Abstract
Background
Data evaluating the impact of objectively measured psoriasis severity on type 2 diabetes mellitus (T2DM) risk are lacking.
Objective
To determine the risk of T2DM in patients with psoriasis compared to adults without psoriasis, stratified by categories of directly assessed body surface area (BSA) affected by psoriasis.
Methods
A prospective, population-based, cohort study from the UK. 8,124 adults with psoriasis and 76,599 adults without psoriasis were followed prospectively for approximately 4 years.
Results
280 (3.44%) incident cases of diabetes in the psoriasis group and 1867 (2.44%) incident cases of diabetes in those without psoriasis. After adjusting for age, sex and body mass index, the hazard ratios (95%CI) for developing incident diabetes were 1.21 (1.01–1.44), 1.01 (0.81–1.26), and 1.64 (1.23–2.18) in the ≤2BSA, 3–10%BSA, and >10%BSA groups compared to those without psoriasis, respectively (p=0.004 for trend). Worldwide, we estimate an additional 125,650 new diagnoses of T2DM per year in patients with psoriasis as compared to those without.
Limitations
Relatively short-term follow-up and exclusion of prevalence cases which may have masked associations in patients with less extensive psoriasis.
Conclusion
Clinicians may measure BSA affected by psoriasis in order to target diabetes prevention efforts for patients with psoriasis.
Keywords: Psoriasis, Diabetes, Cohort study, Epidemiology, Body surface area
Introduction
Psoriasis is a common, chronic inflammatory disease affecting over 125 million people worldwide1,2. A vast and growing body of literature links psoriasis to an excess risk of major medical morbidities, and even mortality3–10. Of special interest is the association of psoriasis and diabetes11,12 as genetic studies have identified shared susceptibility loci and inflammatory cytokines upregulated in psoriasis are known to promote insulin resistance13–17. A recent, small meta-analysis identifying five papers (4 of which did not measure psoriasis severity) assessing incidence showed that psoriasis is associated with a relative risk of 1.27 (95%CI 1.16–1.40) for developing type 2 diabetes mellitus (T2DM)4. Beyond the systematic review, we identified one additional study that used treatment patterns to establish a higher risk of T2DM18. Despite current knowledge, data from population-based, prospective studies evaluating the impact of objectively measured disease severity on T2DM risk are still lacking. Only a handful of studies have assessed risk (incidence) of T2DM while adjusting for confounding variables11,18–20, and even fewer were able to evaluate the effects of psoriasis severity (all of which used treatment patterns) on T2DM association18,19.
Therefore, a better understanding of how physician-reported psoriasis severity affects the risk of T2DM is essential to determine which patients have a clinically important increased risk of diabetes and therefore should be targeted for augmented prevention efforts. While a variety of approaches have been used to define psoriasis severity, categories of body surface area (BSA) affected have been commonly used in epidemiological studies and are clinically intuitive. The National Psoriasis Foundation21,22 and the Centers for Disease Control23 categorize BSA into ≤2% (“mild”), 3–10% (“moderate”), and >10% (“severe”). Furthermore, we have previously shown that these simple categories are positively associated with prevalence of psoriatic arthritis and major medical comorbidities in a dose-response manner12,24. The goal of this study was to determine the risk of T2DM in patients with psoriasis compared to adults without psoriasis, stratified by categories of directly assessed BSA affected by psoriasis.
Methods
Study Design and Data Source
We conducted a prospective, population-based cohort study nested within The Health Improvement Network (THIN) to determine the incidence of diabetes in patients with psoriasis. THIN is a UK EMR database containing patient demographics, diagnostic information, clinical measurements, and prescriptions from general practitioners (GPs) using Vision software (In Practice Systems, Ltd). GPs coordinate almost all patients’ care in the UK healthcare system; hence, medical information from specialists and hospitals is routinely recorded in their EMR. Diagnoses are recorded in THIN through a READ diagnostic code system25 and documented prescriptions are linked to the British National Formulary (BNF)26. This study’s version of THIN included longitudinal data on 7.5 million registered patients from 415 practices, with demographics broadly representative of the general UK population27. Studies have validated the accuracy of THIN data for use in large-scale epidemiology research, particularly in psoriasis research28,29. This study was conducted in compliance with the Declaration of Helsinki30 and manuscript prepared in accordance with the STROBE statement31. The research was approved by the University of Pennsylvania Institutional Review Board and the Cambridgeshire Research Ethics Committee. No consent was required as de-identified data were used.
