Abstract
Purpose
To examine the association between objectively measured physical activity and risk of developing incident knee osteoarthritis (OA) in a community-based cohort of middle-aged and older adults.
Methods
We used data from the Osteoarthritis Initiative (OAI), an ongoing prospective cohort study of adults aged 45 to 83 at initial enrollment with elevated risk of symptomatic knee OA. Moderate-vigorous physical activity (MVPA) was measured by a uniaxial accelerometer for seven continuous days in two data collection cycles, and was categorized as inactive (<10 minutes/week), low activity (10–<150 minutes/week), and active (≥150 minutes/week). Incident knee OA based on radiographic and symptomatic OA and joint space narrowing were analyzed as outcomes over four years of follow-up. Participants free of the outcome of interest in both knees at study baseline were included (sample sizes ranged from 694 to 1,331 for different outcomes). We estimated hazard ratio (HR) and its 95% confidence intervals (CI).
Results
In multivariate adjusted analyses, active MVPA participation was not significantly associated with risk of incident radiographic knee OA (HR: 1.52; 95% CI: 0.68–3.40), symptomatic knee OA (HR: 1.17; 95% CI: 0.44–3.09), or joint space narrowing (HR: 0.87; 95% CI: 0.37–2.06), when compared with inactive MVPA participation. Similar results were found for participants with low activity MVPA.
Conclusion
MVPA was not associated with the risk of developing incident knee OA or joint space narrowing over four years of follow-up among OAI participants who are at increased risk of knee OA.
Keywords: physical activity, accelerometer, knee OA, cohort study
Introduction
Knee osteoarthritis (OA) is a leading cause of disability (1) that limits function and mobility, impairs quality of life, is the primary indication for knee replacement, and is associated with substantial medical expenditure (2). A main factor in the pathogenesis of OA, particularly for the load-bearing knee joint, is excessive mechanical stress (3) which can lead to injury and irreversible damage to the joint. Nevertheless, proper mechanical stimuli, such as regular physical activity, can maintain or improve joint health by strengthening the muscles around the joint, promoting synovial fluid transfer of nutrients (4), and preventing cartilage loss/defects by maintaining optimum physiological processes (5,6).
It is unclear whether the increased joint loading associated with certain types or intensity of physical activity increases the risk of developing incident knee OA, or accelerates the progression of disease. A recent cross-sectional study from OAI showed no association between running and knee OA (7). Evidence from longitudinal studies showed that regular physical activity does not increase the risk for developing knee OA (8, 9,10, 11,12, 13, 14, 15) except among those with the highest activity levels (16, 17). However, these studies used self-reported physical activity, which may be subject to recall and social desirability bias, resulting in over-reporting of exposure and possibly underestimate the risk (18). In addition, a variety of survey instruments have been used to measure physical activity which limits comparison of results across studies. Lastly, physical activity assessment questionnaires typically capture selected types of activities, but rarely measure all domains (i.e. sports/recreational, household, and occupational activities) nor do they capture unstructured activities well. Two recent studies (19, 20) used objectively measured physical activity (via pedometers) to examine the impact of walking on structural changes in the knee joint with one study reporting no association and the other showing an association between structural progression and walking >10 000 steps/day among those with less cartilage volume at baseline. However, these studies did not examine the outcome of greater clinical and public health relevance, knee OA.
To address these limitations, we performed the first study to our knowledge that examines the association between objectively measured physical activity and risk of developing knee OA in a community-based cohort of middle-aged and older adults at high risk for symptomatic knee OA. We hypothesized that moderate-vigorous physical activity (MVPA) was not associated with incident knee OA.
MATERIALS AND METHODS
Data source
We used data from the Osteoarthritis Initiative (OAI), available for public access at (http://www.oai.ucsf.edu/), an ongoing prospective cohort study investigating risk factors and biomarkers associated with the development and progression of knee OA. Participants in the OAI consisted of men and women, ages 45–79 years at enrollment, with or at elevated risk of developing symptomatic knee OA. Elevated risk was defined as frequent knee symptoms without radiographic OA, or two or more eligibility risk factors (e.g. overweight, previous knee injury, hand OA/Heberden’s nodes). The complete inclusion and exclusion criteria were described in detail elsewhere (21). A total of 4,796 eligible participants were recruited in the initial evaluation in 2004–2006 at four clinical sites (Baltimore, Maryland; Columbus, Ohio; Pittsburgh, Pennsylvania; and Pawtucket, Rhode Island). All study participants provided informed consent. The study protocol and consent documentation were approved by the local institutional review boards.
