Abstract
The lack of technology for direct global-scale targeting of the adult mouse nervous system has hindered research on brain processing and dysfunctions. Currently, gene transfer is normally achieved by intraparenchymal viral injections, but these injections target a restricted brain area. Herein, we demonstrated that intravenous delivery of adeno-associated virus (AAV)-PHP.B viral particles permeated and diffused throughout the neural parenchyma, targeting both the central and the peripheral nervous system in a global pattern. We then established multiple procedures of viral transduction to control gene expression or inactivate gene function exclusively in the adult nervous system and assessed the underlying behavioral effects. Building on these results, we established an effective gene therapy strategy to counteract the widespread accumulation of α-synuclein deposits throughout the forebrain in a mouse model of synucleinopathy. Transduction of A53T-SCNA transgenic mice with AAV-PHP.B-GBA1 restored physiological levels of the enzyme, reduced α-synuclein pathology, and produced significant behavioral recovery. Finally, we provided evidence that AAV-PHP.B brain penetration does not lead to evident dysfunctions in blood-brain barrier integrity or permeability. Altogether, the AAV-PHP.B viral platform enables non-invasive, widespread, and long-lasting global neural expression of therapeutic genes, such as GBA1, providing an invaluable approach to treat neurodegenerative diseases with diffuse brain pathology such as synucleinopathies.
Keywords: gene therapy, AAV, synucleinopathy, DREADD receptors, Parkinson’s disease, neurodegenerative diseases, tuberous sclerosis, Tsc1
Graphical Abstract

Extensive gene delivery in the CNS is attainable through a single systemic injection of AAV-PHP.B. The authors exploited this system to simultaneously target different brain areas and modulate their functions but also to unveil its therapeutic potential. Global transduction of a therapeutic gene reversed pathological symptoms in a model of synucleinopathy.
Introduction
Genetic modification of adult brain neurons is an indispensable tool to determine the detailed anatomy and function of defined neuronal circuitries. However, genetic engineering in mice is a laborious and time-consuming technology, and it has grown increasingly challenging with the elevated complexity required for the dynamic assessment of gene function in time and space. Virus-mediated gene transfer has become a fundamental strategy for gene modification in the nervous system. In particular, recombinant adeno-associated viruses (AAVs) are commonly used as gene transfer vehicles in the brain due to their broad range of infectivity, high safety profile, and relatively rapid diffusion.1, 2, 3 However, intraparenchymal injection of AAVs supports robust but relatively localized transduction in the brain tissue.4 Thus, while this approach is meaningful for assessing the function of small neuronal clusters, its application to wider neuronal circuitries or large neural areas up to the entire brain remains unfeasible. More widespread CNS transduction has been achieved through intravenous (i.v.) delivery of AAVs able to cross the blood-brain barrier (BBB).5, 6, 7 Intravenous infusions represent an ideal non-invasive delivery route for viral agents that feature brain tropism.8 Among all the AAV serotypes, AAV9 has shown the greatest ability to permeate the BBB after peripheral vascular administration. In fact, i.v. administration of AAV9 in neonatal mice resulted in extensive and diffuse transduction in the CNS both in neurons and in astrocytes.5, 6, 7 However, the same approach results in a lower transduction efficiency by the AAV9 in adult mice, with increased targeting of the glial cells with respect to the neuronal fraction.5, 7, 9 Thus, recent research has focused on generating novel AAV9 variants through selected mutagenesis of the capsid proteins to obtain more efficient gene transfer in the brain after viral peripheral delivery. In particular, Deverman and colleagues10 conceived a Cre-recombination-based AAV targeted evolution strategy (CREATE) to isolate a novel engineered AAV9 capsid, named PHP.B, with the 7-amino-acid insertion TLAVPFK in the VP1 capsid protein. AAV-PHP.B was shown to outperform the standard AAV9 in transducing neurons after i.v. administration in adult mice. Considering these properties, we sought to use AAV-PHP.B as a common platform for building a set of molecular tools for the straightforward genetic manipulation of the neonatal and adult mouse nervous system on a global scale to interrogate gene function and modulate neuronal activity within the entire nervous system. In addition, this system offers an unprecedented opportunity for treating diseases that globally affect the nervous system. In particular, widespread accumulation of alpha-synuclein (α-syn) protein aggregates in Lewy bodies is a key neuropathological hallmark of Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA), leading to complex and heterogeneous symptomatic manifestations. GBA1 encodes the enzyme lysosomal glucocerebrosidase (GCase), and heterozygous mutations of the gene are the most common genetic risk factor for PD and DLB.11, 12 Mounting evidence suggests that GCase activity impairment is affecting lysosomal activity and overall autophagic flux negatively affecting α-syn aggregates degradation and catabolism.13, 14, 15 Intriguingly, focal expression of exogenous GCase is sufficient to limit α-syn protein inclusions and partially prevent dopaminergic neuronal cell death.16, 17 Nonetheless, an exclusively global approach targeting large brain areas might be considered for therapeutic exploitation, given the pervasive distribution of α-syn deposits throughout the brain. Herein, we showed that AAV-PHP.B-mediated GBA1 overexpression enabled a robust and long-lasting reduction of α-syn inclusions in the whole forebrain accompanied by a significant recovery in lifespan and cognitive performance.