Study Population
The Incident Heath Outcomes and Psoriasis Events (iHOPE) cohort, previously described by our group12, is comprised of individuals randomly sampled from THIN who were, at the time of sampling: aged 25 to 64, with at least 1 psoriasis READ diagnostic code within 2 years before survey administration, and registered in a practice that was participating in THIN’s Additional Information Services (58% of the THIN practices), which involves participants’ GPs completing questionnaires in exchange for financial compensation. As psoriasis was the exposure of interest, GPs were prospectively sent a research survey29 which was collected in subsequent 12 months and involved GPs confirming psoriasis diagnosis and evaluating severity of disease. GPs assessed the extent of psoriasis by evaluating the amount BSA involved and provided this as a continuous estimate (0–100%) and as separate categories: mild (limited disease with ≤2% BSA affected), moderate (scattered disease with 3–10% BSA affected), or severe (extensive disease with >10% BSA affected)32.
Each patient with psoriasis was individually matched with up to ten randomly selected patients without psoriasis (defined by no history of psoriasis READ diagnostic codes) by practice, visit date, and age (within 10-year age categories). The exposed group in our primary analyses required GP confirmation of psoriasis diagnosis and severity assessment by BSA affected from the survey. The unexposed group was also required to be actively registered, with at least 1 visit to their GP within two years, before the time of random sampling. Subjects with and without psoriasis were excluded from analyses if there was a history of T2DM at baseline, which corresponds to the index date.
Type 2 Diabetes Mellitus Outcome and Covariate Definitions
The primary outcome, the incidence of T2DM, was defined by a composite of 1 T2DM READ diagnostic code plus a second READ diagnostic code, or 1 T2DM pharmacologic therapy, or 1 laboratory confirmation, whichever occurred first. This identification approach was adapted from a validation study using THIN by Sharma et al with 100% PPV33. Furthermore, according to the UK National Institute for Clinical Excellence and GP Notebook clinical guidelines, T2DM was defined as ≥6.5% HbA1c or ≥48mmol/mol34,35. T2DM antidiabetic drugs were identified according to the BNF that corresponded with the observation period36.
Multiple potential confounders were identified a priori: age, sex, BMI, smoking and alcohol use, medical comorbidities (hyperlipidemia and hypertension), use of prescription oral and inhaled corticosteroids, and the Townsend deprivation score (which correlates to socioeconomic status). These were measured prior to survey sampling or the corresponding visit date for matched controls. Age was treated as a continuous variable, whereas other covariates were categorical.
Person-Time Calculation
For psoriasis exposed individuals, the index date (cohort entry) was defined by the date the GP survey29 was sent. The index date for unexposed individuals was the corresponding date for the exposed individual to whom they were matched. The event of interest was the incidence of T2DM. Patients were censored at end of follow-up due to patient death or transfer out of practice, end of practice participation in THIN, or the end of the observation period (February 5, 2015).
Statistical Analysis
Baseline patient demographics were summarized with descriptive statistics for patients with and without psoriasis. Differences in baseline demographic and clinical characteristics were assessed using the Student’s t-test for continuous variables and χ2 tests for categorical variables. A raw incidence rate for T2DM was estimated by dividing the number of events (new diagnoses of T2DM) by the total person-time under observation. Cox proportional hazards models were used to estimate hazard ratios (HR) comparing the exposed and unexposed cohorts. We identified covariates for inclusion in a final, adjusted Cox proportional hazards model utilizing a purposeful selection approach. Our initial model included all covariates in Table 1. Variables were then eliminated from the model by sequentially removing covariates starting with the largest p-value (p>0.05). If the HR point estimates for psoriasis did not change by more than 10% in the reduced model as compared to the prior multivariable model, then the covariate was removed. Our final model was adjusted for age, sex, and BMI. The likelihood ratio test was used to evaluate significance of covariates, determining best model fit. Log-log survival plots for psoriasis were constructed to assess the proportional-hazards assumption, which was not violated. All effect measures were reported with 95%CIs. Our sample size, provided power in excess of 98% for HRs of 1.2 or greater, assuming a 2-sided alpha-level of 0.05. Analyses were performed using STATA 13.1 (College Station, Texas, USA).
Table 1.