Moderate-Vigorous Physical Activity (MVPA)
A subgroup of OAI participants participated in an accelerometry study at the 48-month (n=2,127) and the 72-month follow-up visit (n=1,521) (Figure 1). The age, sex, and body mass index (BMI) distribution of this subgroup did not differ from the overall OAI cohort. The baseline of this analysis is the 48-month visit when the first accelerometry study was conducted. All participants with 72-month accelerometer data also had 48 month data. Physical activity was measured using a small uniaxial accelerometer (ActiGraph GT1M, Pensacola, FL) that measures vertical acceleration and deceleration. The validity and reliability of accelerometers under field conditions have been established in many populations including adults with arthritis (22,23,24,25). Participants were given uniform scripted instructions to wear the accelerometer unit on a belt at the natural waistline on the right hip in line with the right axilla, continuously from when they got up in the morning until retiring at night (except water activities) for seven consecutive days. At the end of the seven-day monitoring period, accelerometer data were downloaded using the manufacture’s software, and checked for valid data recording.
Figure 1.
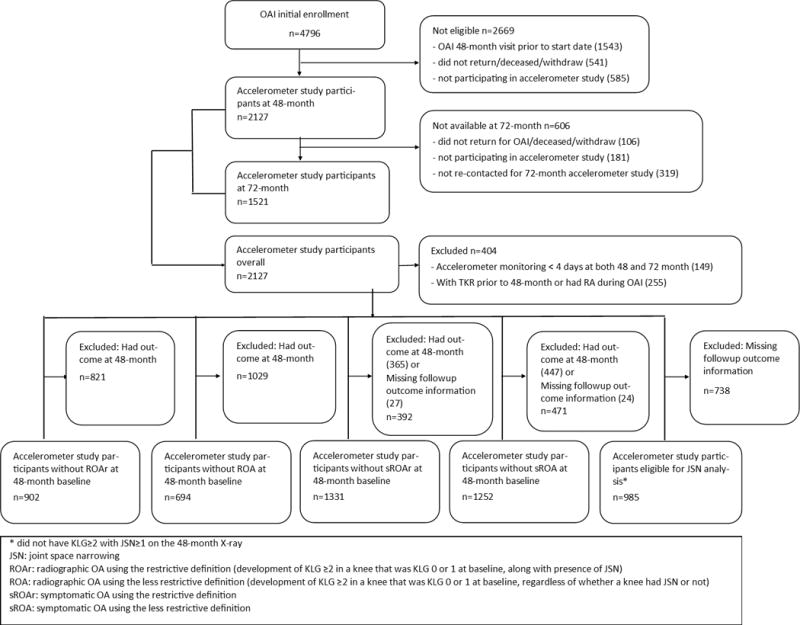
Study population flowchart
Accelerometry data were processed to determine the frequency, intensity, and duration of physical activities for each participant using established and validated methodologies (26). The accelerometer outputs activity counts, which is the weighted sum of the number of accelerations measured in one minute, where the weights are proportional to the magnitude of measured acceleration or deceleration. Weekly MVPA minutes were determined in three steps. First, data were filtered to identify non-wear periods. Non-wear periods were defined as ≥90 minutes with no activity counts (27). A valid day of wearing was defined as ≥10 wear hours in a 24-hour period (28). We included only participants who had at least four valid days of wearing in order to provide a reliable estimate of the physical activity. Second, MVPA occurring in bouts was identified; a bout was defined as ≥10 consecutive minutes above the 2,020 activity count threshold (moderate: 2,020–5,998 counts; vigorous: ≥5,999 counts) with allowance for interruptions of up to two minutes below threshold (29, 30). The average daily minutes of MVPA occurring in bouts was calculated, and the weekly total minutes were estimated as seven times the average daily total minutes. The US Department of Health and Human Services (HHS) recommends that US adults of all ages, including those with arthritis, engage in at least 150 minutes per week of moderate-equivalent intensity aerobic physical activity (31). Thus, for the third step, the weekly MVPA minutes were summed based on the HHS 2008 Physical Activity Guidelines for Americans, which indicated adults should have at least 150 minutes of moderate activity per week, 75 minutes of vigorous activity per week, or a moderate-equivalent combination of the two (vigorous minutes×2 + moderate minutes) (31). We categorized weekly MVPA minutes into three levels: active (≥150 minutes/week), low activity (10 to < 150 minutes/week), and inactive (<10 minutes/week). We analyzed MVPA as a categorical variable described above and a continuous variable using the weekly minutes accrued in bouts of MVPA. The focus on MVPA is primarily because this is the PA intensity and level that has been shown to be associated with a reduced risk of many outcomes including mortality, cardiovascular events, and development of diabetes (31). The 2008 HHS Physical Activity Guidelines indicate that the amount of health benefits attained from physical activity vary by level: inactive adults receive no health benefits, adults who have low activity receive some benefits, and those who meet guidelines receive substantial benefits (31). Such benefits have been also observed in adults with arthritis who participate in moderate-intensity, low-impact activities (e.g., walking, cycling, water exercise), 3 to 5 times per week for 30 to 60 minutes per session (i.e., accumulate approximately 150 minutes of MVPA per week) in ten minute bouts(31, 32, 33). A vexing question is whether health benefits are attained at the potential expense of joint damage. Therefore, our study specifically evaluates the relationship of recommended physical activity with the risk of developing knee osteoarthritis.
