDH2 reversible inhibition (ALDH2i) has been shown to prevent the increase of alcohol-induced rewarding dopamine release in brain, reduce cocaine-induced euphoria and inhibit anxiety-like behaviors.
Liver generated acetaldehyde also enters in the brain, however, it cannot reach considerable levels mostly due to the presence of ALDH in the microvasculature of the blood brain barrier (Eriksson and Sippel, 1977; Westcott et al., 1980). Acetaldehyde possesses reinforcing properties (Karahanian et al., 2011), suggesting that some ethanol-induced behavioral effects may be a result of the formation of acetaldehyde. Interestingly, acetaldehyde injected directly in rodent brains produces ethanol-induced like behaviors (Quintanilla et al., 2002; Smith et al., 1984; Rodd et al., 2005). Preclinical studies have highlighted the importance of considering modulating ethanol PK by inhibiting its metabolism with consequent acetaldehyde accumulation selectively at central (positive reinforcing) levels (Guerrini et al., 2006; Imbert et al., 2015; Kerr et al., 1989; Keung and Vallee, 1993). In the brain, where catalase is the primary ethanol-metabolizing enzyme, ALDH2 is expressed at very low levels. Therefore, modulation of brain catalase activity can change acetaldehyde levels, and possibly influence ethanol-related addictive behaviors. All these observations support the hypothesis that acetaldehyde in the brain may play a role in AUD (Deng and Deitrich, 2008). Therefore, while irreversible inhibition of ALDH2 by disulfiram represents a treatment based on ethanol aversion, selective inhibition of ALDH2, or catalase, in the brain should be further evaluated as a possible new pharmacological CNS approach to probe a PK-based approach to treat AUD. The exact mechanism for acetaldehyde’s behavioral effects, however, is still under investigation, at this stage, and the hypothesis that modulating brain acetaldehyde can be beneficial for treating AUD requires further research and clinical exploration.
Pharmacological approaches that can reduce ethanol cytotoxic effects
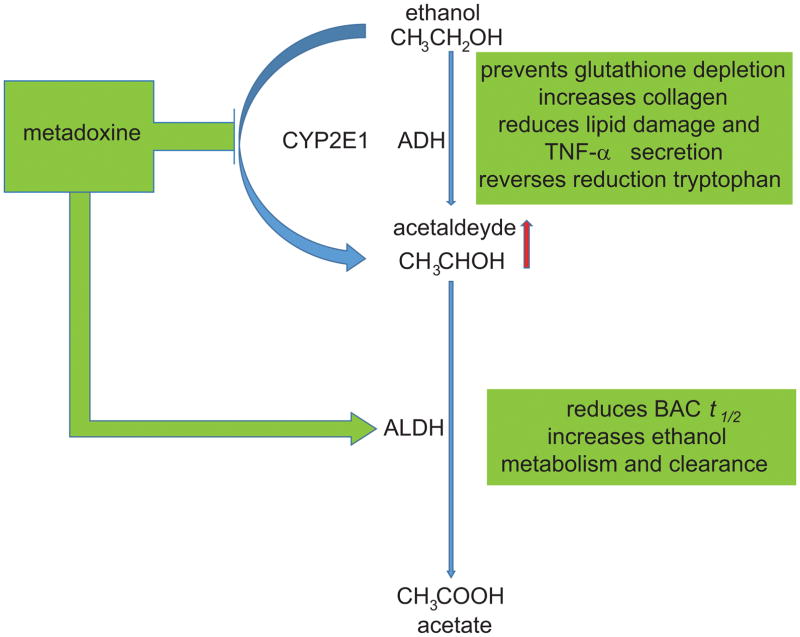
Altering ethanol PK may represent an effective approach for reducing the toxic tissue and organ effects of both acute and chronic exposure to alcohol. Metadoxine, a pyridoxine-pyrrolidone carboxylate, is a medication approved in several European countries (but not in the US) for treating alcohol intoxication. Metadoxine has also been evaluated as a medication effective to reduce the cytotoxic effects of ethanol. In cell culture, metadoxine prevented glutathione depletion, increased collagen, and reduced ethanol-induced lipid peroxidation damage and TNF-α secretion caused by acetaldehyde production (Gutierrez-Ruiz et al., 2001) (Figure 2). Metadoxine appeared to inhibit CYP2E1 (Ki et al., 2007), a specific ethanol-inducible liver isoenzyme (Dey and Cederbaum, 2006) involved in liver injury (steatosis and steatohepatitis) after chronic ethanol ingestion (Ardies et al., 1987). These data support early preclinical studies on ethanol-exposed rats, where pyridoxidine reversed the reduction in the apoenzyme for tryptophan pyrrolase caused by chronic ethanol exposure (Ragusa et al., 1980). Notably, clinical studies support a role of metadoxine in reducing alcohol-related tissue damage, specifically in alcoholic liver disease (Higuera-de la Tijera et al., 2015; Caballeria et al., 1998).