Baseline Characteristics of Psoriasis Patients and Non-Psoriasis Patients (n=84723).
| Non-Psoriasis (n=76599) | Psoriasis (n=8124) | P-Valuea | Standardized Difference | |||
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 35711 | 46.62% | 4088 | 50.32% | <0.001 | 0.07 |
| Female | 40888 | 53.38% | 4036 | 49.68% | ||
| Age, y | ||||||
| Mean | 44.54 | 44.86 | 0.015 | 0.03 | ||
| Median (IQR) | 45 (36–54) | 45 (36–54) | ||||
| BMI, kg/m2 | ||||||
| <18.5 | 1435 | 2.11% | 127 | 1.74% | <0.001 | 0.14 |
| ≥18.5–<25 | 27355 | 40.14% | 2547 | 34.99% | ||
| ≥25–<30 | 23759 | 34.87% | 2547 | 34.99% | ||
| ≥30–<35 | 10313 | 15.13% | 1284 | 17.64% | ||
| >35 | 5280 | 7.75% | 774 | 10.63% | ||
| Missing | 8457 | 11.04% | 845 | 10.40% | ||
| Smoking | ||||||
| Never | 37650 | 49.98% | 3025 | 37.58% | <0.001 | 0.25 |
| Current | 18744 | 24.88% | 2582 | 32.05% | ||
| Former | 18934 | 25.14% | 2442 | 30.34% | ||
| Missing | 1271 | 1.66% | 75 | 0.92% | ||
| Alcohol | ||||||
| Never | 7420 | 11.04% | 654 | 9.09% | <0.001 | 0.07 |
| Current | 55565 | 82.70% | 6026 | 83.76% | ||
| Former | 4206 | 6.26% | 514 | 7.14% | ||
| Missing | 9408 | 12.28% | 930 | 11.45% | ||
| History of Hyperlipidemia | ||||||
| Yes | 5351 | 6.99% | 663 | 8.16% | <0.001 | 0.04 |
| No | 71248 | 93.01% | 7461 | 91.84% | ||
| History of Hypertension | ||||||
| Yes | 9444 | 12.33% | 1084 | 13.34% | 0.008 | 0.03 |
| No | 67155 | 87.67% | 7040 | 86.66% | ||
| History of Systemic Corticosteroids | ||||||
| Yes | 4861 | 6.35% | 636 | 7.83% | <0.001 | 0.06 |
| No | 71738 | 93.65% | 7488 | 92.17% | ||
| History of Inhaled Corticosteroids | ||||||
| Yes | 3941 | 5.14% | 449 | 5.53% | 0.140 | 0.02 |
| No | 72658 | 94.86% | 7675 | 94.47% | ||
| Townsend Score | ||||||
| 1 | 19159 | 26.00% | 1856 | 23.72% | <0.001 | 0.06 |
| 2 | 16024 | 21.75% | 1689 | 21.59% | ||
| 3 | 15407 | 20.91% | 1668 | 21.32% | ||
| 4 | 13228 | 17.95% | 1469 | 18.78% | ||
| 5 | 9865 | 13.39% | 1141 | 14.59% | ||
| Missing | 2916 | 3.81% | 301 | 3.71% | ||
| History of Biologic/Systemic Drugs | 151 | 0.19% | 353 | 4.35% | ||
| History of Phototherapy | 70 | 0.09% | 560 | 6.89% | ||
The χ2test was used for analyses of categorical variables, and t-test was used for analyses of continuous variables. P-values are based on comparisons of the group of patients with psoriasis compared to matched individuals without psoriasis.
