Abstract
Alloparenting, defined as care provided by individuals other than parents, is a universal behavior among humans that has shaped our evolutionary history and remains important in contemporary society. Dysfunctions in alloparenting can have serious and sometimes fatal consequences for vulnerable infants and children. In spite of the importance of alloparenting, they still have much to learn regarding the underlying neurobiological systems governing its expression. Here, they review how a lack of alloparental behavior among traditional laboratory species has led to a blind spot in our understanding of this critical facet of human social behavior and the relevant neurobiology. Based on what is known, they draw from model systems ranging from voles to meerkats to primates to describe a conserved set of neuroendocrine mechanisms supporting the expression of alloparental care. In this review we describe the neurobiological and behavioral prerequisites, ontogeny, and consequences of alloparental care. Lastly, they identified several outstanding topics in the area of alloparental care that deserve further research efforts to better advance human health and wellbeing.
Keywords: alloparenting, oxytocin, prairie vole, child abuse, brain
INTRODUCTION
By the age of 3, over 90% of American children have experienced regular alloparental care (Network NECCR, 2001), which is defined as the provisioning of care by individuals other than the young’s biological parents. The quality and quantity of this alloparental care predicts social–emotional and cognitive–linguistic outcomes and childcare quality may interact with infant temperament to predict behavioral problems and social competence (Pluess and Belsky, 2009). Here, we review the biology of alloparental care, beginning with the significance of alloparenting in shaping human evolutionary history. The majority of what we know concerning the neurobiology of alloparenting derives from work done in the relatively few mammalian species that also demonstrate alloparental care. Research in these select animal models has identified several conserved neurohormonal systems that seem likely to be involved in human alloparenting. We further examine evidence suggesting that the expression of alloparenting and its neurobiological substrates are influenced both by experience during ontogeny, including early family structure, as well as by exposure to exogenous hormones especially in early life. In addition, we discuss findings in traditional laboratory species that do not typically demonstrate alloparental care. Finally, we highlight several unresolved questions that stand between our understanding of alloparental care and bringing this knowledge to bear to improve human health and wellbeing.
By definition, alloparental behavior is similar to parental behavior from the perspective of the recipient of care, and different than parental behavior from the perspective of the caregiver. Often, the line between parenting and alloparenting is further blurred, as in communal breeding/nesting, where adults share the burden of caring for their own offspring and those of other adults simultaneously. It is most likely that alloparenting shares a great deal of neurobiological similarity with parental behavior, although alloparenting is expected to be less tightly coupled to the endocrine processes related to reproduction. Throughout this review, we will attempt to synthesize disparate findings from across the wide variety of studies which touch on alloparenting. However, the terminology of alloparenting research is clouded by a lack of standardization. Too frequently researchers opt for descriptors which combine “virgin” and “parental” in a convoluted and contradictory approach, or resort to “babysitting” in an effort to broaden appeal. When the correct descriptor, “alloparenting” is not included, the literature fragments and scientific progress is slowed. Given the tremendous significance care of others has played throughout human evolution and into the modern day, shaping a wide range of behaviors and neural systems, alloparenting deserves to be studied with far greater appreciation.
THE EVOLUTIONARY SIGNIFICANCE OF ALLOPARENTING IN HUMANS
Alloparental care is a universal behavior among human societies (Sear and Mace, 2008). Meanwhile, in our closest living relative, the chimpanzee, alloparenting is displayed only very rarely (Kishimoto et al., 2014). At some recent point in human evolution, the trend toward increased alloparenting appears to have increased according to a number of lines of evidence. Humans wean their offspring at an average of approximately 2.5 years, while chimpanzees and orangutans wean at about 5 and 7.7 years, respectively (Galdikas and Wood, 1990; Kennedy, 2005). Across 58 traditional societies, the availability of alloparental care is associated with earlier age at weaning (Quinlan and Quinlan, 2008). Further, human neonates are approximately 6% of maternal body mass at birth, while chimpanzee neonates are only approximately 3% (Leutenegger, 1972; Rosenberg and Trevathan, 2007; DeSilva, 2011). In order to accomplish the feat of having large offspring who develop quickly, humans are believed to have employed a communal/cooperative breeding strategy with a high level of alloparenting (Kennedy, 2005; DeSilva, 2011).
Our evolutionary legacy of alloparenting has been hypothesized to explain several fundamental aspects of the human condition. Perhaps most notably, alloparenting has been proposed as having promoted or permitted the emergence of language among early human ancestors (Hrdy, 2009). Cooperation, language and intelligence, particularly social intelligence, may be reinforced by alloparenting. Concurrently, the development of a large neocortex provided a substrate for the emergence of mentalizing, theory-of-mind and language. During human evolution, alloparenting is thought to have helped reduce the energetic demands of a large neocortex (Navarrete et al., 2011). Indeed, across mammalian clades, there exists a positive correlation between brain size and the amount of allocare by non-mothers (Isler and van Schaik, 2012). Interestingly, this pattern is particularly robust for carnivores but does not hold as tightly for contemporary primates. Among primates therefore, humans may resemble carnivores, raising the possibility that our recent adoption of increased meat consumption, alloparenting, and encephalization go hand in hand.
Human alloparenting takes place in the context of cooperative breeding, wherein individuals live in groups and coordinate their efforts to feed, care and protect young to which they themselves did not give birth (Burkart et al., 2009). How then might humans have evolved alloparenting and cooperative breeding? Monogamy is strongly associated with, and typically considered the ancestral state to the evolution of cooperative breeding (Bogin et al., 2014). Cooperative breeding is maximized when sibling relatedness is high, that is, in the context of female monandry (Bogin et al., 2014). Reducing inter-birth interval increases a male’s likelihood of repeat siring with a given female, thus there can be a positive feedback loop between paternal caregiving, sibling relatedness, cooperative breeding, and alloparental care. Cooperative breeding is associated with decreased inter-birth interval (Russell and Lummaa, 2009), and alloparental care in humans both shortens birth intervals and increases child survivorship (Lahdenpera et al., 2004; Sear and Mace, 2008). It has been estimated that human females expend 14%–29% less child-care effort across the lifetime (compared with expectations based on other mammals) due to the intense alloparenting behavior characteristic to human societies (Bogin et al., 2014).
Alloparenting remains critically important in contemporary culture because even today, children are between six (Schnitzer and Ewigman, 2008), eight (Stiffman et al., 2002), fifty (Schnitzer and Ewigman, 2005), or even a hundred (Daly and Wilson, 1988a) times more likely to suffer fatal abuse when living with an unrelated adult, that is, while under alloparental care (reviewed in Daly and Wilson, 1988b) (Also see discussion of pup attackers in animal models below). For example, being an unrelated caregiver (i.e., alloparent) was the most strongly predictive characteristic of fatal child maltreatment perpetrators in a study of cases from Florida during 1999–2002 (Yampolskaya et al., 2009). A more complete knowledge of alloparenting could inform the etiology of its dysfunction. In the case of child abuse, a deeper understanding may inform the prevention of neglect and other deleterious behaviors. Unfortunately, our psychophysiological understanding of alloparenting is still in its own infancy.
MODEL ORGANISMS FOR THE STUDY OF ALLOPARENTAL BEHAVIOR
If we are to understand the neurobiological mechanisms for alloparental behavior, we must have suitable animal models. One reason why alloparenting remains poorly understood is because it is relatively rare among mammalian species. Cooperative breeding exists in only 3% of mammals (Russell, 2004), however, this figure may be an over-estimate inasmuch as it includes species which express shared care but do not share provisioning for the young (Hrdy, 2009). For the purposes of this review, we include cooperatively-breeding, but non-provisioning species, such as prairie voles as examples of alloparenting species.
Cooperative breeding is also rare among non-human primates, with only two New World families, Callitrichidae and Pitheciidae, having independently evolved such a strategy (Garber, 1996). Thus, there is much to learn concerning the behaviors relevant to cooperative breeding (and hence alloparenting) in marmosets, tamarins, and titi (Santos et al., 1997; Nunes et al., 2000). Intriguingly, the neuropeptide oxytocin (discussed in depth below), which is highly conserved across eutherian mammals, has multiple sequence variants among New World primates, and the evolution of social monogamy among these species appears related to phylogenetic differences in the sequence of the oxytocin receptor (Ren et al., 2015). Outside of primates, cooperative breeding is more common among carnivores, though the physiological basis for these behaviors has been less well-characterized. The notable exception to this is found in meerkats, among whom the contributions of oxytocin and prolactin have begun to be studied (see below).
As is often the case in neuroscience, most mechanistic investigations of the neurobiology of alloparenting have been conducted in rodents. In this review we will focus on research in voles, but also will cover research using dwarf hamsters, naked mole rats, and laboratory rats. An interesting comparison can also be made to California mice (Peromyscus californicus), which are monogamous and reliably demonstrate paternal care for young, but are primarily non-alloparental or even infanticidal as virgins (Gubernick et al., 1994).
The best characterized model organisms for the study of alloparental behavior are the monogamous and bi-parental prairie vole (Microtus ochrogaster), and mandarin vole (Lasiopdomys mandarinus). Among male prairie voles 60%–80% of adults (60 days old) are spontaneously alloparental, whereas only roughly 50% of adult females are (Carter and Roberts, 1997). This proportion may further drop in females to less than 20% by 90 days of age (Lonstein and De Vries, 2001). However, female prairie voles will continue to exhibit alloparental behavior as adults if they remain in the natal nest and experience the birth and rearing of the subsequent litter (Lonstein and De Vries, 2001). It has been hypothesized that alloparental males would incur fitness benefits in the form of increased mating opportunities in exchange for alloparental caregiving (Smuts and Gubernick, 1992). However, when empirically tested, female prairie voles showed no preference for males that had previously demonstrated alloparental behavior (Ophir et al., 2008a). Although there is no clear sexual dimorphism in terms of alloparental behavior in sub-adult prairie voles, there do appear to be sex-related differences in terms of the relevant neurobiology. For example, systemic N-methyl-D-aspartate (NMDA) receptor blockade produced opposing effects in males and females at 40–50 days of age (Kirkpatrick and Kakoyannis, 2004).
In nature, only 30% of offspring emigrate from the natal nest to form their own families (Getz et al., 1994). Under field conditions, therefore, most vole offspring remain with their parents at least until approximately 6–7 weeks of age, by which time they will have experienced the birth and rearing of a subsequent litter of siblings by their parents (Carter and Roberts, 1997). The behaviors displayed by the alloparental vole, namely: pup retrieval, licking/grooming, and arched-back huddling, are not qualitatively different from behaviors seen in actual parents (Roberts et al., 1998a; Lonstein and De Vries, 2001). The exact purpose of each of these behaviors has not been elucidated, but we can speculate that they serve to keep the pup in the nest, warm, safe, and calm. The individual contributions of the multiple aspects of motivation which lead the alloparental vole to care for a pup have not been investigated to date. Thus, we do not know how the alloparental vole perceives the pup, whether they care for the pup because it is a rewarding experience or because the distressed pup is perceived as presenting noxious stimuli to be alleviated through the provision of care. In our laboratory, we have twice attempted to ascertain whether pups are rewarding to adult male alloparents using the Conditioned Place Paradigm to pair the experience of alloparenting with a given environment; however, we have not been able to find any such effect (Kenkel, unpublished observations). Among voles that do not display alloparental care toward a novel pup, responses range from ignoring the pup, actively avoiding the pup or even attacking the pup. Here too, little is known about the qualities of either pup or attacker which bring about an instance of pup attack.
