Abstract
Background/Aims
We investigated the time taken for patients with metastatic non-small cell lung cancer (NSCLC) to develop brain metastases (BM), as well as their subsequent overall median survival following diagnosis, considering the epidermal growth factor receptor (EGFR) mutational status.
Methods
We retrospectively investigated the medical records of 259 patients diagnosed with advanced NSCLC from January 2010 to August 2013, who were tested for EGFR mutations. The time from the diagnosis of advanced NSCLC to the development of BM and the overall median survival after BM development (BM-OS) were evaluated and compared by EGFR mutational status.
Results
Sixty-seven patients (25.9%) developed BM. Synchronous BM occurred more often in patients with EGFR mutation type (MT) (n = 20, 27.4%) compared with EGFR wild type (WT) (n = 27, 14.5%, p < 0.009). The median BM-OS was significantly longer in patients with EGFR MT than in those with EGFR WT (25.7 months vs. 3.8 months, p < 0.001), and a similar trend was noticed for patients with synchronous BM (25.7 months for EGFR MT vs. 6.8 months for EGFR WT, p < 0.001). However, in patients with metachronous BM development, the difference in BM-OS between patients with EGFR MT (14.6 months) and EGFR WT (2.5 months) did not reach statistical significance (p = 0.230).
Conclusions
Synchronous BM was more common in NSCLC patients with EGFR MT than in those with EGFR WT. However, EGFR mutations were associated with significantly longer median BM-OS, especially when the brain was the first metastatic site.
Keywords: Receptor, epidermal growth factor; Carcinoma, non-small-cell lung; Brain metastases; Prognosis
INTRODUCTION
Brain metastases (BM) are a critical complication of non-small cell lung cancer (NSCLC), because this is the most common primary cancer leading to BM. The presence of BM in patients with NSCLC is associated with poor prognosis, with a median survival of only 7 months recorded for these patients, despite whole-brain radiation therapy [1].
NSCLC with epidermal growth factor receptor (EGFR) mutations comprises a distinct subgroup of the disease, associated with significant sensitivity to EGFR-tyrosine kinase inhibitors (TKIs) and improved overall and progression-free survival when treated with EGFR-TKIs [2-4]. Although BM occurs in a substantial number of NSCLC cases with EGFR mutations, the relationship between EGFR mutations and the risk of BM occurrence as well as the associated prognosis is not clear, and only retrospective studies in this regard are currently reported in the literature. In several of these retrospective studies, EGFR mutations have been found to be associated with a higher risk of BM development [5,6] as well as longer overall median survival from the time of BM diagnosis than those with EGFR wild type (WT) [6-11]. However, the results from these studies are inconsistent [12,13]. Although relatively few studies have examined the time from the diagnosis of NSCLC to BM development, the time was estimated from the initial diagnosis of NSCLC, irrespective of the stage, and not from the time of diagnosis of metastatic NSCLC.
The aim of this study was to investigate the incidence, timing, and median overall survival (OS) associated with BM in patients with metastatic NSCLC harboring EGFR mutations compared to those exhibiting WT EGFR.
METHODS
Patients
We retrospectively reviewed the medical records of patients from a single healthcare center who were diagnosed with NSCLC stage IV (according to the 7th American Joint Committee on Cancer [AJCC] Cancer Staging System) between January 2010 and August 2013 whose EGFR mutational status was known. Every patient underwent brain magnetic resonance imaging when first diagnosed with metastatic lung cancer, irrespective of the presence of symptoms of BM. During the course of the disease, brain imaging was performed only when BM were suspected. Data of clinical characteristics including sex, age, Eastern Cooperative Oncology Group performance status, history of smoking, the date of diagnosis of BM, symptoms of BM, treatment, and survival time were obtained from medical records or from the records of the national health insurance system. Patient observation continued through May 2015. This study was approved by the Institutional Review Board of Gachon University Gil Medical Center.