Results
Patient Characteristics
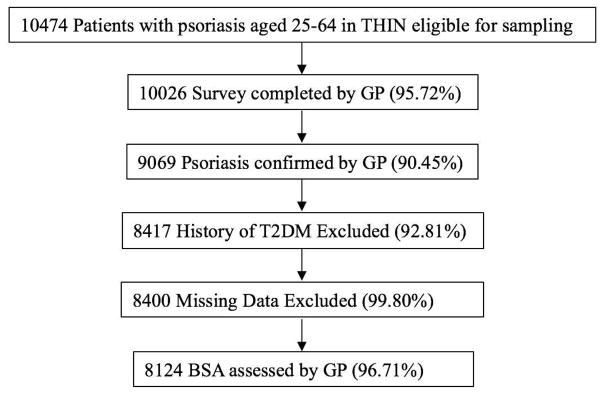
Of the 10474 eligible patients with psoriasis READ codes sampled for survey mailing, 10026 had surveys returned from their GPs, of whom 9069 had confirmed psoriasis diagnosis by their GP. 652 had a history of T2DM and 17 subjects had missing medical records and were therefore excluded from analyses. Our entire study cohort consisted of 84723 individuals of whom 8124 had psoriasis, and 76599 did not have psoriasis. After stratification by physician-reported BSA, 4216 (51.90%), 2915 (35.88%), and 993 (12.22%) had ≤2% BSA, 3–10% BSA, and >10% BSA affected, respectively (Figure 1). Compared to patients without psoriasis, the group with psoriasis had the same median age, slightly more males, and similar follow-up duration. Standardized differences (Table 1), a measure not influenced by sample size, indicated meaningful differences (>0.1) between patients with and without psoriasis for BMI and smoking status37.
Figure 1.
Exposed Population Selection.
Incidence of Type 2 Diabetes Mellitus in Patients with Psoriasis
There were 280 (3.44%) and 1867 (2.44%) incident outcomes of T2DM in patients with psoriasis and without psoriasis, respectively. The unadjusted incidence rate of T2DM was 5.97 new diagnoses of T2DM per 1000 person-years in the unexposed group. The highest incidence rate of T2DM was in patients affected by >10% BSA (12.22 per 1000 person-years) (Table 2). After adjusting for age, sex and BMI, the HRs (95%CI) for developing incident T2DM were 1.21 (1.01–1.44), 1.01 (0.81–1.26), and 1.64 (1.23–2.18) in the ≤2% BSA, 3–10% BSA, and >10% BSA groups compared to patients without psoriasis, respectively (p=0.004 for trend; Table 3). As patients with >10% BSA may be heterogeneous in their disease burden, we further evaluated increases in BSA in this group (based on a continuous measure provided by GPs) and determined that for every 10% increase in BSA affected with the fully adjusted model, the hazard of incident T2DM increased by a factor of 1.193 (95%CI 1.025–1.390). Interactions between age and psoriasis and sex and psoriasis were not statistically significant (p<0.1). Findings were similar using robust standard errors to account for clustering within matched sets of observations.
Table 2.
Incidence of Type 2 Diabetes Mellitusa Rates in Psoriasis Patients Compared to Controls and by Psoriasis Severity.
| Non-Psoriasis (n=76599) | Psoriasis (n=8124) | ≤2% BSA (n=4216) | 3–10% BSA (n=2915) | >10% BSA (n=993) | |
|---|---|---|---|---|---|
| Number of new DM cases | 1867 (2.44%) | 280 (3.44%) | 139 (3.30%) | 92 (3.16%) | 49 (4.93%) |
| Number of person-years | 312682.34 | 33788.32 | 17471.244 | 12308.723 | 4008.356 |
| Average length of follow-up, yrs | 4.08 | 4.16 | 4.14 | 4.22 | 4.04 |
| Incident T2DM rate, per 1000 person-years (95%CI) | 5.97 (5.71–6.25) | 8.29 (7.37–9.32) | 7.96 (6.74–9.40) | 7.47 (6.09–9.17) | 12.22 (9.24–16.17) |
Type 2 Diabetes Mellitus defined by (1) Two Diagnostic READ codes (2) One Diagnostic READ code and 1 Diabetic Drug code, (3) One Diagnostic READ code and 1 Laboratory value of HbA1c ≥6.5% or ≥48mmol/mol.
Table 3.
Hazard of Type 2 Diabetes Mellitus in Psoriasis Patients Compared to Non-Psoriasis Patients.
| Psoriasis, Hazard Ratio (95%CI) | |||||
|---|---|---|---|---|---|
| n | ≤2% BSA | 3–10% BSA | >10% BSA | P-valuea | |
| Unadjusted | 84723 | 1.33 (1.12–1.58) | 1.25 (1.02–1.54) | 2.05 (1.55–2.73) | <0.001 |
| Fully Adjustedb | 75421 | 1.21 (1.01–1.44) | 1.01 (0.81–1.26) | 1.64 (1.23–2.18) | 0.004 |
P-value for test for trend conducted by coding psoriasis severity as a linear variable (0: no psoriasis; 1: mild; 2: moderate; 3: severe)
Fully adjusted model includes age, sex, and body mass index. Type 2 Diabetes Mellitus defined by (1) Two Diagnostic READ codes (2) One Diagnostic READ code and 1 Diabetic Drug code, (3) One Diagnostic READ code and 1 Laboratory value of HbA1c ≥6.5% or ≥48mmol/mol.
