Abstract
Glioma cells are one of the most aggressive and malignant tumors. Following initial surgery, and radio-chemotherapy they progress rapidly, so that patients’ median survival remains under two years. They invade throughout the brain, which makes them difficult to treat, and are universally lethal. Though total resection is always attempted it is not curative. Standard of care in 2016 comprises surgical resection, radiotherapy and chemotherapy (temozolomide). Median survival is currently ~14–20 months post-diagnosis though it can be higher in high complexity medical university centers, or during clinical trials. Why the immune system fails to recognize the growing brain tumor is not completely understood. We believe that one reason for this failure is that the brain lacks cells that perform the role that dendritic cells serve in other organs. The lack of functional dendritic cells from the brain causes the brain to be deficient in priming systemic immune responses to glioma antigens. To overcome this drawback we reconstituted the brain immune system for it to initiate and prime anti-glioma immune responses from within the brain. To achieve brain immune reconstitution adenoviral vectors are injected into the resection cavity or remaining tumor. One adenoviral vector expresses the HSV-1 derived thymidine kinase which converts ganciclovir into phosphoganciclovir which becomes cytotoxic to dividing cells. The second adenovirus expresses the cytokine fms-like tyrosine kinase 3 ligand (Flt3L). Flt3L differentiates precursors into dendritic cells and acts as a chemokine for dendritic cells. This results in HSV-1/ganciclovir killing of tumor cells, and the release of tumor antigens, which are then taken up by dendritic cells recruited to the brain tumor microenvironment by Flt3L. Concomitant release of HMGB1, a TLR2 agonist that activates dendritic cells, stimulates dendritic cells loaded with glioma antigens to migrate to the cervical lymph nodes to prime a systemic CD8+ T cytotoxic killing of brain tumor cells. This induced immune response causes glioma-specific cytotoxicity, induces immunological memory, and does not cause brain toxicity or autoimmunity. A Phase I Clinical Trial, to test our hypothesis in human patients, was opened in December 2013 (see: NCT01811992, Combined Cytotoxic and Immune-Stimulatory Therapy for Glioma, at ClinicalTrials.gov). This trial is a first in person trial to test the whether the re-engineering of the brain immune system can serve to treat malignant brain tumors. The long and winding road from the laboratory to the clinical trial follows below.
Towards Gene Therapy for Brain Tumors: re-engineering the brain immune system to treat highly malignant gliomas (Glioblastoma Multiforme [GBM], WHO Grade IV)
Malignant brain tumors are universally fatal (Omuro & DeAngelis, 2013). Though different views persist concerning the cellular origin of malignant gliomas, it is thought that astrocytes, oligodendrocytes, neuronal progenitors or neural stem cells can originate these tumors (Stiles & Rowitch, 2008). In the early XXth century Hans Scherer described the patients’ mean overall survival as 6 months post-diagnosis (Molinaro, Wrensch, Jenkins, & Eckel-Passow, 2015). At that time the treatment of GBM was surgical as it was thought that total tumor resection could be curative. However, a 50% surgical mortality, a high level of recurrences, and an overall survival below 12 months, challenged this idea. To explain such tumor behavior, in the 1950’s, Bailey suggested that malignant glioma tumors invade far away from the initial tumor location and that these distal sites could initiate tumor regrowth.
Contemporary standard of care for the treatment of malignant brain tumors consists of surgery (Pannullo, Fraser, Moliterno, Cobb, & Stieg, 2011; Sanai & Berger, 2008), chemotherapy (Koukourakis et al., 2009; Pitz, Desai, Grossman, & Blakeley, 2011), and radiotherapy (Rock et al., 2012; Stadlbauer, Buchfelder, Salomonowitz, & Ganslandt, 2010). Surgery reduces tumor mass and brain swelling, one of the reasons that GBM patients are taken to the ER; seizures are the other main reason for emergency treatment. In cases of brain swelling surgery is indicated to avoid brainstem herniation, impaction, and death (Kotsarini, Griffiths, Wilkinson, & Hoggard, 2010). Alternatively, patients may receive dexamethasone or VEGF inhibitors to inhibit tumor induced edema. Chemotherapy, currently represented by temozolomide is the standard chemotherapy for GBM (Brandes et al., 2014; Field, Jordan, Wen, Rosenthal, & Reardon, 2015; Hart, Garside, Rogers, Stein, & Grant, 2013; Olson, Nayak, Ormond, Wen, & Kalkanis, 2014; L. J. Yang, Zhou, & Lin, 2014). Radiotherapy improves survival and is usually administered at short times post-surgery using established standards.
The largest carefully quantified series of extent of glioma resection vs. survival indicated the existence of a survival threshold at ~70% of tumor resection; only above this threshold does survival become proportional to the extent of resection (Mitchell, Ellison, & Mendelow, 2005; Pouratian & Bookheimer, 2010; Sanai & Berger, 2008). Below 70% survival is much reduced. Even if not curative, this work shows that gross total resections carry a strong survival benefit.
There is a critical need to develop new and effective treatments for patients suffering from GBM. The large number of clinical trials for GBM is illustrated by the following ratio: [(number of active clinical trials in the USA in www.clinicaltrials.gov) divided by the prevalence of the disease in USA, ×100], i.e., how many trials are active per patient. For high grade glioma this ratio is 4.85; for ovarian cancer, 11.66; for pancreatic cancer, 4.11; for breast cancer, 2.99; for lung cancer, 2.71; for melanoma, 2.28; for prostate cancer, 2.28. As the frequency of these cancers is: breast cancer > lung cancer > prostate cancer > melanoma > pancreatic cancer > > ovarian cancer, the ratio reflects that the amount of active clinical trials is a reflection of the lethality and lack of treatment for a given cancer, rather than its incidence.
New data obtained from detailed analyses of the genomic landscape of GBM now informs the development of novel chemotherapies. Chemotherapy that is effective in other cancers that share alterations in signaling pathways also altered in GBM, new delivery methods for otherwise unresectable tumors, new treatments targeted to childhood gliomas, are all being explored to move forward the treatment of GBM (Buczkowicz, Bartels, Bouffet, Becher, & Hawkins, 2014; Buczkowicz & Hawkins, 2015; Grimm & Chamberlain, 2013; Jansen, van Vuurden, Vandertop, & Kaspers, 2012; Karsy et al., 2012; Nobusawa, Hirato, & Yokoo, 2014; Panditharatna, Yaeger, Kilburn, Packer, & Nazarian, 2015; Veldhuijzen van Zanten et al., 2015; Z. J. Wang et al., 2015; Wu et al., 2014). Anti-angiogenic agents are also being explored (Chinot et al., 2014; Fine, 2014; Gilbert et al., 2014; Gilbert, Sulman, & Mehta, 2014; Gururangan et al., 2010; Hart, et al., 2013; Raizer et al., 2015), as well as novel surgical techniques designed to increase tumor resection with the aid of new imaging technologies (e.g., MRI (Bohman et al., 2010; Kubben et al., 2011), 5-Aminolevulinic acid (5-ALA (Colditz & Jeffree, 2012; Eljamel, 2015; Hauser, Kockro, Actor, Sarnthein, & Bernays, 2015; Jaber et al., 2015; Lau et al., 2015), Raman spectroscopy (Ji et al., 2015; Ji et al., 2013)). Immunization to treat brain tumors has been explored for the last 15–20 years (e.g., dendritic cells primed with unknown or known tumor antigens; with TLR agonists; with heat shock proteins) (Batich, Swartz, & Sampson, 2015; Bregy, Wong, Shah, Goldberg, & Komotar, 2013; Finocchiaro & Pellegatta, 2014; Mohme, Neidert, Regli, Weller, & Martin, 2014; Reardon et al., 2013; See et al., 2011; X. Wang et al., 2014; Weiss, Weller, & Roth, 2015; Wheeler & Black, 2009), and this now includes the addition of checkpoint inhibitors. It is disappointing that vaccination trials, gene therapy trials, chemotherapy trials, and anti-angiogenic trials have, until now, not performed stronger than controls in Phase III trials (Khasraw, Ameratunga, Grant, Wheeler, & Pavlakis, 2014) (Simonato, et al., 2013). In spite of this fervent activity, GBM remains one of the most lethal cancers. In the 1930’s survival of GBM was ~6 months; as it currently stands at 14–20 months median survival, the last 80 years have only improved survival by ~12 months, i.e., by less than 5 days per year (deSouza et al., 2016).