Outcomes
We analyzed knee radiographic OA, symptomatic OA, and joint space narrowing (JSN) as the outcomes. Starting with the 48-month OAI visit, participants were followed with questionnaires every 12 months, and were invited to attend clinical examinations every 24 months. During clinical visits, participants had bilateral posteroanterior knee radiographs using a Synaflexer frame (Synarc, San Francisco, CA) to create a fixed standard flexed knee position. This protocol provides reproducible estimates of joint space and consistent knee images over time (34). Radiographs were assessed by clinical readers and graded using Kellgren and Lawrence Grades (KLG) (35). In addition, JSN was assessed in the medial and lateral tibiofemoral compartments using the Osteoarthritis Research Society International (OARSI) atlas (36) scored 0 to 3. For each participant, serial radiograph readings had good to excellent test-retest reliability for KLG and JSN both cross-sectionally and longitudinally (kappa≥0.7) (364).
OAI reports incident radiographic OA (ROA) using two definitions. The less restrictive definition is development of KLG ≥2 in a knee that was KLG 0 or 1 at baseline, regardless of whether a knee had JSN or not. These knees could develop ROA simply due to new or enlarging osteophytes in a knee with normal joint space. The restrictive definition required the development of KLG ≥2 in a knee that was KLG 0 or 1 at baseline, along with presence of JSN (OARSI JSN grade ≥1 at or before the visit of onset). Symptomatic OA was defined by the presence of both ROA and joint symptoms in the same knee. Thus, two types of symptomatic OA outcomes were available using either the restrictive or less restrictive ROA definition. Knee symptoms were determined by a “yes” to “During the past 12 months, have you had pain, aching, or stiffness in or around your left/right knee on most days for at least one month?” To be consistent with the biennial radiographic assessment of the knee, we considered knee symptoms present if participants reported “yes” to this question at least once in two consecutive years. We defined knee JSN as ≥1 grade increase in either the medial or lateral tibiofemoral compartments from the previous clinical visit in a knee that did not have KLG≥2 with JSN≥1 on the 48-month X-ray.
We analyzed five knee outcomes: ROAr (radiographic OA using restrictive definition), ROA (radiographic OA using less restrictive definition), sROAr (symptomatic OA using the restrictive ROA definition), sROA (symptomatic OA using the less restrictive ROA definition), and JSN. At the time of the analyses, OAI radiographic outcomes post 48-month baseline were available for the 72-month and 96-month visits, therefore, the longest follow-up period for this analysis was four years.
Study population
All analyses were person-based, and the study population (population at risk) for each outcome included those who were outcome-free in both knees at baseline. A person was considered an incident case if at least one knee developed the outcome of interest during follow-up. Participants free of the disease at baseline, but who had knee replacement during follow-up, were also considered incident cases for all five outcomes.
For each outcome, the at-risk sample was comprised of participants with sufficient accelerometry data (≥4 valid days monitoring), but excluded persons with the outcome in either knee at baseline (Figure 1). Those eligible for incident symptomatic knee OA analyses may have either knee symptoms or ROA at baseline, but not both in the same knee. For instance, a knee with pain at the 48-month baseline but not radiographic OA was eligible to be an incident symptomatic OA knee at 72 months if it developed radiographic OA and still had pain at 60 or 72 month. Participants who had knee replacement prior to the 48-month visit and those with rheumatoid arthritis at any of the OAI visits were excluded. The analytic sample size varied by outcomes (Figure 1).
