Abstract
Objective
To predict the likelihood of hospital-onset Clostridium difficile infection (HO-CDI) based on patient clinical presentations at admission
Design
Retrospective data analysis
Setting
Six US acute care hospitals
Patients
Adult inpatients
Methods
We used clinical data present at the time of admission in electronic health record (EHR) systems to develop and validate a HO-CDI predictive model. The outcome measure was HO-CDI cases identified by a non-duplicate positive C. difficile toxin assay result with stool specimens collected >48 hours after inpatient admission. We fit a logistic regression model to predict the risk of HO-CDI. We validated the model using 1,000 bootstrap simulations.
Results
Among 78,080 adult admissions, 323 HO-CDI cases were identified (4.1/1,000 admissions). The logistic regression model yielded 14 independent predictors, including hospital community onset CDI pressure, patient age ≥65, previous healthcare exposures, CDI in previous admission, admission to the intensive care unit, albumin ≤3 g/dL, creatinine >2.0 mg/dL, bands > 32%, platelets ≤150 or >420 109/L, and WBC >11,000 mm3. The model had a c-statistic of 0.78 (95% CI: 0.76, 0.81) with good calibration. For 79% patients with risk score of 0-7, there were 19 HO-CDIs per 10,000 admissions; for patients with risk score of 20+, there were 623 HO-CDIs per 10, 000 admissions (P<0.0001).
Conclusion
Using clinical parameters available at the time of admission, this HO-CDI model displayed a good predictive ability. It may have utility as an early risk identification tool for HO-CDI preventive interventions and outcome comparisons.
Keywords: Clostridium difficile Infection (CDI), predictive model, CDI risk score, electronic health record (EHR), electronic medical record (EMR), laboratory data, risk adjustment
Clostridium difficile infections (CDI) are one of the most frequent healthcare associated infections (HAI) in the USA,1 having more than doubled in prevalence in U.S. hospitals from 2000 to 2009.2 Meanwhile, the US National Vital Statistics suggest that deaths due to CDI have increased nearly 10-fold from 1999 to 2010, becoming the 18th leading cause of death for people aged 65 and older in 2010.3 In addition, there is a major economic burden from CDI.4,5
Several studies have investigated the risk for developing CDI based on various combinations of patient, treatment, and environmental factors either already present at the time of hospital admission or occurring after admission.6 Factors that reflect ongoing modification in host susceptibility to CDI, such as antibiotic use, and factors that reflect ongoing risk of transmission, such as Clostridium difficile–associated disease (CDAD) pressure, may be followed throughout the entire hospital stay and, as such, may be considered as care process variables that are at least partially under the control of providers and the hospital. Such risk predictive models that incorporate both patient and care process risks throughout the hospital stay can be useful for internal hospital quality improvement purposes. On the other hand, there are advantages to a risk score system that restricts candidate variables to those brought in by patients when they are admitted to an acute care setting.
One use of on-admission risk stratification of individual patients is to assist hospitals in directing more specific, costly, or difficult to implement prevention strategies (e.g. probiotics, passive immunization, or more stringent avoidance of high-risk antibiotics) to those identified as high risk patients. Another use of an on-admission risk score is to adjust rates of hospital-onset (HO)-CDI by underlying patient risks that are beyond the control of hospitals, thereby advancing more fair inter-hospital comparisons. The objective of our study was to devise a HO-CDI risk predictive model using patient data available at the time of acute care admission only.
METHODS
Data Source
We analyzed clinical data in a Clinical Research Database from CareFusion (CareFusion Inc., San Diego, CA, USA). This data set consisted of electronically captured daily census, location, clinical, and microbiology data (e.g., specimen collection date/time, location, and test results), general laboratory test results, demographics, admission sources, and other clinical and administrative data from CareFusion’s electronic HAI surveillance system MedMined® and its former MediQual® research database, which have been previously described elsewhere5,7,8, 9 The data set used for this study was a deidentified limited data set. The study protocols were approved by the New England IRB (Wellesley, MA).
Study Cohort
Adult patients (age 18 or older) discharged from 6 acute care hospitals between January 1, 2007 and June 30, 2008.
Definition of HO-CDI
We defined HO-CDI among adult (≥18 years) inpatients as a non-duplicate positive C. difficile stool diagnostic assay (EIA toxin assay A&B) collected >48 hours after inpatient admission. For practical purposes, non-duplicate CDI was defined as no prior positive results within 14 days per definition of the Centers for Disease Control and Prevention (CDC).10 Non-recurrent CDI cases were defined as a positive result after more than eight weeks without another positive. Recurrent CDI cases were identified as the second positive result between 2 and 8 weeks following any previous positive result.5,8 If a patient had more than one HO-CDI incidences in a single admission, it was counted only once in the model fit process because the analytic unit was hospital admission.