In situ re-engineering the brain immune system to treat malignant brain tumors
The brain immune system
The brain displays a set of particular immune responses that differ from those seen in most other organs. Further, there are two immune compartments within the brain. One resides within the brain parenchyma proper; the other within the ventricles and meninges. They differ structurally, functionally and physiologically. The brain parenchyma lacks proper lymphatic channels, and afferent dendritic cells, i.e., those that can pick up antigens, carry them to lymph nodes and present them to naïve T cells. Composing the second system are the choroid plexus located within the brain ventricles and the meninges of the spinal cord and brain; these contain lymphatics and all immune cells necessary to prime a systemic immune response. Extracellular fluid from the brain drains through well characterized perivascular channels that lead to lymphatics near the olfactory bulb, and then to the cervical lymph nodes (Carare, Hawkes, & Weller, 2014; Clapham, O'Sullivan, Weller, & Carare, 2010; Iliff et al., 2014; Iliff & Nedergaard, 2013; Iliff et al., 2012; Jessen, Munk, Lundgaard, & Nedergaard, 2015; Kida, Pantazis, & Weller, 1993; Kida, Weller, Zhang, Phillips, & Iannotti, 1995; Kress et al., 2014; Laman & Weller, 2013; Weller, 1998; Weller, Djuanda, Yow, & Carare, 2009; Weller, Engelhardt, & Phillips, 1996; Weller, Galea, Carare, & Minagar, 2010; Weller, Kida, & Zhang, 1992; Weller, Subash, Preston, Mazanti, & Carare, 2008; L. Yang et al., 2013).
At the physiological level the brain parenchyma can be the target of an immune response (i.e., in multiple sclerosis, or paraneoplastic syndromes), but it is essentially impossible to stimulate a systemic immune response by carefully delivering a particulate insoluble antigen directly into the brain parenchyma. Conditions which cause release of brain antigens into the systemic circulation (e.g., trauma, stroke, neurosurgery) do not induce autoimmune responses against brain tissue. In multiple sclerosis and paraneoplastic syndromes, and in any situation aiming to induce brain autoimmunity, initial stimulation of the immune system needs to occur systemically. In spite of the failure to induce systemic anti-brain immune responses from the brain parenchyma, i.e., an antigen delivered directly into the brain proper, systemic immune responses will only be triggered once the antigen enters the ventricular system. This is elegantly illustrated by the results from Charles Bangham laboratory, and others. Bangham’s group showed that careful injection of live replication competent influenza virus into the brain caused a major local inflammation, yet, no systemic immune response could be detected until the replicating virus penetrated the ‘ventricular immune system’, and essentially comparable results can be obtained using different experimental approaches (Bell, Taub, & Perry, 1996; Hawke, Stevenson, Freeman, & Bangham, 1998; Matyszak & Perry, 1996a, 1996b, 1998; Perry, 2000; Stevenson, Bangham, & Hawke, 1997; Stevenson, Hawke, & Bangham, 1997; Stevenson, Hawke, Sloan, & Bangham, 1997).
Lymphocytes only persist in the brain if they engage their cognate antigen, which can lead to neuropathology (Hawke, et al., 1998; Reuter, Gomez, Wilson, & Van Den Pol, 2004; van Den Pol, Mocarski, Saederup, Vieira, & Meier, 1999). NK cells, which are not antigen specific, also contribute to neuropathology in stroke (Gan et al., 2014), and in brain tumors (Baker et al., 2014). Immune responses to non-replicating viral vectors injected into the brain parenchyma can be induced, but only following a systemic immunization against the vector. Such antiviral immune responses against vectors injected into the brain have been described for RAdv, AAV, and lentivirus, and can either block transgene expression or even cause overt neuropathology depending on vector dose and experimental design. Diffuse brain autoimmunity is never encountered in these experimental paradigms (Abordo-Adesida et al., 2005; Dewey et al., 1999; Larocque et al., 2010; Lowenstein, 2005; Lowenstein, Mandel, Xiong, Kroeger, & Castro, 2007; Peden et al., 2009; Thomas, Birkett, Anozie, Castro, & Lowenstein, 2001; Thomas, Schiedner, Kochanek, Castro, & Lowenstein, 2000, 2001; Zirger et al., 2012), indicating that such an immune response is specific to the viral antigens, but does not lead to a phenomenon akin to antigen spreading and the priming of an anti-brain parenchyma immune response.
The entirety of brain immune responses described above, and which differ significantly from immune responses elsewhere in the body, is described as ‘the brain immune privilege’ (Bechmann, Galea, & Perry, 2007; Galea, Bechmann, & Perry, 2007). In summary, the brain can be targeted by a systemic immune response, but only if the primary immunization occurs systemically, i.e., outside of the brain. Although an immune response will not be induced following delivery of particulate, insoluble antigen to the brain parenchyma proper, antigens injected directly into the brain parenchyma elicit a transitory and local innate immune response (Barcia et al., 2007; Byrnes, MacLaren, & Charlton, 1996; Byrnes, Rusby, Wood, & Charlton, 1995; Byrnes, Wood, & Charlton, 1996; Kajiwara, Byrnes, Charlton, Wood, & Wood, 1997; Thomas, et al., 2000; Thomas, Schiedner, et al., 2001; Wood, Byrnes, Rabkin, Pfaff, & Charlton, 1994; Wood, Charlton, Wood, Kajiwara, & Byrnes, 1996).
Injection of any type of antigen into the ventricles or meninges will elicit a systemic immune response against the antigen. Equally, injection of a soluble antigen that can diffuse into the ventricular system will also induce immune responses. Careful direct injection of viral vectors into the brain parenchyma is predicted to cause a transitory innate immune inflammation, but no systemic immune reaction. Therefore, potentially therapeutic replication-incompetent viral vectors remain within the brain and express encoded therapeutic proteins long term, as required for long term therapeutic administration, i.e., Parkinson’s Disease. For the treatment of malignant brain cancer shorter expression is sufficient given glioma’s faster progression, with only transient local inflammation.
Combined gene and immunotherapy for brain tumors
The initial experimental therapeutic approach for the treatment of brain tumors employed vectors expressing HSV1-TK delivered to the brain tumors. Both murine retroviral vectors and human Adenoviral vectors were utilized. Adenoviral vectors have a high transduction efficacy in brain tumors, and the combination of HSV1-TK and ganciclovir is a powerful cytotoxin. We tested the efficiency of HSV1-TK/ganciclovir in a syngeneic rat model of glioma (Dewey, et al., 1999). If the therapy was delivered shortly after implantation of the tumor cells, all animals survived. However, if therapy was delivered at 12 days post implantation when tumors occupied the whole rat striatum, Adv-TK/ganciclovir only protected 20% of treated animals. Thus, depending on tumor size the therapy’s efficiency decreased from 100% to 20% (Ali et al., 2004; Ali et al., 2005). In conclusion, directly cytotoxic therapies that necessitate the transduction of ~100% of target cells would be hard pressed to display high efficacy in human clinical trials, as no vector system is yet able to transduce 100% of a target organ. As a consequence clinical trials utilizing strategies that need to transduce a vast majority of tumor cells to be effective therapeutically performed poorly in clinical trials.