NEUROBIOLOGY OF ALLOPARENTING
Based on the limited success of experimental manipulation of the hormones prolactin, estradiol, and testosterone in affecting paternal care, it has been put forth that unlike maternal behavior, paternal caregiving may be independent of hormonal regulation (Wynne-Edwards and Timonin, 2007). However, this conclusion was based chiefly on results from experiments conducted with dwarf hamsters in the genus Phodopus, which may not be representative of paternal behavior across mammals. Additionally, much of this argument is predicated on testing the assumption that paternal behavior would rely on the same systems involved in the initiation of maternal behavior that occurs at the switch from the non-maternal to maternal state in females, rather than those systems involved in the maintenance of maternal behavior (Schradin, 2007). Paternal behavior and alloparental behavior are not so discreetly timed to birth as maternal behavior; therefore the relevant neural mechanisms are most likely established gradually during development. We begin our analysis of candidate neurobiological systems with the expression of oxytocin receptors in the nucleus accumbens, a relevant site of coordination for the incentive to alloparent.
Oxytocin
Oxytocin is perhaps best known as the initiator of maternal behavior (Keverne and Kendrick, 1992), which has inspired much of the work on alloparenting under the hypothesis that alloparenting, like much of pro-social behavior broadly defined, represents a form of the primordial mammalian bond—that between mother and infant (Carter, 1998). Early studies of oxytocin receptor expression in the rat brain revealed a decrease in oxytocin receptors in the nucleus accumbens from day 20 to adulthood (Shapiro and Insel, 1989), which coincides with the decline in responsiveness by mothers toward unfamiliar pups (Mayer et al., 1979; Mayer and Rosenblatt, 1979). Furthermore, oxytocin can increase the holding and licking of pups in pre-weaning, but not post-weaning rats (Peterson et al., 1991).
From these observations, Olazabal and colleagues tested the involvement of nucleus accumbens oxytocin receptors in mediating both juvenile and adult alloparental behavior in prairie voles. The nucleus accumbens processes the salience of rewarding, aversive and novel stimuli, thereby helping to translate emotion and motivation into action (Olazabal et al., 2013). In a cross-species comparison, juvenile female prairie voles were found to have the highest nucleus accumbens oxytocin receptor density, followed by juvenile female rats, and then lastly juvenile female mice and meadow voles (both of which do not display alloparental care) (Olazabal and Young, 2006a,b). Similarly, in a comparison of individual differences among juvenile female prairie voles, alloparental behavior was also positively correlated with oxytocin receptor expression in the nucleus accumbens (Olazabal and Young, 2006a, b). Infusion of an oxytocin receptor antagonist into the nucleus accumbens was able to completely block the expression alloparental behavior in adult female prairie voles (Olazabal and Young, 2006a, b). Conversely, over-expression of oxytocin receptors in the nucleus accumbens of female prairie voles increases the expression of alloparental behavior (Keebaugh and Young, 2011), but only if the manipulation takes place before adulthood (Ross et al., 2009). When oxytocin receptors in the nucleus accumbens of female prairie voles are knocked down, via a short hairpin RNA vector, in the juvenile period through adulthood, alloparenting behavior is diminished (Keebaugh et al., 2015).
These findings seem to generalize to other mammalian species as well. Relative to the solitary Cape mole rat, the eusocial naked mole rat, which does practice alloparenting, has higher levels of oxytocin receptor expression in the nucleus accumbens (Kalamatianos et al., 2010). Likewise, marmosets show both high levels of alloparental care as well as high levels of oxytocin receptor density in the nucleus accumbens (Schorscher-Petcu et al., 2009). Peripheral administration of oxytocin to meerkats results in increased cooperative behaviors, including feeding and associating with pups, both of which could be considered alloparental (Madden and Clutton-Brock, 2011). fMRI work in adult humans supports the idea that oxytocin may be a widely conserved mechanism for the expression of alloparental care in mammals. In adult humans, intranasal oxytocin leads to an increase in finding infants’ faces appealing—an effect which depended on the subjects’ oxytocin receptor genotype (Marsh et al., 2012). Also in humans, intranasal oxytocin administration results in less amygdala activation in response to infant crying, while increasing activation in the insula and inferior frontal gyrus (Riem et al., 2011). Activation of the amygdala can produce feelings of fear, anxiety, and disgust (Stark et al., 2003), while the insula has been implicated in empathy (Bartels and Zeki, 2004). Similarly, intranasal oxytocin prior to infant laughter reduced activation in the amygdala and increased functional connectivity between the amygdala and other brain regions involved in emotion regulation (Riem et al., 2012). However, it should be stated that the reproductive experience of the subjects was left unspecified in the above studies (though subjects were tested in response to stimuli from an unrelated infant, which would meet the definition of alloparental responsiveness). In a female rhesus macaque, pharmacological blockade of the oxytocin receptor reduced interest in an infant; however, this was conducted on only a single subject (Boccia et al., 2007).
Research in adult male prairie voles has supported the importance of oxytocin in alloparental behavior, but also that of the closely related neuropeptide arginine vasopressin (Bales et al., 2004a). At least in the ventral pallidum, however, vasopressin 1a receptors do not appear to play a role in the activation of alloparental behavior (Barrett et al., 2013). But in order to prevent the male voles’ ordinarily high propensity for alloparental care, it was necessary to pharmacologically block both the oxytocin and vasopressin 1a receptors throughout the brain simultaneously (Bales et al., 2004a); treatment with an antagonist for either neuropeptide receptor individually failed to block the expression of alloparental care. However, the designer of that particular oxytocin antagonist has since determined it to be less selective than originally thought (Manning et al., 2008). In fact, the presumed oxytocin antagonist had less affinity for the oxytocin receptor than for the vasopressin receptor 1a, so the matter of whether oxytocin receptor blockade affects male alloparental care required study with a more selective antagonist.
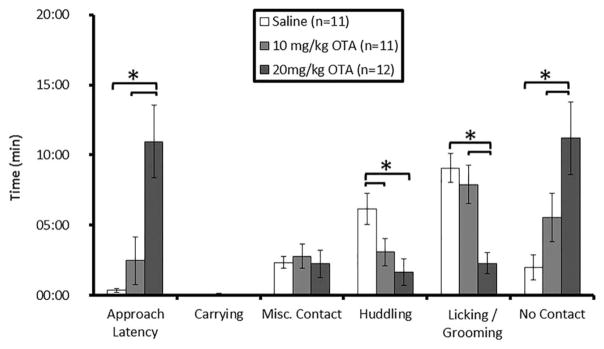
Because previous work had left open the question of oxytocin’s role on alloparental responsiveness in male prairie voles, we sought to investigate the effects of a peripherally administered oxytocin receptor antagonist (OTA, L-368,899)—synthesized and generously provided by Merck Research Laboratories, West Point, PA (Pettibone and Freidinger, 1997), the results of which we present here for the first time. This antagonist readily crosses the blood–brain barrier (Boccia et al., 2007), making peripheral administration a viable approach to block central receptors. Subjects were randomly selected to receive an injection of one of three treatments: (1) a high dose of OTA (OTAHIGH, 20 mg/kg, intraperitoneal) (n = 16), (2) a medium dose of OTA (OTAMED, 10 mg/kg, i.p.) (n = 15), or (3) saline vehicle (n = 17). Following injection, animals were placed into novel cages and allowed to habituate there for 20 minutes. Voles of all three treatments then received an unrelated, 1- to 3-day-old pup and were tested for alloparental behavior.
Within the OTAHIGH group, 7 of 16 animals responded parentally, 5 animals responded non-parentally, and 4 animals attacked the pup. Within the OTAMED group, 9 animals responded parentally, 1 animal responded non-parentally, 3 animals attacked, and 1 was mis-handled (and therefore excluded). Within the saline group, 12 animals responded parentally and 5 attacked the pup. Because the number of pup attacking males was too small to allow group analysis, such animals were not included in the analysis. Results from this experiment are shown in Figure 1. Behavior was scored and compared via ANOVAs, which yielded effects of OTA dose on: approach latency [F(2,32) = 9.206, p = 0.001], huddling [F(2,32) = 5.024, p = 0.013], and licking/grooming [F(2,32) = 11.25, p < 0.001]. Post-hoc analyses revealed that the OTAHIGH group, relative to the saline group, showed significantly greater latency to approach the pup (OTAHIGH = 657.94 ± 155.5 sec, SAL = 21.3 ± 8.2 sec, p = 0.007), less time spent huddling (OTAHIGH = 98.6 ± 56.5 sec, SAL = 370.3 ± 67.4 sec, p = 0.004), and less time spent licking/grooming (OTAHIGH = 137.6 ± 56.5 sec, SAL = 544.5 ± 61.7 sec, p <0.001); relative to the OTAMED group, the OTAHIGH group showed significantly greater latency to approach the pup (OTAHIGH = 657.94 ± 155.5sec, OTAMED = 147.8 ± 101.6 sec, p = 0.003), and less time spent licking/grooming less time (OTAHIGH = 137.6 ± 56.5 sec, OTAMED = 473.9 ± 82.0 sec, p = 0.001). Post-hoc analyses revealed that the OTAMED group, relative to the saline group, spent less time huddling (OTAMED = 185.5 ± 88.0 sec, SAL = 370.3 ± 67.4 sec, p = 0.047). These data suggest that oxytocin may be important for the activation of alloparental care in adult male prairie voles.
Figure 1.
Adult male prairie vole behavior during an alloparental care test following pre-treatment with oxytocin antagonist or vehicle. Systemic treatment with an oxytocin antagonist dose-dependently blocked the expression of alloparental care toward a novel pup. * indicates p <0.05.
Gonadal Steroids
Investigations of male caregiving behavior frequently return to testosterone, as there is a long history of study regarding the negative relationship between testosterone and paternal care in birds, which has only recently begun to be challenged (Lynn, 2016). This has led some to conclude there is no clear relationship between testosterone and alloparental behavior in male mammals (Solomon and Hayes, 2009). Alloparental behavior remains high in male prairie voles after castration as adults (Lonstein and De Vries, 1999). On the other hand, neonatal castration reduced male alloparental behavior when tested as adults (Lonstein et al., 2002). Treatment of male prairie voles with testosterone during early life (postnatal days 1–6) reduces later alloparental behavior (Roberts et al., 1996), whereas neonatal androgen exposure in females does not masculinize alloparental responsiveness (Lonstein et al., 2002).