Sensitivity Analyses and Multiple Imputation
Our observations were robust across a variety of sensitivity analyses and after using multiple imputation for missing BMI data (Supplemental Table 1). Results changed little in response to varying outcome (T2DM) definitions, varying exposure (psoriasis) group definitions, and alternative handling of missing data in BMI. Investigating observation bias and treatment pattern effects did not affect our findings significantly (Supplemental Table 1, #11, #7, #8). To ensure incident T2DM outcomes were not prevalent outcomes, sensitivity analyses excluding patients who had registered with the practice within 9 months prior to the index date38 and those with diagnoses of T2DM within 1 year after index date were conducted, and results were essentially unchanged.
Attributable Risk
Based on our data, we estimate that in individuals with psoriasis, there are 3 additional diagnoses of diabetes per 1000 patient-years compared to patients without psoriasis, after accounting for traditional risk factors such as age, sex and BMI, identified in routine medical practice. Furthermore, the excess risk, or number of extra cases of T2DM, attributable to psoriasis is most clinically significant in patients with >10%BSA affected with 6.25 extra cases per 1000 person-years as compared to those without psoriasis versus those with ≤2% BSA affected, in which the excess risk is 1.99 per 1000 person-years. Using standardized cumulative incidence estimates based on our adjusted Cox model39 an additional estimated 125,650 new diagnoses of T2DM per year worldwide are seen in patients with psoriasis as compared to those without, of which, approximately 25, 000 have psoriasis affecting greater than 10% of their BSA.
Discussion
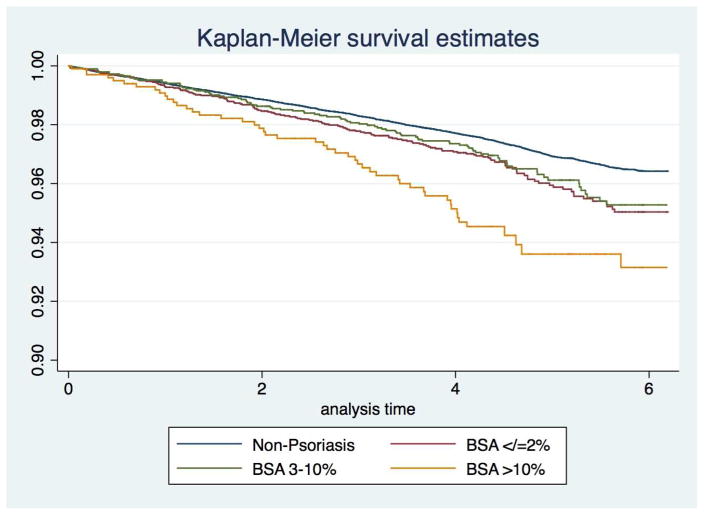
In our large, population-based, prospective cohort study from the UK, we found a positive dose-response relationship between an objective measure of psoriasis severity and the incidence of T2DM. Patients with psoriasis affected by >10%BSA had the highest risk of T2DM, compared to adults without psoriasis. Moreover, for every 10% increase in BSA affected by psoriasis (i.e., 20%, 30%, etc.) there is approximately a 20% higher hazard of diabetes, which emphasizes the dose-response relationship between burden of skin psoriasis and risk of diabetes. Our results accounted for major diabetic risk factors (age, sex, and BMI) routinely collected in clinical practice, were robust across multiple sensitivity analyses, and suggest that psoriasis and its severity are important predictors of diabetes risk. We estimate, based on our data, that a patient with psoriasis affecting 10% or more of their BSA has about a 60% higher risk per year of developing T2DM, which translates into an extra 25,000 new cases of diabetes annually worldwide attributable to severe psoriasis. We also observed an increased risk of diabetes in patients with ≤2% BSA; however, this association was smaller, of lower clinical significance, but still important to note. Interestingly, we did not observe a statistically significantly increased risk in the 3–10% BSA group. We note that based on the 95%CIs and Kaplan-Meier curves (Figure 2) we cannot exclude that this finding is statistically different from the ≤2% BSA group, though it is evidently lower than the >10% BSA group. Furthermore, we have previously demonstrated a dose-response relationship between BSA and prevalent diabetes12 so it is conceivable that our findings with incident T2DM were diluted by prevalent T2DM in patients with varying levels of psoriasis severity. Additionally, the sample size and duration of follow-up may not have been sufficient to detect a prospective association in this subgroup.
