Abstract
Objectives
Auditory verbal hallucinations (AVH) are among the most common symptoms in schizophrenia. Earlier studies suggest changes in the structural connectivity of auditory areas involved in the pathophysiology of auditory hallucinations. Combining diffusion tensor imaging (DTI) and fibre tractography provides a unique opportunity to visualize and quantify entire fibre bundles.
Methods
Fibre tracts connecting homotopic auditory areas via the corpus callosum were identified with DTI in ten first episode paranoid schizophrenia patients and ten healthy controls. Regions of interest were drawn manually, to guide tractography, and fractional anisotropy (FA) – a measure of fibre integrity – was calculated and averaged over the entire tract for each subject.
Results
There was no difference in the FA of the interhemispheric auditory fibres between schizophrenic patients and healthy controls. However, the subgroup of patients hearing conversing voices showed increased FA relative to patients without these symptoms (P = 0.047) and trendwise increased FA relative to healthy controls (P = 0.066). In addition, a trendwise correlation between FA values and AVH symptoms (P = 0.089) was found.
Conclusions
Our findings suggest that in addition to local deficits in the left auditory cortex and disturbed fronto-temporal connectivity, the interhemispheric auditory pathway might be involved in the pathogenesis of AVH.
Keywords: Schizophrenia, brain imaging, MRI, positive symptoms, biological psychiatry
Introduction
Brain imaging has provided a unique and unprecedented opportunity to understand the biological basis of psychopathological symptoms in psychiatric diseases. While it has long been speculated that schizophrenia might be a disease with disturbed connectivity of brain regions (Friston and Frith 1995; Weinberger and Lipska 1995; Andreasen et al. 1998), current methodological advances in Diffusion Tensor Imaging (DTI) and fibre tracking have enabled us now to test specific hypotheses concerning the relationship between the connectivity of distinct brain regions and psychopathological symptoms of interest (for review see (Kubicki et al. 2007). Since auditory verbal hallucinations (AVH) are among the most prominent symptoms in schizophrenia, different brain imaging methods have been used in order to investigate them. For example, Functional Magnetic Resonance Imaging (fMRI) and Positron Emission Tomography (PET) during AVH have both shown that activation of the auditory cortex is observed (Dierks et al. 1999; Shergill et al. 2000), as well as other regions including the inferior frontal cortex and the anterior cingulate cortex (ACC). Interestingly, several authors have described bilateral activation of the auditory cortex during AVH (Copolov et al. 2003; Lennox et al. 2000), while others have described only unilateral activation, typically on the left side (Dierks et al. 1999; Suzuki et al. 1993) or both uni- and bilateral patterns in different patients within the same study (van de Ven et al. 2005) (for a review see Allen et al. 2008). In addition, it has been shown that there is a positive correlation between the subjective reality of hallucinations and the functional coupling of the inferior frontal cortex with the auditory cortex (Raij et al. 2009).
Using DTI, increased anisotropy was shown in a number of white matter structures in patients with AVH. Hubl et al. (2004) showed increased fractional anisotropy (FA) in the lateral temporoparietal section of the arcuate fasciculus. This tract connects language production areas with auditory processing areas. Other findings in this study included increased FA values in the corpus callosum both in anterior parts (where fibres connecting frontal areas are crossing) and posterior parts (in an area where fibres connecting auditory areas are crossing). In another investigation patients with AVH were shown to have increased anisotropy in the superior longitudinal fascicule and the anterior cingulum (Shergill et al. 2007). More recently, increased water diffusivity was reported in the left side superior temporal gyrus in patients with schizophrenia, which was associated with auditory hallucinations (Lee et al. 2009).
Since many brain imaging findings concerning AVH are related to either basic auditory areas or language production and perception areas, it is interesting to note that during more recent years there has been increasing evidence for an important role of the interhemispheric pathway connecting bilateral auditory areas for speech perception. For example, phonological awareness is associated with higher diffusivity perpendicular to the main axis of the corpus callosum in its posterior part that is connecting the temporal lobes (Dougherty et al. 2007). Phonological awareness refers to an individual’s awareness of the sound structure, or phonological structure, of a spoken word. It includes the ability to auditorily distinguish units of speech, such as a word’s syllables and a syllable’s individual phonemes. This finding has been interpreted as suggesting that subjects with high phonological awareness have reduced interhemispheric connectivity and are better at processing rapidly changing auditory stimuli.
In a more recent study using an auditory speech perception task and probabilistic tractography, a significant relationship between the anatomical connectivity between the superior temporal lobe areas and speech perception performance has been described (Westerhausen et al. 2009). It is interesting that in both aforementioned studies specific structure–function associations between phonological awareness/auditory speech perception and the anatomical connections, between the bilateral auditory areas, as assessed by fibre tractography, were found in healthy subjects.
