Abstract
Background
Abnormalities in coherent cortical circuit functioning, reflected in gamma band activity (~40 Hz), may be a core deficit in schizophrenia. The early auditory gamma band response (EAGBR) is a neurophysiologically simple probe of circuit functioning in primary auditory cortex. We examined the EAGBR in first hospitalized schizophrenia to assess whether it was reduced at first hospitalization.
Method
Wavelet evoked power and intertrial phase locking of the EAGBR at Fz to standard tones during an oddball target detection task were examined in 28 first hospitalized schizophrenia patients (10 female) and 44 control subjects (17 female).
Results
At first hospitalization EAGBR trial-to-trial phase locking and evoked power were significantly reduced in patients. Although reduced overall in patients, greater total symptoms were significantly associated with greater gamma phase locking and power. Additionally, greater EAGBR power was marginally associated with greater positive factor scores, hallucinations, and thinking disturbance.
Conclusions
Abnormalities of gamma band functioning in local auditory sensory circuits are present in schizophrenia at first hospitalization further evidence that basic sensory processes are impaired in schizophrenia. It remains to be determined whether the EAGBR becomes permanently impaired with disease progression, and if its reduction is specific to schizophrenia.
Keywords: Schizophrenia, Gamma band response, Oscillation, EEG, Evoked potential, Auditory oddball
9.1. Introduction
9.1.1. Integration and connectivity in schizophrenia
Schizophrenia reflects a disintegration of many facets of distributed and parallel brain functioning, characterized by failure of integration between domains, e.g., poor coordination of mental and emotional processes, and disorder within domains, e.g., cognitive disorganization. Abnormalities of functional and structural connectivity within and between brain regions are apparent (Andreasen et al., 1998; Uhlhaas et al., 2006; Ford et al., 2007; Zhou et al., 2007; Roopun et al., 2008; Stephan et al., 2009), suggesting an underlying pathophysiological and anatomical disconnection. The formation of flexible, dynamic neural assemblies, both local and long-range, is thought to underlie distributed cerebral processing and is likely due to temporal synchrony of neural firing within and between brain regions. There has been particular interest in gamma frequency (30–80 Hz) because of its relation to perceptual binding in animals (Freeman and Van Dijk 1987; Singer 1993; Engel and Singer, 2001) and higher-order cognition in humans (Başar et al., 1999; Rodriguez et al., 1999; Tallon-Baudry and Bertrand, 1999; Herrmann et al., 2004; Kaiser and Lutzenberger, 2005; Jensen et al., 2007).
9.1.2. Wavelet analysis of oscillations
Interest in frequency analysis of the EEG has undergone resurgence in tandem with development of methods that provide amplitude and phase measures superior to windowed Fourier transforms or restricted bandpass techniques. The wavelet method uses windowed or edge-modulated sinusoidals for frequency-in-time analyses, such as the Morlet wavelet which uses a Gaussian-modulated sinusoid. Measures of primary interest within the gamma range are power and the intertrial phase-locking factor (PLF). Power measures the energy within specific frequency bands and is the square of amplitude. PLF is a measure of the variance in phase across trials and thus reflects the temporal stability of oscillatory activity from trial to trial at specific sites. Though phase and amplitude are independent — clearly a low amplitude signal can be in phase from trial to trial — evoked power and PLF interact substantially as a high amplitude signal when averaged can appear smaller if out of phase between trials.
9.1.3. Gamma band responses
Gamma responses have been categorized into evoked and induced forms. Evoked responses are time locked to stimulus onset and generally occur earlier in the processing stream. These types of event-related oscillations (EROs) are thought to reflect basic perceptual mechanisms such as sensory registration. Evoked power is typically extracted from the averaged response across trials (i.e., the wavelet of the averaged response) and is analogous to the stimulus-locked event-related potential (ERP), where temporal variability between trials reduces the signal. Induced power represents the averaged wavelets of trials and thus does not “average out” non-stimulus-locked activity. Induced responses generally occur later and show varying onset times relative to stimulus onset. Like long-latency ERPs, these EROs are thought to reflect greater endogenous cognitive activity related to more complex internal operations and transforms of perceptual information. For an excellent review of gamma and other oscillations in cognitive and psychiatric disorders, see Başar and Güntekin (2008).
9.1.4. Gamma band responses in schizophrenia
Although recent work has focused on later induced gamma activity associated with complex perceptual phenomena (e.g., illusory contours) in schizophrenia (Spencer et al., 2003; Uhlhaas et al., 2006), it is important to determine whether more basic sensory processes are also impaired in the disorder. Coordinated activity within local processing circuits in sensory cortex is not only necessary for perception, but also for subsequent complex cognition. Recent ERP work has provided evidence of abnormalities in early sensory ERPs in schizophrenia (Leavitt et al., 2007; Javitt, 2009; Salisbury et al., 2010). One type of sensory-driven or evoked gamma oscillation, the steady-state evoked potential, uses repetitive click stimuli to cause the EEG to oscillate at the driving frequency. Deficits exist in gamma driving in schizophrenia (Kwon et al., 1999a,b; Light et al., 2006) even at first hospitalization (Spencer et al., 2008b). However, this response reflects the superposition of many responses to many stimuli and a subsequent reverberation of local circuits which cannot be purely sensory in nature.
Another gamma burst occurs early within the auditory system to discrete stimuli and may serve as a simple probe of coherent cortical circuit functioning. This early auditory gamma band response (EAGBR) to simple stimuli occurs early in the information processing stream and allows for a more reductionistic probing of basic cortical architecture in primary sensory areas (Brosch et al., 2002). Like other sensory processes, the EAGBR appears to be enhanced by selective attention (Tiitinen et al., 1993; Debener et al., 2003), and possibly task difficulty, perhaps through anterior cingulate modulation (Mulert et al., 2007). EAGBR abnormalities in schizophrenia would be consistent with basic low level processing deficits in schizophrenia as suggested by some recent studies (Leavitt et al., 2007; Javitt 2009).