It is usually thought that the brain’ immune privilege impedes an immune attack against brain tumors. Thus, it was postulated that T cells could not cross the BBB. Interestingly however, the BBB appeared very early in evolution and is thought to have facilitated the development and growth of nervous systems in early animal lineages. As these were evolving in the sea, the BBB maintained ionic levels within limits permissive to neuronal function. Immune cells, especially activated immune cells, can enter the brain even if no disruption of the BBB is induced. This explains why in multiple sclerosis or paraneoplastic syndromes the priming of immune responses occurs outside the brain. Immune surveillance of the brain further suggests that the low number of immune cells necessary for immune surveillance can also enter the brain. As activated lymphocytes, including activated and cytotoxic lymphocytes, can enter the brain suggests that the failure to mount therapeutic immune responses against brain tumors is likely to lie in the failure of brain tumors to stimulate an active and systemic immune response. Further, if an immune response against brain tumors could be induced, activated cytotoxic lymphocytes ought to be able to target glioma cells. A number of mechanisms could be invoked to explain the failure to activate the systemic anti-tumor immune response. A lack of priming from within the brain parenchyma could be explained by a downregulation of immune responses (i.e., T-regs, MDSCs, etc.), the inability of antigen containing cells – not dendritic cells- to exit the brain, or, the absence of afferent antigen presenting dendritic cells from the brain parenchyma proper. Therefore, a systemic immunization against brain tumors could be active in inducing a therapeutic anti-glioma immune response.
Various types of systemic anti-glioma immunization have been attempted. Disappointingly the results have not demonstrated consistent anti-glioma immune responses and extension of patient’s survival beyond individual cases. Several of these approaches are now in Phase III clinical trials. Though some of these have presented data suggesting an increase in progression free survival, none have been able to demonstrate an unequivocal increase in overall patient survival in Phase III randomized, double blinded, clinical trials, the gold standard in medical treatment. Given the excitement generated by these approaches, reasons for the underperformance of these trials are not yet completely understood.
The physiology of brain immune responses (also referred to as the brain immune privilege) could be explained by assuming that the brain lacks functional dendritic cells; i.e., a cell that takes up antigens, travels to the lymph nodes to present antigens to antigen-specific naïve T cells to stimulate their expansion and effector mechanisms. Even if cells expressing dendritic cell markers have been described in the brain we postulate that given the physiology of brain immune responses described above there is no cell in the brain that acts as a peripheral dendritic cell. Injection of an insoluble, particulate antigen into the skin (an organ that contains proper dendritic cells) will induce the stimulation of a systemic adaptive immune response. Careful injection of an identical antigen into the brain parenchyma will fail to induce a systemic adaptive immune response (Bechmann, et al., 2007; Galea, et al., 2007; Lowenstein, 2002). We assume that this difference is caused by the presence (skin) or absence (brain) of functional dendritic cells. The differential distribution of functional dendritic cells can be explained through a detailed study of the co-evolution of the brain and the immune system (Lowenstein, 2002). The brain and its constituent cells, including neurons and microglia, appear early in evolution and also during development, while immune cells and lymphatic channels appear late. We postulated that the late appearance of lymphatic vessels restricts their growth in the brain, and precludes the colonization of the brain by dendritic cells (Lowenstein, 2002). As a consequence immune responses in the brain, i.e. no priming of a systemic immune response, yet being a target of an immune attack), are the result of the parallel evolution and development of the brain and the immune system. Our hypothesis can thus explain the physiology and pathology of brain immune responses. We also postulated that recruiting dendritic cells to the brain tumors, could result in antigen uptake, activation, and migration to the draining lymph nodes, and thus prime a systemic adaptive immune response against brain tumors. Our work has shown this to be the case. Our strategy, described below, results in the shifting of brain immune function to being able to prime a systemic immune response against brain tumor antigens. In consequence, we refer to our therapeutic strategy as the re-engineering of the brain immune system.
Another clinically relevant characteristic of malignant brain tumors, and which is beyond clinical treatment at this time, is their high mutation rate (Brat et al., 2015; Brennan et al., 2013; Buczkowicz et al., 2014; Galvao et al., 2014; Kim et al., 2015; Noushmehr et al., 2010; Taylor et al., 2014; Verhaak et al., 2010). The molecular makeup of these tumors at first resection differs significantly from tumors resected at recurrence. A capacity to recognize tumor neo-antigens is a central determinant of the power of immune rejection of tumors (Kreiter et al., 2015; Linnemann et al., 2015; Yadav et al., 2014). However, if immunization is performed with pre-identified antigens, it is likely that the tumor could escape such immune responses. Checkpoint inhibitors are now being exploited to stimulate endogenous pre-existing anti-tumor immune responses, yet are ineffective due to T cell exhaustion (Hamid et al., 2013; Kroemer & Galluzzi, 2015; Larkin et al., 2015; Page, Postow, Callahan, Allison, & Wolchok, 2014; Robert et al., 2015; Romano et al., 2015; Spain & Larkin, 2016; Weber et al., 2015; Wolchok et al., 2013).
Re-engineering the brain immune system to treat malignant brain tumors
To overcome tumor escape from the activated immune system we designed a therapy that would re-engineer the brain immune system in such a way that immune cells, i.e., dendritic cells, entering the brain tumor microenvironment would sample glioma antigens present therein. In the absence of an experimental bias of tumor antigens made available to the dendritic cells, upon tumor progression immune cells ought to identify tumor neo-antigens and continue mounting an immune response against an evolving antigenic landscape of gliomas.
We proposed to re-engineer the brain tumor microenvironment to recognize brain glioma antigens to mount a systemic cytotoxic immune response, induce immunological memory, and continue sampling the brain tumor microenvironment to recognize glioma neo-antigens that appear during tumor evolution. To do so, based on the pathophysiological understanding of the physiology and evolution of the brain immune system described above (Lowenstein, 2002), we concluded that recruiting dendritic cells to the brain could serve to simulate an anti-glioma immune response and be of therapeutic benefit (Curtin et al., 2006).
Dendritic cells need to sample tumor antigens to then carry them to lymph nodes in order to present such antigens to naïve T cells. To promote the capacity of dendritic cells to sample the brain tumor microenvironment we constructed an Adv expressing HSV1-TK. Upon the systemic administration of ganciclovir (GCV), TK would kill transduced and actively proliferating GBM cells releasing antigens within an immunogenic cell death process. To attract DCs to the brain we constructed an Adv expressing Flt3L, the most powerful inducer of DCs. DCs would then be capable of carrying out their normal function, i.e., uptake GBM antigens, migrate to the draining lymph node where they would present the GBM antigens to T cells. Injections of Adv-Flt3L on its own into large experimental brain tumors induced an antitumor response, albeit as a single agent, it had limited therapeutic efficacy. Importantly, expression of Flt3L by itself within the brain tumor microenvironment demonstrated the appearance of cells whose morphology was compatible with that of DCs. These experiments demonstrated that even if Flt3L by itself was able to induce entry of DCs into the brain, and provide a significant therapeutic response, this was not sufficient to induce complete tumor rejection. We concluded from these experiments that there was a factor missing which was necessary in order to elicit a significant improvement in median survival and yield long term survivors. To evaluate our hypothesis we tested the combination of the conditional cytotoxic (Ad-TK) and immune-stimulatory (Ad-Flt3L) approach. Only 10–15% of animals implanted with large tumors and treated with Adv-TK+GCV alone showed a beneficial effect. Importantly, as predicted, the addition of Adv-Flt3L increased survival of animals treated with Adv.HSV1-TK to 70–80%. This demonstrated that Adv-Flt3L added a major potentially synergistic effect to the GBM cell death induced by Adv-TK.
Brain tumors can be multifocal at diagnosis, and they always recur, which poses a formidable therapeutic challenge. We thus tested experimentally whether our therapy would be able to treat gliomas both in a model of recurrence and in a model of a multifocal tumor. Multifocal tumors were modeled by injecting tumor cells into both hemispheres, whereas the combination of therapeutic adenoviruses was injected into only one tumor mass. The treatment protected a high percentage of animals (~70%) demonstrating that the combined TK + Flt3L gene therapy approach induced a systemic immune response capable of recognizing, attacking and destroying a second tumor which had not previously been treated directly (King, Muhammad, et al., 2008). To model tumor recurrence after treatment, animals were allowed t o survive for two months to assure that they were tumor free. At this time they were challenged with a tumor injected into the contralateral brain hemisphere. All animals that survived the initial tumor also survived the second tumor challenge demonstrating that anti-GBM systemic immunity had been induced in response to the initial treatment.