Accordingly, it was the aim of the present study to specifically test the hypothesis of whether or not anatomical connections between bilateral auditory areas are related to the presence of AVH in schizophrenic patients. Since increased anisotropy in posterior parts of the corpus callosum, probably connecting the bilateral auditory areas, was described earlier in schizophrenic patients with AVH (Hubl et al. 2004), we predicted increased anisotropy in schizophrenic patients with AVH across the connecting interhemispheric auditory pathway. We used a combination of DTI and fibre tractography that was recently introduced in order to allow a quantification of fractional anisotropy values across a fibre tract of interest (Oh et al. 2009).
Materials and methods
Subjects
Ten subjects with paranoid schizophrenia and ten healthy controls participated in this study (see Table I). The patients were recruited from inpatients at McLean Hospital, a private psychiatric hospital affiliated with Harvard Medical School. Healthy control subjects were recruited through newspaper and online advertisement. After a complete description of the study, written informed consent was obtained from all participants. The study was approved by the local institutional review boards at McLean Hospital, Brigham and Women’s Hospital, the VA Boston Healthcare System, and Harvard Medical School. The protocols for diagnosis and clinical evaluations have been previously described in detail (see (Salisbury et al. 2007) and Supplementary Material available online). This study was conducted in accordance with the guidelines of the current version of the “Declaration of Helsinki”.
Table I.
Demographic and clinical characteristics of subjects.
| Demographic | SZ (total) n = 10 Mean ± SD |
SZ (AVH) n = 5 Mean ± SD |
SZ (NAVH) n = 5 Mean ± SD |
HC n = 10 Mean ± SD |
P value |
|---|---|---|---|---|---|
| Age (years) | 26.9 ± 8.6 | 28.8 ± 2.3 | 25.0 ± 7.1 | 22.4 ± 2.2 | 0.126 |
| Sex, male/female | 8/2 | 4/1 | 4/1 | 8/2 | 1 |
| Handedness | 0.69 ± 0.36 | 0.86 ± 0.19 | 0.52 ± 0.43 | 0.66 ± 0.21 | 0.828 |
| Socioeconomic status | 2.8 ± 1.14 | 2.8 ± 1.10 | 2.8 ± 1.30 | 2.2 ± 0.63 | 0.162 |
| Parents’ socioeconomic status | 1.2 ± 0.45 | 1.2 ± 0.45 | 1.2 ± 0.45 | 1.5 ± 0.71 | 0.264 |
| Education (years) | 14.0 ± 2.3 | 14.2 ± 1.8 | 13.8 ± 2.9 | 14.4 ± 1.8 | 0.635 |
| Mini-Mental State Examination score | 28.2 ± 1.75 | 28.2 ± 1.92 | 28.2 ± 1.79 | 29.8 ± 0.42 | 0.012 |
| WAIS-III score | |||||
| Information | 12.7 ± 2.70 | 14.6 ± 1.67 | 10.8 ± 2.17 | 13.9 ± 1.66 | 0.248 |
| Digits span | 9.8 ± 2.57 | 8.8 ± 1.79 | 10.8 ± 3.03 | 11.3 ± 2.16 | 0.175 |
| Global Assessment Scale score | 31.67 ± 5.82 | 30.0 ± 2.0 | 33.33 ± 8.50 | N/A | N/A |
| Total Positive and Negative | 73.5 ± 14.6 | 67.0 ± 8.8 | 80.0 ± 17.2 | N/A | N/A |
| Syndrome Scale score | |||||
| Negative Syndrome Scale score | 16.1 ± 6.2 | 13.6 ± 4.3 | 18.6 ± 7.2 | N/A | |
| Positive Syndrome Scale score | 20.5 ± 3.0 | 19.2 ± 3.6 | 21.8 ± 1.6 | N/A | |
| Medication dose (chlorpromazine equivalent/mg/d) | 239.6 ± 122.1 | 254.2 ± 71.2 | 225 ± 170.8 | N/A | N/A |
| Duration of illness from first hospitalization to scan, median (range) (mo) | 5.27 ± 6.21 (0.17–16) | 5.70 ± 7.35 (1–16) | 4.83 ± 5.68 (1–12) | N/A | N/A |
| Duration of medication use, median (range) (mo) | 0.9 ± 0.44 (0.4–1.4) | 1.12 ± 1.09 (0.3–2.7) | 1.0 ± 0.75 (0.3–2.7) | N/A | N/A |
SZ, schizophrenia; HC, healthy control; N/A, not applicable; WAIS, Wechsler Adult Intelligence Scale; mo, months; AVH, auditory verbal hallucinations; SD, standard deviation.