Statistical analysis
Because outcomes were evaluated biennially at the 72-month and 96-month visits, we used survival analysis for discrete data which is an analog of the Cox proportional hazard model for continuous data. This method estimates the discrete hazard rate, which is the probability of developing the outcome of interest in each subsequent time interval given a person’s risk factors at the previous visit. We estimated a discrete hazards model, which accounts for repeated measures on the same individual and does not require a proportional hazard assumption. We fit a generalized linear model with a complementary log-log link using SAS Proc GenMod procedure (SAS 9.3, Carey, NC). We reported results as hazard ratios (HR) with 95% confidence intervals (CI). Because the weekly minutes were positively skewed towards zero (41% of participants were inactive, Table 1), we log transformed the continuous MVPA variable using the base of two (the hazard ratio for the continuous MVPA variable can be interpreted as the ratio of developing the outcome when doubling the amount of physical activity). Statistical testing used α=0.05 level.
Table 1.
Distribution of MVPA by baseline (OAI 48-month visit) characteristics among participants at risk for ROAr.
| MVPA categorya (n [%])
|
MVPA weekly minutes (median [IQR]) | |||||
|---|---|---|---|---|---|---|
| Baseline characteristics | n | Inactive | Low | Active | P valueb | |
| Number of participants | 879c | 357 (40.6) | 372 (42.3) | 150 (17.1) | 24 (102) | |
| ROAr incidence | 51 | 22 | 19 | 10 | ||
| Age | <0.01 | |||||
| 49–64 year | 502 | 171 (34.1) | 227 (45.2) | 104 (20.7) | 37 (131) | |
| ≥ 65 year | 377 | 186 (49.3) | 145 (38.5) | 46 (12.2) | 10 (65) | |
| Sex | <0.01 | |||||
| Men | 364 | 114 (31.3) | 166 (45.6) | 84 (23.1) | 43 (141) | |
| Women | 515 | 243 (47.2) | 206 (40.0) | 66 (12.8) | 13 (76) | |
| BMI categoryd | <0.01 | |||||
| Under/healthy weight | 277 | 87 (31.4) | 119 (43.0) | 71 (25.6) | 47 (151) | |
| Overweight | 339 | 135 (39.8) | 149 (44.0) | 55 (16.2) | 24 (102) | |
| Obesity | 259 | 132 (51.0) | 103 (39.8) | 24 (9.2) | 9 (60) | |
| Knee Injury | <0.01 | |||||
| No | 506 | 229 (45.3) | 202 (39.9) | 75 (14.8) | 19 (84) | |
| Yes | 373 | 128 (34.3) | 170 (45.6) | 75 (20.1) | 32 (130) | |
Weekly moderate-equivalent intensity physical activity <10 min: inactive; 10–149 min: low activity; ≥150 min: active
Chi-Square test for difference in proportion by groups
23 participants did not have valid accelerometry data at 48-month, but had valid data at 72-month
Under/healthy weight: BMI<25 kg/m2; Overweight: 25≤BMI<30 kg/m2; Obesity: BMI≥30 kg/m2. The BMI values were missing for four participants.
Abbreviations: MVPA=moderate-vigorous physical activity; BMI: body mass index; IQR= interquartile range; OAI: Osteoarthritis Initiative; ROAr: radiographic OA using the restrictive definition (i.e. development of KLG ≥2 in a knee that was KLG 0 or 1 at baseline, along with presence of JSN)
Both unadjusted and adjusted analyses were performed to investigate the association between MVPA and incident knee OA outcomes. Age, sex, BMI, and knee injury history were adjusted for in the multivariate model when examining the association between MVPA and radiographic knee OA and JSN. These covariates were selected based on their potential to confound the association between MVPA and our study outcomes, and have been used in prior related studies (9, 11, 12, 16). BMI was calculated from measured height and weight. Prior knee injury was assessed by asking participants if their left or right knee was “ever injured badly enough to limit ability to walk for at least two days?” For symptomatic knee OA, medication use for knee symptoms was also included as a covariate which was assessed by asking if participants “used medication for pain, aching or stiffness more than half the days of a month in the past 12 months”. All covariates were assessed every 12 months.
MVPA, age, BMI, injury history, and medications for knee symptoms were included in the statistical model as time-varying variables, and sex was included as a time-independent variable. For example, physical activity, age, and BMI at 48-month baseline, and binary (yes, no) status of knee injury and medication use for knee symptoms up to 72-month were used for analyzing outcomes at 72-month.
RESULTS
Among participants at risk for ROAr, 41%, 42%, and 17% of participants were physically inactive, low active, and active at baseline, respectively (Table 1). Participants who were more physically active were younger, more likely to be men, had lower BMI, and had prior knee injury. These patterns were similar across the other four knee outcomes (data not shown).