EGFR mutational analysis
Tumor DNA was acquired from paraffin-embedded cancer tissue and amplified by polymerase chain reaction (PCR). For the mutational analysis of the EGFR gene (exons 18-21) in the latter samples, direct sequencing was performed on samples collected in 2011 and 2012. In 2010, EGFR mutation analysis was not routine and was performed at the physician’s discretion. Since 2011, it has been performed in nearly every patient with metastatic NSCLC with sufficient tumor DNA. Prior to 2013, the Big Dye Terminator v 1.1 kit, together with an ABI 3130xl genetic analyzer (both from Applied Biosystems, Foster City, CA, USA), was used for bidirectional sequencing of the tumor DNA samples. Since 2013, the peptide nucleic acid (PNA)-mediated real-time PCR clamping method was used, involving the PNA Clamp TM EGFR Mutation Detection Kit (PANAGENE Inc., Daejeon, Korea).
Statistical analysis
Categorical variables were compared using a chi-square test. For the patients who did not have BM at initial diagnosis, time to brain metastases (TTBM) was calculated from the date of metastatic NSCLC diagnosis to the date of the first occurrence of BM. OS of a patient was defined as the time between diagnosis of metastatic NSCLC and death of the patient (from any cause) or last date of clinic visit. BM-OS, on the other hand, was calculated from the time of diagnosis of the first BM to the time of death of the patient (from any cause) or last date of clinic visit. Survival time was estimated by the Kaplan-Meier method and was compared with a log-rank test. Follow-up duration was estimated using the Kaplan-Meier estimate of potential follow-up [14].
RESULTS
Patient characteristics
We retrospectively identified 259 patients with metastatic NSCLC with known EGFR mutation status using available medical records. EGFR mutations were found in 73 patients. The most common EGFR mutations were exon 19 deletions (n = 41, 56.2%) and the exon 21 point mutation L858R (n = 23, 31.5%). Most patients had adenocarcinoma (n = 180, 70.0%). The clinical characteristics of these patients are listed in Table 1.
Table 1.
Patient characteristics
| Characteristic | Total (n = 259) | EGFR MT (n = 73) | EGFR WT (n = 186) | p value |
|---|---|---|---|---|
| Sex | ||||
| Male | 167 (64.5) | 27 (37.0) | 140 (75.3) | < 0.001 |
| Female | 92 (35.5) | 46 (63.0) | 46 (24.7) | |
| Age, yr | 68 (37–89) | 66 (37–87) | 68 (37–89) | 0.155 |
| Smoking history | < 0.001 | |||
| Never-smoker | 106 (40.9) | 48 (65.8) | 58 (31.2) | |
| Former smoker | 68 (26.3) | 15 (20.5) | 53 (28.5) | |
| Current smoker | 85 (32.8) | 10 (13.7) | 75 (40.3) | |
| Pathologic histology | < 0.001 | |||
| Adenocarcinoma | 180 (70.0) | 64 (87.7) | 116 (62.4) | |
| Squamous | 40 (15.4) | 2 (2.7) | 38 (20.4) | |
| Large cell | 27 (10.4) | 6 (8.2) | 21 (11.3) | |
| Other | 12 (4.6) | 1 (1.4) | 11 (5.9) | |
| Type of EGFR mutation | ||||
| Deletion in exon 19 | 41 (56.2) | |||
| L858R | 23 (31.5) | |||
| Others | 9 (12.3) | |||
| Brain metastases | 67 (25.9) | 27 (37.0) | 40 (21.5) | < 0.006 |
| Synchronous BM | 20 (27.4) | 27 (14.5) | < 0.009 | |
| Metachronous BM | 7 (9.6) | 13 (7.0) | < 0.578 |
Values are presented as number (%) or median (range).
EGFR, epidermal growth factor receptor; MT, mutation type; WT, wild type; BM, brain metastasis.
BM development and EGFR mutations
Sixty-seven patients were diagnosed with BM. The median estimated potential follow-up duration for those patients with BM was 41.4 months. During the disease course, 37.0% (n = 27) of patients with EGFR mutations and 21.5% (n = 40) of those with EGFR WT developed BM (p < 0.006). Among 27 BM patients with EGFR mutations, 25 patients had the L858R mutation or deletion in exon 19, while the remaining two patients had the G719X mutation in exon 18.