Results
Global-Scale Neural Transduction and Single Neuronal Cell Labeling by AAV-PHP.B i.v. Delivery in Neonatal and Adult Mice
An AAV2 transfer plasmid was used to clone the GFP cDNA downstream of a constitutive chicken β-actin (CBA) promoter and combined with the PHP.B rep/cap and helper plasmids for productive viral infection. Viral particles were then harvested from both cells and supernatants, separately concentrated, and finally mixed together in order to obtain high-titer viral preparations. A dose of 2 × 1012 viral genomes (vg) AAV-PHP.B-GFP was administered by tail vein injection into 8-week-old mice (Figure 1A). Transduction efficiency was evaluated between 3 and 5 weeks post-injection by assessing GFP expression in various organs. As previously reported,10 GFP signal was widely detected in all CNS regions, with diffuse and robust staining in the forebrain, midbrain, and cerebellum and along the entire spinal cord axis (Figures 1B–1E and S1). Co-labeling for regional neuronal markers and GFP expression revealed that a very significant fraction of neurons, generally higher than 65%, was targeted by AAV-PHP.B in these regions (Figures 1F–1J). Interestingly, beyond the CNS, robust GFP expression was detected in the dorsal root (DRG) and sympathetic (SG) ganglia as well (Figures 1K–1M). In fact, the majority of beta-III-tubulin+ DRG and tyrosine hydroxylase (TH)+ thoracic SG neurons were effectively transduced by the virus (Figures 1K–1M and S2). Therefore, a single i.v. administration of AAV-PHP.B-GFP is sufficient for global and robust transduction of the adult mouse CNS and provides new evidence of efficient tropism for peripheral nervous system (PNS) structures as well. Furthermore, we assessed the AAV-PHP.B viral distribution after i.v. injection in neonatal mice. Interestingly, 3 weeks after viral administration, global targeting of the nervous system was confirmed, with a pattern similar but not identical to that obtained in adult mice (Figure S3). In fact, the GFP signal was particularly strong in selected glial populations in the cerebral cortex, hippocampus, and striatum (arrows in Figure S3). Although neuronal transduction was generally very efficient, especially in the cortex, hippocampus, cerebellum, and spinal cord, it was very limited in the substantia nigra, revealing notable differences with respect to the transduction pattern obtained through i.v. delivery in adult animals (Figure S3). Thus, at the perinatal stage, although administered by a comparable systemic delivery route, the AAV-PHP.B had a close but distinct tropism for glial and neuronal cells, probably as a result of some phenotypic differences between neonatal and adult cells likely associated with their maturation state.
Figure 1.
Global GFP Expression in the Central and Peripheral Nervous System with a Single AAV-PHP.B Intravenous Injection
(A) Schematic view depicting the transgenic cassette integrated in the AAV-PHP.B vector and injection of the viral particles into the tail vein of an adult mouse. (B) GFP immunofluorescence in a brain transduced with an empty AAV-PHP.B (negative control). (C–E) GFP localization on coronal hemi-sections at different rostro-caudal levels of the AAV-PHP.B-GFP-transduced brain, including the anterior (C) and posterior forebrain (D) and the cerebellum (E). (F–H) High-magnification images of the cerebral cortex (F), substantia nigra (G), and cerebellum (H) showing the double staining for GFP and the neuronal marker NeuN, TH, and Calb1, respectively. (I) Bar graph showing the fraction of cells positive for a specific neuronal marker (NeuN, TH, and Calb1) and expressing the viral GFP transgene. (J) GFP staining on a coronal section of the AAV-PHP.B-GFP-transduced spinal cord. (K and L) Section of thoracic dorsal root (DRG) or sympathetic (SG) ganglion co-stained for GFP and beta-III-tubulin (K) or TH (L), respectively. (M) Bar graph showing the fraction of cells positive for a specific neuronal marker (Hb9, beta-III-tubulin, and TH) and expressing the viral GFP transgene. (n = 12 mice for B–J; n = 4 mice for K–M). Scale bars are 500 μm (B–E and J), 100 μm (G and H), and 50 μm (K and L).