The proportions (number with outcome) of participants who developed knee ROAr, ROA, sROAr, sROA, and JSN during four years of follow-up were 5.7% (51), 8.1% (56), 4.4% (59), 4.8% (60), and 8.1% (80), respectively (Table 2). When MVPA was analyzed as a categorical variables, those who had low activity or were active did not have statistically significant differences in the hazard of developing ROAr compared with those who were physically inactive in univariate analysis (HR: 0.90 [95% CI: 0.46–1.74] and HR: 1.02 [95% CI: 0.44–2.35], respectively), or in multivariate analysis (HR: 1.15 [95% CI: 0.58–2.30] and HR: 1.74 [95% CI: 0.73–4.16], respectively). When analyzed as a continuous variable, doubling the amount of MVPA again was not associated with the risk of developing ROAr in univariate (HR: 0.98 [95% CI: 0.89–1.07]) or multivariate analyses (HR: 1.03 [95% CI: 0.94–1.14]). The lack of significant association was also observed for other radiographic and symptomatic knee OA outcomes (Table 2).
Table 2.
Association (Hazard ratio and 95% CI) between MVPA (categorical and continuous) and five knee OA outcomes
| ROAr | ROA | sROAr | sROA | JSN | |
|---|---|---|---|---|---|
| At risk population | 902 | 694 | 1331 | 1252 | 985 |
| Incident cases (proportion) | 51 (5.7%) | 56 (8.1%) | 59 (4.4%) | 60 (4.8%) | 80 (8.1%) |
| MVPA as categorical | |||||
| Univariate analysis | |||||
| Inactive | ref | ref | ref | ref | ref |
| Low | 0.90 (0.46–1.74) | 0.59 (0.31–1.14) | 1.03 (0.56–1.89) | 0.76 (0.41–1.42) | 0.97 (0.59–1.60) |
| Active | 1.02 (0.44–2.35) | 0.90 (0.42–1.95) | 1.03 (0.45–2.32) | 0.88 (0.40–1.97) | 0.59 (0.27–1.27) |
| Test-of-trend p-value | 0.935 | 0.282 | 0.996 | 0.694 | 0.381 |
| Multivariate analysisa | |||||
| Inactive | ref | ref | ref | ref | ref |
| Low | 1.15 (0.58–2.30) | 0.77 (0.39–1.49) | 1.28 (0.64–2.58) | 0.92 (0.45–1.88) | 1.21 (0.71–2.06) |
| Active | 1.74 (0.73–4.16) | 1.52 (0.68–3.40) | 1.46 (0.54–3.90) | 1.17 (0.44–3.09) | 0.87 (0.37–2.06) |
| Test-of-trend p-value | 0.453 | 0.264 | 0.711 | 0.858 | 0.622 |
| MVPA as continuousb | |||||
| Univariate analysis | |||||
| MVPA | 0.98 (0.89–1.07) | 0.95 (0.86–1.04) | 0.99 (0.91–1.08) | 0.96 (0.88–1.05) | 0.97 (0.90–1.04) |
| p-value | 0.639 | 0.236 | 0.868 | 0.366 | 0.356 |
| Multivariate analysisa | |||||
| MVPA | 1.03 (0.94–1.14) | 1.00 (0.91–1.11) | 1.04 (0.93–1.15) | 1.00 (0.89–1.11) | 1.02 (0.94–1.10) |
| p-value | 0.512 | 0.988 | 0.518 | 0.942 | 0.715 |
For ROAs, ROA, and JSN, adjusted for age, sex, BMI, and prior knee injury. For sROAr and sROA, adjusted for age, sex, BMI, prior knee injury, and medication use for knee symptoms.
Physical activity was transformed as log base 2 of weekly minutes in bouts of moderate-equivalent intensity activity. The hazard ratio for the analysis which uses MVPA as a continuous variable can be interpreted as the ratio of developing the outcome when doubling the amount of MVPA.
Abbreviations: MVPA: Moderate-Vigorous Physical activity; JSN: joint space narrowing; ROAr: radiographic OA using the restrictive definition (development of KLG ≥2 in a knee that was KLG 0 or 1 at baseline, along with presence of JSN); ROA: radiographic OA using the less restrictive definition (development of KLG ≥2 in a knee that was KLG 0 or 1 at baseline, regardless of whether a knee had JSN or not); sROAr: symptomatic OA using the restrictive definition; sROA: symptomatic OA using the less restrictive definition; HRs: hazard ratios; CI: confidence interval
DISCUSSION
Using data from the OAI longitudinal cohort, we investigated the association over four years between objectively measured MVPA and five incident knee OA outcomes: combinations of radiographic and symptomatic knee OA, and knee joint space narrowing. The risk of developing any of the outcomes did not differ across MVPA levels, even after controlling for age, sex, BMI, knee injury history, and medication used for knee symptoms.