More fruitful investigations are to be found in the domain of estradiol receptors (ER), the expression and manipulation of which have been explored in voles. In prairie voles, daily administration of a selective ERα agonist during the juvenile period (postnatal days 8–14) results in increased aggression directed at pups in both sexes, and decreased alloparental care as well as increased ERα expression in the bed nucleus of the stria terminalis in males (Perry et al., 2015). Blocking all estrogen receptors during this same juvenile time window decreases alloparental behavior in males (Kramer et al., 2009). However, increasing ERα expression within the medial amygdala of adult male voles leads to an inhibition of alloparental behavior (Lei et al., 2010). It uncertain what factor(s) explain these seemingly contradictory findings, though this could be a case of organizational versus activational differences in the actions of ER signaling. Treating adult female prairie voles with estradiol increases their alloparental responsiveness (Lonstein and De Vries, 1999).
Hypothalamic Pituitary Axis
There have been several different lines of investigation regarding the functional interconnectedness between social behavior and the hypothalamic pituitary axis. Maternal behavior has been found to produce anxiolytic effects on the mother, reducing stress reactivity (Slattery and Neumann, 2008), suggesting the hypothesis that alloparenting might be similarly anxiolytic. The effects of stress and glucocorticoid exposure on alloparental responsiveness in prairie voles are sexually dimorphic and age dependent. Treatment of infant female prairie voles (postnatal days 1–6) with corticosterone reduces later alloparental behavior (Roberts et al., 1996). Adult males but not females increase their alloparental behavior following either a surgical stress or a brief swim stress (Bales et al., 2004a, 2006) (see also “The Anxiolytic Pup?” section below).
Prolactin
Prolactin was originally hypothesized to play a causal role in the activation of paternal caregiving, and many investigations into its functions included alloparental behavior as well. The first such observation between prolactin and male parental behavior in a mammal was made in the common marmoset (Dixson and George, 1982). Among biparental primates, peripheral prolactin levels are increased in fathers and non-breeding alloparents, relative to adult males without infant experience (Ziegler et al., 1996; Soltis et al., 2005). Male meerkats who opted to remain at the nest and alloparent on a given day were found to have higher plasma levels of prolactin earlier in the day (and lower levels of cortisol) (Carlson et al., 2006). However, in that same study of meerkats, cortisol levels were more predictive of pup feeding behavior than either prolactin or testosterone (Carlson et al., 2006).
Experimental manipulations have, however, struggled to find a consistent causal role for prolactin in the production of male parental behavior. Pharmacological blockade of prolactin signaling failed to reduce paternal behavior in hamsters (Brooks et al., 2005), though reducing circulating prolactin via administration of a dopamine agonist did lead to reduced infant-carrying by non-breeding male and female common marmosets (Roberts et al., 2001). A different study found prolactin levels unrelated to the birth of an infant or previous experience in marmoset fathers or alloparents, although physical effort carrying infants produced increased prolactin levels (Mota et al., 2006). Lastly, in male rats, which do not typically display either paternal nor alloparental behavior, prolactin administration promotes maternal behavior-like responses to pups (Sakaguchi et al., 1996). Thus, across taxa prolactin has not consistently been associated with paternal or alloparental behavior.
REACTION OF THE ALLOPARENT
Caregiving is a significant energetic expenditure on the part of the alloparent. Beyond the acute mobilization in response to a vulnerable infant though, the experience of alloparenting produces a number of long-lasting changes in the brain and behavior of the caregiver. The landmark example of this phenomenon recorded in the literature is the process of maternal sensitization described in nulliparous rats by Rosenblatt (1967). In both male and female virgins, repeated exposure to pups gradually induces the expression of behaviors that resemble those displayed by maternal rat dams, hence these virgins are induced to become alloparents. These induced virgins show equally high levels of forebrain prolactin receptors (Sugiyama et al., 1996) and preoptic dopamine transporter (Akbari et al., 2013) compared with lactating females. Pup-induced maternal behavior is also associated with increased neurogenesis in the subventricular zone (Furuta and Bridges, 2009) and enhanced spatial learning-dependent foraging skills (Lambert et al., 2005). We see both acute and long-lasting effects of alloparenting across several domains and multiple species, although the effects of alloparental experience on humans remain largely unexplored.
Acute Effects of Being an Alloparent
The acute consequences of alloparental experience have been investigated primarily in rodent species that reliably show spontaneous alloparental behavior, that is, the prairie and mandarin voles. Such acute effects can be seen across a wide range of physiology and behavior in domains that may speak to the mechanisms necessary to produce alloparenting or may be more consequential in nature.
In adult, virgin male prairie voles, exposure to a novel, unrelated pup for 3 hours resulted in increased c-Fos expression in brain regions also found to be activated during maternal behavior (Kirkpatrick et al., 1994a). Exposure to a pup produced increased neuronal activity in the accessory olfactory bulb, lateral septum, medial amygdala, medial preoptic area, medial portion of the bed nucleus of the stria terminalis, nucleus reuniens, and paraventricular nucleus of the thalamus (Kirkpatrick et al., 1994a).
In a similar study of adult male prairie voles the medial amygdala and bed nucleus of the stria terminalis were also activated following exposure to a pup for 30 minutes (Northcutt and Lonstein, 2009). In P. californicus subjects that responded alloparentally, exposure to a pup for only 10 minutes produced increased c-Fos expression in the reward-sensitive nucleus accumbens along with several nuclei involved in social behavior, fear, and anxiety (the cingulate cortex, lateral septum, medial pre-optic area, and paraventricular nucleus) (Lambert et al., 2011). It is worth noting that a 3-hour long pup exposure did not produce any effects of neural activation in the paraventricular nucleus in adult prairie voles of either sex (Kirkpatrick et al., 1994a). However, a 20-minute pup exposure resulted in increased c-Fos specifically within oxytocin and vasopressin neurons of the paraventricular nucleus along with a concomitant decrease in c-Fos within corticotrophin-releasing hormone producing neurons of that same nucleus, potentially offering an explanation for the lack of an overall effect (Kenkel et al., 2012). The accumulating neuroanatomical evidence points to a circuit of brain regions involved in male alloparental behavior that includes the BNST, LS, MeA, MPOA, NAcc, and PVN and suggests that connections among these may be mediated by neuropeptides, including oxytocin, vasopressin and corticotrophin-releasing hormone.
This same 20-minute pup exposure paradigm has been found to produce a number of other changes in neurobiology and behavior in prairie voles. For instance, this pup exposure paradigm increased cell proliferation within the dentate gyrus of the hippocampus of adult voles of both sexes, however, this effect was more pronounced in animals that did not respond alloparentally than in those that did (Ruscio et al., 2008). A 20-minute pup exposure also facilitates the formation of a subsequent pair-bond when the male alloparent is introduced to a female following the pup exposure (Kenkel et al., 2012). This effect was again made stronger by the inclusion of animals that responded aggressively toward the pup. This latter response may be analogous to the pair-bond facilitating effect of stressors in male prairie voles (DeVries et al., 1996, 2002). We are left with the conclusion that in prairie voles, the pup represents a stimulus that is both arousing and potentially aversive, capable of producing heightened emotional responses. Exaggeration of the emotional response to a pup may underlie the behavior of pup attackers, about which we know very little.
The Anxiolytic Pup?
Several lines of evidence point toward alloparenting being an anxiolytic experience in male prairie voles. In order to test the effects of neuroendocrine manipulation on alloparenting, male prairie voles were fitted with intracerebroventricular cannulae, however, the stress of this surgery led to an unexpected increase in the expression of alloparental care (Bales et al., 2004a). One interpretation of this result was that the alloparents were seeking out the pup in order to cope with the surgical stress. This hypothesis led to a follow-up study which showed exposure of male prairie voles to a 3 minute swim stressor 45 minutes prior to alloparental testing significantly increased the time spent arched-back huddling over pups and tended to increase the time spent licking and grooming pup (Bales et al., 2006). Furthermore, plasma levels of corticosterone were inversely related to licking/grooming and positively related to the number of pup retrievals (Bales et al., 2006), which also suggested that a pup might be perceived as an anxiolytic stimulus to the alloparent.
Intraperitoneal injection with urocortin II increases the time adult voles spent huddling over pups at both 2 and 4 hours after injection (Samuel et al., 2008). The urocortins are members of the corticotropin-releasing hormone family, which also activate the hypothalamic-pituitary-adrenal axis (Reyes et al., 2001). Urocortin II in particular acts selectively on the corticotropin-releasing hormone type 2 receptor (Hsu and Hsueh, 2001). Conversely, chronic treatment with a selective serotonin re-uptake inhibitor (an anti-depressant) delayed approach to a pup in adult voles of both sexes, though those animals were already parents (Villalba et al., 1997). Thus, hormones related to anxiety (e.g., urocortin II) seem to facilitate alloparenting, while anxiolytic treatments reduce caregiving responsiveness (though these results remain to be extended to alloparents).
In the time during and immediately following a pup exposure, there are several further indications that either the pup or the act of alloparenting may be anxiolytic. At 10 minutes into a pup exposure, plasma corticosterone remains low, that is, at baseline levels, relative to voles that were similarly transferred to a new cage but not given a pup to interact with (Kenkel et al., 2012). At this same 10 minute time point, plasma oxytocin levels are increased (Kenkel et al., 2012), and there has been much work to suggest that oxytocin can suppress the activity of the hypothalamic-pituitary-adrenal axis (Neumann et al., 2000).
We followed up on this work directly by assaying anxiety-like behavior in adult male prairie voles immediately after a pup exposure. Subjects consisted of adult male voles that were randomly exposed to either a pup (PUP; n = 15) or dowel (DOW; n = 12) stimulus for 20 minutes in a novel cage to undergo pup exposure. Following the removal of the stimulus, males were placed immediately into an open field that consisted of an open-top plexiglas box, 50 cm in length and width, 20 cm in height. Lighting was maintained at normal levels and subjects always began the open field by being placed in the same corner of the arena. The subjects were left in the open field for 10 minutes (while the pup was returned to its nest); during this time the subjects’ behavior was recorded for later analysis. The center was defined as one quarter of the total area. Locomotor activity and time in the center were both measured by means of a grid overlay during video analysis. Behaviors scored during the open field test included: lines crossed, time spent in center, digging, climbing, and auto-grooming.
Within the PUP group, 12 of the 15 males responded parentally and so were included in subsequent analyses; 3 males attacked the pup. Following pup exposure, no differences were found between the two groups in any of the open field behaviors measured across the full 10 minute test. However, when the observations were constrained to the first 5 minutes of testing, PUP animals were found to have spent more time in the center (15.22 ± 3.66 sec) than DOW animals (5.15 ± 1.31 sec) (F = 6.152, p = 0.028) as shown in Figure 2. Both groups crossed a similar number of lines (PUP = 136.5 ± 20.8, DOW = 117.7 ± 10.6), indicating that the difference with respect to time in the center was not a function of overall locomotion.
Figure 2.