Candidate Predictor Variables
Candidate predictor variables included patients’ intrinsic risk that decreased the immune function, increased risk of C. difficile colorization, and exposure to antibiotics use: 1) demographics (age, gender); 2) previous healthcare exposure (admitted from another acute care hospital, a skilled nursing facility, and discharge from the same hospital within 30 days); 3) CDI during previous hospital stay; 4) potential infections in the current or previous hospitalization (identified with Nosocomial Infection Marker [NIM®] or Community Infection Marker [CIM®] status. A NIM or CIM is defined as a positive microbiologic specimen with a non-duplicate hospital isolate, where the specimen is collected from a single source. This algorithm-based system accounts for timing of specimen collection in relation to patient admission and location. It excludes common contaminations. For NIM, the specimen is collected three days after admission. For CIM, the specimen is collected within three days from admission);7 5) need for intensive care (admission to an intensive care unit (ICU), need for mechanical ventilation); and 6) admission laboratory testing results within 24 hours (serum chemistry, blood gas, cardiac markers, hematology, and coagulation parameters) as additional indicators of clinical severity. For continuous laboratory variables, we used previously published cuts for the categorization.9,11,12 Patients who did not have a particular laboratory measure on admission were treated as not clinically indicated and hence, would be in the reference group.
Previous studies found that CO-CDI pressure was associated with increased risk of HO-CDI.6,8 It is one of the hospital-level risk factors adjusted by the CDC National Healthcare Safety Network.13 Hence, in addition to the individual’s potential intrinsic risk as measured by the above clinical presentation, we also devised two community onset (CO) CDI pressure variables, defined a priori, to represent the environmental risk. (1) CO-CDI Pressure: rolling average number of CO-CDI 14 days prior to and 3 days after the patient’s index admission divided by the total number of hospital admissions during this period. The CO-CDI Pressure only accounted for CO-CDIs in patients who did not have previous discharge from the same hospitals within 12 weeks. (2) All CO-CDI Pressure: this variable counts for all CO-CDIs, including those patients who were discharged from the same hospitals within 12 weeks. We evaluated the distribution of the CDI pressure in relation to the CDI rate and determined the cutpoint for categorizing this variable to simplify the model application.
Development and Validation of Predictive Model
We conducted univariable analysis of candidate variables. Variables with significant association to HO-CDI (P<0.05) were included in the multivariable logistic regression analysis. We also tested model using a P-value <0.10 for eligible candidate variable. We fit the logistic regression model using the step-wise approach. We also tested backwards section approach. We reiterated the model fitting process and reviewed models among authors, including a CDI expert, a gastroenterologist, an intensivist, and two biostatisticians. We combined multiple levels of certain general laboratory data to create a more robust estimate in the model when the coefficients were similar and the numbers were small. The final model was determined by clinical plausibility and statistical significance.14,15 We used a P-value of <0.05 as a general criterion for model variable retention, but also forced in certain variables with a P-value nearly significant for the final model based on theoretical importance. We converted the model coefficients into a risk score system. Specifically, we divided each variable coefficient by the smallest coefficient in the model and rounded this ratio to the nearest integer.16 We summed up all pertinent variable points for each patient into an aggregated HO-CDI risk score. We then validated this risk model by conducting 1,000 bootstrap simulations.17 The bootstrap process randomly samples with replacement the study cohort. It fits the model and generates the c-statistic for each random sample. We used the 2.5th and 97.5th percentiles as the lower and upper limits of c-statistic 95% confidence intervals from the 1,000 simulations.
To simplify the clinical application, we devised a risk score grouping that approximated a linear relationship between the HO-CDI risk and the HO-CDI rates by varying the cut points of the groups.
RESULTS
Patient Characteristics
Among 78,080 adult admissions, 323 HO-CDI cases (including 310 non-recurrent and 13 recurrent CDIs) were identified (4.1/1,000 admissions) (Table 1). Patients with HO-CDI were older, more likely to have previous healthcare exposures, to be admitted to the ICU, and more likely to have abnormal laboratory test results on admission.
Table 1.