Our findings are consistent with several other prospective cohort studies, all of which used self-reported physical activity. A study that combined data from the OAI and the Multicenter Osteoarthritis (MOST) study (12) reported no association between community-based physical activity (occupational, household, and leisure) and the development of knee ROA, sROA, or JSN over 30 to 48 months. In a population-based prospective cohort (9), MVPA levels were not associated with knee ROA or sROA. Other longitudinal studies also observed no association between recreational physical activity and risk of knee ROA, sROA, or JSN over 9 years (11), between long-distance running and knee ROA among community middle- to older-aged adults over two decades (10), and between leisure time physical activity and total knee replacement due to OA over 11 years (8).
The relation between physical activity and knee OA varies by individual knee radiographic features. Specifically, physical activity has a more established positive association with osteophyte formation (11, 16, 17), but little to no evidence of an association with JSN as a surrogate measure of cartilage loss and degeneration (10, 11, 13). Although not statistically significant, we observed elevated adjusted hazard ratios for radiographic knee OA among physically active participants compared with those who were inactive, whereas the opposite was observed for JSN. Many studies used the KLG in defining knee OA which relies heavily on the presence of osteophytes that could develop as a functional adaptation to mechanical stimuli (37). Osteophytes may enhance the functional property of the joint by increasing the joint surface area thus decreasing stress or by reducing motion at a joint and improving joint stability (37). Osteophytes alone, in the absence of cartilage degeneration and symptoms, may not be a sufficient reason to prohibit individuals from physical activity, given its tremendous health benefits.
The federal physical activity guidelines utilize a legacy metric requiring activity bouts of moderate or vigorous activity lasting at least 10 minutes, based on studies demonstrating cardiovascular benefits from aerobic activity (31). However, physical activity studies of adults based on objective accelerometer monitoring indicate only 10–15% of community-dwelling adults meet national guidelines (38). Similarly, only 17% in our study participants met aerobic physical activity guidelines. Previous studies using OAI data have shown that physical activity at lower intensity or less than the guideline recommended threshold have health benefits for adults with osteoarthritis of the knee or risk factors for knee osteoarthritis. Specifically, greater time spent in light intensity physical activities was associated with reduced risk of onset and progression of disability, and this relation was independent of the time spent in moderate or vigorous activities (32). In addition, a minimum of ≥45 minutes/week of moderate-vigorous intensity physical activity promotes improved or sustained high function for adults with lower-extremity joint symptoms (39).
This study is subject to at least five limitations. First, the requirement that only MVPA occurring in bouts of at least 10 consecutive minutes may underestimate total exposure for some participants. The methodology we used for accelerometer data processing is consistent with the U.S. National Health and Nutrition Examination Survey (27), however, different cutoffs for light, moderate, and vigorous physical activity using accelerometry data exist and may influence results. Second, the overall MVPA levels in OAI participants were low with over 40% of them being physically inactive, and the percentage of our study population that participated in vigorous physical activities was only 6%. Third, the number of participants who developed the outcomes during four years of follow-up was relatively small and the confidence intervals for the hazard ratios were wide due to limited sample size. The lower statistical power precluded more extensive analyses, for instance, testing effect measure modification between MVPA and malalignment, and/or MVPA and BMI, and the risk of knee OA. However, this study provide some of the first data on association between objectively measured physical activity and risk of knee OA, which can be used for future meta-analysis or systematic review when more data become available. Fourth, although those selected for the accelerometry study did not differ from the overall cohort, participants excluded at 48 months due to having the outcome of interest at study baseline were older and had greater BMI indicating potential selection bias for all five outcomes, and more women remained in the final analytic sample than men for the ROAr and sROAr outcomes. However, there is no indication what the direction of these selection bias, if any, could be, and how it could impact the findings. Though one would suspect that participants with greater BMI and/or age would have lower physical activity. Finally, we adjusted for several important confounders, but residual confounding is a possibility of all observational studies. For example, we do not have information of bone mineral density or nutritional factors that could affect joint health.