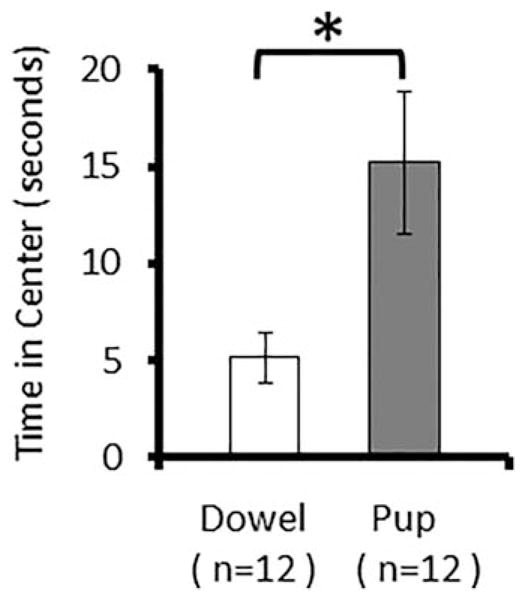
Adult male prairie vole behavior during an open field test of anxiety-related behavior following exposure to a pup. Subjects that had been exposed to a pup for 20 minutes immediately prior to open field testing spent more time in the center of the arena, indicative of less anxiety-related behavior. * indicates p <0.05.
From this collection of findings, there is strong evidence that stressors both acute and chronic facilitate alloparental care in male prairie voles, and that this may be due to anxiolytic properties of the pup and/or the act of alloparenting the pup. Indeed, the expression of alloparental care in prairie voles appears to be relaxed to a casual observer. However, when we examined the autonomic state of alloparental voles during this seemingly calm behavior, we encountered an unanticipated response: a sustained increase in heart rate (Kenkel et al., 2013, 2014, 2015).
Cardioacceleration and Alloparenting
When we first implanted adult prairie voles with radiotelemetry devices to measure their heart rate, locomotor activity and temperature while alloparenting, we hypothesized based on the results described above, that an anxiolytic alloparenting experience would manifest a state of lower physiological arousal in the caregiving adult. Recall that pup exposure produced an attenuation of corticosterone in adult males, and that the activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system are highly correlated across a variety of stressors (among 60 studies, the magnitude of plasma norepinephrine and ACTH responses during stress correlate with an r = 0.93 (Goldstein and Kopin, 2008)). Thus, the first observation that alloparenting represented an apparent divergence of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system was unexpected.
Throughout the entirety of a pup exposure episode, adult prairie voles demonstrate a sustained increase in heart rate (Kenkel et al., 2013). A typical baseline heart rate for an adult prairie vole is 360–380 beats per minute, but heart rate rises to 500+ beats per minute in the presence of a pup. This response persists throughout a typical 20 minute pup exposure, and does not attenuate nor habituate upon repeated exposures across 3 days, nor extended exposures lasting 60 minutes (Kenkel et al., 2013). Pup-induced cardioacceleration can be seen in virgin males (Kenkel et al., 2013), virgin females (Kenkel et al., 2015), and sexually experienced fathers (Kenkel et al., 2014). Pup-induced cardioacceleration coincided with a decrease in locomotor activity and was dependent on close physical proximity to the pup; merely experiencing pup stimuli was insufficient (Kenkel et al., 2013). Furthermore, the phenomenon is specific to interaction with a pup, as the cardioacceleration seen in response to exposure to a novel adult subsides within 5–10 minutes (Kenkel et al., 2013).
An initial first clue to the underlying function of this cardioacceleratory property of vole alloparenting came from the observation that the effect diminishes with advancing pup age (Kenkel et al., 2015). The isolation-induced vocalizations emitted by pups also diminish with advancing pup age, which suggested the pups were becoming more independent and less reliant on alloparental care. Isolation-induced vocalizations were almost completely abolished in the presence of an alloparent or when the ambient temperature was raised to a thermoneutral 36° (Kenkel et al., 2015). This then led us to test for pup-induced cardioacceleration in thermoneutral conditions, where we found it too was greatly diminished. Thus, we were able to hypothesize that the most likely function for pup-induced cardioacceleration is to serve as a means by which the alloparent can convey body warmth to the pup and thereby meet its thermo-regulatory needs.
One intriguing aspect of the physiological state of the alloparent during pup-induced cardioacceleration is the maintenance of parasympathetic cardiac tone. Typically, increases in heart rate are achieved through increases in sympathetic drive as well as release of the so-called “vagal brake,” which constitutes parasympathetic regulation of heart rate. However, in the alloparental vole, we observed less reduction in cardiac vagal tone than expected, and using pharmacological blockade, we were able to determine that the cardioacceleration resulted primarily from an increase in sympathetic tone (Kenkel et al., 2013). This raises the question of what sort of autonomic state pup attackers are in upon presentation with a pup. The heightened state of arousal brought on by a pup may play a role in the etiology of such attacks.
Exposure to a pup is a significant event in the life of a prairie vole. The alloparental response seems to represent an emotionally arousing state, one that is facilitated by pre-existing arousal in the form of a stressor (at least for males). We hypothesize that dysregulation of arousal during pup exposure may predispose some animals to respond aggressively toward the pup. This offers at least face validity for cases of child abuse in humans, as abuse typically takes place in the context of high arousal states.
Long-Lasting Effects of Being an Alloparent
One hypothesis for the evolution of alloparenting is that it serves as parenting “practice” for younger offspring prior to having infants of their own—remaining in the natal nest for a period of time following weaning and caring for the next litter of infants from the breeding pair gives young animals experience in caring for offspring that should make them better prepared to raise their own offspring later. This hypothesis has been put forward to explain heightened interest in infants among juvenile female primates (Lancaster, 1971), however, the data instead support an alternative explanation that juvenile female interest in infants could be a non-adaptive by-product of intense selection for maternal behavior (Jamieson, 1989; Paul and Kuester, 1996; Silk, 1999; Meredith, 2015). Thus, such an interest in infants may have served as a spandrel for the eventual adoption of alloparental behavior in our own species.
There is also conflicting support for the “practice” hypothesis in the prairie vole, where offspring who are exposed to pups as adolescents show lower levels of parental care toward their own offspring than do breeding pairs where neither partner has prior experience alloparenting (Stone et al., 2010) yet exhibit greater amounts of alloparenting behavior toward unrelated offspring as subadults (Roberts et al., 1998a). This increase in alloparenting of unrelated pups is also seen in male mandarin voles after prior experience with infants (Song et al., 2010) as well as in Mongolian gerbils (Salo and French, 1989). Pups of breeding pairs where at least one parent has prior alloparenting experience do, however, show increased weight gain, suggesting that while less time is spent caring for offspring, parents may be more efficient or effective with the time they do spend with pups. These perhaps contradictory findings support the need to understand not only the neurobiology that underlies alloparental behavior in both males and females but also how this circuitry overlaps with that which regulates paternal and maternal behavior.
Given the differences in parental care with and without prior alloparenting experience, there also needs to be more work examining how alloparenting experience itself shapes these neural circuits in both sexes. For example, remaining in the natal nest to alloparent a subsequent litter results in increased anxiety-like behavior and decreased exploration of a novel environment and also leads to decreases in brain-derived neurotrophic factor (BDNF) in the CA1 region of the hippocampus and sex-specific increases in BDNF in the bed nucleus of the stria terminalis in voles (Greenberg et al., 2012). Lower levels of BDNF in CA1 are associated with increases in anxiety (Groves, 2007). That alloparenting experience produces changes in BDNF and this associated behavior suggests that it does have long-term consequences for animals. Thus, we are left with divergent effects of acute versus extensive alloparenting on prairie voles’ anxiety.
On the other hand, extensive alloparental experience in virgin female rats for either 3 or 14 days reduces the expression of anxiety-related behaviors as well as increases the expression of the rate-limiting enzyme for serotonin synthesis in the 14 day condition (Harding and Lonstein, 2016). Similar results were seen in the alloparental African striped mouse (Rhabdomys pumilio), where alloparenting experience resulted in less anxiety-related behavior in females (Pillay and Rymer, 2015). Other consequences of alloparenting experience in female African striped mice include heightened competitiveness in a social competence test and improved working and long-term memory. These effects were specific to the caregiving behaviors of alloparenting, as they were not seen in subjects merely exposed to pup stimuli without the option to physically interact. Unfortunately, the underlying factors in the species differences in the effects of extensive alloparenting on anxiety have not been addressed. In both prairie voles and African striped mice, philopatry (remaining with the natal nest so as to serve as an alloparent) is associated with reproductive suppression (Carter et al., 1995; Schradin and Pillay, 2004).
Studies in mandarin voles reinforce the notion that alloparenting experience produces long-lasting changes in the brain and behavior of alloparents. Mandarin voles with long-term alloparental experience displayed significantly more locomotor activity in a novel environment and were more investigatory of a novel same-sex conspecific (Wu et al., 2013). Prior experience with pups facilitates alloparental responsiveness in adult male mandarin voles, yet intriguingly also leads to an upregulation in ERα expression within the medial amygdala (Song et al., 2010). This same pattern of ERα expression in the medial amygdala was negatively associated with alloparental responsiveness in prairie voles (Lei et al., 2010). Alloparental experience in male mandarin voles also leads to upregulation of ERα expression within the arcuate nucleus. A single 10-minute exposure to a novel pup results in an increased number of oxytocin neurons in the hypothalamus (PVN and SON) of adult male mandarin voles 1 week later (Song et al., 2010). This finding deserves replication as it may speak to a profound and long-lasting change in brain and behavior triggered by only a brief social experience.
The psychological consequences of alloparenting experience in humans has received some attention, but primarily thus far as it relates to subsequent parental behavior, and not whether alloparenting experience may impact other social/affective traits. Prior experience caring for infants does not correlate with cortisol, estradiol, testosterone, progesterone, nor attachment to one’s own infant in postpartum women, although such experience does boost the confidence of would-be mothers (Fleming et al., 1997), as well as prenatal feelings of competence, positive attitude (Fleming et al., 1988) and efficacy in childcare (Leerkes and Burney, 2007).
ONTOGENY OF ALLOPARENTING
Experiences during early development alter a number of outcomes in offspring, including displays of social behavior. In the prairie vole variation in early family structure, early manipulation and parental care, and early neuropeptide exposure all have lasting, often sex-specific consequences on alloparental behavior.