9.1.5. Auditory pathophysiology in schizophrenia
Gray matter deficits throughout the auditory cortex have been noted in schizophrenia, even at first hospitalization (Kwon et al., 1999a,b; Hirayasu et al., 2000; Rojas et al., 2002; Kasai et al., 2003; Whitford et al., 2005). Moving down from complex to simple auditory processes, it is apparent that auditory system dysfunction in schizophrenia spans multiple levels of processing complexity. The presence of auditory hallucinations is a hallmark of schizophrenia and has been associated with abnormal primary auditory cortex activation (Lennox et al., 2000; Wible et al., 2009) and abnormal temporo-frontal white matter connectivity (Hubl et al., 2004). Abnormalities in the P300 to auditory oddballs (Roth et al., 1980; Bramon et al., 2004; Van der Stelt et al., 2005) and in the P200 and N100 to standard and oddball stimuli (Ford et al., 2001; O’Donnell et al., 2004) in schizophrenia are well established and present even at first hospitalization for schizophrenia (Salisbury et al., 1999, 2010). The P50/P1 auditory ERP is abnormal in schizophrenia (Freedman et al., 1987). Thus, ERPs spanning late endogenous and early exogenous processes are abnormal in schizophrenia.
9.1.6. EAGBR in schizophrenia
Consequently, it seems likely that the EAGBR would be reduced in schizophrenia. Results to date have been inconsistent. Six studies have used odd-ball-type target detection paradigms to examine the EAGBR. Gallinat et al. (2004) used a modified paradigm with click pairs as standard stimuli (500 ms separation) and tones as targets. Significant differences between patients and controls for EAGBR activity were not detected, although activity at Cz to standard clicks appears to be approximately 40% smaller in patients than controls. PLF was not reported. Spencer et al. (2008a) measured EAGBR power and PLF in 23 schizophrenics versus 21 controls. They did not detect significant EAGBR reductions in schizophrenia. Roach and Mathalon (2008) suggested that wavelet parameters might play a role in detection of group differences and detected reduced EAGBR PLF in 21 patients relative to 22 controls. Power was not assessed. Hall et al. (2011) reported reduced evoked EAGBR power and PLF in schizophrenics and their ill and well twins, leading Hall et al. (2011) to propose EAGBR as a putative endophenotype for the disorder. Leicht et al. (2010) found both reduced evoked power and PLF of EAGBR using wavelets in 90 patients versus 90 healthy comparison subjects. A recent report by Başar-Eroğlu et al. (2011) compared averaged and single trial responses to ignored single tone trains and to attended oddball trains. They reported that the averaged EAGBR did not differ between 10 patients and 10 controls, but that EAGBR power was increased in patients on individual trials over occipital areas. They suggested that trial-to-trial variability might be a crucial parameter for understanding the basic underlying pathophysiology of schizophrenia. Another extant report of early gamma activity in schizophrenia did not, in fact, measure the same phenomenon. Symond et al. (2005) used a standard two-tone oddball task and reported gamma phase across a windowed Fast Fourier Transform (FFT) from 37 to 41 Hz from −150 to 150 ms post stimulus. They did not examine intertrial PLF at specific sites, but measured synchrony between sites (anterior vs. posterior, left vs. right). They detected lower synchrony in 40 first episode schizophrenia subjects versus 40 controls, but they did not measure EAGBR per se with, for example, some peaks preceding stimulus onset. Thus, six studies have assessed the EAGBR in response to attended oddball-type tasks in schizophrenia, with equivocal results: half found reductions of the averaged responses, half did not.
Several studies examined EAGBR to clicks in the P50-gating paradigm, where click pairs (~500 ms ISI) are passively attended with a long ITI (~10 s). The EAGBR to clicks may not be identical to the EAGBR to tones (clicks include broad spectrum energy unlike pure tones), but it is likely that these EROs are highly similar. Click-gating tasks have generally reported lower but non-significantly different EAGBR to the first click (S1) and relatively normal gamma gating to the second click (S2). Using selective bandpass methods (not frequency in time methods like wavelets), Clementz et al. (1997) reported normal MEG and EEG measurement of S1 EAGBR, and that MEG detected an S2 EAGBR-gating defect in schizophrenia, but ERP measures (recorded from Cz) did not. Clementz and Blumenfeld (2001) used multi-channel EEG to determine if more dense recordings might detect ERP differences in the EAGBR and associated gating. They found no group differences in EAGBR between groups, but rather found differences in the low frequency component (LFR; theta and beta ranges). Johannesen et al. (2005) reported reduced spectral power of the EAGBR responses in schizophrenia (PCA-extracted component of restricted bandpass data submitted to FFT). They did not find any differences in EAGBR power between paranoid versus non-paranoid schizophrenia. Like Clementz and Blumenfeld (2001), they found reductions in the LFR. Later, Johannesen et al. (2008) used the Sensory Gating Inventory (Hetrick et al., 2012), to classify schizophrenia patients with perceptual and attentional anomalies versus those without, and found that EAGBR power was smaller to S1 in schizophrenia subjects with high SGI scores than in controls, but greater in those with normal range scores than in controls, although only the two patient subgroups were significantly different. Hong et al. (2004) used a restricted bandpass method and found no difference in S1 gamma between schizophrenics and controls. Brockhaus-Dumke et al. (2008) reported similar phase locking in patients and controls to the first of two paired clicks.