DTI acquisition
Line-scan DTIs (Kubicki et al. 2004) were acquired with a quadrature head coil on a 1.5-Tesla GE Echo-speed system (General Electric Medical Systems, Milwaukee, WI) using the following scanning parameters: six gradient directions at b = 1000 s/mm2, two baseline images (b = 5 s/mm2), FOV 220 × 165 mm; 128 × 96 scan matrix; slice thickness = 4 mm; inter-slice distance = 1 mm; receiver bandwidth ± 4 kHz; echo time = 64 ms; effective repetition time = 2592 ms; scan time = 60 s per slice selection.
ROI definition and preprocessing
In order to extract the fibre tracts connecting the bilateral auditory areas, we used a seeding tractography technique, followed by a selection process that only preserves tracts going through specific combinations of regions of interest (ROIs) (Conturo et al. 1999; Mori et al. 1999). Initial seeding was done in a midsagittal ROI covering the posterior third of the corpus callosum where the auditory fibres crossed. A single rater (V.K.), blind to diagnosis, gender and age drew the ROIs for all subjects.
Diffusion-tensor imaging and preprocessing
Diffusion Tensor Images (DTIs) were constructed from the Diffusion-Weighted Images using a least-squares estimation. Deterministic (streamline) tractography was implemented in the Slicer 2.7. software package (www.slicer.org), as described previously (Oh et al. 2007). Tractography was initiated from every voxel defined by the ROI (see above), and followed the direction defined by the principal eigenvector. A step size of 0.5 mm was employed, with a radius of curvature >0.87 mm. Tractography was terminated upon reaching a voxel of FA <0.15 (the stopping criterion). A coronal ROI was defined for each subject at the level of the splenium (i.e. where the tapetum could be discretely identified (Huang et al. 2005), and fibres were excluded if they failed to pass thorough this ROI. The purpose of the exclusion ROI was to ensure that only CC fibres connecting superior temporal cortices remained in the analysis. Figure 1 illustrates the auditory CC fibres extracted from one representative subject. For each subject, FA was calculated for every voxel through which any of the auditory CC fibres passed, and mean FA was calculated by averaging the FA of these voxels (Basser and Pierpaoli 1996).
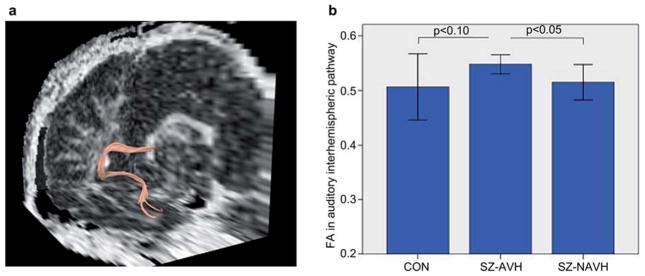
Figure 1.
(a) 3D reconstruction of the right-hemisphere fibre tracking (temporo-callosal pathway) for a single subject in native space. (b) Group comparisons of fractional anisotropy (FA) ± 1 SD in the auditory interhemispheric pathway in healthy controls (CON), schizophrenic patients with auditory verbal hallucinations (SZ-AVH) and schizophrenic patients without auditory verbal hallucinations (SZ-NAVH). Mann–Whitney U-tests showed significant increased FA values in SZ-AVH in comparison to SZ-NAVH (P = 0.047) and trendwise increased FA relative to healthy controls (P = 0.066).
Statistical analysis
Statistical analyses were performed using the SPSS-software package (16.0) with a personal computer. Due to the small sample size Kruskal–Wallis tests were performed to assess group differences between the three subgroups in demographic and clinical characteristics except for gender. We used Mann–Whitney U-tests in order to compare mean FA values between patients with schizophrenia and healthy controls. Correlation analyses between AVH symptoms and FA values were performed as Spearman correlation analyses. The significance level was set at α = 0.05 in all analyses.
Results
The demographic and clinical characteristics of the present samples are shown in Table I. There were no significant differences among the three groups in terms of age, gender, or years of education.
There was no difference in mean FA values in the comparison of all schizophrenic patients to the healthy control group. However, schizophrenic patients with AVH demonstrated increased FA values in the inter-hemispheric auditory pathway in comparison to patients without AVH (Mean FA = 0.514 ± 0.032; Z = −1.984; P = 0.047, see Figure 1). Moreover, schizophrenic patients with AVH demonstrated marginally increased FA values compared to healthy controls (Mean FA = 0.547 ± 0.175 vs. 0.506 ± 0.060; Z = −1.837; P = 0.066).