Among 67 patients with BM, the brain was the first site of metastasis in 47 patients (70.1%). Synchronous BM was significantly more common in patients with EGFR mutations (n = 20, 27.4% of all 73 patients with EGFR mutations) than in patients with EGFR WT (n = 27, 14.5% of 186 patients with EGFR WT, p < 0.009) (Table 1). The prevalence of metachronous BM, however, did not appear to differ according to EGFR mutational status. In patients with metachronous development of BM, TTBM did not differ significantly according to EGFR mutational status (median TTBM 13.4 months for EGFR mutations vs. 8.8 months for EGFR WT, p < 0.229) (Fig. 1).
Figure 1.
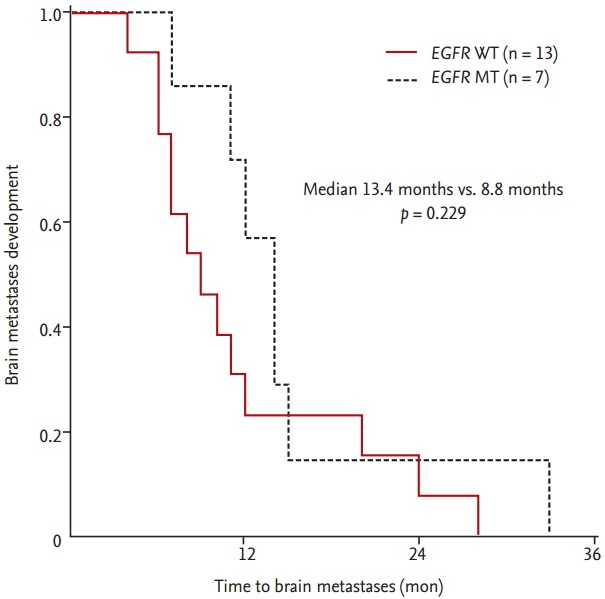
Time to brain metastases compared by epidermal growth factor receptor (EGFR) mutational status in 20 nonsmall cell lung cancer patients with metachronous brain metastases. EGFR mutation type (MT, dashed line) vs. EGFR wild type (WT, solid line).
The characteristics of BM by EGFR mutational status are displayed in Table 2. Patients with EGFR mutation type (MT) were more likely to belong to the female sex, and had better performance status compared to patients with EGFR WT. The presence of symptoms of BM, the number of BM lesions, and the treatment were not significantly different between EGFR MT and EGFR WT patients.
Table 2.
Characteristics of patients developed brain metastases
| Characteristic | Total (n = 67) | EGFR MT (n = 27) | EGFR WT (n = 40) | p value |
|---|---|---|---|---|
| Sex | 0.014 | |||
| Male | 35 (52.2) | 9 (33.3) | 26 (65.0) | |
| Female | 32 (47.8) | 18 (66.6) | 14 (35.0) | |
| Age, yr | 68 (41–85) | 64 (45–83) | 68 (41–85) | 0.335 |
| ECOG PS | 0.040 | |||
| 0–1 | 56 (83.6) | 26 (96.3) | 30 (75.0) | |
| 2–3 | 11 (16.4) | 1 (3.7) | 10 (25.0) | |
| Smoking history | 0.013 | |||
| Never-smoker | 31 (46.3) | 17 (63.0) | 14 (35.0) | |
| Former smoker | 16 (23.9) | 7 (25.9) | 9 (22.5) | |
| Current smoker | 20 (29.9) | 3 (11.1) | 17 (42.5) | |
| Pathologic histology | 0.085 | |||
| Adenocarcinoma | 47 (70.1) | 23 (85.2) | 24 (60.0) | |
| Squamous | 6 (9.0) | 0 | 6 (15.0) | |
| Large cell | 10 (14.9) | 3 (11.1) | 7 (17.5) | |
| Other | 4 (6.0) | 1 (3.7) | 3 (7.5) | |
| Symptoms of BM | 0.803 | |||
| Absent | 30 (44.8) | 13 (48.1) | 17 (42.5) | |
| Present | 37 (55.2) | 14 (51.9) | 23 (57.5) | |
| EGFR TKI treatment | ||||
| 1st line | 13 (48.1) | |||
| 2nd line | 11 (40.7) | |||
| 3rd line | 2 (7.4) | |||
| None | 1 (3.7) | |||
| Timing of BM | 0.599 | |||
| Synchronous | 47 (70.1) | 20 (74.1) | 27 (67.5) | |
| Metachronous | 20 (29.9) | 7 (25.9) | 13 (32.5) | |
| No. of lesions | 0.741 | |||
| Single | 19 (28.4) | 6 (22.2) | 13 (32.5) | |
| 2–4 | 11 (16.4) | 5 (18.5) | 6 (15.0) | |
| ≥ 5 | 37 (55.2) | 16 (59.3) | 21 (52.5) | |
| Local treatment of BM | 0.896 | |||
| WBRT | 37 (55.2) | 15 (55.6) | 22 (55.0) | |
| SRS | 11 (16.4) | 5 (18.5) | 6 (15.0) | |
| Surgery | 4 (6.0) | 2 (7.4) | 2 (5.0) | |
| None | 15 (22.4) | 5 (18.5) | 10 (25.0) |
Values are presented as number (%) or median (range).