Figure 2.
Kaplan-Meier survival estimates of time to T2DM diagnosis for psoriasis cohort stratified according to the Body Surface Area (BSA): ≤2% (mild), 3–10% (moderate), and >10%(severe); and matched patients without psoriasis.
Our study advances existing literature by demonstrating that a simple phenotypic objective measure of disease severity has clinical significance beyond the skin in that it predicts risk of developing T2DM. It also builds upon our recent work in which we demonstrated that mortality risk among patients with psoriasis followed prospectively for a period of approximately 4 years is restricted to patients with greater than 10% BSA affected10. Current literature uses treatment patterns (i.e., systemic therapy) to define severity, which is problematic, as treatment may alter the risk of diabetes (either positively or negatively), and it is well known that psoriasis is widely undertreated21. Thus, it is necessary to determine if the findings generalize to patients with extensive psoriasis who are not receiving systemic agents or phototherapy. Most studies that assessed incidence4,18,19 did not adjust for confounders and all used psoriasis treatment as a surrogate marker for psoriasis severity. Moreover, our broadly representative, population-based study design and high GP survey response rate, suggest that our findings are likely generalizable to the psoriasis population (i.e., strong external validity).
Overall limitations to our study include selection bias and information bias. However, exposed and unexposed matched individuals were randomly drawn from the same population (minimizing selection bias) and data were collected in by the same practices within a similar timeframe (minimizing information bias). It is possible that patients with severe psoriasis visit their GP more frequently, especially with existing comorbidities, although our findings were robust to observation bias (Supplemental Table 1, #11). Finally, as with all observational studies, the possibility of unmeasured or unknown confounders exist. However, we tested and accounted for numerous confounders in our primary analyses, and our findings were robust to multiple sensitivity analyses.
Conclusion
Our findings demonstrate that psoriasis is a significant risk factor for incident T2DM beyond age, sex and BMI and that the risk of developing T2DM increases with increasing BSA affected. Clinicians may consider measuring BSA affected by psoriasis as part of standard of care since it has important prognostic implications. Patients with psoriasis affecting >10% BSA should be targeted for diabetes prevention efforts.
Supplementary Material
Abbreviations
- BMI
Body Mass Index
- BNF
British National Formulary
- BSA
Body Surface Area
- CIs
Confidence Intervals
- EMR
Electronic Medical Records
- GPs
General Practitioners
- T2DM
Type 2 Diabetes Mellitus
- THIN
The Health Improvement Network
- UK
United Kingdom
- USA
United States of America
Footnotes
Conflicts of Interest
In the previous 12 months, Dr. Gelfand served as a consultant for BMS, Coherus (DSMB), Dermira, GSK, Janssen Biologics, Menlo Therapeutics, Novartis Corp, Regeneron, Dr Reddy’s labs, Sanofi and Pfizer Inc., receiving honoraria; and receives research grants (to the Trustees of the University of Pennsylvania) from Abbvie, Janssen, Novartis Corp, Regeneron, Sanofi, Celgene, and Pfizer Inc.; and received payment for continuing medical education work related to psoriasis that was supported indirectly by Lilly, Valeant, and Abbvie. Dr. Gelfand is a co-patent holder of resiquimod for treatment of cutaneous T cell lymphoma
Dr. Mehta is a full-time US government employee and has received research grants to NIH from Abbvie, Celgene, Novartis and Janssen.