We found a statistical trend towards a positive correlation between FA values and AVH symptoms (Spearman ρ = 0.46, P = 0.089).
In an exploratory analysis of correlations between FA and psychopathological measures and between FA and the chlorpromazine equivalents, we found no statistically significant correlations between FA reduction and factors or items from the Positive and Negative Syndrome Scale nor between FA values and chlorpromazine equivalent values.
Discussion
This study investigated the relationship between the auditory interhemispheric pathway and AVH in patients with a first episode of schizophrenia. We used a combination of DTI and fibre tractography in order to quantify mean diffusion values specifically across the fibre bundles of interest. In line with our hypothesis we found increased FA values in the interhemispheric pathway in patients with AVH, but not in patients without AVH.
While this is the first study to specifically test this hypothesis, a previous study using DTI also showed (among other findings) increased FA values in the posterior part of the corpus callosum in schizophrenic patients (in an area where the interhemispheric auditory fibres cross) (Hubl et al. 2004), and a more recent study showed a positive correlation between auditory hallucination scores and FA values in the superior temporal gyrus (Lee et al. 2009).
According to an influential concept, hallucinations result from attributing one’s own inner speech to an external agency (Frith 1992). This has been hypothesized to be related to a functional disconnection of frontal brain areas and brain areas concerned with perception (Friston and Frith 1995). This concept is consistent with the finding of increased FA values in the arcuate fasciculus (Hubl et al. 2004), a tract that connects language production areas in the frontal lobe (e.g., Broca’s area) with auditory processing and language perception areas in the temporal lobe.
While the interhemispheric pathway has been investigated with respect to phonological awareness and speech perception, it has (to the best of our knowledge) not been investigated with respect to its association with AVH. However, our finding is in line with a recent electroencephalography (EEG) study describing a positive correlation between synchronization of bilateral auditory areas and hallucination symptom scores (Mulert et al. 2010). Apart from that, the relationship to phonological awareness and speech perception might help to understand the role of the interhemispheric pathway the pathophysiology of AVH. The interhemispheric auditory pathway, crossing in the posterior third of the corpus callosum, is responsible for the interhemispheric interplay of prosodic information and syntactic information that is necessary for speech comprehension. For example, patients with a lesion in the posterior third of the corpus callosum do not show an event-related N400 potential in a speech comprehension task requiring the interaction of prosodic and syntactic information (Friederici et al. 2007). There are now also DTI tractography studies demonstrating a structure–function relationship between the auditory interhemispheric pathway and phonological awareness or speech comprehension (Dougherty et al. 2007; Westerhausen et al. 2009).
The overall picture concerning the relationship between DTI findings and psychopathological symptoms in schizophrenia such as AVH is far from being completely understood. While the present finding is in line with previous findings of positive correlations between FA values in different fibre tracts and positive symptoms such as AVH (Seok et al. 2007) there are also reports of reduced FA values in speech related fibre tracts and negative correlations between FA values and psychopathological measurements (Phillips et al. 2009). In a recent DTI study, schizophrenia patients exhibited FA reductions in their frontal fibres crossing via the corpus callosum but at the same time significant positive correlations were observed to the severity of their psychotic symptoms such as hallucinations and delusions (Whitford et al. 2010). These results have been interpreted in such a way that mild asynchronies between the activities of spatially discrete brain regions might give rise to psychotic symptoms. However, severe asynchronies, caused by severe white matter damage might not be incorporable into a coherent phenomenological framework and thus not give rise to psychotic symptoms (Whitford et al. 2010).
Limitations
Since the sample size in the present study is small, our results in ten first episode patients need to be viewed as preliminary, and require replication with increased sample sizes.
In summary, our results suggest that the inter-individual variability of the auditory interhemispheric pathway, known to be related to phonological awareness and speech perception, might also play an important role in the pathogenesis of AVH.
Acknowledgments
This work was supported by a grant of the German Society for Psychiatry, Psychotherapy and Neurology (Imaging Award 2007)(CM), by an Overseas-Based Biomedical Training Fellowship from the National Health and Medical Research Council of Australia (NHMRC 520627), administered through the Melbourne Neuropsychiatry Centre at the University of Melbourne (TW), by. the Department of Veterans Affairs (Merit Award to RWM and to MES, Schizophrenia Center Award to RWM and MES, Middleton Award to RWM), by the National Institute of Health (R01 MH 40799, R01 MH 052807, P50MH080272 to RWM; R01 MH58704 to DFS, and R01 MH 50740 to MES and a K05 MH 070047 award to MES), and NARSAD awards (DFS and MES). Kathrin Holzschneider was involved in the final editing of the paper.