EGFR, epidermal growth factor receptor; MT, mutation type; WT, wild type; ECOG PS, Eastern Cooperative Oncology Group Performance status; BM, brain metastasis; TKI, tyrosine kinase inhibitor; WBRT, whole brain radiotherapy; SRS, stereotactic radiosurgery.
Association between EGFR mutations and survival following BM
At the time of this analysis, 52 of the 67 patients (77.6%) with BM had died, 14 patients remained alive, and one patient was lost to follow-up. The median OS of all 67 BM patients was 13.7 months. OS did not differ significantly according to the timing of BM development (median OS 12.1 months for the synchronous BM group vs. 14.0 months for the metachronous BM group). However, the median BM-OS in those patients who displayed metachronous BM (n = 20) was only 2.5 months.
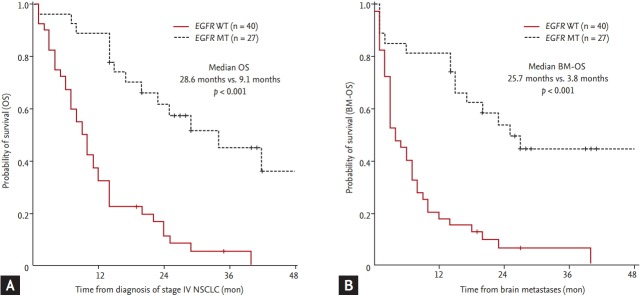
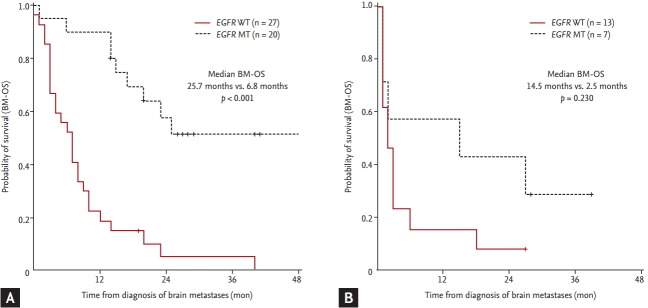
EGFR mutations were associated both with longer OS (median OS 28.6 months in EGFR MT vs. 9.1 months in EGFR WT, p < 0.001) (Fig. 2A) and longer BM-OS (median BM-OS 25.7 months in EGFR mutations vs. 3.8 months in EGFR WT, p < 0.001) (Fig. 2B). In patients with synchronous BM, the survival after BM development was longer in EGFR MT than in EGFR WT patients (median BM-OS 25.7 months vs. 6.8 months, respectively; p < 0.001) (Fig. 3A). In patients with metachronous BM, BM-OS in patients with EGFR mutations tended towards longer OS (median BM-OS 14.5 months for EGFR MT vs. 2.5 months for EGFR WT, p = 0.230) (Fig. 3B), but it did not reach statistical significance. Cause of death could be determined for 28 (53.8%) of the original cohort of NSCLC patients with BM. Most patients (n = 12) had systemic progression with stable BM at the time of death. Seven patients died of progression of BM at time of death (one patient with EGFR MT vs. six with EGFR WT).
Figure 2.
Investigating the relationship between epidermal growth factor receptor (EGFR) status and overall survival (OS) of non-small cell lung cancer (NSCLC) patients. OS of patients from the time of diagnosis of stage IV NSCLC (A) or diagnosis of brain metastases (BM-OS) (B) compared by EGFR mutational status. EGFR mutation type (MT, dashed line) vs. EGFR wild type (WT, solid line).