Early Family Structure
In the field, prairie voles family units may include single females rearing offspring alone, a single male/female breeding pair, or a communal group comprised of a breeding pair as well as non-reproductive animals who serve as alloparents (Getz and Carter, 1996). Differences in family structure are observed across different populations of voles, where those originating from territories in Kansas do not typically form communal groups like those that are seen in voles originating from Illinois (Danielson and Gaines, 1987). This variation in family structure and therefore variation in the number of caretakers available in early life results in long term consequences on alloparenting behavior in offspring. From a population perspective, increased alloparenting is seen in Illinois voles in the lab, modeling what is likely seen in the field based on family structure (Roberts and Carter, 1997). In fact, the presence of the father in the nest in Kansas voles decreases alloparenting in juveniles (Roberts and Carter, 1997; Roberts et al., 1998b), while a reduction in alloparenting is seen in Illinois offspring with the absence of the father (Wang and Novak, 1994; Roberts and Carter, 1997). This dichotomy between populations reinforces the notion that variation in habitat and resource availability, demonstrated in these two populations, alters behavior of animals, even under laboratory conditions, potentially through impacts on neural circuits. However, more recent studies on these regional populations have called overt differences between them into question (Ophir et al., 2007) and prairie voles in general are far from genetically monogamous (Ophir et al., 2008b).
Along with effects of geography, family structure variation also results in sex-specific differences in alloparenting that appear to vary by age. Rearing by a single mother decreases subsequent alloparenting behaviors, including licking and grooming, huddling, and time in the nest, in females as adults with no apparent impact on males (Ahern and Young, 2009) while single-mother rearing produces similar effects in juvenile male offspring (Wang and Novak, 1994). That effects of early family structure are sex- and age-dependent are interesting—the rate of spontaneous alloparenting is high in male voles throughout adolescence and adulthood while females are likely to become more infanticidal as they age (Lonstein and De Vries, 1999). However, females who remain in the natal nest post-weaning into adulthood, even without the presence of younger pups to care for, tend to show high levels of alloparenting when exposed to a pup but only if both the mother and father remain in the nest with them (Lonstein and De Vries, 2001). It may be that biparental rearing early in life acts as a signal to offspring that the environment may not be well-suited for dispersal upon weaning and females instead maintain a higher level of alloparenting that would be appropriate in caring for younger siblings, and subsequent cooperative living.
Early Handling and Parental Care
Early handling in rats has life-long consequences for behavior and neurobiology of offspring, including the later display of maternal behavior (Levine et al., 1967; Meaney and Aitken, 1985; Ladd et al., 1996; Boccia and Pedersen, 2001). Work in the prairie vole has demonstrated effects of early manipulation on species-typical social behaviors, including alloparenting. A single manipulation by being picked up in a cup on PND 1 (MAN0 condition) resulted in decreases in displays of alloparental behavior in adolescent male offspring compared with behavior of animals that experienced a single manipulation by a gloved hand (MAN1 condition) at the same time point in early life (Bales et al., 2007a, 2011). Offspring appear to be especially sensitive to a single manipulation on PND 1, as manipulation with a gloved hand only on PND 7 does not have the same effect on alloparenting (Bales et al., 2011). Likewise, additional disturbance on PND 1, including the MAN1 manipulation occurring 3 times on PND 1, led to an overall reduction in alloparental behavior and an increase in pup attacks in male offspring (Boone et al., 2006). The effects of this early manipulation are transmitted to the next generation as well—offspring of pairs where at least one parent experienced the early life MAN0 manipulation show decreased alloparenting in adolescence (Stone and Bales, 2010). These pairs that included a MAN0 animal also showed a remarkable decrease in successful breeding, indicating that this early handling experience has lasting impacts not only on behavior of offspring but also on reproductive fitness.
Differences in alloparenting behavior as well as other offspring outcomes following early handling in voles may be mediated by changes in parental behavior due to the manipulation itself. Indeed, the MAN1 condition results in an increase in parental pup-directed behavior (Tyler et al., 2005) similar to that observed in rat models of early handling (Smotherman et al., 1977; Smotherman and Bell, 1980). Naturally occurring variation in unmanipulated breeding pairs leads to very similar behavioral phenotypes in offspring. Offspring of parents displaying high levels of early postpartum infant care engage in greater amounts of alloparenting as adolescents then do offspring of parents that engage in decreased amounts of parental care (Perkeybile et al., 2013). Cross-fostering shows this behavior is transmitted from parent to offspring via non-genomic mechanisms—alloparenting behavior of offspring is predicted by the parental care received as an infant (Perkeybile et al., 2015), indicating that at least some components of this behavior are gained through early experience. The exact mechanisms by which this occurs are not yet known. Early MAN0 handling, in addition to decreasing alloparental behavior, also disrupts partner preference formation (Bales et al., 2007a) and leads to increased OTR binding in several regions associated with social behavior, including the BNST and NAcc (Bales et al., 2011). Increased OTR binding is also seen in the BNST following decreased early care (Perkeybile and Bales, unpublished data). Variation in OTR binding in the NAcc has been repeatedly associated with variation in alloparenting, as discussed previously (Olazabal and Young, 2006a, b; Keebaugh and Young, 2011; Keebaugh et al., 2015). It is likely that early handling and early parental care, then, result in altered alloparental behavior through altering OTR density in neural circuitry necessary for social behavior.
Early Neuropeptide Exposure
Exposure to OT early in life alters the developmental trajectories of offspring, including alloparenting at both adolescent and adult ages. Blocking OT on PND 1 serves to decrease alloparental behavior in males at weaning, including increasing attack rates (Bales et al., 2004b). Administration of OT on PND1, meanwhile, increases alloparenting in adult females, but the effect was dose-dependently (Bales et al., 2007c). These same early manipulations also alter neuropeptide availability, as measured by OT and AVP immunoreactivity (Yamamoto et al., 2004; Kramer et al., 2006), and binding of their associated receptor systems (Bales et al., 2007b). While we know that these neuropeptides are involved in regulating alloparental behavior, the differing effects of different doses of OT suggest that more work needs to be done to fully understand the mechanisms that drive this behavior. In addition, there appear to be differences between males and females in the role neuropeptides play in shaping alloparenting. The neurobiology of this behavior has been characterized in females, but given the high rates of alloparenting seen in male prairie voles throughout adolescence and into adulthood, it will be important to also characterize this system in males and identify differences or correspondence with the circuitry seen in females.
Communal Nesting
While not always strictly alloparenting in the sense that unrelated animals are caring for younger offspring, communal nesting is a common form of “community care” for rodent offspring. Communal nesting occurs when females combine their pups into a single next and share rearing responsibilities between each other (Crowcroft and Rowe, 1963; Sayler and Salmon, 1969; Branchi, 2009). This style of offspring rearing is highly common in mice in the field, with estimates of prevalence up to 90% (Crowcroft and Rowe, 1963; Manning et al., 1992; Branchi, 2009). There are a number of reported benefits to offspring being born and reared in a communal nest, including increased rates of survival (Mennella et al., 1990; Konig, 1994), faster rates of development, indexed often by weight gain (Sayler and Salmon, 1969; Mennella et al., 1990; Hayes and Solomon, 2004), and decreased rates of infanticide (Schultz and Lore, 1993; Manning et al., 1995).
Recently, communal nesting in mice has been developed into a model of social enrichment in the laboratory in an effort to understand the role social complexity in the early environment plays in shaping developmental trajectories in offspring (Branchi, 2009). In this model, offspring are reared in a nest with their mother and littermates and two additional lactating dams and their respective litters. Compared with offspring reared under standard laboratory conditions with a single mother and litter, communally nesting offspring receive greater amounts of maternal care and display higher levels of social interaction (Branchi et al., 2006a; Curley et al., 2009). They also show a higher level of social competency, forming social hierarchies quicker (Branchi et al., 2006a) and maintaining them under varying social conditions more appropriately (D’Andrea et al., 2007). These behaviors appear to be responsive to both maternal and peer interactions—offspring with greater amounts of combined early maternal and peer contact demonstrate higher social competency levels (Branchi et al., 2013a). That social complexity in the early environment, in the form of increased maternal care and increased peer interaction, appears to consistently result in animals that are able to more appropriately interact with their social environment indicated that communal nesting may benefit offspring not just during development, but also into adulthood.
Effects of communal nesting on offspring behavior extend to their response to a stressor, where offspring from communal nests show increases in anxiety-like behavior in an elevated plus maze (Branchi et al., 2006b; D’Andrea et al., 2010) that can be ameliorated if tested with a conspecific (Branchi and Alleva, 2006), suggesting that social support has the potential to regulate stress response in these animals. In fact, the type of stressor used results in differing outcomes. Communally nested offspring show less anhedonia and a decreased corticosteroid response during a social stressor compared with offspring from single mother nests (Branchi et al., 2010, 2013b) and a faster recovery from this stressor (D’Andrea et al., 2010), yet do not differ in response to a physical stressor. It may be that rearing in an environment with a high degree of social contact and interaction programs offspring toward a decreased and potentially more adaptive response to social stress in adulthood.
This early rearing also leads to increases in BDNF levels in a number of regions, including the striatum, hippocampus, hypothalamus, and frontal cortex (Branchi et al., 2006a, b, 2013a). Reduced BDNF has been associated with an increased risk for developing psychopathology (Duman et al., 1997; Nestler et al., 2002). These offspring of communal nests also consistently show decreases in depressive-like behavior (Branchi et al., 2006a, 2010; D’Andrea et al., 2010). Communal nesting, then, may be acting on BDNF and neurotrophins more broadly in early life to regulate circuitry associated with vulnerability to psychopathologies—early social complexity in the form of multiple caregivers and a high degree of peer interaction may serve as a protective measure for later development of psychiatric dysfunction and disorder.
UNRESOLVED TOPICS IN ALLOPARENTING
We are left with several unresolved topics, the addressing of which we believe will lead to greater translatability between animal models of alloparenting and human health and wellbeing. To wit, we know very little regarding the actual benefits of alloparenting beyond very gross measures such as increased weight gain and survival. What social, emotional, and neuroendocrine differences are present in pups who experienced alloparental care in early life? Mandarin vole pups that were raised with older sibling alloparents present demonstrated less aggression and anxiety-related behavior as adults, as well as increased parenting behavior toward their own pups (Wu et al., 2013), which is a promising start to this line of research.
We also have very little data as to the difference between fatherhood and alloparenting. How does the paternal experience differ from an extended bout of alloparenting? Ultimately, paternity uncertainty means this may be a matter of semantics, but there are still important implications for the neurobiology of male caregiving. This has so far been difficult to address in prairie voles because on the one hand there does not appear to be any difference in caregiving behaviors between these two conditions, while on the other fatherhood requires mating, which is accompanied by substantial endocrine changes in prairie voles. Fathers responded to pups with a similar degree of cardioacceleration compared with virgin males, however their baseline heart rate was lower at rest, suggesting adaptation to the chronic condition of provisioning care for pups. Ideally, future work would compare sexually experienced males with and without pup exposure.