Patient Characteristics
| Variable | Non-Cases, n (Column %) | HO-CDI Cases, n (Column %) | Univariate P-Value |
|---|---|---|---|
| Total n | 77,757 | 323 | |
| Demographics | |||
| Age <65 | 35,331 (45.4) | 84 (26.0) | <0.0001 |
| 65-84 | 32,776 (42.2) | 187 (57.9) | |
| >84 | 9,650 (12.4) | 52 (16.1) | |
| Female | 42,225 (54.3) | 173 (53.6) | 0.789 |
| Male | 35,532 (45.7) | 150 (46.4) | |
| Medicaid | 4,695 (6.0) | 15 (4.6) | 0.0004 |
| Medicare | 29,201 (37.6) | 156 (48.3) | |
| Other payers | 43,861 (56.4) | 152 (47.1) | |
| Previous Healthcare Exposure | |||
| Transferred from other acute care hospital | 4,057 (5.2) | 35 (10.8) | <0.0001 |
| Transferred from skilled nursing facilities | 1,919 (2.5) | 42 (13.0) | <0.0001 |
| Same hospital discharge during previous 30 days | 9,916 (12.8) | 94 (29.1) | <0.0001 |
| CDI during previous hospitalization | 306 (0.4) | 10 (3.1) | <0.0001 |
| NIM in previous hospitalization | 1,144 (1.5) | 11 (3.4) | 0.0041 |
| CIM in previous hospitalization | 3,174 (4.1) | 29 (9.0) | <0.0001 |
| Clinical Presentation at Admission | |||
| Admitted to intensive care unit | 12,452 (16.0) | 115 (35.6) | <0.0001 |
| Mechanic ventilation on admission | 2,268 (2.9) | 52 (16.1) | <0.0001 |
| CIM on admission | 8,570 (11.0) | 82 (25.4) | <0.0001 |
| Albumin <= 2.4 g/dL | 2,123 (2.7) | 36 (11.1) | <0.0001 |
| Albumin 2.5 - 2.7 g/dL | 1,822 (2.3) | 30 (9.3) | |
| Albumin 2.8 - 3 g/dL | 3,126 (4.0) | 39 (12.1) | |
| Albumin <= 3 g/dL | 7,071 (9.1) | 105 (32.5) | <0.0001 |
| Creatinine > 2.0 mg/dL | 6,280 (8.1) | 72 (22.3) | <0.0001 |
| Pro BNP > 8000 | 104 (0.1) | 4 (1.2) | <0.0001 |
| Bands > 32% | 886 (1.1) | 16 (5.0) | <0.0001 |
| Platelets <= 115 10^9/L | 5,590 (7.2) | 44 (13.6) | <0.0001 |
| Platelets 115.1 - 150 10^9/L | 5,539 (7.1) | 41 (12.7) | |
| Platelets > 420 10^9/L | 3,191 (4.1) | 27 (8.4) | |
| Platelets <=150 or >420 10^9/L | 14,320 (18.4) | 112 (34.7) | <0.0001 |
| WBC 11 - 14.1 ×1,000/mm3 | 12,010 (15.4) | 72 (22.3) | <0.0001 |
| WBC 14.2 - 19.8 ×1,000/mm3 | 8,169 (10.5) | 59 (18.3) | |
| WBC 1>19.8 ×1,000/mm3 | 4,092 (5.3) | 36 (11.1) | |
| WBC > 11 ×1,000mm3 | 24,271 (31.2) | 167 (51.7) | <0.0001 |
| Troponin I >0.1 or CPK MB >=6 ng/mL | 6,863 (8.8) | 54 (16.7) | <0.0001 |
| Community-Onset CDI Pressure, per 10,000 admissions, median (1st, 3rd quartile) | 19.3 (0, 38.9) | 20.9 (0, 41.6) | 0.2551 |
| All Community-Onset CDI Pressure, per 10,000 admissions, median (1st, 3rd quartile) | 52.7 (27.4, 85.7) | 59.5 (30.7, 94.0) | 0.0499 |
Note: NIM: nosocomial infection marker; CIM: community infection marker; WBC: white blood cell count
HO-CDI Model
The logistic regression model yielded 14 independent predictors (Table 2). Risk factors included CO-CDI Pressure greater than 60th percentile, age >64, transferred from another hospital or skilled nursing facilities, CDI during previous hospital stay, previous hospital discharge within 30 days, admission to the intensive care unit, albumin ≤3 g/dL, creatinine >2.0 mg/dL, bands >32%, platelets ≤150 or >420 10^9/L, and white blood cell counts >11,000mm3. The model had a c-statistic of 0.78 (95% CI: 0.76, 0.81) (Figure 1). The Hosmer-Lemeshow χ2 was12.6 (P=0.13), indicating goodness of fit (Figure 2).