Figure 3.
Overall survival of non-small cell lung cancer patients according to timing of brain metastases. Overall survival from the time of diagnosis of brain metastases (BM-OS) by epidermal growth factor receptor (EGFR) mutational status among patients with synchronous (A) or metachronous (B) development of brain metastases. EGFR mutant type (MT, dashed line) vs. EGFR (WT, solid line).
DISCUSSION
The current study demonstrated that EGFR mutations in metastatic NSCLC patients were associated with a high likelihood of BM, and that such mutations were linked to higher median survival after BM development, especially in patients with synchronous BM development. On the other hand, in patients with metachronous BM, TTBM was not significantly different according to EGFR mutation status.
Most studies on BM and EGFR mutations, including ours, assess EGFR mutations from extracranial tumor tissue, which has been validated by Luo et al. [13]. They demonstrated a high concordance rate (93.3%) of EGFR mutational status between BM and extracranial tumor tissue.
In line with studies [5,6,10] that reported synchronous BM presence in 11% to 16% of stage I to IV patients, more commonly in patients with EGFR mutations, our study showed that EGFR mutations were associated with a higher incidence of BM. Synchronous BM was present in 18.1% of the patients with stage IV NSCLC and was more common in patients with EGFR mutations (27.4%) than in those with EGFR WT (14.5%). This increased risk of BM associated with EGFR mutations has also previously been observed in patients with NSCLC of earlier stages who underwent curative resection [5,6]. The reasons for the high propensity of BM in EGFR mutant NSCLC remain unclear. Plausible underlying mechanisms for the increase in BM include activation of EGFR or MET receptor tyrosine kinase-associated signaling pathways, as has previously been reported in both NSCLC and breast cancer. In particular, EGFR activation in breast cancer cells has been shown to be associated with a higher capability of migration and invasion to the brain [15], and Met protein activation has been demonstrated to be associated with a higher risk of BM in patients with NSCLC [16].
In agreement with our results, for patients with BM from primary NSCLC, EGFR mutations have been reported to be associated with better survival from the time of BM development (15 to 25 months) [6-10] than patients with WT EGFR (7 months) [1]. It is not clear whether the prolonged BM-OS in patients with EGFR mutations is due to the improved OS from better control of extracranial disease with EGFR-TKIs, due to the favorable role of EGFR-TKIs in treating BM itself, or due to differences in the biology and behavior between EGFR mutated and WT NSCLC cells. Regarding the efficacy of EGFR-TKIs on intracranial disease in NSCLC, there is a substantial intracranial response [17,18]; however, central nervous system (CNS) penetration of gefitinib or erlotinib is limited in pharmacokinetic studies [19,20]. The role of EGFR-TKIs in the prevention of BM progression is supported by an observation of a lower rate of CNS progression (hazard ratio, 0.56) in NSCLC patients with activating EGFR mutations treated with EGFR-TKIs compared with those treated with conventional chemotherapy [21]. It must be stated; however, that it is difficult to distinguish between the effects of systemic and local treatment in published retrospective studies.
Although EGFR mutations are associated with better survival in populations with BM, BM itself remains associated with lower survival compared to those without BM in the patient group with EGFR mutations [22]. Prognostic assessment is especially important in patients with BM in order to implement suitable treatment strategies. EGFR mutations have an additional prognostic impact independent of well-known prognostic indices such as the lung-specific graded prognostic assessment (GPA) index [8] or recursive partitioning analysis class [7]. Incorporation of EGFR mutational status into the prognostic estimation of BM from NSCLC should be considered in the future, in the same manner as for the molecular subtypes of breast cancer, which has been included in breast-specific GPA scoring criteria [1].