Figure 2. Pharmacological approaches that can reduce ethanol cytotoxic effects.
The pyridoxine-pyrrolidone carboxylate metadoxine can accelerate ethanol metabolism by increasing the activity of ALDH, inhibiting CYP2E1, and accelerating plasma and urinary clearance of ethanol. Metadoxine may also reduce cytotoxic effect of ethanol, reduce intoxication and improve recovery.
Metadoxine appears to accelerate ethanol metabolism by increasing the activity of ALDH and by accelerating plasma and urinary clearance of ethanol (Pares et al., 1991; Calabrese et al., 1995). Furthermore, in a double-blind, randomized controlled trial with patients with acute ethanol intoxication a single intravenous (IV) administration of metadoxine, compared to placebo, significantly increased ethanol elimination and decreased blood alcohol content (BAC) (Shpilenya et al., 2002). This also resulted in decreased symptoms of ethanol intoxication (e.g.: agitation, impairment). Additionally, preliminary clinical data suggest that metadoxine may reduce alcohol drinking in alcohol-dependent individuals (Leggio et al., 2011; Guerrini et al., 2006). However, the data generated until now are very preliminary and the possible mechanisms of action of how metadoxine may work are not fully understood. Therefore, additional work is needed to evaluate metadoxine and other novel pharmacotherapies that may be beneficial in reducing alcohol use by modulating ethanol PK.
Another strategy to reduce the cytotoxic effect of alcohol focuses on the utilization of energy substrate in the brain. Early brain imaging studies have shown that alcohol-induced decreases in brain activity (Volkow et al., 1990; de Wit et al., 1990) may be due to the effect of alcohol in decreasing glucose metabolism (Volkow et al., 2006). More recently, a positron emission tomography (PET) study showed that during heavy alcohol consumption there is a shift from brain glucose metabolism to acetate or other ketones (Volkow et al., 2013; Jiang et al., 2013). As such the use of acetate, as an alternative energy substrate during acute intoxication, was seen as a vulnerable pathophysiological effect during alcohol intoxication and replacement during withdrawal. The sudden deprivation of brain glucose (favored energy substrate) and acetate (alternative energy substrate) may contribute to the negative symptoms during alcohol withdrawal. This withdrawal effect raises the possibility of a therapeutic intervention where increasing plasma acetate concentration (ketogenic diet) could help minimize adverse effects from acute alcohol withdrawal in alcohol abusers undergoing detoxification (Volkow et al., 2015).
Pharmacological approaches that target the biphasic effects of ethanol
A pharmacotherapy for AUD able to alter ethanol PK might represent an interesting mechanism to facilitate reduction in ethanol use by altering the pleasurable acute effects of ethanol. Alcohol consumption influences executive cognitive functioning differentially on the ascending, compared to the descending, limb of the BAC curve (Pihl et al., 2003), As such, the co-administration of a medication for AUD with ethanol should ideally affect the biphasic effects of ethanol by reducing the stimulant and/or increasing the sedative effects in the alcohol ascending limb. For example, the FDA-approved medication naltrexone has shown to reduce the stimulant effects of alcohol (Setiawan et al., 2011; Swift et al., 1994). Naltrexone seems to mediate this effect by disrupting the connection between alcohol-induced stimulation and further alcohol consumption(Anton et al., 2004).