The efficiency of the combined Ad-TK and Ad-Flt3L gene therapy in eliminating large experimental gliomas, including multifocal gliomas, and a recurring glioma model, in the absence of long term behavioral (King, Kroeger, et al., 2008) or inflammatory side effects (Barcia et al., 2006; Barcia, et al., 2007; Gerdes, Castro, & Lowenstein, 2000; Larocque, et al., 2010; Thomas, Birkett, et al., 2001; Thomas, et al., 2000; Thomas, Schiedner, et al., 2001) indicated the induction of systemic anti-glioma immunity. In experiments performed in a rat model of recurrent GBM, animals that survived the first tumor (i.e., responded to the combined gene therapy), were challenged with a second tumor implanted in the contralateral brain hemisphere and, were injected systemically with antibodies to deplete various populations of immune cells. Depletion of CD4+ and CD8+ T cells abolished the efficiency of the GBM immune memory response. Depletion of macrophages and other immune cells had very minor effects on the treatment. The treatment thus eliminates primary tumors, multifocal tumors, and upon tumor recurrences through a systemic cytotoxic immune response mediated by CD4+ and CD8+ T cells.
In subsequent experiments we also tested whether our hypothesis concerning the proposed mechanism of action was supported by the behavior of the immune cells involved. To this effect, we were able to demonstrate that upon the intratumoral injection of pDCs (plasmacytoid dendritic cells) were recruited to the tumoral microenvironment. Recruited pDCs also took up fluorescent microbeads as a surrogate for the uptake of tumoral antigens, and were detectable within the draining cervical lymph. Subsequently, we also followed the increase in circulating CD8+ cytotoxic T cells, whose depletion blocked the anti-tumoral effect of the gene/immunotherapy (Curtin, et al., 2006; Curtin et al., 2009).
An unresolved issue remaining was whether any innate immune mechanisms were also necessary for the combined Ad-TK and Ad-Flt3L gene/immunotherapy to be effective. Adenoviruses and recombinant adenovirus vectors (Ads) are known to be able to stimulate innate immune responses which in the brain include the release of IL8, IL1α/β, and TNFα, amongst other cytokines. In the brain innate immune responses are short lived and are accompanied by cellular activation of microglia and astrocytes. We assessed the role of Ad-TK mediated GBM cell death to uncover the role played by Toll Receptor signaling in mediating anti-GBM immunity. The hypothesis we sought out to test, was that TLR signaling was necessary for Ad-TK and Ad-Flt3L gene/immunotherapy effectiveness. We implemented experiments identical to those described above, but performed in animals deficient for various TLRs. GBM bearing mice treated with Ad-TK and Ad-Flt3L gene/immunotherapy, but lacking TLR2, succumbed to tumor burden. Thus, indicating that TLR2 signaling was necessary for the effectiveness of the treatment. We subsequently identified HMGB1 as an endogenous ligand for TLR2, and showed that it was released from dying glioma cells (Curtin, et al., 2009). Though HMGB1 has been described as a potential ligand also for TLR4, we did not observe inhibition of our treatment in animals lacking TLR4. Even if a potential interaction of HMGB1 with RAGE has not been formally rejected, the elimination of anti-tumor responses in TLR2 animals, and the need for TLR2 on dendritic cells, reduces the odds that RAGE binding plays a significant role in our paradigm (Sims, et al., 2010). The essential need for HMGB1 as part of our treatment was further elucidated by inhibiting HMGB1. Using specific polyclonal anti-HMGB1 antibodies, or through the injection of glycyrrhizin, a small molecule inhibitor of HMGB1, we showed that blocking HMGB1 completely abolishes the efficacy of our treatment. Both approaches blunted the therapeutic effect of Ad-TK and Ad-Flt3L gene/immunotherapy indicating that release of HMGB1 from dying glioma cells is necessary for tumor regression and the generation of anti-GBM immunity. TLR2 signaling was required on dendritic cells originating from the bone marrow. The absence of TLR2 from dendritic cells also eliminated the efficiency of Ad-TK and Ad-Flt3L gene/immunotherapy (Candolfi et al., 2012; Candolfi et al., 2009; Curtin, et al., 2006; Curtin, et al., 2009).
In summary, in situ re-engineering of the brain immune response to develop a therapy for deadly malignant brain tumors can be described as the induction of glioma cell death, and release of HMGB1, by Ad-TK/ganciclovir, and the recruitment of DCs to the glioma microenvironment by Ad-Flt3L. DCs can then sample the tumor microenvironment and take up tumor antigens. Upon activation of DCs by HMGB1 acting on TLR2, DCs migrate to the draining lymph nodes where they induce a CD8+ cytotoxic immune response which eliminates glioma growth from the brain. This strategy also generates anti-GBM immunological memory, which eliminates GBM recurrence. This therapy exhibits a very high safety profile without any evidence of toxic adverse effects disseminated throughout the brain such as generalized autoimmunity (Dewey, et al., 1999; Larocque, et al., 2010), provided a systemic anti-viral immune response does not occur (Zirger, et al., 2012).
The holy grail: endogenous immunotherapy trials in human patients suffering from glioblastoma multiforme
At this time, we decided to move forward with the implementation of a Phase I clinical trial using Ad-TK (+GCV) in combination with Ad-Flt3l in patients suffering from glioblastoma grade IV (WHO). Following the production and quality control checks of the clinical grade recombinant adenoviruses, their toxicity testing, and bio-distribution studies, we submitted our application for an IND to the FDA. The letter from the FDA allowing us to proceed with the Phase I Clinical Trial described by our IND #14574 was received in 2011. The first patient to the trial was recruited at the beginning of 2014. To inspect the trial’s detail the trial’s identifier NCT# NCT01811992 allows its review at https://clinicaltrials.gov/ct2/show/NCT01811992, an NIH supported website describing ongoing clinical trials. The trial involves 6 cohorts of 3 patients each, for a total of 18 patients, we have already completed treatment of the first cohort and treated on patient in the second cohort. We are aiming to complete the trial in 2018. Preliminary data of this ongoing trial will be presented during 2018.
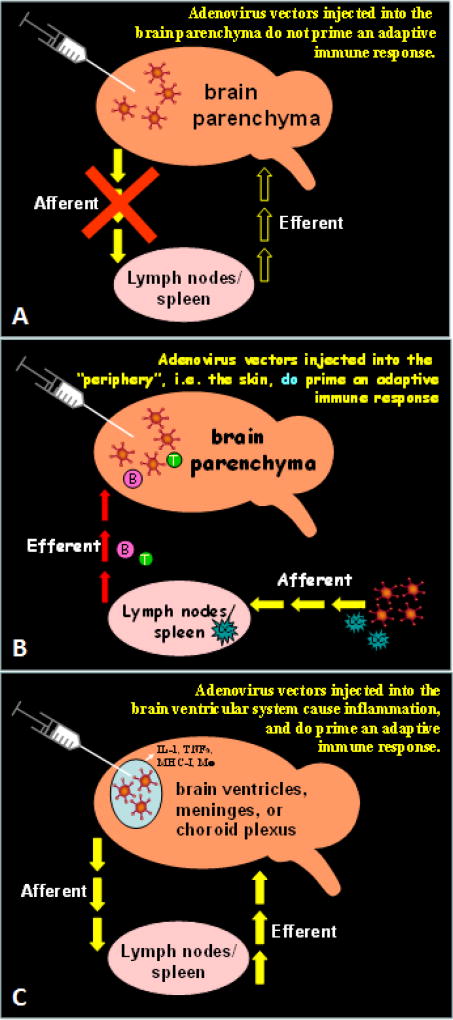
FIGURE 1.
This figure illustrates in schematic fashion the neuroimmune structure underlying the phenomenology known as the brain’s immune privilege. In this figure the antigen model used to explore the brain’s immune responses are non-replicating adenoviral vectors. (A) illustrates the condition in which adenoviral vectors are injected carefully only into the brain parenchyma proper. Under these conditions given the absence of afferent dendritic cells from the brain parenchyma, viral vectors have been shown to remain in the brain for 12 months and more. A systemic anti-adenoviral immune response will not be induced. (B) If however, the systemic immune system is primed, an immune response will be generated, brain inflammation will ensue, and the viral vectors will be eliminated. (C) Direct administration of vectors into the brain ventricles will induce a systemic anti-adenoviral immune response, as the ventricular immune system has all necessary cells and vessels to do so. Therefore, in our novel therapeutic strategy, we recruit dendritic cells to the brain to implement those essential aspects of immune function which are missing from the brain.