Interestingly, we found that the favorable effect of EGFR mutations on survival from BM diagnosis was lacking in NSCLC patients who developed metachronous BM, which was in agreement with previous findings by Shin et al. [5]. This phenomenon may be due to different mechanisms or drug sensitivities between synchronous and metachronous BM or it may be because diagnosis of metachronous BM usually accompanies CNS symptoms, while synchronous BMs are often asymptomatic. In contrast, overall risk of BM was higher in EGFR mutant NSCLC patients [5,6,10]. In those with metachronous BM, TTBM development seemed to be longer in EGFR mutant NSCLC than in EGFR WT [6,7], which was observed in this study too. In former studies [6,7], TTBM was calculated from the initial diagnosis of NSCLC stage I to IV, which was estimated more uniformly from the initial diagnosis of metastatic stage in this study. The opposite characteristics of EGFR MT, namely, a higher risk of synchronous BM yet a longer TTBM, may be due to delayed BM progression owing to EGFR-TKI treatment in EGFR mutant NSCLC [7,21].
In conclusion, we report that EGFR mutations in metastatic NSCLC were associated with a greater frequency of synchronous BM and with significantly longer survival from the time of BM diagnosis when compared with EGFR WT, the latter trend being more pronounced in those patients with synchronous versus metachronous BM. We therefore propose that EGFR mutational status should be considered when assessing possible treatment strategies for BM in patients with NSCLC.
KEY MESSAGE
1. Synchronous brain metastases were more common in metastatic non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations than in those with wild type EGFR.
2. EGFR mutations were associated with significantly longer overall survival from the time of brain metastases diagnosis in metastatic NSCLC patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 5.Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9:195–199. doi: 10.1097/JTO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 6.Stanic K, Zwitter M, Hitij NT, Kern I, Sadikov A, Cufer T. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol. 2014;48:173–183. doi: 10.2478/raon-2014-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichler AF, Kahle KT, Wang DL, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–1199. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DW, Shin DY, Kim JW, et al. Additional prognostic role of EGFR activating mutations in lung adenocarcinoma patients with brain metastasis: integrating with lung specific GPA score. Lung Cancer. 2014;86:363–368. doi: 10.1016/j.lungcan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Mak KS, Gainor JF, Niemierko A, et al. Significance of targeted therapy and genetic alterations in EGFR, ALK, or KRAS on survival in patients with non-small cell lung cancer treated with radiotherapy for brain metastases. Neuro Oncol. 2015;17:296–302. doi: 10.1093/neuonc/nou146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekine A, Satoh H, Iwasawa T, et al. Prognostic factors for brain metastases from non-small cell lung cancer with EGFR mutation: influence of stable extracranial disease and erlotinib therapy. Med Oncol. 2014;31:228. doi: 10.1007/s12032-014-0228-9. [DOI] [PubMed] [Google Scholar]
- 11.Lee HL, Chung TS, Ting LL, et al. EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in nonsmall cell lung cancer patients with brain metastases. Radiat Oncol. 2012;7:181. doi: 10.1186/1748-717X-7-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendriks LE, Smit EF, Vosse BA, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer. 2014;84:86–91. doi: 10.1016/j.lungcan.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Luo D, Ye X, Hu Z, et al. EGFR mutation status and its impact on survival of Chinese non-small cell lung cancer patients with brain metastases. Tumour Biol. 2014;35:2437–2444. doi: 10.1007/s13277-013-1323-9. [DOI] [PubMed] [Google Scholar]
- 14.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 15.Nie F, Yang J, Wen S, et al. Involvement of epidermal growth factor receptor overexpression in the promotion of breast cancer brain metastasis. Cancer. 2012;118:5198–5209. doi: 10.1002/cncr.27553. [DOI] [PubMed] [Google Scholar]
- 16.Benedettini E, Sholl LM, Peyton M, et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am J Pathol. 2010;177:415–423. doi: 10.2353/ajpath.2010.090863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceresoli GL, Cappuzzo F, Gregorc V, Bartolini S, Crino L, Villa E. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol. 2004;15:1042–1047. doi: 10.1093/annonc/mdh276. [DOI] [PubMed] [Google Scholar]
- 18.Porta R, Sanchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 19.Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non-small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24:4517–4520. doi: 10.1200/JCO.2006.06.6126. [DOI] [PubMed] [Google Scholar]
- 21.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–4414. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noronha V, Joshi A, Gokarn A, et al. The importance of brain metastasis in EGFR mutation positive NSCLC patients. Chemother Res Pract. 2014;2014:856156. doi: 10.1155/2014/856156. [DOI] [PMC free article] [PubMed] [Google Scholar]