Table 2.
Hospital-onset Clostridium difficile Infection Risk Score Model
| Variable | Model with CO-CDI pressure | Model with All-CO-CDI pressure | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | aOR (95%CI) | P value | Score | Estimate (SE) | aOR (95%CI) | P value | Score | |
| CO-CDI pressure > 60th percentile* | 0.23 (0.12) | 1.26 (1.00,1.59) | 0.0534 | 1 | 0.45 (0.19) | 1.56 (1.07,2.27) | 0.0201 | 2 |
| Age ≥65 | 0.65 (0.14) | 1.92 (1.47,2.51) | <.0001 | 3 | 0.65 (0.14) | 1.91 (1.46,2.49) | <.0001 | 3 |
| Transferred from other acute care hospital | 0.57 (0.20) | 1.77 (1.20,2.62) | 0.0041 | 3 | 0.57 (0.20) | 1.77 (1.20,2.62) | 0.004 | 3 |
| Transferred from skilled nursing facilities | 0.96 (0.18) | 2.61 (1.82,3.75) | <.0001 | 4 | 0.94 (0.18) | 2.57 (1.79,3.68) | <.0001 | 4 |
| Mechanic ventilation on admission | 0.90 (0.19) | 2.47 (1.70,3.60) | <.0001 | 4 | 0.91 (0.19) | 2.49 (1.71,3.63) | <.0001 | 4 |
| Admitted to intensive care unit | 0.43 (0.15) | 1.54 (1.16,2.05) | 0.003 | 2 | 0.43 (0.15) | 1.54 (1.16,2.05) | 0.0029 | 2 |
| Hospital discharge during previous 30 days | 0.64 (0.14) | 1.89 (1.44,2.48) | <.0001 | 3 | 0.64 (0.14) | 1.89 (1.44,2.49) | <.0001 | 3 |
| CIM on admission | 0.44 (0.15) | 1.55 (1.17,2.06) | 0.0025 | 2 | 0.44 (0.15) | 1.56 (1.17,2.07) | 0.0023 | 2 |
| CDI during previous hospitalization | 1.18 (0.36) | 3.25 (1.61,6.57) | 0.001 | 5 | 1.18 (0.36) | 3.24 (1.60,6.55) | 0.0011 | 5 |
| Albumin ≤ 3 g/dL | 0.80 (0.14) | 2.23 (1.70,2.93) | <.0001 | 4 | 0.80 (0.14) | 2.22 (1.69,2.91) | <.0001 | 4 |
| Creatinine > 2.0 mg/dL | 0.45 (0.15) | 1.57 (1.17,2.10) | 0.0027 | 2 | 0.46 (0.15) | 1.58 (1.18,2.12) | 0.0022 | 2 |
| Bands > 32% | 0.67 (0.27) | 1.96 (1.15,3.34) | 0.0136 | 3 | 0.69 (0.27) | 1.98 (1.16,3.38) | 0.0117 | 3 |
| Platelets ≤150 or >420 10^9/L | 0.49 (0.13) | 1.63 (1.28,2.09) | 0.0001 | 2 | 0.48 (0.13) | 1.62 (1.26,2.07) | 0.0001 | 2 |
| WBC > 11 ×1,000mm3 | 0.41 (0.12) | 1.50 (1.18,1.91) | 0.0009 | 2 | 0.40 (0.12) | 1.49 (1.17,1.89) | 0.0012 | 2 |
CO-CDI Pressure was dichotomized at 60th percentile (>24.8 CO-CDI per 10,000 admissions); All-CO-CDI Pressure was dichotomized at 60th percentile (>64.1 of all CO-CDI per 10,000 admissions).
Figure 1.