We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal. Supported in part by a grant (K24 AR064310) from NIH/NIAMS (JMG) and a medical dermatology fellowship from the National Psoriasis Foundation (MTW). This study does not require IRB review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
- 2.(WHO). WHO. Global Report on Psoriasis. Geneva: World Health Organization; [Google Scholar]
- 3.Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143(12):1493–1499. doi: 10.1001/archderm.143.12.1493. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149(1):84–91. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 5.Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi: 10.1136/bmj.f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeshita J, Wang S, Shin DB, et al. Effect of psoriasis severity on hypertension control: a population-based study in the United Kingdom. JAMA Dermatol. 2015;151(2):161–169. doi: 10.1001/jamadermatol.2014.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong AW, Lin SW, Chambers CJ, Sockolov ME, Chin DL. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6(3):e18227. doi: 10.1371/journal.pone.0018227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 9.Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74(2):326–332. doi: 10.1136/annrheumdis-2014-205675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noe MH, Shin DB, Wan MT, Gelfand JM. Objective Measures of Psoriasis Severity Predict Mortality: A Prospective Population-Based Cohort Study. J Invest Dermatol. 2017 doi: 10.1016/j.jid.2017.07.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Han J, Hu FB, Curhan GC, Qureshi AA. Psoriasis and risk of type 2 diabetes among women and men in the United States: a population-based cohort study. J Invest Dermatol. 2012;132(2):291–298. doi: 10.1038/jid.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149(10):1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelfand JM. Psoriasis, Type 2 Diabetes Mellitus, and Obesity: Weighing the Evidence. JAMA Dermatol. 2016;152(7):753–754. doi: 10.1001/jamadermatol.2016.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316(5829):1336–1341. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quaranta M, Burden AD, Griffiths CE, et al. Differential contribution of CDKAL1 variants to psoriasis, Crohn’s disease and type II diabetes. Genes Immun. 2009;10(7):654–658. doi: 10.1038/gene.2009.51. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Wang Z, Rani PL, et al. Identification of PTPN22, ST6GAL1 and JAZF1 as Psoriasis Risk Genes Demonstrates Shared Pathogenesis between Psoriasis and Diabetes. Exp Dermatol. 2017 doi: 10.1111/exd.13393. [DOI] [PubMed] [Google Scholar]
- 17.Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130(7):1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 18.Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148(9):995–1000. doi: 10.1001/archdermatol.2012.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brauchli YB, Jick SS, Meier CR. Psoriasis and the risk of incident diabetes mellitus: a population-based study. Br J Dermatol. 2008;159(6):1331–1337. doi: 10.1111/j.1365-2133.2008.08814.x. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AA, Choi HK, Setty AR, Curhan GC. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145(4):379–382. doi: 10.1001/archdermatol.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the National Psoriasis Foundation surveys, 2003–2011. JAMA Dermatol. 2013;149(10):1180–1185. doi: 10.1001/jamadermatol.2013.5264. [DOI] [PubMed] [Google Scholar]
- 22.Horn EJ, Fox KM, Patel V, Chiou CF, Dann F, Lebwohl M. Are patients with psoriasis undertreated? Results of National Psoriasis Foundation survey. J Am Acad Dermatol. 2007;57(6):957–962. doi: 10.1016/j.jaad.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003–2004. J Am Acad Dermatol. 2009;60(2):218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: Epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chisholm J. The Read clinical classification. BMJ (Clinical research ed) 1990;300(6732):1092. doi: 10.1136/bmj.300.6732.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed May 23, 2016];THIN Database. https://www.ucl.ac.uk/pcph/research-groups-themes/thinpub/database.
- 27.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 28.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 29.Seminara NM, Abuabara K, Shin DB, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164(3):602–609. doi: 10.1111/j.1365-2133.2010.10134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 31.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 32.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: a study from the US population. J Am Acad Dermatol. 2004;51(5):704–708. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Sharma M, Petersen I, Nazareth I, Coton SJ. An algorithm for identification and classification of individuals with type 1 and type 2 diabetes mellitus in a large primary care database. Clin Epidemiol. 2016;8:373–380. doi: 10.2147/CLEP.S113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Excellence NIfC. Type 2 diabetes: The management of type 2 diabetes (CG87) 2009 [Google Scholar]
- 35.WHO. Use of Glycated Haemoglobin (HbA1c) in the Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation. Geneva: 2011. [PubMed] [Google Scholar]
- 36.Committee JF. British National Formulary. 62. London: BMJ Group and Pharmaceutical Press; 2011. p. 1052. [Google Scholar]
- 37.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation. 2009;38(6):1228–1234. [Google Scholar]
- 38.Mamtani R, Haynes K, Finkelman BS, Scott FI, Lewis JD. Distinguishing incident and prevalent diabetes in an electronic medical records database. Pharmacoepidemiol Drug Saf. 2014;23(2):111–118. doi: 10.1002/pds.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata Journal. 2012;12(2):308–331. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.