What factors among either pups or adults predispose the adult to attacking a pup? This is critical if behavioral neuroendocrinologists wish to inform human child abuse. Unhealthy children and children with disabilities are more likely to suffer maltreatment (Daly and Wilson, 1988b) and, on a population level, disease burden (“parasite stress”) is positively correlated with rates of child abuse across the U.S. (Thornhill and Fincher, 2011) (however, see also Hackman and Hruschka, 2013 for discussion of how fast life history may be a better predictor of outcome). Furthermore, young children are more likely to suffer both lethal and non-lethal maltreatment (Daly and Wilson, 1988a, b; Thornhill and Fincher, 2011) (see also the U.S. National Child Abuse and Neglect Data System (NCANDS), http://www.childwelfare.gov/systemwide/statistics/can.cfm). Thus, we would predict that younger, less healthy pups will be more likely to suffer attack. Upon establishing such an effect, interventions could then be targeted at the adult animal with the goal of reducing aggressive behavior. Interestingly, bilaterally bulbectomized, virgin adult male prairie voles were significantly more likely to attack pups than were sham-lesioned males (Kirkpatrick et al., 1994b), suggesting that olfactory cues normally inhibit pup-directed aggression.
There is also a certain practice in the research community which deserve to be addressed. The majority of prairie vole labs typically wean offspring at 20–21 days of age, precluding older siblings from expressing alloparental care toward the subsequent litter. This deviation from ethological norms poses serious consequences beyond merely the realm of alloparental behavior. We have detailed above the vast and robust changes alloparenting brings about upon the caregiver, and research has only begun to describe the effects upon the recipient, but we can already conclude that depriving animals of this experience alters their social, emotional and neuroendocrine development. Whether we can reasonably accommodate the constraints of animal husbandry while staying true to the natural history of the prairie vole will be a challenge for future research.
Acknowledgments
The authors would like to acknowledge that this work was supported by a grant from the NIH: HD075750 (CSC).
References
- Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari EM, Shams S, Belay HT, Kaiguo M, Razak Z, Kent CF, Westwood T, et al. The effects of parity and maternal behavior on gene expression in the medial preoptic area and the medial amygdala in postpartum and virgin female rats: A microarray study. Behav Neurosci. 2013;127:913–922. doi: 10.1037/a0034884. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kim AJ, Lewis-Reese AD, Carter CS. Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm Behav. 2004a;45:354–361. doi: 10.1016/j.yhbeh.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev Psychobiol. 2004b;44:123–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- Bales KL, Kramer KM, Lewis-Reese AD, Carter CS. Effects of stress on parental care are sexually dimorphic in prairie voles. Physiol Behav. 2006;87:424–429. doi: 10.1016/j.physbeh.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, Carter CS. Early experience affects the traits of monogamy in a sexually dimorphic manner. Dev Psychobiol. 2007a;49:335–342. doi: 10.1002/dev.20216. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors. Neuroscience. 2007b;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, van Westerhuyzen JA, Lewis-Reese AD, Grotte ND, Lanter JA, Carter CS. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav. 2007c;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Boone E, Epperson P, Hoffman G, Carter CS. Are behavioral effects of early experience mediated by oxytocin? Front Psychiatry. 2011;2:24. doi: 10.3389/fpsyt.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CE, Keebaugh AC, Ahern TH, Bass CE, Terwilliger EF, Young LJ. Variation in vasopressin receptor (Avpr1a) expression creates diversity in behaviors related to monogamy in prairie voles. Horm Behav. 2013;63:518–526. doi: 10.1016/j.yhbeh.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Pedersen CA. Brief vs. long maternal separations in infancy: Contrasting relationships with adult maternal behavior and lactation levels of aggression and anxiety. Psychoneuroendocrinology. 2001;26:657–672. doi: 10.1016/s0306-4530(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Boccia ML, Goursaud AP, Bachevalier J, Anderson KD, Pedersen CA. Peripherally administered non-peptide oxytocin antagonist, L368,899, accumulates in limbic brain areas: A new pharmacological tool for the study of social motivation in non-human primates. Horm Behav. 2007;52:344–351. doi: 10.1016/j.yhbeh.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B, Bragg J, Kuzawa C. Humans are not cooperative breeders but practice biocultural reproduction. Ann Hum Biol. 2014;41:368–380. doi: 10.3109/03014460.2014.923938. [DOI] [PubMed] [Google Scholar]
- Boone E, Sanzenbacher LL, Carter CS, Bales KL. Society for Neurosci. Atlanta, GA: 2006. Sexually dimorphic effects of early experience on alloparental care and adult social behavior in voles. [Google Scholar]
- Branchi I. The mouse communal nest: Investigating the epigenetic influences of the early social environment on brain and behavior development. Neurosci Biobehav Rev. 2009;33:551–559. doi: 10.1016/j.neubiorev.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Branchi I, Alleva E. Communal nesting, an early social enrichment, increases the adult anxiety-like response and shapes the role of social context in modulating the emotional behavior. Behav Brain Res. 2006;172:299–306. doi: 10.1016/j.bbr.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006a;60:690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment augments adult hippocampal BDNF levels and survival of BRDU-positive cells while increasing anxiety- and depression-like behavior. J Neurosci Res. 2006b;83:965–973. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- Branchi I, D’Andrea I, Cirulli F, Lipp HP, Alleva E. Shaping brain development: Mouse communal nesting blunts adult neuroendocrine and behavioral response to social stress and modifies chronic antidepressant treatment outcome. Psychoneuroendocrinology. 2010;35:743–751. doi: 10.1016/j.psyneuen.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Branchi I, Curley JP, D’Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013a;38:522–532. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Santarelli S, D’Andrea I, Alleva E. Not all stressors are equal: Early social enrichment favors resilience to social but not physical stress in male mice. Horm Behav. 2013b;63:503–509. doi: 10.1016/j.yhbeh.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Brooks PL, Vella ET, Wynne-Edwards KE. Dopamine agonist treatment before and after the birth reduces prolactin concentration but does not impair paternal responsiveness in Djungarian hamsters, Phodopus campbelli. Horm Behav. 2005;47:358–366. doi: 10.1016/j.yhbeh.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Burkart JM, Hrdy SB, Van Schaik CP. Cooperative breeding and human cognitive evolution. Evol Anthropol. 2009;18:175–186. [Google Scholar]
- Carlson AA, Russell AF, Young AJ, Jordan NR, McNeilly AS, Parlow AF, Clutton-Brock T. Elevated prolactin levels immediately precede decisions to babysit by male meerkat helpers. Horm Behav. 2006;50:94–100. doi: 10.1016/j.yhbeh.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Roberts RL. The psychobiological basis of cooperative breeding. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. New York: Cambridge Press; 1997. pp. 231–266. [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: The prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Crowcroft P, Rowe FP. Social organization and territorial behaviour in the wild house mouse (Mus musculus L.) Proc Zool Soc Lond. 1963;140:517–531. [Google Scholar]
- Curley JP, Davidson S, Bateson P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Behav Neurosci. 2009;3:25. doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea I, Alleva E, Branchi I. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behav Brain Res. 2007;183:60–66. doi: 10.1016/j.bbr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- D’Andrea I, Gracci F, Alleva E, Branchi I. Early social enrichment provided by communal nest increases resilience to depression-like behavior more in female than in male mice. Behav Brain Res. 2010;215:71–76. doi: 10.1016/j.bbr.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Daly M, Wilson M. Evolutionary social psychology and family homicide. Science. 1988a;242:519–524. doi: 10.1126/science.3175672. [DOI] [PubMed] [Google Scholar]
- Daly M, Wilson M. Homicide. New York: Aldine de Gruyter; 1988b. [Google Scholar]
- Danielson BJ, Gaines MS. Spatial patterns in 2 syntopic species of microtines - Microtus ochrogaster and Synaptomys cooperi. J Mamm. 1987;68:313–322. [Google Scholar]
- DeSilva JM. A shift toward birthing relatively large infants early in human evolution. Proc Natl Acad Sci U S A. 2011;108:1022–1027. doi: 10.1073/pnas.1003865108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci U S A. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, Carter CS. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology. 2002;27:705–714. doi: 10.1016/s0306-4530(01)00073-7. [DOI] [PubMed] [Google Scholar]
- Dixson AF, George L. Prolactin and parental behaviour in a male New World primate. Nature. 1982;299:551–553. doi: 10.1038/299551a0. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Ruble D, Flett GL, Shaul DL. Postpartum adjustment in first-time mothers: Relations between mood, maternal attitudes, and mother-infant interactions. Dev Psychol. 1988;24:71–81. [Google Scholar]
- Fleming AS, Ruble D, Krieger H, Wong PY. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm Behav. 1997;31:145–158. doi: 10.1006/hbeh.1997.1376. [DOI] [PubMed] [Google Scholar]
- Furuta M, Bridges RS. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res Bull. 2009;80:408–413. doi: 10.1016/j.brainresbull.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galdikas BM, Wood JW. Birth spacing patterns in humans and apes. Am J Phys Anthropol. 1990;83:185–191. doi: 10.1002/ajpa.1330830207. [DOI] [PubMed] [Google Scholar]
- Garber PA. One for all and breeding for one: Cooperation and competition as a tamarin reproductive strategy. Evol Anthropol. 1996;5:187–199. [Google Scholar]
- Getz LL, Carter CS. Prairie-vole partnerships. Am Sci. 1996;84:56–62. [Google Scholar]
- Getz LL, McGuire B, Hofmann J, Pizzuto T, Frase B. Natal dispersal and philopatry in the prairie vole (Microtus ochrogaster): Settlement, survival, and potential reproductive success. Ethol Ecol Evol. 1994;6:267–284. [Google Scholar]
- Goldstein DS, Kopin IJ. Adrenomedullary, adrenocortical, and sympathoneural responses to stressors: A meta-analysis. Endocr Regul. 2008;42:111–119. [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, van Westerhuyzen JA, Bales KL, Trainor BC. Is it all in the family? The effects of early social structure on neural-behavioral systems of prairie voles (Microtus ochrogaster) Neuroscience. 2012;216:46–56. doi: 10.1016/j.neuroscience.2012.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Schneider KA, Jeannotte LA. Individual differences in the mechanisms underlying the onset and maintenance of paternal behavior and the inhibition of infanticide in the monogamous biparental California mouse, Peromyscus californicus. Behav Ecol Sociobiol. 1994;34:225–231. [Google Scholar]