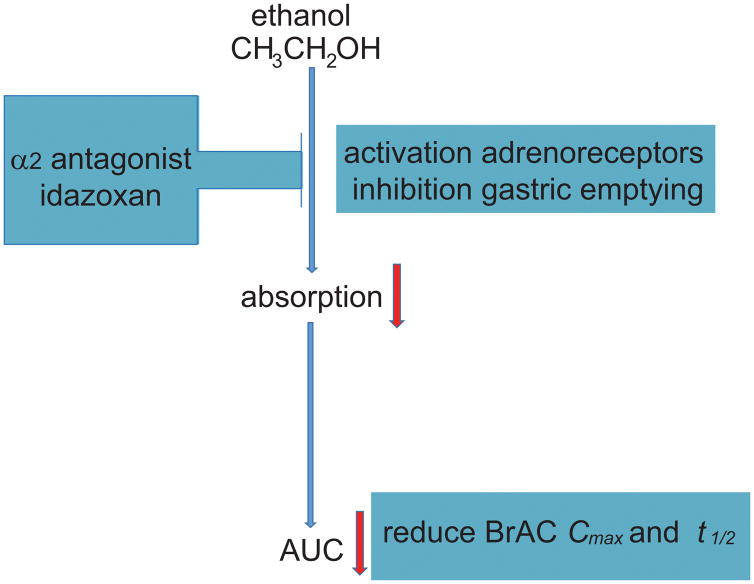
Recently, a double-blind, placebo-controlled, human laboratory pilot study evaluated ethanol PK when co-administered with idazoxan, an α2-adrenergic antagonist (Haass-Koffler et al., 2015). This study showed that a single dose of idazoxan (40 mg) produced a reduction of peak concentration (Cmax), delay of time of maximum concentration (tmax), and altered the biphasic effects of ethanol by decreasing stimulation and increasing sedation in social drinkers. A pharmacokinetics/pharmacodynamics (PK/PD) model that linked ethanol concentration (PK) and ethanol effects (PD) (Wright et al., 2011) was used to evaluate ethanol dose-concentration-response relationships after medication administration (Holford, 1997; Taylor et al., 2008). The PK/PD model adopted in this study (Haass-Koffler et al., 2015) was useful since PK levels (Cmax and tmax) and PD symptoms (stimulation effects) influence each other. While reduction of Cmax may represent the reduction of BAC, delayed tmax typically accompanies delays in gastric emptying (Wright et al., 2011) (Figure 3). The PK effects of idazoxan may be due to the selective activation of the α2A-adrenoceptor subtype, which has been shown to be responsible for the inhibition of gastric emptying in preclinical models (Tsukada et al., 2003), thereby reducing ethanol bioavailability.
Figure 3. Pharmacological approaches that target the biphasic effects of ethanol.
The α2-adrenergic antagonist idazoxan can reduce subjective effects secondary to ethanol intoxication.
Pharmacological approaches with additional effects on alcohol PK
A variety of antioxidant isoflavones (puerarin, daidzin, and daidzein), extracted from the kudzu plant (Pueraria lobata), have been used as antidipsotropic treatments in Chinese medicine, for review see (McGregor, 2007). Daidzin is a highly potent inhibitor of the ALDH2 isozyme (IC50, 80-nM) (Lowe et al., 2008). It also seems to reduce BAC levels by a delay of stomach emptying in rats (Lin and Li, 1998). Other daidzin analogs have been developed with more selective and reversal inhibitors specifically for ALDH2 (Arolfo et al., 2009). These compounds have also been shown to reduce ethanol consumption and increase abstinence in non-treatment-seeking heavy drinkers (Lukas et al., 2013; Penetar et al., 2012).
Another flavonoid, dihydromyricitin, a compound purified from the plant Hovenia, has also been used in traditional Chinese medicine for treating alcohol abuse. Dihydromyricitin also appears to antagonize alcohol effects, although less is known about its mechanism of action (Shen et al, 2012). Other flavonoids compounds, including genistein and resveratrol may reduce alcoholic fatty liver (Tang et al., 2016) and have been studied for the treatment of AUD for years.