Thus, analyses using click stimuli have generally not found reductions of EAGBR. Three of the six studies examining EAGBR to tones have reported EAGBR reductions. It is not clear why EAGBR results have been discrepant. In addition to the differences in the physical characteristics of the stimuli, one notable difference is that three positive tone studies used continuous wavelet analyses, whereas most of the gating data used restricted bandpass analyses. Wavelets are thought to be more sensitive to EROs than other methods (Lee and Yamamoto, 1994), but it is likely that Roach and Mathalon’s (2008) caveat about wavelet parameters influencing measures is also important. Too, oddball tasks generally demand selective attention, but gating paradigms are passive, but this cannot account for inconsistent results in oddball tasks. Other factors such as prolonged medication, illness progression, and increased auditory thresholds in chronic schizophrenic populations (Rabinowicz et al., 2000) may affect EAGBR measures. Diagnostic subtype may play a role, although Johannesen et al. (2005) did not detect an effect. We suspect the actual paradigms used play a great role, as the low signal-to-noise ratio of the EAGBR makes it difficult to detect. Tasks that use many pure tone pip stimuli should be best able to visualize the < 1 μV response. In addition, studies with large samples have generally been better able to detect group differences in this small response, as the effect size is very small.
9.1.7. Current aims
The present study examined the EAGBR in subjects at or near first hospitalization, free from potential chronicity-related confounds. The presence of EAGBR abnormalities early in the disease is crucial if the EAGBR is truly an endophenotype. Studying subjects at first hospitalization reduces the contribution of prolonged medication and progressive cortical reduction and allows for longitudinal study early in the disease to evaluate changes reflective or predictive of deterioration.
9.2. Methods
9.2.1. Subjects
Twenty-eight first hospitalized schizophrenia subjects (FHSz) at or within 1 year of first hospitalization (mean (S.D.) 18.4 (19.9) days), diagnosed via SCID-P interview for DSM-IV criteria and chart review (15 paranoid, 1 disorganized, 2 undifferentiated, 8 schizoaffective (5 bipolar subtype, 3 depressed subtype), 1 schizophreniform, and 1 delusional disorder, paranoid subtype), participated. Forty-four healthy control subjects (HC) were recruited from the local community through newspaper and online advertisements and were free of Axis I or II disorders (structured clinical interview for DSM-IV non-patient edition (SCID-NP); structured clinical interview for DSM-IV Axis II personality disorders (SCID II)), as well as a history of Axis I disorders in first-degree relatives by report. Groups were matched for age, handedness, WAIS information and vocabulary scaled scores, gender distributions, and parental SES. Subjects met these inclusion criteria: (1) right-handed (Edinburgh Handedness Inventory); (2) no ECT; (3) no history of neurological illness; (4) no alcohol or drug dependence or “detox” within the last 5 years; and (5) estimated verbal IQ above 75. Patients had slightly lower Mini-Mental State Exam (MMSE) and WAIS working memory task scores than controls, consistent with effects of acute psychosis, but were generally bright and high functioning (Table 1). The McLean Hospital IRB approved this study. After complete description of the study to the subjects, written informed consent was obtained. Subjects were paid $15/h for their participation.
TABLE 1.
DEMOGRAPHIC AND CLINICAL INFORMATION
| Patients | HC | Statistic | p | |
|---|---|---|---|---|
| Gender (M/F) | 18/10 | 27/17 | χ2 = 0.15 | 0.70 |
| Age | 24.8 (7.6) | 26.3 (8.0) | t (70) = 0.80 | 0.42 |
| Handedness | 0.77 (0.21) | 0.81 (0.14) | t (65) = 0.76 | 0.45 |
| SES | 3.4 (1.3) | 2.2 (0.8) | t (66) = 4.56 | <0.0012 |
| PSES | 1.70 (1.0) | 1.70 (1.1) | t (65) = 0.03 | 0.97 |
| Minimental State | 28.7 (1.3) | 29.4 (0.6) | t (65) = 2.91 | 0.009 |
| WAIS info | 12.8 (2.4) | 13.0 (2.3) | t (65) = 0.29 | 0.77 |
| WAIS vocab | 13.4 (2.3) | 14.2 (2.8) | t (65) = 1.17 | 0.25 |
| WAIS digit span | 9.9 (2.3) | 12.0 (2.2) | t (65) = 3.81 | <0.001 |
| WAIS symbol digit D | 7.8 (2.0) | 13.0 (8.9) | t (65) = 2.75 | 0.008 |
| MEDS | 299.9 (252.0) | |||
| GAS | 34.0 (8.2) | |||
| PANSS total | 75.6 (14.0) | |||
| PANSS positive | 20.4 (4.3) | |||
| PANSS negative | 18.7 (6.6) | |||
| PANSS td | 11.2 (3.8) | |||
| SAPS | 10.3 (6.3) | |||
| SANS | 11.5 (5.9) |
Note: values are mean (S.D.). Less df reflects data unavailable for some subjects. SES = socio-economic status; PSES = parental SES; WAIS info = Wechsler Adult Intelligence Scale information scaled score; WAIS vocab = Wechsler Adult Intelligence Scale vocabulary scaled score; WAIS digit span = Wechsler Adult Intelligence Scale digit span scaled score; WAIS symbol digit = Wechsler Adult Intelligence Scale digit symbol scaled score; MEDS = daily antipsychotic medication dosage, chlorpromazine equivalents; GAS = global assessment scale, equivalent to global assessment of functioning scale; PANSS = positive and negative syndrome scale; PANSS positive = PANSS positive symptom factor; PANSS negative = PANSS negative symptom factor; PANSS td = PANSS thought disturbance factor; SAPS = scale for the assessment of positive symptoms; SANS = scale for the assessment of negative symptoms.