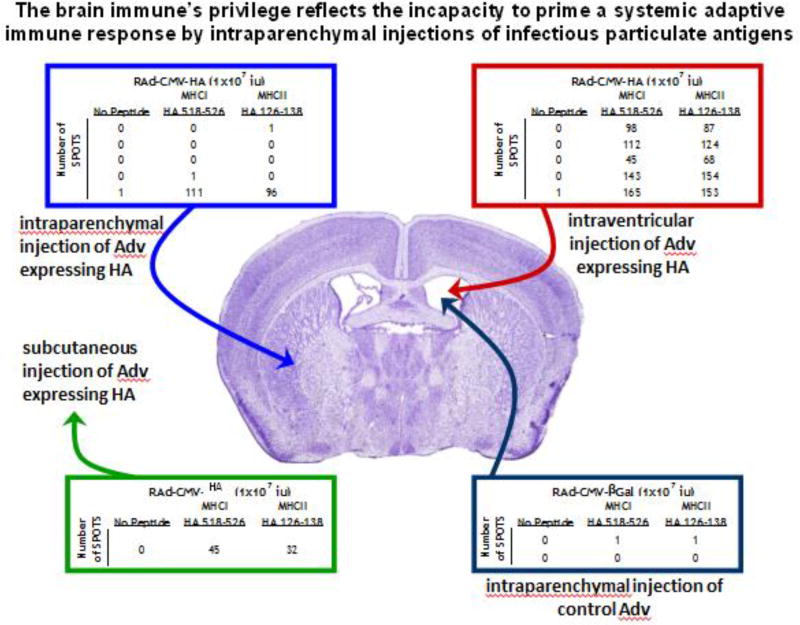
FIGURE 2.
This figure exemplifies in practical fashion the neuroimmune structure underlying the phenomenology known as the brain’s immune privilege. Injection of an adenovirus expressing influenza hemagglutinin (HA) into the brain parenchyma does not cause a systemic anti-adenoviral, or anti-HA immune response. However, if the same virus is injected into the brain ventricles or subcutaneously a systemic immune response against HA can be detected. As a negative control, injection into the ventricles of a virus not expressing HA, does not cause systemic anti-HA immunity.
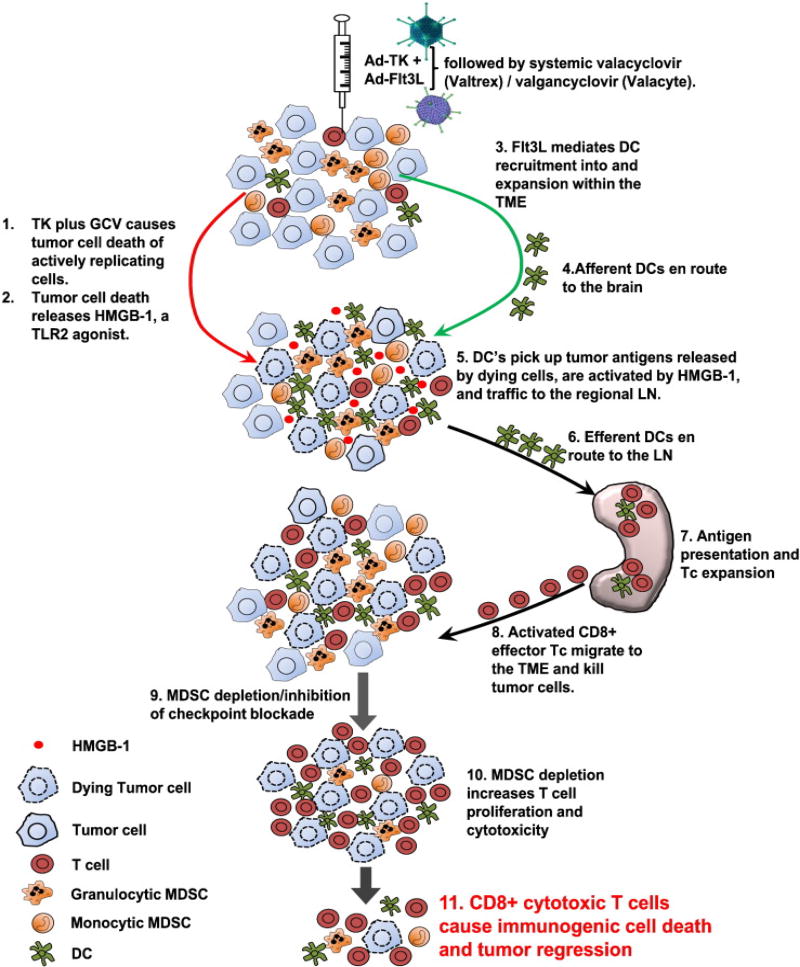
FIGURE 3.
This figure illustrates the mechanism of action of our novel approach to re-design the brain immune system to allow it to recognize novel tumor antigens. Ad-TK+GCV kills tumor cells and releases HMGB1. Ad-Flt3L recruits dendritic cells to the brain. These take up tumor antigens, and upon stimulation of TLR2 by HMGB1, they induce a systemic anti brain tumor immune response.
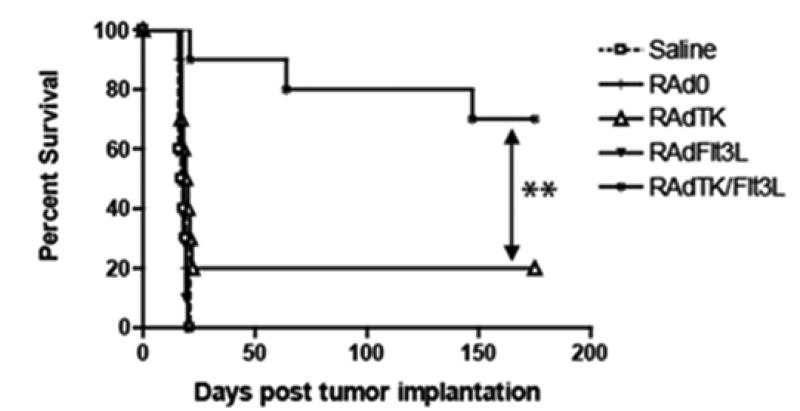
FIGURE 4.
This figure shows that the addition of RAd-Flt3L increases the efficiency of RAd-TK from 20% of long term animal survival to 75% of animals survival, demonstrating the efficiency of using both adenoviral vectors in a stringent model of glioblastoma.
TABLE 1.
Characteristics of the two CNS immune compartments: (a) the brain ventricular immune system, and (ii) parenchymal immune systems
| (a) The brain ventricular immune system |
Anatomy:
|
Physiology:
|
|
|
| (b) The brain parenchymal immune system |
Anatomy:
|
Physiology:
|
HIGHLIGHTS.
Immune responses against brain tumors are usually limited and insufficient for therapeutic effect.
We hypothesized that the absence of proper afferent dendritic cells from the brain parenchyma underlies the failure of the brain to mount immune responses against malignant brain tumors.
A method was developed to attract afferent dendritic cells to the brain, through the intraparenchymal delivery of adenoviral-mediated expression of fms-related tyrosine kinase 3 ligand (Flt3L). This was then combined with a method to kill tumor cells, through the delivery of a second adenovirus expressing HSV1-thymidine kinase, followed by systemic ganciclovir administration to activate TK killing of dividing tumor cells.
Delivery of both viral vectors into experimental malignant brain tumors causes death of tumor cells and release of the endogenous TLR agonist HMGB1. Afferent dendritic cells in the brain take up tumor antigens, and then become activated by HMGB1 binding to TLR2 receptors on dendritic cells. Once activated, dendritic cells migrate to cervical lymph nodes where they stimulate a systemic anti-glioma immune response. This immune response is mediated by CD4+ and CD8+ T cells, induces immunological anti-glioma memory, and is able to recognize tumor neo-antigens.