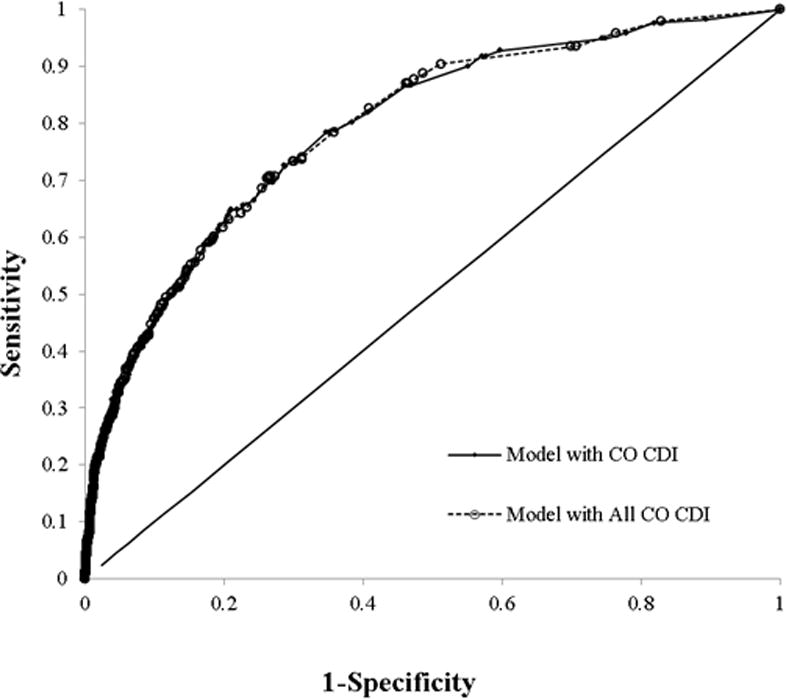
Receiver Operating Curve for HO-CDI Risk Score Models. Model with CO-CDI Pressure had a c-statistic of 0.78. Model with All-CO-CDI Pressure had a c-statistic of 0.79.
Figure 2.
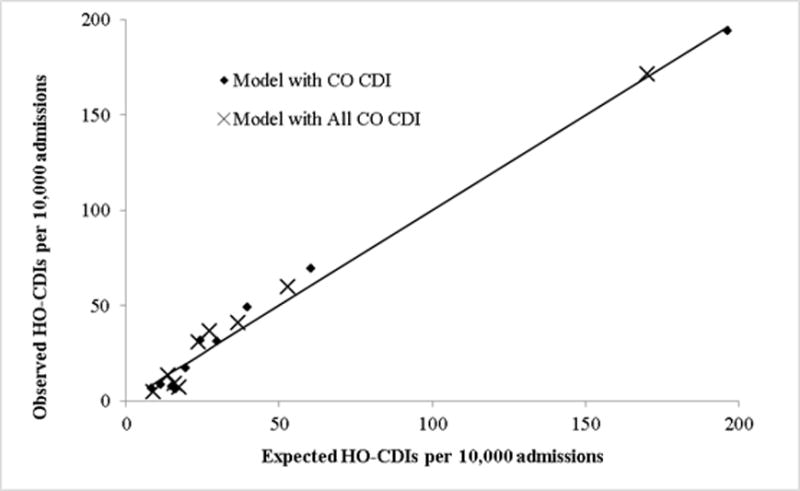
Hosmer-Lemeshow Calibration
The model that incorporated the All CO-CDI Pressure yielded a slighted greater measure of association than the model using the CO-CDI pressure based only on those patients not recently discharged from the measurement hospital. The remaining variables displayed similar estimates. The model c-statistic was 0.79 and the Hosmer-Lemeshow χ2 was15.8 (P=0.03).
The risk score ranged from 0 to 28, with a median of 4 and interquartile range of 2 and 7 for the model with CO-CDI pressure variable. The risk score distribution and corresponding admission volume were in Figure 3. The linearization of the risk score resulted in a nearly linear relationship of increased scores and higher observed HO-CDI rates (Figure 4). The Cochran-Armitage trending test was significant (P<0.0001). For patients with risk score of 0-7, the observed HO-CDI attack rate during hospital stay was 19/10,000 admissions. In contrast, for patients with a risk score of 20 or higher, the observed HO-CDI attack rate was 623/10,000 admissions. Approximately 79% of all admissions had a HO-CDI risk score of 7 or less. They accounted for 35% of all HO-CDI cases. Patients with a HO-CDI score 8 or more accounted for 21% of all admissions but 65% of all HO-CDI cases.
Figure 3.