Challenges in medication development that alter ethanol PK
Pharmacological interventions that alter ethanol PK, without producing the disulfiram adverse reaction to alcohol, might represent a novel approach to evaluate AUD pharmacotherapies. However, there are several clinical challenges with this approach.
First, PK pathways are very difficult to assess accurately in a heterogeneous AUD population. One of the main sources of inter-individual variability in ethanol metabolism is the genetic polymorphisms in the ethanol metabolizing enzymes (Li et al., 2001). There are five functional classes of cytosolic ADH encoded by seven human genes. Polymorphism occurs at the ADH2 and ADH3 loci, which account for the significant differences in alcohol metabolism observed in different ethnic and racial groups: ADH2*1 (White and Black), ADH2*2 (Asian), and ADH2*3 (~15% African American). Compared to the more common ADH2*1 allele, the ADH2*3 isozyme has a higher metabolic rate, which may result in a protective effect. Polymorphisms also occur at mitochondrial ALDH. Approximately one-third of Asians possess a slow variant of ALDH (ALDH*2) that results in accumulation of acetaldehyde, producing the uncomfortable flushing due to vasodilation (Takeshita and Morimoto, 2000; Higuchi et al., 1995).
Medications that affect alcohol metabolism have so far shown limited efficacy in clinical practice. Clinical trials of disulfiram have yielded negative results (Jorgensen et al., 2011; Fuller and Gordis, 2004; Jonas et al., 2014). Variability in medication compliance and responses to acetaldehyde accumulation between individuals (Substance Abuse and Mental Health Services Administration, 2009) introduces inconsistent patient response to treatment using this approach. The variable acetaldehyde response produced by disulfiram appears to be due to the fact that disulfiram is a pro-drug that has to be enzymatically converted to active metabolites, notably N-acetyl-S-(N,N-diethylcarbamoyl) cysteine and the amount of this metabolite produced varies across individuals (Winefield et al., 2015).
Altering the function of alcohol metabolizing enzymes can have other effects, as ethanol is not the exclusive substrate of these enzymes. ADH enzymes interact with many different molecules depending on the enzymes’ location within the cell. For example, ADH and ALDH are involved in retinoic acid synthesis and metabolism. Interestingly, ADH enzymes are primarily found in liver and kidney cells, but are also expressed in other cells throughout the body. Within cells, ADH enzymes are located in the endoplasmic reticulum (protein processing and transport), mitochondria (energy), cytosol (cellular internal fluid), and the nucleus. As such, any manipulation affecting acetaldehyde accumulation should be considered carefully, since acetaldehyde accumulation is connected with serious pathologies including cancers and neurodegenerative diseases; for an extensive review, see (Chen et al., 2014).
The downside of developing medications that can reduce BAC is a potential misuse of these medications in binge drinking individuals. For example, on college campuses, students or drivers could use such a drug before a binge drinking episode, and favor high level of ethanol consumption without reaching the BAC levels considered legally drunk. Therefore, while pharmacotherapies for AUD that lower BAC levels may be beneficial by reducing tissue chemical toxicity and by lowering the intoxication effect, this effect may encourage drinkers to consume more ethanol to achieve the expected pleasurable effects. A combined biobehavioral model that assesses alcohol concentration (PK) and alcohol effects (pharmacodynamics, PD) (Wright et al., 2011) should be developed to provide a conceptual framework in human studies. A PK/PD paradigm may be helpful to evaluate ethanol dose-concentration-response relationships (Holford, 1997; Taylor et al., 2008; Haass-Koffler et al., 2015). Finally, in addition to the heterogeneity of this disease (early versus late onset of alcoholism, comorbidities, severe withdrawal, etc.), the PK parameters (absorption, distribution, metabolism, and excretion), and variations between individuals may present a significant challenge for developing medications that target ethanol PK.