- Hackman J, Hruschka D. Fast life histories, not pathogens, account for state-level variation in homicide, child maltreatment, and family ties in the U.S. Evol Hum Behav. 2013;34:118–124. [Google Scholar]
- Harding KM, Lonstein JS. Extensive juvenile baby-sittingfacilitates later adult maternal responsiveness, decreases anxiety, and increases dorsal raphe tryptophan hydroxylase-2 expression in female laboratory rats. Dev Psychobiol. 2016;58:492–508. doi: 10.1002/dev.21392. [DOI] [PubMed] [Google Scholar]
- Hayes LD, Solomon NG. Costs and benefits of communal rearing to female prairie voles (Microtus ochrogaster) Behav Ecol Sociobiol. 2004;56:585–593. [Google Scholar]
- Hrdy SB. Mothers and Others: The Evolutionary Origins of Mutual Understanding. Cambridge, MA: Belknap Press of Harvard University Press; 2009. [Google Scholar]
- Hsu SY, Hsueh AJ. Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat Med. 2001;7:605–611. doi: 10.1038/87936. [DOI] [PubMed] [Google Scholar]
- Isler K, van Schaik CP. Allomaternal care, life history and brain size evolution in mammals. J Hum Evol. 2012;63:52–63. doi: 10.1016/j.jhevol.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Jamieson IG. Behavioral heterochrony and the evolution of birds’ helping at the nest: An unselected consequences of communal breeding? Am Nat. 1989;133:394–406. [Google Scholar]
- Kalamatianos T, Faulkes CG, Oosthuizen MK, Poorun R, Bennett NC, Coen CW. Telencephalic binding sites for oxytocin and social organization: A comparative study of eusocial naked mole-rats and solitary cape mole-rats. J Comp Neurol. 2010;518:1792–1813. doi: 10.1002/cne.22302. [DOI] [PubMed] [Google Scholar]
- Keebaugh AC, Young LJ. Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm Behav. 2011;60:498–504. doi: 10.1016/j.yhbeh.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, Barrett CE, Laprairie JL, Jenkins JJ, Young LJ. RNAi knockdown of oxytocin receptor in the nucleus accumbens inhibits social attachment and parental care in monogamous female prairie voles. Soc Neurosci. 2015;10:561–570. doi: 10.1080/17470919.2015.1040893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J Neuroendocrinol. 2012;24:874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Lewis GF, Yee JR, Pournajafi-Nazarloo H, Grippo AJ, Porges SW, et al. Autonomic substrates of the response to pups in male prairie voles. PLoS One. 2013;8:e69965. doi: 10.1371/journal.pone.0069965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Suboc G, Carter CS. Autonomic, behavioral and neuroendocrine correlates of paternal behavior in male prairie voles. Physiol Behav. 2014;128:252–259. doi: 10.1016/j.physbeh.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Yee JR, Porges SW, Ferris CF, Carter CS. Cardioacceleration in alloparents in response to stimuli from prairie vole pups: The significance of thermoregulation. Behav Brain Res. 2015;286:71–79. doi: 10.1016/j.bbr.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GE. From the ape’s dilemma to the weanling’s dilemma: Early weaning and its evolutionary context. J Hum Evol. 2005;48:123–145. doi: 10.1016/j.jhevol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Kendrick KM. Oxytocin facilitation of maternal behavior in sheep. Ann N Y Acad Sci. 1992;652:83–101. doi: 10.1111/j.1749-6632.1992.tb34348.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Kakoyannis A. Sexual dimorphism and the NMDA receptor in alloparental behavior in juvenile prairie voles (Microtus ochrogaster) Behav Neurosci. 2004;118:584–589. doi: 10.1037/0735-7044.118.3.584. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Kim JW, Insel TR. Limbic system fos expression associated with paternal behavior. Brain Res. 1994a;658:112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Williams JR, Slotnick BM, Carter CS. Olfactory bulbectomy decreases social behavior in male prairie voles (M. ochrogaster) Physiol Behav. 1994b;55:885–889. doi: 10.1016/0031-9384(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Ando J, Tatara S, Yamada N, Konishi K, Kimura N, Fukumori A, et al. Alloparenting for chimpanzee twins. Sci Rep. 2014;4:6306. doi: 10.1038/srep06306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig B. Components of lifetime reproductive success in communally and solitarily nursing house mice - A laboratory study. Behav Ecol Sociobiol. 1994;34:275–283. [Google Scholar]
- Kramer KM, Choe C, Carter CS, Cushing BS. Developmental effects of oxytocin on neural activation and neuropeptide release in response to social stimuli. Horm Behav. 2006;49:206–214. doi: 10.1016/j.yhbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Perry AN, Golbin D, Cushing BS. Sex steroids are necessary in the second postnatal week for the expression of male alloparental behavior in prairie voles (Microtus ochragaster) Behav Neurosci. 2009;123:958–963. doi: 10.1037/a0016927. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Lahdenpera M, Lummaa V, Helle S, Tremblay M, Russell AF. Fitness benefits of prolonged post-reproductive lifespan in women. Nature. 2004;428:178–181. doi: 10.1038/nature02367. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Berry AE, Griffin G, Amory-Meyers E, Madonia-Lomas L, Love G, Kinsley CH. Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol Behav. 2005;84:799–806. doi: 10.1016/j.physbeh.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Franssen CL, Bardi M, Hampton JE, Hainley L, Karsner S, Tu EB, et al. Characteristic neurobiological patterns differentiate paternal responsiveness in two Peromyscus species. Brain Behav Evol. 2011;77:159–175. doi: 10.1159/000326054. [DOI] [PubMed] [Google Scholar]
- Lancaster JB. Play-mothering: The relations between juvenile females and young infants among free-ranging vervet monkeys (Cercopithecus aethiops) Folia Primatol (Basel) 1971;15:163–182. [PubMed] [Google Scholar]
- Leerkes EM, Burney RV. The development of parenting efficacy among new mothers and fathers. Infancy. 2007;12:45–67. doi: 10.1111/j.1532-7078.2007.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Lei K, Cushing BS, Musatov S, Ogawa S, Kramer KM. Estrogen receptor-alpha in the bed nucleus of the stria terminalis regulates social affiliation in male prairie voles (Microtus ochrogaster) PLoS One. 2010;5:e8931. doi: 10.1371/journal.pone.0008931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutenegger W. Newborn size and pelvic dimensions of Australopithecus. Nature. 1972;240:568–569. doi: 10.1038/240568a0. [DOI] [PubMed] [Google Scholar]
- Levine S, Haltmeyer GC, Kargs GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiol Behav. 1967;2:5. [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behaviour of adult virgin prairie voles: Independence from gonadal hormones and vasopressin. J Neuroendocrinol. 1999;11:441–449. doi: 10.1046/j.1365-2826.1999.00361.x. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Social influences on parental and nonparental responses toward pups in virgin female prairie voles (Microtus ochrogaster) J Comp Psychol. 2001;115:53–61. doi: 10.1037/0735-7036.115.1.53. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, De Vries GJ. Parental responsiveness is feminized after neonatal castration in virgin male prairie voles, but is not masculinized by perinatal testosterone in virgin females. Horm Behav. 2002;41:80–87. doi: 10.1006/hbeh.2001.1740. [DOI] [PubMed] [Google Scholar]
- Lynn SE. Endocrine and neuroendocrine regulation of fathering behavior in birds. Horm Behav. 2016;77:237–248. doi: 10.1016/j.yhbeh.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Madden JR, Clutton-Brock TH. Experimental peripheral administration of oxytocin elevates a suite of cooperative behaviours in a wild social mammal. Proc Biol Sci. 2011;278:1189–1194. doi: 10.1098/rspb.2010.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning CJ, Wakeland EK, Potts WK. Communal nesting patterns in mice implicate MHC genes in kin recognition. Nature. 1992;360:581–583. doi: 10.1038/360581a0. [DOI] [PubMed] [Google Scholar]
- Manning CJ, Dewsbury DA, Wakeland EK, Potts WK. Communal nesting and communal nursing in house mice, Mus musculus domesticus. Anim Behav. 1995;50:741–751. [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: Research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Gorodetsky EK, Goldman D, Blair RJR. The influence of oxytocin administration on responses to infant faces and potential moderation by OXTR genotype. Psychopharmacology. 2012;224:469–476. doi: 10.1007/s00213-012-2775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AD, Rosenblatt JS. Ontogeny of maternal behavior in the laboratory rat: Early origins in 18- to 27-day-old young. Dev Psychobiol. 1979;12:407–424. doi: 10.1002/dev.420120502. [DOI] [PubMed] [Google Scholar]
- Mayer AD, Freeman NC, Rosenblatt JS. Ontogeny of maternal behavior in the laboratory rat: Factors underlying changes in responsiveness from 30 to 90 days. Dev Psychobiol. 1979;12:425–439. doi: 10.1002/dev.420120503. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations - Temporal parameters. Dev Brain Res. 1985;22:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Blumberg MS, McClintock MK, Moltz H. Inter-litter competition and communal nursing among Norway rats: Advantages of birth synchrony. Behav Ecol Sociobiol. 1990;27:183–190. [Google Scholar]
- Meredith SL. Anchoring the clade: Primate-wide comparative analysis supports the relationship between juvenile interest in infants and adult patterns of infant care. Folia Primatol (Basel) 2015;86:117–123. doi: 10.1159/000368356. [DOI] [PubMed] [Google Scholar]
- Mota MTDS, Franci CR, de Sousa MBC. Hormonal changes related to paternal and alloparental care in common marmosets (Callithrix jacchus) Horm Behav. 2006;49:293–302. doi: 10.1016/j.yhbeh.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480:91–93. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Network NECCR. Nonmaternal care and family factors in early development: An overview of the NICHD Study of Early Child Care. J Appl Dev Psychol. 2001;22:457–492. [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: Partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Northcutt KV, Lonstein JS. Social contact elicits immediate-early gene expression in dopaminergic cells of the male prairie vole extended olfactory amygdala. Neuroscience. 2009;163:9–22. doi: 10.1016/j.neuroscience.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Anim Behav. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate spontaneousmaternal behavior in adult female prairie voles. Neuroscience. 2006a;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Horm Behav. 2006b;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, Gonzalez-Mariscal G, Levy F, et al. New theoretical and experimental approaches on maternal motivation in mammals. Neurosci Biobehav Rev. 2013;37:1860–1874. doi: 10.1016/j.neubiorev.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Morphological, genetic, and behavioral comparisons of two prairie vole populations in the field and laboratory. J Mamm. 2007;88:989–999. [Google Scholar]
- Ophir AG, Crino OL, Wilkerson QC, Wolff JO, Phelps SM. Female-directed aggression predicts paternal behavior, but female prairie voles prefer affiliative males to paternal males. Brain Behav Evol. 2008a;71:32–40. doi: 10.1159/000108609. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Anim Behav. 2008b;75:1143–1154. [Google Scholar]
- Paul A, Kuester J. Infant handling by female Barbary macaques (Macaca sylvanus) at Affenberg Salem: Testing functional and evolutionary hypotheses. Behav Ecol Sociobiol. 1996;39:133–145. [Google Scholar]