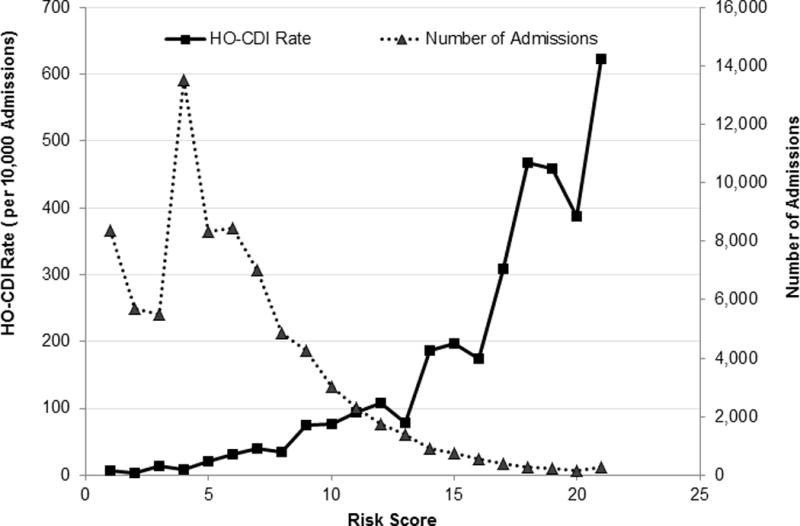
Distribution of HO-CDI Risk Score and Corresponding Admission Volume. *Score 20 and above were collapsed because of small numbers. **Model with CO-CDI Pressure was presented. Model with All-CO-CDI Pressure yielded similar distribution. Data were not shown.
Figure 4.
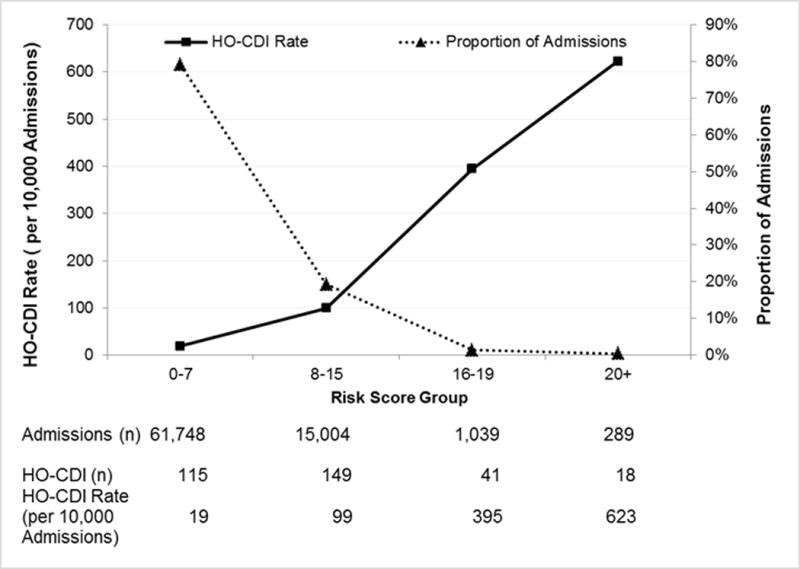
Linearization of HO-CDI Risk and Corresponding Proportion of Admission. * Model with CO-CDI Pressure was presented. Model with All-CO-CDI Pressure yielded similar distribution. Data were not shown.
DISCUSSION
We have developed a risk stratification score based upon parameters available at the time of admission. We found that the 21% patients within the higher risk strata accounted for 65% of all HO-CDI cases. While validation of this scoring system should be performed in other hospitalized patient populations, preferably in settings with available electronic health data to allow automation, this scoring system could be an important tool for prioritizing more specialized, resource-intensive prevention strategies. If aggregated across a facility, it could also function as a risk adjustment tool for HO-CDI rates to allow inter-hospital comparisons that better reflect the effectiveness of intra-hospital prevention efforts.
The principal epidemiologic risk factors for CDI arise from antibiotic exposure, which perturbs the lower intestinal microbiota impairing an important host defense mechanism, and factors that reflect the likelihood of acquiring C. difficile such as admission to a ward with a high colonization pressure.6 There are also other risk factors such as age and underlying illness that may be markers of decreased immunocompetence. Among the 14 factors in our risk scoring system, several may be surrogates for both increased transmission risk and likely exposure to antibiotics. For example, both admission to an ICU and mechanical ventilation may serve as dual surrogates. The ICU is a location of increased exposure to antibiotics as well as to other patients who are either colonized or infected with C. difficile; mechanical ventilation is an additional marker of disease severity that increases likely exposure to antibiotics and is associated with longer ICU stays.