9.2.2. Procedure
Subjects detected low probability Target tones (15%, 1.2 kHz, 73 dB SPL, 50 ms pips, 5 ms rise/fall times) among Standard tones (1 kHz, 73 dB SPL, 50 ms pips, 5 msc rise/fall times) presented over insert headphones (Etymotic/Earlink) with an ISI jittered equally between 800, 900, 1000, 1100, and 1200 ms. A total of 200 tones were presented with the exception of latter subjects to whom 400 tones were presented (for increased signal-to-noise ratios). Only the first 200 trials for these subjects were used in the present study so that averages had the same inherent signal-to-noise-ratio and equal habituation and refractory effects. Stimulus delivery and digital triggers were generated with Superlab Pro v.2 (Cedrus). Subjects kept a mental count of the Target tones.
9.2.3. EEG recording and processing
EEG activity was recorded from 60 scalp sites and the nose tip using a 64-channel cap (custom designed Electro-Cap International sintered Ag-AgCl caps). Activity was recorded continuously using SynAmps and Scan Acquire (Neuroscan/Compumedics, USA). The right mastoid served as the reference, except for two bipolar electrooculogram channels. Two electrodes medial to the right eye, one above and one below, monitored vertical eye movements and blinks. Electrodes at the outer canthi of the eyes monitored horizontal eye movements. The forehead served as ground. Electrode impedances were below 5 kO. The EEG bandpass was 0.10 (6 dB/octave roll-off) to 100 Hz (24 dB/octave roll-off). EEG was digitized at 500 Hz.
Off-line processing was performed with BrainVision Analyzer (Brain Products GMBH). EEG was rereferenced to averaged mastoids and segmented for Standard stimuli into epochs of 1100-ms duration, including a 100 ms pre-stimulus baseline.
To illustrate the major time-averaged ERP correlates of the EAGBR ERO, voltage in time averages was constructed for controls using restricted passband filtering (Clementz and Blumenfeld, 2001). The EAGBR was visualized by restricted filtering from 35 to 45 Hz. Middle and high frequency contributions to the standard ERPs were visualized by high pass filtering at 8 Hz. Low frequency contributions to the ERP were visualized by low pass filtering at 8.5 Hz, with eye movement correction using the method of Gratton et al. (1983). Epochs were baseline corrected by subtraction of the average pre-stimulus voltage at each site. Subsequently, epochs containing eye movements exceeding ± 50 mV at F7, F8, FP1, or FP2 were rejected.
For wavelet-derived frequency in time measures, the EEG was digitally high pass filtered at 8 Hz (24 dB/octave roll-off) to remove eye movement artifact, drift, and low frequency components. Standard tone epochs (processed as above) underwent time frequency analysis in Matlab for intertrial phase locking and evoked power (software provided by C. Torrence and G. Compo; http://atoc.colorado.edu/research/wavelets). A complex Morlet wavelet with Morlet’s constant f/sf = 6 and a fixed cycle length of 6 was used over the 20–80 Hz range with 11 frequency bins. Baseline correction for each frequency bin used the 100 ms pre-stimulus interval. Evoked power was derived from the squared amplitude coefficient of the wavelet transform of the ERP. Phase was calculated as the arc tangent of the ratio of the imaginary and real coefficients of the transform for each individual trial. PLF was calculated as 1 minus the variance of phase across trials, the circular variance, for each time-frequency point (Tallon-Baudry et al., 1996). A peak-picking method based on Mulert et al. (2007), modified to diminish the likelihood of missing a peak on either side of 40 Hz, was used. Based on the grand average maximum ± 25 ms, the peak value from 50 to 100 ms post stimulus among 3 frequency bins centered at 40, 46, and 53 Hz was selected for each individual. Analysis focused on the Fz and Cz chains, including left (F1, C1), midline (Fz, Cz), and right (F2, C2) sites for standard stimuli. Repeated measures ANOVA utilizing Huynh-Feldt epsilon for position (left, middle, right) was used to test for effects. Follow-up tests used t tests. Correlations used Pearson correlations. Significance was achieved at p ≤ 0.05.
9.3. Results
9.3.1. Morphology
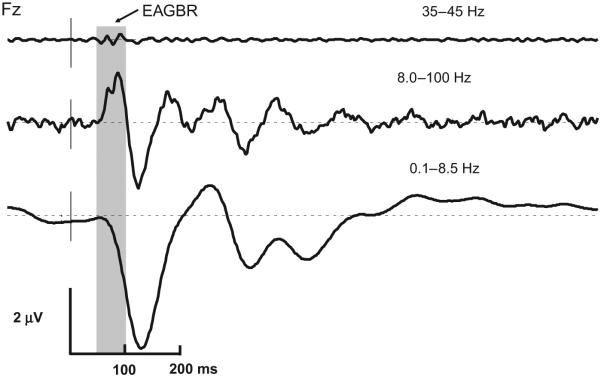
Comparison of HC grand averaged standard stimuli waveforms (Fig. 1) shows that the EAGBR overlaps with the P1/P50 response, as expected (Pantev and Elbert, 1994; Clementz et al., 1997; Clementz and Blumenfeld, 2001). Both the EAGBR and the P1 are partially overlapped by the descending slope of the N100.
Fig. 1.
Voltage in time restricted passband control subject grand averaged ERPs to standard stimuli illustrating different neurophysiological signals and their overlap. The shaded box indicates 50–100 ms post stimulus. The top trace shows the averaged EAGBR response. The middle trace highlights the P1/P50 response, which appears to overlap to a great degree with the EAGBR. The bottom trace shows N1/N100 and P2/P200. Note that the N1 response begins at approximately 75 ms and overlaps partially with the EAGBR and P1.