Conclusions
Disulfiram, the first medication approved by the FDA for AUD acts via altering ethanol metabolism. The use of disulfiram for AUD, however, has generated conflicting results in clinical practice and a recent meta-analysis does not support its efficacy for AUD patients (Jonas et al., 2014). However, medications that alter ethanol PK, without the adverse symptoms when co-administered with alcohol, may represent a novel approach to elucidate the complex mechanisms of AUD and develop novel therapeutic approaches. For example, selective ALDH2 reversible inhibitors have been evaluated as potential novel approaches for the treatment of AUD. Also, the use of medications that reduce BAC would limit the delivery of chronic ethanol chemical toxic effects at the tissue level and would reduce the undesirable behavioral effects produced by acute intoxication. Finally, incorporating PK parameters into neurobiological analyses may shed light on the positive/stimulant effects of ethanol.
While manipulating ethanol PK represents an intriguing and potentially exciting approach to investigate novel medications for AUD, there are several challenges with this approach that must be taken into account. Including (but not limited to) the genetic variability in the enzymes that affect ethanol metabolism, the variability in the time between ethanol ingestion and medication response between individuals, and the potential risk of excessive alcohol use to overcome the effects of the putatively effective medication.
Acknowledgments
CLH-K is currently supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) 1K01AA023867 and previously by the training grant 5T32AA007459-28. LL is supported by the National Institutes of Health (NIH) intramural funding ZIA-AA000218 (Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology; PI: Leggio), jointly supported by the NIAAA Division of Intramural Clinical and Biological Research and by the National Institute on Drug Abuse Intramural Research Program (NIDA IRP). FA is supported by grant number 1UH3TR000963 (PIs: Akhlaghi and Leggio) from the National Center for Advancing Translational Sciences (NCATS).
The authors also thank Karen Smith, NIH Library for bibliographic assistance. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Conflict of interest
Dr. Swift has received travel and honorarium from D&A Pharma, Lundbeck and consultant fees from CT Laboratories. The other authors report no biomedical financial interests or potential conflicts of interest. The other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Amir S. Brain and liver aldehyde dehydrogenase activity and voluntary ethanol consumption by rats: relations to strain, sex, and age. Psychopharmacology (Berl) 1978;57:97–102. doi: 10.1007/BF00426964. [DOI] [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, et al. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Ardies CM, Lasker JM, Lieber CS. Characterization of the cytochrome P-450 monooxygenase system of hamster liver microsomes. Effects of prior treatment with ethanol and other xenobiotics. Biochem Pharmacol. 1987;36:3613–3619. doi: 10.1016/0006-2952(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Overstreet DH, Yao L, et al. Suppression of heavy drinking and alcohol seeking by a selective ALDH-2 inhibitor. Alcohol Clin Exp Res. 2009;33:1935–1944. doi: 10.1111/j.1530-0277.2009.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow RA, Dong C, White A. Prevalence of alcohol-interactive prescription medication use among current drinkers: United States, 1999 to 2010. Alcohol Clin Exp Res. 2015;39:371–379. doi: 10.1111/acer.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballeria J, Pares A, Bru C, et al. Metadoxine accelerates fatty liver recovery in alcoholic patients: results of a randomized double-blind, placebo-control trial. Spanish Group for the Study of Alcoholic Fatty Liver. J Hepatol. 1998;28:54–60. doi: 10.1016/s0168-8278(98)80202-x. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Calderone A, Ragusa N, et al. Effects of metadoxine on cellular formation of fatty acid ethyl esters in ethanol treated rats. International journal of tissue reactions. 1995;17:101–108. [PubMed] [Google Scholar]