In a first in person clinical trial at The University of Michigan we are testing the hypothesis that Ad-Flt3L in combination with Ad-HSV1.TK (+ ganciclovir) can be potentially therapeutic in human patients suffering from high grade malignant brain tumors, glioblastoma multiforme, WHO grade IV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, Lowenstein PR. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16(6):741–751. doi: 10.1089/hum.2005.16.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, Castro MG. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10(6):1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, Castro MG. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65(16):7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker GJ, Chockley P, Yadav VN, Doherty R, Ritt M, Sivaramakrishnan S, Castro MG, Lowenstein PR. Natural killer cells eradicate galectin-1-deficient glioma in the absence of adaptive immunity. Cancer Res. 2014;74(18):5079–5090. doi: 10.1158/0008-5472.CAN-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Gerdes C, Xiong WD, Thomas CE, Liu C, Kroeger KM, Lowenstein PR. Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glia Biol. 2006;2(4):309–322. doi: 10.1017/S1740925X07000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Jimenez-Dalmaroni M, Kroeger KM, Puntel M, Rapaport AJ, Larocque D, Castro MG, Lowenstein PR. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol Ther. 2007;15(12):2154–2163. doi: 10.1038/sj.mt.6300305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batich KA, Swartz AM, Sampson JH. Enhancing dendritic cell-based vaccination for highly aggressive glioblastoma. Expert Opin Biol Ther. 2015;15(1):79–94. doi: 10.1517/14712598.2015.972361. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28(1):5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bell MD, Taub DD, Perry VH. Overriding the brain's intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience. 1996;74(1):283–292. doi: 10.1016/0306-4522(96)00083-8. [DOI] [PubMed] [Google Scholar]
- Bohman LE, Swanson KR, Moore JL, Rockne R, Mandigo C, Hankinson T, Bruce JN. Magnetic resonance imaging characteristics of glioblastoma multiforme: implications for understanding glioma ontogeny. Neurosurgery. 2010;67(5):1319–1327. doi: 10.1227/NEU.0b013e3181f556ab. discussion 1327-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes AA, Franceschi E, Ermani M, Tosoni A, Albani F, Depenni R, Vanzo C. Pattern of care and effectiveness of treatment for glioblastoma patients in the real world: Results from a prospective population-based registry. Could survival differ in a high-volume center? Neurooncol Pract. 2014;1(4):166–171. doi: 10.1093/nop/npu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brat DJ, Verhaak RG, Aldape KD, Yung WK, Salama SR, Cooper LA, Radenbaugh AJ. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N Engl J Med. 2015;372(26):2481–2498. doi: 10.1056/NEJMoa1402121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ. Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev. 2013;39(8):891–907. doi: 10.1016/j.ctrv.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Chin L. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczkowicz P, Bartels U, Bouffet E, Becher O, Hawkins C. Histopathological spectrum of paediatric diffuse intrinsic pontine glioma: diagnostic and therapeutic implications. Acta Neuropathol. 2014;128(4):573–581. doi: 10.1007/s00401-014-1319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczkowicz P, Hawkins C. Pathology, Molecular Genetics, and Epigenetics of Diffuse Intrinsic Pontine Glioma. Front Oncol. 2015;5:147. doi: 10.3389/fonc.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, Hawkins C. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46(5):451–456. doi: 10.1038/ng.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, MacLaren RE, Charlton HM. Immunological instability of persistent adenovirus vectors in the brain: peripheral exposure to vector leads to renewed inflammation, reduced gene expression, and demyelination. J Neurosci. 1996;16(9):3045–3055. doi: 10.1523/JNEUROSCI.16-09-03045.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, Rusby JE, Wood MJ, Charlton HM. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66(4):1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, Wood MJ, Charlton HM. Role of T cells in inflammation caused by adenovirus vectors in the brain. Gene Ther. 1996;3(7):644–651. [PubMed] [Google Scholar]
- Candolfi M, King GD, Yagiz K, Curtin JF, Mineharu Y, Muhammad AK, Foulad D, Kroeger KM, Barnett N, Josien R, Lowenstein PR, Castro MG. Plasmacytoid dendritic cells in the tumor microenvironment: immune targets for glioma therapeutics. Neoplasia. 2012;14(8):757–770. doi: 10.1593/neo.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candolfi M, Yagiz K, Foulad D, Alzadeh GE, Tesarfreund M, Muhammad AK, Castro MG. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res. 2009;15(13):4401–4414. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carare RO, Hawkes CA, Weller RO. Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav Immun. 2014;36:9–14. doi: 10.1016/j.bbi.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- Clapham R, O'Sullivan E, Weller RO, Carare RO. Cervical lymph nodes are found in direct relationship with the internal carotid artery: significance for the lymphatic drainage of the brain. Clin Anat. 2010;23(1):43–47. doi: 10.1002/ca.20887. [DOI] [PubMed] [Google Scholar]
- Colditz MJ, Jeffree RL. Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 1: Clinical, radiological and pathological studies. J Clin Neurosci. 2012;19(11):1471–1474. doi: 10.1016/j.jocn.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C, Castro MG. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176(6):3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Castro MG. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deSouza RM, Shaweis H, Han C, Sivasubramiam V, Brazil L, Beaney R, Ashkan K. Has the survival of patients with glioblastoma changed over the years? Br J Cancer. 2016;114(2):146–150. doi: 10.1038/bjc.2015.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey RA, Morrissey G, Cowsill CM, Stone D, Bolognani F, Dodd NJ, Lowenstein PR. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5(11):1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- Eljamel S. 5-ALA Fluorescence Image Guided Resection of Glioblastoma Multiforme: A Meta-Analysis of the Literature. Int J Mol Sci. 2015;16(5):10443–10456. doi: 10.3390/ijms160510443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field KM, Jordan JT, Wen PY, Rosenthal MA, Reardon DA. Bevacizumab and glioblastoma: scientific review, newly reported updates, and ongoing controversies. Cancer. 2015;121(7):997–1007. doi: 10.1002/cncr.28935. [DOI] [PubMed] [Google Scholar]
- Fine HA. Bevacizumab in glioblastoma--still much to learn. N Engl J Med. 2014;370(8):764–765. doi: 10.1056/NEJMe1313309. [DOI] [PubMed] [Google Scholar]
- Finocchiaro G, Pellegatta S. Perspectives for immunotherapy in glioblastoma treatment. Curr Opin Oncol. 2014;26(6):608–614. doi: 10.1097/CCO.0000000000000135. [DOI] [PubMed] [Google Scholar]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28(1):12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Galvao RP, Kasina A, McNeill RS, Harbin JE, Foreman O, Verhaak RG, Zong H. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc Natl Acad Sci U S A. 2014;111(40):E4214–4223. doi: 10.1073/pnas.1414389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, Liu Q, Wu W, Yin JX, Bai XF, Shen R, Shi FD. Ischemic neurons recruit natural killer cells that accelerate brain infarction. Proc Natl Acad Sci U S A. 2014;111(7):2704–2709. doi: 10.1073/pnas.1315943111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes CA, Castro MG, Lowenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol Ther. 2000;2(4):330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Mehta MP. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(21):2048–2049. doi: 10.1056/NEJMc1403303. [DOI] [PubMed] [Google Scholar]
- Grimm SA, Chamberlain MC. Brainstem glioma: a review. Curr Neurol Neurosci Rep. 2013;13(5):346. doi: 10.1007/s11910-013-0346-3. [DOI] [PubMed] [Google Scholar]
- Gururangan S, Chi SN, Young Poussaint T, Onar-Thomas A, Gilbertson RJ, Vajapeyam S, Kun LE. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Oncol. 2010;28(18):3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MG, Garside R, Rogers G, Stein K, Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev. 2013;4:CD007415. doi: 10.1002/14651858.CD007415.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SB, Kockro RA, Actor B, Sarnthein J, Bernays RL. Combining 5-ALA Fluorescence and Intraoperative MRI in Glioblastoma Surgery: A Histology-Based Evaluation. Neurosurgery. 2015 doi: 10.1227/NEU.0000000000001035. [DOI] [PubMed] [Google Scholar]