To our knowledge this is the first study to examine the association between objectively measured physical activity and incident knee OA outcomes. Although self-reported assessment may be better at measuring longer term activity levels and the only way of assessing relatively uncommon activities (12), objectively measured physical activity eliminates reporting bias, provides a more complete and accurate assessment of overall activity level, and allows for comparisons across studies. In addition, we analyzed objectively measured physical activity at two follow-up visits as a time-varying variable which captured changes over time.
In conclusion, we found no evidence suggesting an association between MVPA and risk of developing five knee OA outcomes over four years among OAI participants. These findings need to be confirmed in future research using objectively measured MVPAin larger samples and with longer follow-up, and among populations with higher MVPA levels and younger ages. Improved knowledge on the optimal amount of MVPA that maximize health benefits while minimizing potential adverse effect on the joint is essential for OA prevention, intervention, and rehabilitation.
Acknowledgments
Supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants R01-AR054155, R21-AR059412, P60-AR48098, and R01-AR055287). The Osteoarthritis Initiative is a public-private partnership comprised of 5 contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262) funded by the NIH, a branch of the Department of Health and Human Services, and conducted by the Osteoarthritis Initiative Study Investigators.
Footnotes
CDC Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The results of the present study do not constitute endorsement by ACSM. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Conflict of Interest
All authors have no conflict of interest to declare.
References
- 1.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323–30. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter DJ, Wilson DR. Imaging the role of biomechanics in osteoarthritis. Rheum Dis Clin North Am. 2009;35(3):465–83. doi: 10.1016/j.rdc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 5.Racunica TL, Teichtahl AJ, Wang Y, et al. Effect of physical activity on articular knee joint structures in community-based adults. Arthritis and Rheumatism. 2007;57(7):1261–8. doi: 10.1002/art.22990. [DOI] [PubMed] [Google Scholar]
- 6.Urquhart DM, Wluka AE, Teichtahl AJ, Cicuttini FM. The effect of physical activity on the knee joint: is it good or bad? Br J Sports Med. 2007;41(9):546–7. doi: 10.1136/bjsm.2007.037416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guideline for the prevention of falls in older persons American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49(5):664–72. [PubMed] [Google Scholar]
- 9.Stenholm S, Koster A, Valkeinen H, et al. Association of Physical Activity History With Physical Function and Mortality in Old Age. J Gerontol A Biol Sci Med Sci. 2016;71(4):496–501. doi: 10.1093/gerona/glv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo GH, Driban JB, Kriska AM, et al. Is There an Association Between a History of Running and Symptomatic Knee Osteoarthritis? A Cross-Sectional Study From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2017 Feb;69(2):183–191. doi: 10.1002/acr.22939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ageberg E, Engstrom G, Gerhardsson de Verdier M, Rollof J, Roos EM, Lohmander LS. Effect of leisure time physical activity on severe knee or hip osteoarthritis leading to total joint replacement: a population-based prospective cohort study. BMC Musculoskelet Disord. 2012;13:73. doi: 10.1186/1471-2474-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour KE, Hootman JM, Helmick CG, et al. Meeting physical activity guidelines and the risk of incident knee osteoarthritis: a population-based prospective cohort study. Arthritis Care Res (Hoboken) 2014;66(1):139–46. doi: 10.1002/acr.22120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarty EF, Hubert HB, Lingala VB, Zatarain E, Fries JF. Long distance running and knee osteoarthritis. A prospective study. American journal of preventive medicine. 2008;35(2):133–8. doi: 10.1016/j.amepre.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felson DT, Niu J, Clancy M, Sack B, Aliabadi P, Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis and Rheumatism. 2007;57(1):6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 12.Felson DT, Niu J, Yang T, et al. Physical activity, alignment and knee osteoarthritis: data from MOST and the OAI. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2013;21(6):789–95. doi: 10.1016/j.joca.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannan MT, Felson DT, Anderson JJ, Naimark A. Habitual physical activity is not associated with knee osteoarthritis: the Framingham Study. The Journal of Rheumatology. 1993;20(4):704–9. [PubMed] [Google Scholar]