- Chen CH, Ferreira JC, Gross ER, et al. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Metz J, Wagner N, et al. Behavioral and subjective effects of ethanol: relationship to cerebral metabolism using PET. Alcohol Clin Exp Res. 1990;14:482–489. doi: 10.1111/j.1530-0277.1990.tb00508.x. [DOI] [PubMed] [Google Scholar]
- Deng XS, Deitrich RA. Putative role of brain acetaldehyde in ethanol addiction. Curr Drug Abuse Rev. 2008;1:3–8. doi: 10.2174/1874473710801010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- Edwards S, Kenna GA, Swift RM, et al. Current and promising pharmacotherapies, and novel research target areas in the treatment of alcohol dependence: a review. Curr Pharm Des. 2011;17:1323–1332. doi: 10.2174/138161211796150765. [DOI] [PubMed] [Google Scholar]
- Eriksson CJ, Sippel HW. The distribution and metabolism of acetaldehyde in rats during ethanol oxidation-I. The distribution of acetaldehyde in liver, brain, blood and breath. Biochem Pharmacol. 1977;26:241–247. doi: 10.1016/0006-2952(77)90310-0. [DOI] [PubMed] [Google Scholar]
- Falk D, Wang XQ, Liu L, et al. Percentage of subjects with no heavy drinking days: evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:2022–2034. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]
- Fuller RK, Gordis E. Does disulfiram have a role in alcoholism treatment today? Addiction. 2004;99:21–24. doi: 10.1111/j.1360-0443.2004.00597.x. [DOI] [PubMed] [Google Scholar]
- Guerrini I, Gentili C, Nelli G, et al. A follow up study on the efficacy of metadoxine in the treatment of alcohol dependence. Subst Abuse Treat Prev Policy. 2006;1:35. doi: 10.1186/1747-597X-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Ruiz MC, Bucio L, Correa A, et al. Metadoxine prevents damage produced by ethanol and acetaldehyde in hepatocyte and hepatic stellate cells in culture. Pharmacological research : the official journal of the Italian Pharmacological Society. 2001;44:431–436. doi: 10.1006/phrs.2001.0883. [DOI] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L, Davidson D, et al. Effects of idazoxan on alcohol pharmacokinetics and intoxication: a preliminary human laboratory study. Alcohol Clin Exp Res. 2015;39:594–602. doi: 10.1111/acer.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald J, Jacobsen E. The Formation of Acetaldehyde in the Organism after Ingestion of Antabuse (Tetraethylthiuramdisulphide) and Alcohol. Acta Pharmacol Toxicol. 1948;4:305–310. doi: 10.1111/j.1600-0773.1949.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Matsushita S, Murayama M, et al. Alcohol and aldehyde dehydrogenase polymorphisms and the risk for alcoholism. Am J Psychiatry. 1995;152:1219–1221. doi: 10.1176/ajp.152.8.1219. [DOI] [PubMed] [Google Scholar]
- Higuera-de la Tijera F, Servin-Caamano AI, Serralde-Zuniga AE, et al. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol. 2015;21:4975–4985. doi: 10.3748/wjg.v21.i16.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipolito L, Sanchez MJ, Polache A, et al. Brain metabolism of ethanol and alcoholism: an update. Curr Drug Metab. 2007;8:716–727. doi: 10.2174/138920007782109797. [DOI] [PubMed] [Google Scholar]
- Holford NH. Complex PK/PD models--an alcoholic experience. International journal of clinical pharmacology and therapeutics. 1997;35:465–468. [PubMed] [Google Scholar]
- Imbert B, Alvarez JC, Simon N. Anticraving Effect of Baclofen in Alcohol-Dependent Patients. Alcohol Clin Exp Res. 2015;39:1602–1608. doi: 10.1111/acer.12823. [DOI] [PubMed] [Google Scholar]
- Jiang L, Gulanski BI, De Feyter HM, et al. Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest. 2013;123:1605–1614. doi: 10.1172/JCI65153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Jorgensen CH, Pedersen B, Tonnesen H. The efficacy of disulfiram for the treatment of alcohol use disorder. Alcohol Clin Exp Res. 2011;35:1749–1758. doi: 10.1111/j.1530-0277.2011.01523.x. [DOI] [PubMed] [Google Scholar]