- Hawke S, Stevenson PG, Freeman S, Bangham CR. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187(10):1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34(49):16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44(6 Suppl 1):S93–95. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, Wolfer J, Ewelt C, Holling M, Hasselblatt M, Niederstadt T, Stummer W. The Value of 5-ALA in Low-grade Gliomas and High-grade Gliomas Lacking Glioblastoma Imaging Features: An Analysis Based on Fluorescence, MRI, 18F-FET PET, and Tumor Molecular Factors. Neurosurgery. 2015 doi: 10.1227/NEU.0000000000001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MH, van Vuurden DG, Vandertop WP, Kaspers GJ. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38(1):27–35. doi: 10.1016/j.ctrv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The Glymphatic System: A Beginner's Guide. Neurochem Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji M, Lewis S, Camelo-Piragua S, Ramkissoon SH, Snuderl M, Venneti S, Orringer DA. Detection of human brain tumor infiltration with quantitative stimulated Raman scattering microscopy. Sci Transl Med. 2015;7(309):309ra163. doi: 10.1126/scitranslmed.aab0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji M, Orringer DA, Freudiger CW, Ramkissoon S, Liu X, Lau D, Xie XS. Rapid, label-free detection of brain tumors with stimulated Raman scattering microscopy. Sci Transl Med. 2013;5(201):201ra119. doi: 10.1126/scitranslmed.3005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K, Byrnes AP, Charlton HM, Wood MJ, Wood KJ. Immune responses to adenoviral vectors during gene transfer in the brain. Hum Gene Ther. 1997;8(3):253–265. doi: 10.1089/hum.1997.8.3-253. [DOI] [PubMed] [Google Scholar]
- Karsy M, Gelbman M, Shah P, Balumbu O, Moy F, Arslan E. Established and emerging variants of glioblastoma multiforme: review of morphological and molecular features. Folia Neuropathol. 2012;50(4):301–321. doi: 10.5114/fn.2012.32361. [DOI] [PubMed] [Google Scholar]
- Khasraw M, Ameratunga MS, Grant R, Wheeler H, Pavlakis N. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. 2014;9:CD008218. doi: 10.1002/14651858.CD008218.pub3. [DOI] [PubMed] [Google Scholar]
- Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19(6):480–488. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Kida S, Weller RO, Zhang ET, Phillips MJ, Iannotti F. Anatomical pathways for lymphatic drainage of the brain and their pathological significance. Neuropathol Appl Neurobiol. 1995;21(3):181–184. doi: 10.1111/j.1365-2990.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Zheng S, Amini SS, Virk SM, Mikkelsen T, Brat DJ, Verhaak RG. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res. 2015;25(3):316–327. doi: 10.1101/gr.180612.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Kroeger KM, Bresee CJ, Candolfi M, Liu C, Manalo CM, Castro MG. Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol Ther. 2008;16(4):682–690. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GD, Muhammad AK, Curtin JF, Barcia C, Puntel M, Liu C, Castro MG. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10(1):19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotsarini C, Griffiths PD, Wilkinson ID, Hoggard N. A systematic review of the literature on the effects of dexamethasone on the brain from in vivo human-based studies: implications for physiological brain imaging of patients with intracranial tumors. Neurosurgery. 2010;67(6):1799–1815. doi: 10.1227/NEU.0b013e3181fa775b. discussion 1815. [DOI] [PubMed] [Google Scholar]
- Koukourakis GV, Kouloulias V, Zacharias G, Papadimitriou C, Pantelakos P, Maravelis G, Kouvaris J. Temozolomide with radiation therapy in high grade brain gliomas: pharmaceuticals considerations and efficacy; a review article. Molecules. 2009;14(4):1561–1577. doi: 10.3390/molecules14041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiter S, Vormehr M, van de Roemer N, Diken M, Lower M, Diekmann J, Sahin U. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature. 2015;520(7549):692–696. doi: 10.1038/nature14426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76(6):845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L. Combinatorial immunotherapy with checkpoint blockers solves the problem of metastatic melanoma-An exclamation sign with a question mark. Oncoimmunology. 2015;4(7):e1058037. doi: 10.1080/2162402X.2015.1058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol. 2011;12(11):1062–1070. doi: 10.1016/S1470-2045(11)70130-9. [DOI] [PubMed] [Google Scholar]
- Laman JD, Weller RO. Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol. 2013;8(4):840–856. doi: 10.1007/s11481-013-9470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocque D, Sanderson NS, Bergeron J, Curtin JF, Girton J, Wibowo M, Lowenstein PR. Exogenous fms-like tyrosine kinase 3 ligand overrides brain immune privilege and facilitates recognition of a neo-antigen without causing autoimmune neuropathology. Proc Natl Acad Sci U S A. 2010;107(32):14443–14448. doi: 10.1073/pnas.0913496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau D, Hervey-Jumper SL, Chang S, Molinaro AM, McDermott MW, Phillips JJ, Berger MS. A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg. 2015:1–10. doi: 10.3171/2015.5.JNS1577. [DOI] [PubMed] [Google Scholar]
- Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, Schumacher TN. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21(1):81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23(1):23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR. The case for immunosuppression in clinical gene transfer. Mol Ther. 2005;12(2):185–186. doi: 10.1016/j.ymthe.2005.06.439. [DOI] [PubMed] [Google Scholar]
- Lowenstein PR, Mandel RJ, Xiong WD, Kroeger K, Castro MG. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7(5):347–360. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyszak MK, Perry VH. Delayed-type hypersensitivity lesions in the central nervous system are prevented by inhibitors of matrix metalloproteinases. J Neuroimmunol. 1996a;69(1–2):141–149. doi: 10.1016/0165-5728(96)00082-3. [DOI] [PubMed] [Google Scholar]
- Matyszak MK, Perry VH. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience. 1996b;74(2):599–608. doi: 10.1016/0306-4522(96)00160-1. [DOI] [PubMed] [Google Scholar]
- Matyszak MK, Perry VH. Bacillus Calmette-Guerin sequestered in the brain parenchyma escapes immune recognition. J Neuroimmunol. 1998;82(1):73–80. doi: 10.1016/S0165-5728(97)00190-2. [DOI] [PubMed] [Google Scholar]
- Mitchell P, Ellison DW, Mendelow AD. Surgery for malignant gliomas: mechanistic reasoning and slippery statistics. Lancet Neurol. 2005;4(7):413–422. doi: 10.1016/S1474-4422(05)70118-6. [DOI] [PubMed] [Google Scholar]
- Mohme M, Neidert MC, Regli L, Weller M, Martin R. Immunological challenges for peptide-based immunotherapy in glioblastoma. Cancer Treat Rev. 2014;40(2):248–258. doi: 10.1016/j.ctrv.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Molinaro AM, Wrensch MR, Jenkins RB, Eckel-Passow JE. Statistical considerations on prognostic models for glioma. Neuro Oncol. 2015 doi: 10.1093/neuonc/nov255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobusawa S, Hirato J, Yokoo H. Molecular genetics of ependymomas and pediatric diffuse gliomas: a short review. Brain Tumor Pathol. 2014;31(4):229–233. doi: 10.1007/s10014-014-0200-6. [DOI] [PubMed] [Google Scholar]
- Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Aldape K. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–522. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JJ, Nayak L, Ormond DR, Wen PY, Kalkanis SN. The role of cytotoxic chemotherapy in the management of progressive glioblastoma : a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):501–555. doi: 10.1007/s11060-013-1338-5. [DOI] [PubMed] [Google Scholar]
- Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- Panditharatna E, Yaeger K, Kilburn LB, Packer RJ, Nazarian J. Clinicopathology of diffuse intrinsic pontine glioma and its redefined genomic and epigenomic landscape. Cancer Genet. 2015;208(7–8):367–373. doi: 10.1016/j.cancergen.2015.04.008. [DOI] [PubMed] [Google Scholar]
- Pannullo SC, Fraser JF, Moliterno J, Cobb W, Stieg PE. Stereotactic radiosurgery: a meta-analysis of current therapeutic applications in neuro-oncologic disease. J Neurooncol. 2011;103(1):1–17. doi: 10.1007/s11060-010-0360-0. [DOI] [PubMed] [Google Scholar]