- Perkeybile AM, Griffin LL, Bales KL. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster) Front Behav Neurosci. 2013;7:21. doi: 10.3389/fnbeh.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Delaney-Busch N, Hartman S, Grimm KJ, Bales KL. Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Front Behav Neurosci. 2015;9:191. doi: 10.3389/fnbeh.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Carter CS, Cushing BS. Effects of postnatal estrogen manipulations on juvenile alloparental behavior. Horm Behav. 2015;75:11–17. doi: 10.1016/j.yhbeh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G, Mason GA, Barakat AS, Pedersen CA. Oxytocin selectively increases holding and licking of neonates in preweanling but not postweanling juvenile rats. Behav Neurosci. 1991;105:470–477. doi: 10.1037//0735-7044.105.3.470. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Freidinger RM. Discovery and development of non-peptide antagonists of peptide hormone receptors. Biochem Soc Trans. 1997;25:1051–1057. doi: 10.1042/bst0251051. [DOI] [PubMed] [Google Scholar]
- Pillay N, Rymer TL. Alloparenting enhances the emotional, social and cognitive performance of female African striped mice, Rhabdomys pumilio. Anim Behav. 2015;99:43–52. [Google Scholar]
- Pluess M, Belsky J. Differential susceptibility to rearing experience: The case of childcare. J Child Psychol Psychiatry. 2009;50:396–404. doi: 10.1111/j.1469-7610.2008.01992.x. [DOI] [PubMed] [Google Scholar]
- Quinlan RJ, Quinlan MB. Human lactation, pair-bonds, and alloparents. Hum Nat. 2008;19:87–102. doi: 10.1007/s12110-007-9026-9. [DOI] [PubMed] [Google Scholar]
- Ren D, Lu G, Moriyama H, Mustoe AC, Harrison EB, French JA. Genetic diversity in oxytocin ligands and receptors in New World monkeys. PLoS One. 2015;10:e0125775. doi: 10.1371/journal.pone.0125775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, et al. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van Ijzendoorn MH, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biol Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MM, van IMH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ. No laughing matter: Intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology. 2012;37:1257–1266. doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Carter CS. Intraspecific variation and the presence of a father can influence the expression of monogamous and communal traits in prairie voles. Ann N Y Acad Sci. 1997;807:559–562. doi: 10.1111/j.1749-6632.1997.tb51968.x. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Zullo A, Gustafson EA, Carter CS. Perinatal steroid treatments alter alloparental and affiliative behavior in prairie voles. Horm Behav. 1996;30:576–582. doi: 10.1006/hbeh.1996.0060. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Miller AK, Taymans SE, Carter CS. Role of social and endocrine factors in alloparental behavior of prairie voles (Microtus ochrogaster) Can J Zool. 1998a;76:1862–1868. [Google Scholar]
- Roberts RL, Williams JR, Wang AK, Carter CS. Cooperative breeding and monogamy in prairie voles: Influence of the sire and geographical variation. Anim Behav. 1998b;55:1131–1140. doi: 10.1006/anbe.1997.0659. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Jenkins KT, Lawler T, Jr, Wegner FH, Newman JD. Bromocriptine administration lowers serum prolactin and disrupts parental responsiveness in common marmosets (Callithrix j. jacchus) Horm Behav. 2001;39:106–112. doi: 10.1006/hbeh.2000.1639. [DOI] [PubMed] [Google Scholar]
- Rosenberg KR, Trevathan WR. An anthropological perspective on the evolutionary context of preeclampsia in humans. J Reprod Immunol. 2007;76:91–97. doi: 10.1016/j.jri.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–1513. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav Brain Res. 2008;187:9–16. doi: 10.1016/j.bbr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AF. Mammals: Comparisons and contrasts. In: Koenig W, Dickinson J, editors. Ecology and Evolution of Cooperative Breeding in Birds. Cambridge: Cambridge University Press; 2004. pp. 210–227. [Google Scholar]
- Russell AF, Lummaa V. Maternal effects in cooperative breeders: From hymenopterans to humans. Philos Trans R Soc Lond B Biol Sci. 2009;364:1143–1167. doi: 10.1098/rstb.2008.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi K, Tanaka M, Ohkubo T, Doh-ura K, Fujikawa T, Sudo S, Nakashima K. Induction of brain prolactin receptor long-form mRNA expression and maternal behavior in pup-contacted male rats: Promotion by prolactin administration and suppression by female contact. Neuroendocrinology. 1996;63:559–568. doi: 10.1159/000127085. [DOI] [PubMed] [Google Scholar]
- Salo AL, French JA. Early experience, reproductive success, and development of parental behaviour in Mongolian gerbils. Anim Behav. 1989;38:693–702. [Google Scholar]
- Samuel PA, Hostetler CM, Bales KL. Urocortin II increases spontaneous parental behavior in prairie voles (Microtus ochrogaster) Behav Brain Res. 2008;186:284–288. doi: 10.1016/j.bbr.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos CV, French JA, Otta E. Infant carrying behavior in callitrichid primates: Callithrix and leontopithecus. Int J Primatol. 1997;18:889–907. [Google Scholar]
- Sayler A, Salmon M. Communal nursing in mice -Influence of multiple mothers on growth of young. Science. 1969;164:1309. doi: 10.1126/science.164.3885.1309. [DOI] [PubMed] [Google Scholar]
- Schnitzer PG, Ewigman BG. Child deaths resulting from inflicted injuries: Household risk factors and perpetrator characteristics. Pediatrics. 2005;116:e687–e693. doi: 10.1542/peds.2005-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer PG, Ewigman BG. Household composition and fatal unintentional injuries related to child maltreatment. J Nurs Scholar. 2008;40:91–97. doi: 10.1111/j.1547-5069.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Dupre A, Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci Lett. 2009;461:217–222. doi: 10.1016/j.neulet.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Schradin C. Comments to K.E. Wynne-Edwards and M.E. Timonin 2007. Paternal care in rodents: Weakening support of hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:557–559. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Schradin C, Pillay N. The striped mouse (Rhabdomys pumilio) from the succulent karoo, South Africa: A territorial group-living solitary forager with communal breeding and helpers at the nest. J Comp Psychol. 2004;118:37–47. doi: 10.1037/0735-7036.118.1.37. [DOI] [PubMed] [Google Scholar]
- Schultz LA, Lore RK. Communal reproductive success in rats (Rattus norvegicus) - Effects of group composition and prior social experience. J Comp Psychol. 1993;107:216–222. doi: 10.1037/0735-7036.107.2.216. [DOI] [PubMed] [Google Scholar]
- Sear R, Mace R. Who keeps children alive? A review of the effects of kin on child survival. Evol Hum Behav. 2008;29:1–18. [Google Scholar]
- Shapiro LE, Insel TR. Ontogeny of oxytocin receptors in rat forebrain: A quantitative study. Synapse. 1989;4:259–266. doi: 10.1002/syn.890040312. [DOI] [PubMed] [Google Scholar]
- Silk JB. Why are infants so attractive to others? The form and function of infant handling in bonnet macaques. Anim Behav. 1999;57:1021–1032. doi: 10.1006/anbe.1998.1065. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Bell RW. Maternal mediation of early experience. In: Smotherman WP, Bell RW, editors. Maternal Influence and Early Behavior. New York: Spectrum Publishing; 1980. [Google Scholar]
- Smotherman WP, Brown CP, Levine S. Maternal responsiveness following differential pup treatment and mother-pup interactions. Horm Behav. 1977;8:242–253. doi: 10.1016/0018-506x(77)90041-1. [DOI] [PubMed] [Google Scholar]
- Smuts BB, Gubernick DJ. Male-infant relationships in nonhuman primates: Paternal investment or mating effort. In: Hewlett BS, editor. Father-Child Relations: Cultural and Biosocial Contexts. New York: Aldine de Gruyter; 1992. pp. 1–30. [Google Scholar]
- Solomon NG, Hayes LD. The biological basis of alloparental behaviour in mammals. In: Bentley G, Mace R, editors. Substitute Parents: Biological and Social Perspectives on Alloparenting in Human Societies. New York: Berghahn Books; 2009. [Google Scholar]
- Soltis J, Wegner FH, Newman JD. Urinary prolactin is correlated with mothering and allo-mothering in squirrel monkeys. Physiol Behav. 2005;84:295–301. doi: 10.1016/j.physbeh.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Song Z, Tai F, Yu C, Wu R, Zhang X, Broders H, He F, et al. Sexual or paternal experiences alter alloparental behavior and the central expression of ER alpha and OT in male mandarin voles (Microtus mandarinus) Behav Brain Res. 2010;214:290–300. doi: 10.1016/j.bbr.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Stark R, Schienle A, Walter B, Kirsch P, Sammer G, Ott U, Blecker C, et al. Hemodynamic responses to fear and disgust-inducing pictures: An fMRI study. Int J Psychophysiol. 2003;50:225–234. doi: 10.1016/s0167-8760(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Stiffman MN, Schnitzer PG, Adam P, Kruse RL, Ewigman BG. Household composition and risk of fatal child maltreatment. Pediatrics. 2002;109:615–621. doi: 10.1542/peds.109.4.615. [DOI] [PubMed] [Google Scholar]
- Stone AI, Bales KL. Intergenerational transmission of the behavioral consequences of early experience in prairie voles. Behav Process. 2010;84:732–738. doi: 10.1016/j.beproc.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AI, Mathieu D, Griffin L, Bales KL. Alloparenting experience affects future parental behavior and reproductive success in prairie voles (Microtus ochrogaster) Behav Process. 2010;83:8–15. doi: 10.1016/j.beproc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Minoura H, Toyoda N, Sakaguchi K, Tanaka M, Sudo S, Nakashima K. Pup contact induces the expression of long form prolactin receptor mRNA in the brain of female rats: Effects of ovariectomy and hypophysectomy on receptor gene expression. J Endocrinol. 1996;149:335–340. doi: 10.1677/joe.0.1490335. [DOI] [PubMed] [Google Scholar]
- Thornhill R, Fincher CL. Parasite stress promotes homicide and child maltreatment. Philos Trans R Soc B: Biol Sci. 2011;366:3466–3477. doi: 10.1098/rstb.2011.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler AN, Michel GF, Bales KL, Carter CS. Do brief early disturbances of parents affect parental care in the biparental prairie vole (Microtus ochrogaster)? Dev Psychobiol. 2005;47:451. [Google Scholar]
- Villalba C, Boyle PA, Caliguri EJ, De Vries GJ. Effects of the selective serotonin reuptake inhibitor fluoxetine on social behaviors in male and female prairie voles (Microtus ochrogaster) Horm Behav. 1997;32:184–191. doi: 10.1006/hbeh.1997.1420. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Novak MA. Alloparental care and the influence of father presence on juvenile prairie voles, Microtus ochrogaster. Anim Behav. 1994;47:281–288. [Google Scholar]
- Wu R, Song Z, Tai F, An X, Yu P, Li Y. The effect of alloparental experience and care on anxiety-like, social and parental behaviour in adult mandarin voles. Anim Behav. 2013;85:61–69. [Google Scholar]
- Wynne-Edwards KE, Timonin ME. Paternal care in rodents: Weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Cushing BS, Kramer KM, Epperson PD, Hoffman GE, Carter CS. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience. 2004;125:947–955. doi: 10.1016/j.neuroscience.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Yampolskaya S, Greenbaum PE, Berson IR. Profiles of child maltreatment perpetrators and risk for fatal assault: A latent class analysis. J Fam Viol. 2009;24:337–348. [Google Scholar]
- Ziegler TE, Wegner FH, Snowdon CT. Hormonal responses to parental and nonparental conditions in male cotton-top tamarins, Saguinus oedipus, a New World primate. Horm Behav. 1996;30:287–297. doi: 10.1006/hbeh.1996.0035. [DOI] [PubMed] [Google Scholar]