- Karahanian E, Quintanilla ME, Tampier L, et al. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcohol Clin Exp Res. 2011;35:606–612. doi: 10.1111/j.1530-0277.2011.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JT, Maxwell DS, Crabb DW. Immunocytochemistry of alcohol dehydrogenase in the rat central nervous system. Alcohol Clin Exp Res. 1989;13:730–736. doi: 10.1111/j.1530-0277.1989.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Keung WM, Vallee BL. Daidzin: a potent, selective inhibitor of human mitochondrial aldehyde dehydrogenase. Proc Natl Acad Sci U S A. 1993;90:1247–1251. doi: 10.1073/pnas.90.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SH, Choi JH, Kim CW, et al. Combined metadoxine and garlic oil treatment efficaciously abrogates alcoholic steatosis and CYP2E1 induction in rat liver with restoration of AMPK activity. Chemico-biological interactions. 2007;169:80–90. doi: 10.1016/j.cbi.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Koppaka V, Thompson DC, Chen Y, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev. 2012;64:520–539. doi: 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Kenna GA, Ferrulli A, et al. Preliminary findings on the use of metadoxine for the treatment of alcohol dependence and alcoholic liver disease. Hum Psychopharmacol. 2011;26:554–559. doi: 10.1002/hup.1244. [DOI] [PubMed] [Google Scholar]
- Li TK, Yin SJ, Crabb DW, et al. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res. 2001;25:136–144. [PubMed] [Google Scholar]
- Lin RC, Li TK. Effects of isoflavones on alcohol pharmacokinetics and alcohol-drinking behavior in rats. The American journal of clinical nutrition. 1998;68:1512S–1515S. doi: 10.1093/ajcn/68.6.1512S. [DOI] [PubMed] [Google Scholar]
- Lowe ED, Gao GY, Johnson LN, et al. Structure of daidzin, a naturally occurring anti-alcohol-addiction agent, in complex with human mitochondrial aldehyde dehydrogenase. J Med Chem. 2008;51:4482–4487. doi: 10.1021/jm800488j. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Penetar D, Su Z, et al. A standardized kudzu extract (NPI-031) reduces alcohol consumption in nontreatment-seeking male heavy drinkers. Psychopharmacology. 2013;226:65–73. doi: 10.1007/s00213-012-2884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan A, Faiman MD. Diethyldithiocarbamate methyl ester sulfoxide, an inhibitor of rat liver mitochondrial low Km aldehyde dehydrogenase and putative metabolite of disulfiram. Alcohol Clin Exp Res. 1994;18:1013–1017. doi: 10.1111/j.1530-0277.1994.tb00075.x. [DOI] [PubMed] [Google Scholar]
- Mays DC, Ortiz-Bermudez P, Lam JP, et al. Inhibition of recombinant human mitochondrial aldehyde dehydrogenase by two intermediate metabolites of disulfiram. Biochem Pharmacol. 1998;55:1099–1103. doi: 10.1016/s0006-2952(97)00686-2. [DOI] [PubMed] [Google Scholar]
- McGregor NR. Pueraria lobata (Kudzu root) hangover remedies and acetaldehyde-associated neoplasm risk. Alcohol. 2007;41:469–478. doi: 10.1016/j.alcohol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR, et al. A selective ALDH-2 inhibitor reduces anxiety in rats. Pharmacol Biochem Behav. 2009;94:255–261. doi: 10.1016/j.pbb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pares X, Moreno A, Peralba JM, et al. Action of metadoxine on isolated human and rat alcohol and aldehyde dehydrogenases. Effect on enzymes in chronic ethanol-fed rats. Methods and findings in experimental and clinical pharmacology. 1991;13:37–42. [PubMed] [Google Scholar]
- Penetar DM, Toto LH, Farmer SL, et al. The isoflavone puerarin reduces alcohol intake in heavy drinkers: a pilot study. Drug and alcohol dependence. 2012;126:251–256. doi: 10.1016/j.drugalcdep.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl RO, Paylan SS, Gentes-Hawn A, et al. Alcohol affects executive cognitive functioning differentially on the ascending versus descending limb of the blood alcohol concentration curve. Alcohol Clin Exp Res. 2003;27:773–779. doi: 10.1097/01.ALC.0000065434.92204.A1. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME, Callejas O, Tampier L. Aversion to acetaldehyde: differences in low-alcohol-drinking (UChA) and high-alcohol-drinking (UChB) rats. Alcohol. 2002;26:69–74. doi: 10.1016/s0741-8329(01)00197-5. [DOI] [PubMed] [Google Scholar]