The incubation period of C. difficile (i.e., the period between the most recent exposure and symptomatic infection) is generally thought to be less than a week and, except for those patients previously symptomatic with CDI (who are at risk for recurrence), patients who remain asymptomatically colonized for a prolonged period with C. difficile are thought to be at paradoxically decreased, rather than increased, risk for developing symptomatic infection.18 This is thought due to a boosting of anti-toxin antibodies in such asymptomatically colonized patients. Because of this phenomenon, it appears most likely that the association of HO-CDI with previous healthcare exposures in our model (i.e. previous admission, transfer from a nursing home or outside hospital), independent of previous CDI, reflect preceding antibiotic exposures. Metagenomic studies of the lower intestinal microbiota demonstrate dysbiosis that persists for up to 6 months after relatively short single antibiotic exposures; repeated or more intense exposure lead to even more severe and persistent dysbiosis.19 Meanwhile, epidemiologic studies suggest the risk for CDI increases 7-10 fold while patients are on antibiotics and in the month following cessation, and that the risk remains elevated 2-3 fold in the two months after that.20
Predicting the likelihood of HO-CDI at the time of admission may help clinicians to devise early preventative strategies. Although application of our risk score needs to be tested prospectively, preferably in hospitals with advanced electronic health records, restricting data requirements to patient risk factors at the time of admission simplifies risk score application. Incorporation of environmental risk factors, such as the risk of exposure to C. difficile during the entire hospital stay, would be more complex and challenging in terms of implementation, especially across institutions. Nonetheless, development of such a dynamic risk index, even to the point of daily calculation of CDI risk, could be an important future demonstration of how an advanced electronic health record contributes to risk reduction.
Dubberke, et al. showed that “CDAD pressure”, measured as each patient’s total exposure to infectious CDI patients divided by the patient’s length of stay at risk for CDI, was the most predictive factor for HO-CDI with an odds ratio of 13.6 We restricted the CDI pressure to community-onset only which is more suitable for aggregating patient risk at the facility-level so as to assist with inter-hospital comparisons; CO-CDI unrelated to a previous hospitalization is outside the control of a given hospital and therefore is more appropriately included in facility-level risk adjustment. In the future, if a more dynamic CDI patient-focused risk model were developed that accounted for daily intra-hospital exposures, it could incorporate the HO-CDI rate on a given patient ward so as to more precisely predict daily transmission risk. However, including the HO-CDI rate would render the model no longer appropriate for risk adjustment at the facility level as hospital-onset infections reflect, in addition to a facilities baseline risk, the degree to which intra-hospital prevention strategies have been successfully implemented.
In addition to factors that serve as surrogates for either antibiotic exposure or exposure to patients who are colonized or infected with C. difficile, our model included several factors that reflect an increased intrinsic patient risk for infection following colonization. Given the aforementioned importance of humoral immunity as a host defense, this intrinsic infection risk is likely mediated via compromising host immune-responsiveness. Previous studies have found that advanced age, hypoalbuminemia, and an elevated creatinine were associated with HO-CDI.6 Meanwhile, abnormal platelets, elevated bands, and white blood cell counts may reflect underlying infections at other body sites that increase the likelihood of the patient becoming exposed to antibiotics.
Limitations
Our study has limitations. First, although we used data from multiple sites, which is an improvement over a single center study in terms of diversity of patients, the number of hospitals and number of HO-CDI cases were still relatively small. The small number of hospitals may not represent the diversity of nationwide CO-CDI pressure. This may partially explain the relatively small impact of this variable in the model. More representative data are needed to further validate the HO-CDI risk score. Second, application of the HO-CDI risk score needs to be tested in a prospective setting, preferably in automated electronic medical record environment. Third, our risk score restricted data use to electronically captured data that are widely available, such as microbial results, general laboratory test results, previous hospitalization status, and source of admissions. Future studies may test additional predictive ability when more patient clinical data at admission, such as vital signs, become more readily available through electronic automation process. Fourth, the database didn’t have outpatient information regarding antibiotic use prior to admission, a known risk for C. difficile. We opted to identify surrogates of potential antibiotic exposure, such as clinical severity, ICU admissions, exposure to healthcare prior to admission, and infection markers, which were found to be significant risk factors. Finally, for use as a risk identification tool, both the number of patients needed to screen, and, based upon the estimated prevention efficacy of an intervention, the number needed to treat with an intervention to prevent one case of HO-CDI, will be required to determine the overall cost effectiveness of the tool.
CONCLUSIONS
Using patient clinical parameters available at the time of admission, this HO-CDI model displays a good predictive ability. Although further analyses will be required to determine its feasibility and cost effectiveness, it may have utility as a real-time early risk identification tool for HO-CDI preventive interventions. Once aggregated across a facility, it could also function to improve risk adjustment for inter-facility comparisons.