9.3.2. Power
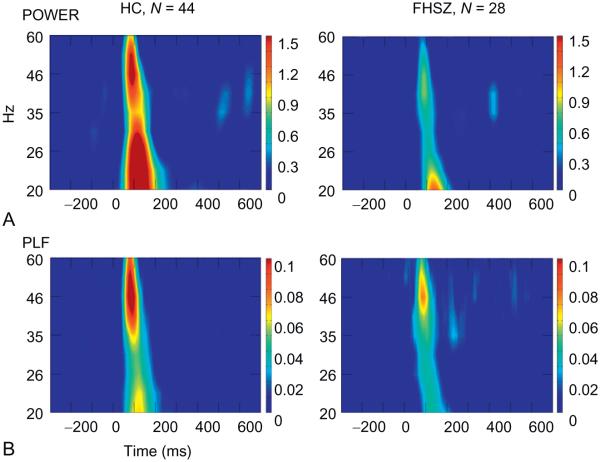
Mean central (f) of EAGBR power tended to be ~46 Hz in both groups. HC showed a well defined burst of EAGBR evoked power, by contrast with patients who showed a weaker signal (Fig. 2A presents frequency-in-time maps for Fz; mean peak values of EAGBR evoked power for each site are presented in Table 2). Patients showed an evoked power reduction (F1, 70 = 3.89, p = 0.05). Hemispheres differed in evoked power (F2, 140 = 5.03, p = 0.01, ε = 0.88), which interacted with chain location (F2, 140 = 4.36, p = 0.03, ε = 0.74). Power was greatest at the midline for both chains, but was relatively larger over the left hemisphere than the right for the frontal chain, and relatively larger over the left hemisphere than the right for the central chain (Table 2). There was no significant main effect for chain location and no other significant interactions.
Fig. 2.
(A) Frequency in time maps of evoked power at Cz for each group for standard stimuli. Note that patients show attenuated evoked power in that time interval relative to controls. The lower (f) activity reflects N1 and P2. (B) Phase-locking factor at Cz for each group for standard stimuli. Although maximum evoked power was observed at 46 Hz in controls, their maximum PLF was lower, closer to 40 Hz. Patients showed very little phase consistency across trials.
TABLE 2.
MEAN PEAK EAGBR EVOKED POWER (70–90 ms, μV)
| F1 | Fz | F2 | |
|---|---|---|---|
| HC | 1.32 (1.77) | 1.42 (1.73) | 1.17 (1.46) |
| Patients | 0.60 (1.00) | 0.67 (1.11) | 0.61 (1.04) |
|
|
|||
| C1 | Cz | C2 | |
|
|
|||
| HC | 1.03 (1.78) | 1.20 (1.84) | 1.18 (2.05) |
| Patients | 0.33 (0.65) | 0.49 (1.03) | 0.57 (0.97) |
9.3.3. Phase
HC showed greater trial-to-trial phase synchrony of the EAGBR than patients (F1, 70 = 5.74, p = 0.02; Fig. 2B presents frequency-in-time maps for Fz PLF; Table 3 presents PLF means for all sites). Additionally, frontal sites showed greater PLF than central sites (F1, 70 = 24.49, p < 0.001) in both groups. PLF was largest at midline sites (F2, 140 = 7.78, p = 0.001, ε = 0.97), but the laterality of PLF interacted with chain location (F2, 140 = 4.12, p = 0.02, ε = 0.94), with the left frontal site larger than the right, whereas left and right central sites were symmetrical (Table 3).
TABLE 3.
MEAN PEAK EAGBR PLF (70–90 ms)
| F1 | Fz | F2 | |
|---|---|---|---|
| HC | 0.10 (0.08) | 0.10 (0.07) | 0.07 (0.07) |
| Patients | 0.06 (0.06) | 0.06 (0.06) | 0.05 (0.06) |
|
|
|||
| C1 | Cz | C2 | |
|
|
|||
| HC | 0.07 (0.07) | 0.08 (0.07) | 0.07 (0.06) |
| Patients | 0.03 (0.06) | 0.05 (0.07) | 0.04 (0.06) |
9.3.4. Correlations with symptoms
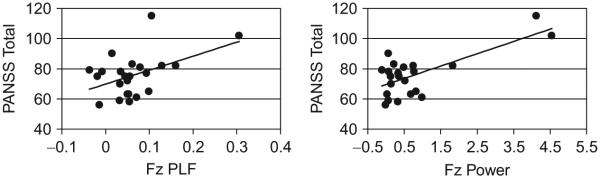
EAGBR evoked power and PLF measures were highly correlated at all sites in both groups. Controls showed the weakest correlation between power and PLF at C1 (r = 0.63, p < 0.001) and greatest at C2 (r = 0.71, p < 0.001). Correlations between different sites ranged from 0.35 to 0.75. Patients showed the weakest correlation between power and PLF at F2 (r = 0.77, p < 0.001) and greatest at C1 (r = 0.85, p < 0.001). Correlations between different sites ranged from 0.61 to 0.87. Evoked gamma power and PLF at Fz and Cz in control subjects were not correlated with WAIS information, vocabulary, or symbol digit scores, but EAGBR PLF at Fz correlated marginally with digit span (r = 0.29, p = 0.056). There were no significant correlations with EAGBR evoked power or PLF and semantic or working memory measures in patients. Within patients, PLF at Fz and Cz was correlated positively with PANSS total scores (r = 0.47, p = 0.024, r = 0.43, p = 0.04, respectively, Fig. 3) but not with positive, negative, or thought disturbance PANSS factors, SANS, SAPS, GAS, or medication dosages (chlorpromazine equivalents). EAGBR power at Fz and Cz was correlated positively with PANSS total scores (r = 0.71, p < 0.001, r = 0.61, p = 0.002, respectively, Fig. 3), but not with SANS, SAPS, GAS, or medication dosages. In addition, EAGBR evoked power at Fz correlated marginally with PANSS hallucinations (r = 0.39, p = 0.066), PANSS positive factor (r = 0.39, p = 0.065), and PANSS thought disturbance factor (r = 0.41, p = 0.051).