- Ragusa N, Zito D, Bondi C, et al. Effects of pyridoxine on hepatic tryptophan pyrrolase activity in rat during chronic ethanol administration. Biochemistry and experimental biology. 1980;16:391–396. [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GL, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, et al. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Pihl RO, Cox SM, et al. The effect of naltrexone on alcohol’s stimulant properties and self-administration behavior in social drinkers: influence of gender and genotype. Alcohol Clin Exp Res. 2011;35:1134–1141. doi: 10.1111/j.1530-0277.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- Sham HL, Kempf DJ, Molla A, et al. ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease. Antimicrobial agents and chemotherapy. 1998;42:3218–3224. doi: 10.1128/aac.42.12.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Gonzalez C, et al. Dihydromyricetin as a novel anti-alcohol intoxication medication. J Neurosci. 2012;32:390–401. doi: 10.1523/JNEUROSCI.4639-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilenya LS, Muzychenko AP, Gasbarrini G, et al. Metadoxine in acute alcohol intoxication: a double-blind, randomized, placebo-controlled study. Alcohol Clin Exp Res. 2002;26:340–346. [PubMed] [Google Scholar]
- Smith BR, Amit Z, Splawinsky J. Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol. 1984;1:193–195. doi: 10.1016/0741-8329(84)90097-1. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Incorporating alcohol pharmacotherapies into medical practice. Rockville, MD: 2009. [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, et al. Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Takeshita T, Morimoto K. Accumulation of hemoglobin-associated acetaldehyde with habitual alcohol drinking in the atypical ALDH2 genotype. Alcohol Clin Exp Res. 2000;24:1–7. [PubMed] [Google Scholar]
- Tang L, Yang F, Fang Z, et al. Resveratrol Ameliorates Alcoholic Fatty Liver by Inducing Autophagy. Am J Chin Med. 2016;44:1207–1220. doi: 10.1142/S0192415X16500671. [DOI] [PubMed] [Google Scholar]
- Taylor RE, Raysor BR, Kwagyan J, et al. Alterations in ethyl alcohol pharmacokinetics during oral consumption of malt liquor beverages in African Americans. Alcohol Clin Exp Res. 2008;32:2074–2080. doi: 10.1111/j.1530-0277.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada F, Nagura Y, Abe S, et al. Effect of restraint and footshock stress and norepinephrine treatment on gastric emptying in rats. Biol Pharm Bull. 2003;26:368–370. doi: 10.1248/bpb.26.368. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res. 1990;35:39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Kim SW, Wang GJ, et al. Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage. 2013;64:277–283. doi: 10.1016/j.neuroimage.2012.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, et al. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage. 2006;29:295–301. doi: 10.1016/j.neuroimage.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Shokri Kojori E, et al. Alcohol decreases baseline brain glucose metabolism more in heavy drinkers than controls but has no effect on stimulation-induced metabolic increases. J Neurosci. 2015;35:3248–3255. doi: 10.1523/JNEUROSCI.4877-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott JY, Weiner H, Shultz J, et al. In vivo acetaldehyde in the brain of the rat treated with ethanol. Biochem Pharmacol. 1980;29:411–417. doi: 10.1016/0006-2952(80)90521-3. [DOI] [PubMed] [Google Scholar]
- Williams E. Effects of alcohol on workers with carbon disulfide. JAMA. 1937;109:1472–1473. [Google Scholar]
- Winefield RD, Heemskerk AA, Kaul S, et al. N-acetyl-S-(N,N-diethylcarbamoyl) cysteine in rat nucleus accumbens, medial prefrontal cortex, and in rat and human plasma after disulfiram administration. J Pharm Biomed Anal. 2015;107:518–525. doi: 10.1016/j.jpba.2015.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DF, Winter HR, Duffull SB. Understanding the time course of pharmacological effect: a PKPD approach. British journal of clinical pharmacology. 2011;71:815–823. doi: 10.1111/j.1365-2125.2011.03925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Fan P, Arolfo M, et al. Inhibition of aldehyde dehydrogenase-2 suppresses cocaine seeking by generating THP, a cocaine use-dependent inhibitor of dopamine synthesis. Nat Med. 2010;16:1024–1028. doi: 10.1038/nm.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]