Acknowledgments
The preliminary data were presented in part as a poster at the IDWEEK, October, 2012, San Diego, CA. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial Support:
No extramural funding was provided to support this analysis other than employment status of authors acknowledged. There is no writing assistance for this manuscript.
Footnotes
Conflict of Interest:
Dr. McDonald has no conflict of interest to disclose.
Drs. Tabak, Johannes, Sun, and Nunez are employees of CareFusion. No other conflict of interest to disclose.
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014 Mar 27;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucado J, Gould C, Elixhauser A. HCUP Statistical Brief #124. Agency for Healthcare Research and Quality; Rockville, MD: Jan, 2012. Clostridium difficile Infections (CDI) in Hospital Stays, 2009. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf. [PubMed] [Google Scholar]
- 3.Murphy SL, Xu J, Kochanek KD. Deaths: Final Data for 2010. National Vital Statistics Reports. 2013;61(4) http://www.cdc.gov/nchs/data/nvsr/nvsr61/nvsr61_04.pdf. Accessed January 9, 2014. [PubMed] [Google Scholar]
- 4.Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009 Jan;30(1):57–66. doi: 10.1086/592981. [DOI] [PubMed] [Google Scholar]
- 5.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol. 2013 Jun;34(6):588–596. doi: 10.1086/670621. [DOI] [PubMed] [Google Scholar]
- 6.Dubberke ER, Yan Y, Reske KA, et al. Development and validation of a Clostridium difficile infection risk prediction model. Infect Control Hosp Epidemiol. 2011 Apr;32(4):360–366. doi: 10.1086/658944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brossette SE, Hacek DM, Gavin PJ, et al. A laboratory-based, hospital-wide, electronic marker for nosocomial infection: the future of infection control surveillance? Am J Clin Pathol. 2006 Jan;125(1):34–39. [PubMed] [Google Scholar]
- 8.Zilberberg MD, Tabak YP, Sievert DM, et al. Using electronic health information to risk-stratify rates of Clostridium difficile infection in US hospitals. Infect Control Hosp Epidemiol. 2011 Jul;32(7):649–655. doi: 10.1086/660360. [DOI] [PubMed] [Google Scholar]
- 9.Tabak YP, Sun X, Nunez CM, Johannes RS. Using electronic health record data to develop inpatient mortality predictive model: Acute Laboratory Risk of Mortality Score (ALaRMS) J Am Med Inform Assoc. 2014 May-Jun;21(3):455–463. doi: 10.1136/amiajnl-2013-001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. Multidrug-Resistant Organism & Clostridium difficile Infection (MDRO/CDI) Module. http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf. Accessed Januray 16, 2015.
- 11.Tabak YP, Sun X, Derby KG, Kurtz SG, Johannes RS. Development and validation of a disease-specific risk adjustment system using automated clinical data. Health Serv Res. 2010 Dec;45(6 Pt 1):1815–1835. doi: 10.1111/j.1475-6773.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabak YP, Johannes RS, Silber JH. Using automated clinical data for risk adjustment: development and validation of six disease-specific mortality predictive models for pay-for-performance. Med Care. 2007 Aug;45(8):789–805. doi: 10.1097/MLR.0b013e31803d3b41. [DOI] [PubMed] [Google Scholar]
- 13.Dudeck MA, Weiner LM, Malpiedi PJ, Edwards JR, Peterson KD, Sievert DM. Risk Adjustment for Healthcare Facility-Onset C difficile and MRSA Bacteremia Laboratory-identified Event Reporting in NHSN. 2013 http://www.cdc.gov/nhsn/pdfs/mrsacdi/RiskAdjustment-MRSA-CDI.pdf. Accessed Janurary 16, 2015.
- 14.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd. New York: John Wiley & Sons, Inc; 2000. [Google Scholar]
- 15.Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source code for biology and medicine. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullivan LM, Massaro JM, D’Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004 May 30;23(10):1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 17.Efron B, Tibshirani R. An Introduction to the Bootstrap. London, England: Chapman & Hall; 1993. [Google Scholar]
- 18.Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011 Aug;60(Pt 8):1070–1079. doi: 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 19.Huse SM, Dethlefsen L, Huber JA, Mark Welch D, Relman DA, Sogin ML. Exploring microbial diversity and taxonomy using SSU rRNA hypervariable tag sequencing. PLoS genetics. 2008 Nov;4(11):e1000255. doi: 10.1371/journal.pgen.1000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. The Journal of antimicrobial chemotherapy. 2012 Mar;67(3):742–748. doi: 10.1093/jac/dkr508. [DOI] [PubMed] [Google Scholar]