- 14.Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis and Rheumatism. 1999;42(1):17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 15.Lane NE, Oehlert JW, Bloch DA, Fries JF. The relationship of running to osteoarthritis of the knee and hip and bone mineral density of the lumbar spine: a 9 year longitudinal study. The Journal of Rheumatology. 1998;25(2):334–41. [PubMed] [Google Scholar]
- 16.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis and Rheumatism. 1997;40(4):728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 17.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106(2):151–7. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 18.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1–14. [PubMed] [Google Scholar]
- 19.Doré DA, Winzenberg TM, Ding C, et al. The association between objectively measured physical activity and knee structural change using MRI. Ann Rheum Dis. 2013 Jul;72(7):1170–5. doi: 10.1136/annrheumdis-2012-201691. [DOI] [PubMed] [Google Scholar]
- 20.Øiestad BE, Quinn E, White D, et al. No Association between Daily Walking and Knee Structural Changes in People at Risk of or with Mild Knee Osteoarthritis. Prospective Data from the Multicenter Osteoarthritis Study. J Rheumatol. 2015 Sep;42(9):1685–93. doi: 10.3899/jrheum.150071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevitt MC, Felson DT, Lester G. The Osteoarthritis Initiative: Protocol for the cohort study. 2006 [Google Scholar]
- 22.Brage S, Wedderkopp N, Franks PW, Andersen LB, Froberg K. Reexamination of validity and reliability of the CSA monitor in walking and running. Med Sci Sports Exerc. 2003;35(8):1447–54. doi: 10.1249/01.MSS.0000079078.62035.EC. [DOI] [PubMed] [Google Scholar]
- 23.Hendelman D, Miller K, Baggett C, Debold E, Freedson P. Validity of accelerometry for the assessment of moderate intensity physical activity in the field. Med Sci Sports Exerc. 2000;32(9 Suppl):S442–9. doi: 10.1097/00005768-200009001-00002. [DOI] [PubMed] [Google Scholar]
- 24.Munneke M, de Jong Z, Zwinderman AH, Tijhuis GJ, Hazes JM, Vliet Vlieland TP. The value of a continuous ambulatory activity monitor to quantify the amount and intensity of daily activity in patients with rheumatoid arthritis. The Journal of Rheumatology. 2001;28(4):745–50. [PubMed] [Google Scholar]
- 25.Welk GJ, Schaben JA, Morrow JR., Jr Reliability of accelerometry-based activity monitors: a generalizability study. Med Sci Sports Exerc. 2004;36(9):1637–45. [PubMed] [Google Scholar]
- 26.Dunlop DD, Song J, Semanik PA, Chang RW, Sharma L, Bathon JM, et al. Objective physical activity measurement in the osteoarthritis initiative: Are guidelines being met? Arthritis and rheumatism. 2011;63(11):3372–82. doi: 10.1002/art.30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song J, Semanik P, Sharma L, et al. Assessing physical activity in persons with knee osteoarthritis using accelerometers: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2010;62(12):1724–32. doi: 10.1002/acr.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 29.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 30.Nevitt MC, Peterfy C, Guermazi A, et al. Longitudinal performance evaluation and validation of fixed-flexion radiography of the knee for detection of joint space loss. Arthritis and Rheumatism. 2007;56(5):1512–20. doi: 10.1002/art.22557. [DOI] [PubMed] [Google Scholar]
- 31.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. 2008 Available from: https://health.gov/paguidelines/pdf/paguide.pdf. Accessed June 12, 2017.
- 32.Dunlop DD, Song J, Semanik PA, et al. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: prospective cohort study. BMJ. 2014;348:g2472. doi: 10.1136/bmj.g2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley GA, Kelley KS, Hootman JM, et al. Effects of community-deliverable exercise on pain and physical function in adults with arthritis and other rheumatic diseases: a meta-analysis. Arthritis Care Res (Hoboken) 2011 Jan;63(1):79–93. doi: 10.1002/acr.20347. [DOI] [PubMed] [Google Scholar]
- 34.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osteoarthritis Initiative (OAI) Test-Retest Reliability of Semi-quantitative Readings from Knee Radiographs. 2012 Available from: https://oai.epi-ucsf.org/datarelease/SASDocs/kXR_SQ_Rel_BU_Descrip.pdf. Accessed June 12, 2017.
- 36.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis and cartilage/OARS. Osteoarthritis Research Society. 1995;3(Suppl A):3–70. [PubMed] [Google Scholar]
- 37.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2007;15(3):237–44. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adults compliance with the Physical Activity Guidelines for Americans. American Journal of Preventive Medicine. 2011;40(4):454–61. doi: 10.1016/j.amepre.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Dunlop DD, Song J, Lee J, et al. Physical Activity Minimum Threshold Predicting Improved Function in Adults With Lower-Extremity Symptoms. Arthritis Care Res (Hoboken) 2017;69(4):475–83. doi: 10.1002/acr.23181. [DOI] [PMC free article] [PubMed] [Google Scholar]