Fig. 3.
Correlations between EAGBR power and PLF to standard tones and total PANSS scores in first hospitalized schizophrenia.
9.4. Discussion
9.4.1. Precis
The current study showed reduction in EAGBR evoked power and phase locking in first hospitalized schizophrenia. These findings support the presence of functional local circuit deficits in schizophrenia early in the sensory processing stream and early during disease course. Deficits at first hospitalization rather than emergence with disease course are consistent with the EAGBR’s identification as a candidate endophenotype.
9.4.2. Underlying pathophysiology
It is not clear whether EAGBR deficits reflect cortical or subcortical processing abnormalities. The EAGBR is contemporaneous with the high frequency component of the P1 (Fig. 1). Some evidence exists suggesting subcortical, thalamic sources for the P1 (Velasco and Velasco, 1986), although most data suggest sources in Heschl’s gyrus (Liégeois-Chauvel et al., 1994). EAGBR deficits may reflect local abnormalities in primary auditory cortex in schizophrenia, but may also be related to feedforward and feedback communication between thalamus (medial geniculate nucleus) and primary auditory cortex. Recent fMRI work suggests co-activation of auditory cortex and thalamus during the EAGBR (Mulert et al., 2010). In terms of pathophysiology, when coupled with other gamma band defects spanning sensory-perceptual and cognitive tasks, it is possible that wide-range gamma band deficits reflect an ubiquitous cortical circuit deficit in schizophrenia that spans various types of cortical architecture, even sensory (Javitt, 2009). Dysfunction of coherent synchrony in cell assemblies of varying complexity may be a core deficit in schizophrenia. It is not clear if such deficits in local circuit function lead to an overall reduction of gamma power, reduced time-locking of gamma band response which leads to apparent power reduction following averaging (Başar-Eroğlu et al., 2011), or some combination of both defects.
9.4.3. Endophenotypes
Deficits in early primary sensory processes in the visual system P1 have recently been demonstrated in schizophrenia patients (Foxe et al., 2001) and their relatives (Yeap et al., 2006) and may serve as an endophenotype. Several other ERPs have been identified via family and twin studies as putative endophenotypes for schizophrenia (Hall et al., 2007). Following up the demonstration of reduced auditory N1 in family members (Force et al., 2008), we suggested that auditory N1 reduction may be endophenotypic for schizophrenia due to its presence at first hospitalization (Salisbury et al., 2010). Our recent collaborative study (Hall et al., 2011) showed reductions in EAGBR evoked power and PLF in schizophrenia, and their ill and well twins, suggesting that the EAGBR is endophenotypic. The current demonstration of EAGBR deficits in first hospitalized schizophrenia strengthens the contention that this simple ERO is an endophenotype for schizophrenia. Neurophysiological measures of auditory sensory processing as early as the P1 and the EAGBR (and analogues in the visual system) may be valid biomarkers for genetic risk factors for schizophrenia. It is important to examine EAGBR in other major psychiatric illnesses, such as bipolar disorder, to determine whether the reduction is specific to schizophrenia or associated with psychosis or some other pathophysiological condition more generally.
9.4.4. Sensory or cognitive deficit?
Although neurophysiological deficits exist in schizophrenia early in auditory sensory processing, it is unclear whether this reduction reflects a true sensory deficit or a deficit in the ability to use top-down attention to modulate auditory signals. Most oddball tasks demonstrating EAGBR deficits in schizophrenia used an active oddball target detection task, while most P50 gating studies that have not seen EAGBR reductions have used a passive paired-click task. Force et al. (2008) indicated that family members did modulate auditory N1 with attention in contrast to schizophrenic and bipolar subjects, and Hall et al. (2011) showed reduced EAGBR in family members on an oddball task (where presumably they modulated sensory signals as a function of selective attention). These findings are consistent with a true sensory defect, but attention effects need to be quantified. Currently, we are recording EAGBR during attend and ignore conditions to assess selective attention effects in first hospitalized subjects and matched controls.
9.4.5. Variability in literature
Among tasks using oddball paradigms, the present study supports the findings of Roach and Mathalon (2008), Leicht et al. (2010), and Hall et al. (2011). Nevertheless, there have been two negative studies looking at the auditory EAGBR on oddball tasks. Gallinat et al. (2004) used click stimuli as standards and a restricted wavelet analysis, which may have affected results (note that their response looks attenuated in midline and left hemisphere sites in their Fig. 3). Spencer et al. (2008a) did not find reductions in a chronic population in the auditory EAGBR, though early gamma band abnormalities were present to visual stimuli. In addition to analytical difference, as discussed by Roach and Mathalon (2008), we note here that measurement methods of wavelets may affect results. For example, our use of peak picking resulted in larger values than when a static interval was used. There is a substantial amount in individual variability in the EAGBR and different measurement schemes may be differentially sensitive to this variability. Finally, the between-group EAGBR differences are quite small in magnitude, and the effect size is not large. Both Leicht et al. (2010) and Hall et al. (2011) tested large numbers of subjects, with effect sizes around 0.3 and 0.4, respectively. The utility of the EAGBR may be somewhat lessened by this fact, which indicates substantial group distribution overlap.