Sparse and selective labeling of distinct neuronal populations is a prerequisite for accurate tracing of nerve projections. To facilitate morphological analysis, we infused the AAV-PHP.B-Cre at different doses in adult Ai9 reporter mice18 and evaluated the extent of transduction in the brain parenchyma (Figure S4A). Interestingly, a low viral dose enabled sparse to single-cell labeling in the brain tissue as detected by tdTomato immunofluorescence imaging (Figures S4B–S4K). Given the whole-brain targeting profile of this virus, single-cell labeling was simultaneously obtained in different brain areas including the cortex, hippocampus, and cerebellum (Figures S4E, S4H, and S4K). These results demonstrate that a single administration of this virus at a low titer enables single-neuron visualization in multiple brain regions simultaneously.
Facile AAV-PHP.B-Based Cre-loxP Conditional Gene Activation and Control of Neuronal Activity in Selected Neuronal Subtypes throughout the Brain
Global targeting of the mouse nervous system by AAV-PHP.B has considerable implications, but it might represent a drawback when the aim is to study more specific neuronal targets or circuits. Thus, we conceived the idea to combine the widespread targeting of AAV-PHP.B with Cre-loxP technology in order to target specific neuronal subtypes in large brain areas (Figure 2A). Initially, we confirmed that systemic transduction of Cre-expressing AAV-PHP.B into Ai9 mice carrying a fluorescent tdTomato protein downstream of a loxP-flanked STOP cassette triggered very efficient Cre excision of the cassette and subsequent expression of the reporter throughout the brain (Figure 2B). Next, we generated an AAV-PHP.B carrying GFP in a FLEX switch cassette whose expression is activated by Cre recombinase. Thus, AAV-PHP.B-FLEX-GFP was infused in different transgenic mouse strains expressing Cre in specific neural subtypes (Figure 2A). Interestingly, this configuration enabled very selective expression of the GFP transgene according to the Cre expression pattern for each transgenic line. In infused NeuroD6-Cre mice, GFP expression was specifically confined to neurons in the cortex and hippocampus (Figures 2C and 2D and data not shown). Co-staining with NeuN and GFP revealed that more than 80% of all the neurons in the aforementioned territories expressed GFP (Figures 2E and 2F). Conversely, neither S100+ nor GFAP+ glial cells were found to co-express GFP (data not shown). Likewise, systemic viral transduction of parvalbumin (PV)-Cre and dopamine transporter (DAT)-Cre transgenic mice led to a very specific pattern of GFP expression restricted to the forebrain GABAergic interneurons or the midbrain dopaminergic neurons, respectively (Figures 2G–2O). In both cases, GFP expression was detected in the majority of these neuronal cell populations and not in other neuronal or glial cell types (Figures 2J and 2O). However, a similar strategy could be equally employed to selectively target glial cells. In fact, i.v. viral transduction of Olig2-Cre mice enabled specific GFP labeling of CC1+ oligodendrocytes but not GFAP+ astrocytes (Figures 2P–2R). Although the overall viral targeting was not as efficient as for neurons, approximately 60% of the oligodendrocyte lineage was targeted (Figure 2S).
Figure 2.