Footnotes
Statement of Interest
None to declare.
References
- Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, Akbudak E, Snyder AZ, Shimony JS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copolov DL, Seal ML, Maruff P, Ulusoy R, Wong MT, Tochon-Danguy HJ, Egan GF. Cortical activation associated with the experience of auditory hallucinations and perception of human speech in schizophrenia: a PET correlation study. Psychiatry Res. 2003;122:139–152. doi: 10.1016/s0925-4927(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W. Activation of Heschl’s gyrus during auditory hallucinations [see comments] Neuron. 1999;22:615–621. doi: 10.1016/s0896-6273(00)80715-1. [DOI] [PubMed] [Google Scholar]
- Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci USA. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, von Cramon DY, Kotz SA. Role of the corpus callosum in speech comprehension: interfacing syntax and prosody. Neuron. 2007;53:135–145. doi: 10.1016/j.neuron.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Frith CD. The cognitive neuropsychology of schizophrenia. Hove: Lawrence Erlbaum; 1992. [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, et al. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Maier SE, Westin CF, Mamata H, Ersner-Hershfield H, Estepar R, et al. Comparison of single-shot echo-planar and line scan protocols for diffusion tensor imaging. Acad Radiol. 2004;11:224–232. doi: 10.1016/s1076-6332(03)00563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Yoshida T, Kubicki M, Bouix S, Westin CF, Kindlmann G, et al. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a Diffusion Tensor Imaging study. Schizophr Res. 2009;108:33–40. doi: 10.1016/j.schres.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox BR, Park SB, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol. 2011;79:55–63. doi: 10.1016/j.ijpsycho.2010.08.004. Epub 2010 Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JS, Song IC, Lee JS, Kang H, Park KS, Kang E, Lee DS. Tractography-guided statistics (TGIS) in diffusion tensor imaging for the detection of gender difference of fiber integrity in the midsagittal and parasagittal corpora callosa. Neuroimage. 2007;36:606–616. doi: 10.1016/j.neuroimage.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Oh JS, Kubicki M, Rosenberger G, Bouix S, Levitt JJ, McCarley RW, et al. Thalamo-frontal white matter alterations in chronic schizophrenia: a quantitative diffusion tractography study. Hum Brain Mapp. 2009;30:3812–3825. doi: 10.1002/hbm.20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips OR, Nuechterlein KH, Clark KA, Hamilton LS, Asarnow RF, Hageman NS, et al. Fiber tractography reveals disruption of temporal lobe white matter tracts in schizophrenia. Schizophr Res. 2009;107:30–38. doi: 10.1016/j.schres.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij TT, Valkonen-Korhonen M, Holi M, Therman S, Lehtonen J, Hari R. Reality of auditory verbal hallucinations. Brain. 2009;132:2994–3001. doi: 10.1093/brain/awp186. Epub 2009 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok JH, Park HJ, Chun JW, Lee SK, Cho HS, Kwon JS, Kim JJ. White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res. 2007;156:93–104. doi: 10.1016/j.pscychresns.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Kanaan RA, Chitnis XA, O’Daly O, Jones DK, Frangou S, et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164:467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yuasa S, Minabe Y, Murata M, Kurachi M. Left superior temporal blood flow increases in schizophrenic and schizophreniform patients with auditory hallucination: a longitudinal case study using 123I-IMP SPECT. Eur Arch Psychiatry Clin Neurosci. 1993;242:257–261. doi: 10.1007/BF02190383. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Roder CH, Prvulovic D, Bittner RA, Dietz MG, et al. The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. Neuroimage. 2005;27:644–655. doi: 10.1016/j.neuroimage.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Lipska BK. Cortical maldevelopment, anti-psychotic drugs, and schizophrenia: a search for common ground. Schizophr Res. 1995;16:87–110. doi: 10.1016/0920-9964(95)00013-c. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Gruner R, Specht K, Hugdahl K. Functional relevance of interindividual differences in temporal lobe callosal pathways: a DTI tractography study. Cereb Cortex. 2009;19:1322–1329. doi: 10.1093/cercor/bhn173. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Kubicki M, Schneidermann JS, O’Donnell LJ, King R, Alvarado JL, et al. Corpus callosum abnormalities and their association with psychotic symptoms in patients with schizophrenia. Biol Psychiatry. 2010;68:70–77. doi: 10.1016/j.biopsych.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]