9.4.6. EAGBR and symptoms
Correlations between PANSS totals and EAGBR evoked power and PLF were found, but were paradoxically positive. This indicates that the most symptomatic patients showed the greatest power and trial-to-trial temporal stability. Remember, however, that the patient means were overall lower than control means. Although somewhat counter-intuitive, this finding appears to be consistent with previous studies showing positive correlations between hallucinations and steady-state gamma parameters (Spencer et al., 2003, 2008a). The observed relationships were influenced somewhat by two outliers in the patient group, although the correlations remained (albeit weaker) using Spearman’s rank order methods. Further research is needed to determine if, within overall reduced patient gamma measures, higher values are associated with greater symptoms.
9.4.7. Caveats
Several issues related to the EAGBR need to be clarified. The reliability of the EAGBR, its variability over the course of disease, and its specificity to schizophrenia need to be assessed. Further, correlations with baseline temporal lobe auditory gray matter volumes and progressive auditory cortex gray matter changes during the early course of the disease need to be determined. Wavelet-derived measures of EEG and their application to psychiatry is an emerging and developing field. Roach and Mathalon (2008) have recently reviewed neural synchrony measures and indicated several methodological issues that the field is still addressing, such as the most appropriate baseline correction method. Comparison of baseline in HC and first hospitalized schizophrenia indicated that the choice used in the current study did not influence the results, but the field has not adopted a standard method. Evoked power and intertrial phase-locking are highly correlated. Nevertheless, they are not entirely redundant measures. It remains to be determined what neurophysiological processes are manifested in these two measures.
9.4.8. Summary
Evoked power and PLF of the EAGBR were reduced in schizophrenia patients at their first hospitalization for psychosis. Cortical dysfunctions related to abnormalities of local circuit synchrony are present in schizophrenia relatively early in cortical sensory processing. This may relate to a ubiquitous defect in GABA and glutaminergic regulation of cell assemblies, although several different neuro-transmitters and neuromodulators are involved in gamma oscillations (Uhlhaas et al., 2008). When coupled with our demonstration of similarly reduced EAGBR measures in the well twins of schizophrenic individuals (Hall et al., 2011), and EAGBR reductions in long-term schizophrenia subjects (Roach and Mathalon, 2008; Leicht et al., 2010), the presence of this deficit in first hospitalized subjects suggests that EAGBR may be a physiologically simple, yet potentially powerful, endophenotype for detecting genetic risk variants associated with schizophrenia, particularly if a means of increasing group differences and hence effect size can be developed.
Acknowledgments
The Authors would like to thank our Research Assistants Rachel Berman, Akanksha Thakur, Courtney Brown, KC Collins, Katherine Tyler, Diane Ventura, and Toni Mahowald for help in collecting and analyzing the data. Drs Taylor and Salisbury had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by the National Institutes of Health R01 MH58704 (D.F.S.) and R01 MH40799 (R.W.M.), and the Department of Veterans Affairs Merit Award, Schizophrenia Center Award, and Middleton Award (R.W.M.).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical–subcortical–cerebellar circuitry? Schizophr. Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Başar E, Güntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Başar E, Başar-Eroğlu C, Karakas S, Schürmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci. Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Başar-Eroğlu C, Mathesa B, Brand A, Schmiedt-Fehra C. Occipital gamma response to auditory stimulation in patients with schizophrenia. Int. J. Psychophysiol. 2011;79:3–8. doi: 10.1016/j.ijpsycho.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr. Res. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Schultze-Lutter F, Mueller R, Tendolkar I, Bechdolf A, Pukrop R, Klosterkoetter J, Ruhrmann S. Sensory gating in schizophrenia: P50 and N100 gating in antipsychotic-free subjects at risk, first-episode, and chronic patients. Biol. Psychiatry. 2008;64:376–384. [Google Scholar]
- Brosch M, Budinger E, Scheich H. Stimulus-related gamma oscillations in primate auditory cortex. J. Neurophysiol. 2002;87:2715–2725. doi: 10.1152/jn.2002.87.6.2715. [DOI] [PubMed] [Google Scholar]
- Clementz B, Blumenfeld L. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp. Brain Res. 2001;139:377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. NeuroReport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK. Top-down attentional processing enhances auditory evoked gamma band activity. NeuroReport. 2003;14:683–686. doi: 10.1097/00001756-200304150-00005. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn. Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Force RB, Venables NC, Sponheim SR. An auditory processing abnormality specific to liability for schizophrenia. Schizophr. Res. 2008;103:298–310. doi: 10.1016/j.schres.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Marsh L, Pfefferbaum A. N1 and P300 abnormalities in patients with schizophrenia, epilepsy, and epilepsy with schizophrenia-like features. Biol. Psychiatry. 2001;49:848–860. doi: 10.1016/s0006-3223(00)01051-9. [DOI] [PubMed] [Google Scholar]
- Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr. Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. NeuroReport. 2001;12:3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, Drebing C, Nagamoto H, Bickford-Wimer P, Franks R. Neurobiological studies of sensory gating in schizophrenia. Schizophr. Bull. 1987;13:669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Van Dijk BW. Spatial patterns of visual cortical fast EEG during conditioned reflex in a rhesus monkey. Brain Res. 1987;422:267–276. doi: 10.1016/0006-8993(87)90933-4. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin. Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hall MH, Rijsdijk F, Picchioni M, Schulze K, Ettinger U, Toulopoulou T, Bramon E, Murray RM, Sham P. Substantial shared genetic influences on schizophrenia and event-related potentials. Am. J. Psychiatry. 2007;164:804–812. doi: 10.1176/ajp.2007.164.5.804. [DOI] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Toulopoulou T, Ettinger U, Bramon E, Murray RM, Salisbury DF. The early auditory gamma-band response is heritable and a putative endophenotype of schizophrenia. Schizophr. Bull. 2011;37:778–787. doi: 10.1093/schbul/sbp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Munk MHJ, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn. Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hetrick WP, Erickson MA, Smith DA. Phenomenological dimensions of sensory gating. Schizophr. Bull. 2012;38(1):178–191. doi: 10.1093/schbul/sbq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch. Gen. Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Buchanan RW, Thaker GK. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr. Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch. Gen. Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu. Rev. Clin. Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr. Res. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Johannesen J, Bodkins M, O’Donnell B, Shekhar A, Hetrick W. Perceptual anomalies in schizophrenia co-occur with selective impairments in the gamma frequency component of midlatency auditory ERPs. J. Abnorm. Psychol. 2008;117:106–118. doi: 10.1037/0021-843X.117.1.106. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Human gamma-band activity: a window to cognitive processing. NeuroReport. 2005;16:207–211. doi: 10.1097/00001756-200502280-00001. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, Yurgelun-Todd DA, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch. Gen. Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, McCarley RW, Hirayasu Y, Anderson JE, Fischer IA, Kikinis R, Jolesz FA, Shenton ME. Left planum temporale volume reduction in schizophrenia. Arch. Gen. Psychiatry. 1999a;56:142–148. doi: 10.1001/archpsyc.56.2.142. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch. Gen. Psychiatry. 1999b;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt VM, Molholm S, Ritter W, Shpaner M, Foxe JJ. Auditory processing in schizophrenia during the middle latency period (10–50 ms): high-density electrical mapping and source analysis reveal subcortical antecedents to early cortical deficits. J. Psychiatry Neurosci. 2007;32:339–353. [PMC free article] [PubMed] [Google Scholar]
- Lee D, Yamamoto A. Wavelet analysis: theory and applications. Hewlett-Packard J. 1994:44–52. [Google Scholar]
- Leicht G, Kirsch V, Giegling I, Karch S, Hantschk I, Möller HJ, Pogarell O, Hegerl U, Rujescu D, Mulert C. Reduced early auditory evoked gamma-band response in patients with schizophrenia. Biol. Psychiatry. 2010;67:224–231. doi: 10.1016/j.biopsych.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Lennox BR, Park SBG, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Liégeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. Electroenceph. Clin. Neurophysiol. 1994;92:204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol. Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Mulert C, Leicht G, Pogarell O, Mergl R, Karch S, Juckel G, Möller H-J, Hegerl U. Auditory cortex and anterior cingulate cortex sources of the early evoked gamma-band response: relationship to task difficulty and mental effort. Neuropsychologia. 2007;45:2294–2306. doi: 10.1016/j.neuropsychologia.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Mulert C, Leicht G, Hepp P, Kirsch V, Karch S, Pogarell O, Reiser M, Hegerl U, Jäger L, Möller HJ, McCarley RW. Single-trial coupling of the gamma-band response and the corresponding BOLD signal. NeuroImage. 2010;49:2238–2247. doi: 10.1016/j.neuroimage.2009.10.058. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int. J. Psychophysiol. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Pantev C, Elbert T. The transient auditory evoked gamma-band field. In: Pantev C, editor. Proceedings of NATO Advanced Research Workshop on Oscillatory Event-Related Brain Dynamics. Plenum Press; New York: 1994. pp. 219–230. [Google Scholar]
- Rabinowicz EF, Silipo G, Goldman R, Javitt DC. Auditory sensory dysfunction in schizophrenia: imprecision or distractibility? Arch. Gen. Psychiatry. 2000;57:1149–1155. doi: 10.1001/archpsyc.57.12.1149. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr. Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature (Lond.) 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Bawn SD, Carlson JP, Arciniegas DB, Teale PD, Reite ML. Alterations in tonotopy and auditory cerebral asymmetry in schizophrenia. Biol. Psychiatry. 2002;52:32–39. doi: 10.1016/s0006-3223(01)01365-8. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr. Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT, Pfefferbaum A, Horvath TB, Kopell BS. P300 and reaction time in schizophrenics and controls. Prog. Brain Res. 1980;54:522–525. doi: 10.1016/S0079-6123(08)61670-2. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biol. Psychiatry. 1999;45:98–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophr. Bull. 2010;36:991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu. Rev. Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J. Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol. Psychiatry. 2008a;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol. Psychiatry. 2008b;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder of temporal connectivity? Am. J. Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitinen HT, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Näätänen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature (Lond.) 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J. Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stelt O, Lieberman JA, Belger A. Auditory P300 in high-risk, recent-onset and chronic schizophrenia. Schizophr. Res. 2005;77:309–320. doi: 10.1016/j.schres.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Velasco M, Velasco F. Subcortical correlates of the somatic, auditory and visual vertex activities. II. Referential EEG responses. Electroenceph. Clin. Neurophysiol. 1986;63:62–67. doi: 10.1016/0013-4694(86)90063-5. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Farrow TF, Gomes L, Brennan J, Harris AW, Williams LM. Grey matter deficits and symptom profile in first episode schizophrenia. Psychiatry Res. 2005;139:229–238. doi: 10.1016/j.pscychresns.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Wible CG, Lee K, Molina I, Hashimoto R, Preus AP, Roach BJ, Ford JM, Mathalon DH, McCarthey G, Turner JA, Potkin SG, O’Leary D, Belger A, Diaz M, Voyvodic J, Brown GG, Notestine R, Greve D, Lauriello J. fMRI activity correlated with auditory hallucinations during performance of a working memory task: data from the FBIRN consortium study. Schizophr. Bull. 2009;35:47–57. doi: 10.1093/schbul/sbn142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, Thakore JH, Foxe JJ. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch. Gen. Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci. Lett. 2007;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]