- Peden CS, Manfredsson FP, Reimsnider SK, Poirier AE, Burger C, Muzyczka N, Mandel RJ. Striatal readministration of rAAV vectors reveals an immune response against AAV2 capsids that can be circumvented. Mol Ther. 2009;17(3):524–537. doi: 10.1038/mt.2008.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH. Persistent pathogens in the parenchyma of the brain. J Neurovirol. 2000;6 Suppl 1:S86–89. [PubMed] [Google Scholar]
- Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104(3):629–638. doi: 10.1007/s11060-011-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouratian N, Bookheimer SY. The reliability of neuroanatomy as a predictor of eloquence: a review. Neurosurg Focus. 2010;28(2):E3. doi: 10.3171/2009.11.FOCUS09239. [DOI] [PubMed] [Google Scholar]
- Raizer JJ, Giglio P, Hu J, Groves M, Merrell R, Conrad C, Gilbert MR. A phase II study of bevacizumab and erlotinib after radiation and temozolomide in MGMT unmethylated GBM patients. J Neurooncol. 2015 doi: 10.1007/s11060-015-1958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Wucherpfennig KW, Freeman G, Wu CJ, Chiocca EA, Wen PY, Dranoff G. An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines. 2013;12(6):597–615. doi: 10.1586/erv.13.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter JD, Gomez DL, Wilson JH, Van Den Pol AN. Systemic immune deficiency necessary for cytomegalovirus invasion of the mature brain. J Virol. 2004;78(3):1473–1487. doi: 10.1128/JVI.78.3.1473-1487.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Ribas A. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- Rock K, McArdle O, Forde P, Dunne M, Fitzpatrick D, O'Neill B, Faul C. A clinical review of treatment outcomes in glioblastoma multiforme--the validation in a non-trial population of the results of a randomised Phase III clinical trial: has a more radical approach improved survival? Br J Radiol. 2012;85(1017):e729–733. doi: 10.1259/bjr/83796755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, Speiser DE. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112(19):6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–764. doi: 10.1227/01.neu.0000318159.21731.cf. discussion 264–756. [DOI] [PubMed] [Google Scholar]
- See AP, Pradilla G, Yang I, Han S, Parsa AT, Lim M. Heat shock protein-peptide complex in the treatment of glioblastoma. Expert Rev Vaccines. 2011;10(6):721–731. doi: 10.1586/erv.11.49. [DOI] [PubMed] [Google Scholar]
- Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, Gary SJ, Lowenstein PR, Vandenberghe LH, Wilson JT, Wolfe HJ, Glorioso JC. Progress in gene therapy for neurological disorders. Nature Review Neurology. 2013;9(5):277–291. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in Inflammation and Cancer. Annu Rev Immunol. 2010;28:367–88. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- Spain L, Larkin J. Combination immune checkpoint blockade with ipilimumab and nivolumab in the management of advanced melanoma. Expert Opin Biol Ther. 2016 doi: 10.1517/14712598.2016.1141195. [DOI] [PubMed] [Google Scholar]
- Stadlbauer A, Buchfelder M, Salomonowitz E, Ganslandt O. Fiber density mapping of gliomas: histopathologic evaluation of a diffusion-tensor imaging data processing method. Radiology. 2010;257(3):846–853. doi: 10.1148/radiol.10100343. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Bangham CR, Hawke S. Recruitment, activation and proliferation of CD8+ memory T cells in an immunoprivileged site. Eur J Immunol. 1997;27(12):3259–3268. doi: 10.1002/eji.1830271225. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Hawke S, Bangham CR. Protection against influenza virus encephalitis by adoptive lymphocyte transfer. Virology. 1997;232(1):158–166. doi: 10.1006/viro.1997.8535. [DOI] [PubMed] [Google Scholar]
- Stevenson PG, Hawke S, Sloan DJ, Bangham CR. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol. 1997;71(1):145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles CS, Rowitch CD Glioma Stem Cells. A midterm exam. Neuron. 2008;58(6):832–46. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Taylor KR, Mackay A, Truffaux N, Butterfield YS, Morozova O, Philippe C, Grill J. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat Genet. 2014;46(5):457–461. doi: 10.1038/ng.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001;3(1):36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci U S A. 2000;97(13):7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001;12(7):839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- van Den Pol AN, Mocarski E, Saederup N, Vieira J, Meier TJ. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J Neurosci. 1999;19(24):10948–10965. doi: 10.1523/JNEUROSCI.19-24-10948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuijzen van Zanten SE, Jansen MH, Sanchez Aliaga E, van Vuurden DG, Vandertop WP, Kaspers GJ. A twenty-year review of diagnosing and treating children with diffuse intrinsic pontine glioma in The Netherlands. Expert Rev Anticancer Ther. 2015;15(2):157–164. doi: 10.1586/14737140.2015.974563. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Hayes DN. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhao HY, Zhang FC, Sun Y, Xiong ZY, Jiang XB. Dendritic cell-based vaccine for the treatment of malignant glioma: a systematic review. Cancer Invest. 2014;32(9):451–457. doi: 10.3109/07357907.2014.958234. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Rao L, Bhambhani K, Miller K, Poulik J, Altinok D, Sood S. Diffuse intrinsic pontine glioma biopsy: a single institution experience. Pediatr Blood Cancer. 2015;62(1):163–165. doi: 10.1002/pbc.25224. [DOI] [PubMed] [Google Scholar]
- Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- Weiss T, Weller M, Roth P. Immunotherapy for glioblastoma: concepts and challenges. Curr Opin Neurol. 2015;28(6):639–646. doi: 10.1097/WCO.0000000000000249. [DOI] [PubMed] [Google Scholar]
- Weller RO. Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J Neuropathol Exp Neurol. 1998;57(10):885–894. doi: 10.1097/00005072-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117(1):1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- Weller RO, Engelhardt B, Phillips MJ. Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways. Brain Pathol. 1996;6(3):275–288. doi: 10.1111/j.1750-3639.1996.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Weller RO, Galea I, Carare RO, Minagar A. Pathophysiology of the lymphatic drainage of the central nervous system: Implications for pathogenesis and therapy of multiple sclerosis. Pathophysiology. 2010;17(4):295–306. doi: 10.1016/j.pathophys.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Weller RO, Kida S, Zhang ET. Pathways of fluid drainage from the brain--morphological aspects and immunological significance in rat and man. Brain Pathol. 1992;2(4):277–284. doi: 10.1111/j.1750-3639.1992.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18(2):253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler CJ, Black KL. DCVax-Brain and DC vaccines in the treatment of GBM. Expert Opin Investig Drugs. 2009;18(4):509–519. doi: 10.1517/13543780902841951. [DOI] [PubMed] [Google Scholar]
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MJ, Byrnes AP, Rabkin SD, Pfaff DW, Charlton HM. Immunological consequences of HSV-1-mediated gene transfer into the CNS. Gene Ther. 1994;1(Suppl 1):S82. [PubMed] [Google Scholar]
- Wood MJ, Charlton HM, Wood KJ, Kajiwara K, Byrnes AP. Immune responses to adenovirus vectors in the nervous system. Trends Neurosci. 1996;19(11):497–501. doi: 10.1016/S0166-2236(96)10060-6. [DOI] [PubMed] [Google Scholar]
- Wu G, Diaz AK, Paugh BS, Rankin SL, Ju B, Li Y, Baker SJ. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat Genet. 2014;46(5):444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Delamarre L. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- Yang LJ, Zhou CF, Lin ZX. Temozolomide and radiotherapy for newly diagnosed glioblastoma multiforme: a systematic review. Cancer Invest. 2014;32(2):31–36. doi: 10.3109/07357907.2013.861474. [DOI] [PubMed] [Google Scholar]
- Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Nedergaard M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med. 2013;11:107. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirger JM, Puntel M, Bergeron J, Wibowo M, Moridzadeh R, Bondale N, Lowenstein PR. Immune-mediated loss of transgene expression from virally transduced brain cells is irreversible, mediated by IFNgamma, perforin, and TNFalpha, and due to the elimination of transduced cells. Mol Ther. 2012;20(4):808–819. doi: 10.1038/mt.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]