AAV-PHP.B Transduction Associated with Cre-loxP Technology Enables the Labeling of a Specific Neural Subpopulation throughout the Brain
(A) Schematic view depicting the GFP FLEX cassette integrated in the AAV-PHP.B vector and injection of the viral particles into the tail vein of Cre-expressing transgenic mouse strains. (B) tdTomato staining on a forebrain coronal section of an AAV-PHP.B-Cre-transduced Ai9 tdTomato reporter strain. The highly diffuse activation of the reporter reflects the highly efficient Cre-mediated recombination occurred after viral transduction (positive control). (C and D) GFP localization in the transduced NeuroD6-Cre forebrain (C) and cortical tissue (D). (E) Co-labeling of GFP and NeuN in NeuroD6-Cre-transduced cortical tissue. (F) Bar graph showing the percentage of cortical GFP+ cells on total NeuN+ cells. (G–I) Single staining for GFP (G) and parvalbumin (PV) (H) and relative co-labeling (I) in the PV-Cre transduced cortical tissue. (J) Bar graph depicting the fraction of GFP+ neurons on total PV-expressing neurons in the cortex. (K–N) Single staining for GFP (K) and TH (L) and relative co-labeling (M and N) on infected DAT-Cre ventral midbrain tissue. (O) Quantification of the percentage of GFP-expressing cells within the TH cellular fraction. (P) GFP immunofluorescence on infected Olig2-Cre cortical tissue. (Q and R) GFP-transduced cells co-express the oligoglial CC1 (Q) but not astrocytic GFAP (R) marker, identifying them as oligodendrocytes. (S) Bar graph quantifying the percentage of transduced GFP cells within the CC1-expressing cellular fraction. (n = 3 mice for each Cre transgenic line). Scale bars are 200 μm (B and C), 200 μm (K–M), 100 μm (D), 50 μm (G–I, N, and P), 30 μm (E), and 20 μm (Q and R).
Thus, combining Cre-loxP cell lineage specificity with spatially broad AAV-PHP.B transduction enabled the targeting of a specific neural subtype in wide regions up to the whole brain. This is a favorable setting for evaluating the function of a specific neuronal cell type within the brain and its resulting effects in live animals. Current optogenetic methods evaluate the effects of altering a specific neuronal circuit, but only in a confined brain territory limited by the intraparenchymal spreading of conventional viruses.19 To move beyond this, we conceived the idea to combine the AAV-PHP.B/Cre-loxP system with the chemogenetic DREADD technology to obtain global modulation of the neuronal activity.20 Moreover, the DREADD receptors are activated by the brain-penetrant small-molecule clozapine N-oxide (CNO), which additionally provides fine temporal control of this system. Thus, as a proof of concept, we sought to test the effects of altering PV+ neuronal function in the whole brain. Although PV has some expression in subcortical regions, especially in cerebellar Purkinje neurons, its major expression selectively localizes to the forebrain fast-spiking inhibitory interneurons. Therefore, we transduced adult PV-Cre transgenic mice with an AAV-PHP.B-FLEX-DREADDM4-mCherry (PHP.B-FLEX-M4C) to inhibit activity exclusively in the PV-expressing neurons (Figure 3A). Double immunofluorescence for the viral mCherry reporter and PV showed a common pattern of staining in the somatosensory cortex (sCx) (Figures 3B and 3C). Quantitative analysis confirmed that the mCherry reporter was restricted exclusively to the PV+ interneurons (Figure 3E). Conversely, a small fraction of PV+ cells did not express mCherry, probably because they were not transduced by the virus (Figure 3E). To determine whether the system was functional, we acutely sliced brains from transduced mice and the recorded the electrical activity of mCherry+ neurons during patch-clamp experiments. Soon after CNO was added to the slice extracellular medium, recorded neurons silenced their activity with an abrupt loss of action potentials and membrane potential hyperpolarization (Figure 3F). Next, PHP.B-FLEX-M4C-transduced PV-Cre mice were implanted with epidural electrodes and electroencephalogram (EEG) recordings performed before and after CNO injection. Interestingly, after CNO, the EEG showed slowed background activity and mild epileptic abnormalities (sharp waves), with no clear epileptic behavior in mice recorded for 12 hr (n = 3) (Figure 3G). We then hypothesized that loss of PV forebrain interneuron activity could lead to increased seizure susceptibility. Accordingly, kainic acid-induced seizure activity was strongly enhanced in PHP.B-FLEX-M4C, leading to animal death immediately after treatment (5 of 5), while the majority of control mice treated with PHP.B-GFP survived the same treatment (4 of 5) (Figure 3H). These findings exemplify a strategy, sophisticated yet extremely easy to implement, by which to control neuronal activity in the whole brain and determine its underlying behavioral consequences in live animals.
Figure 3.
AAV-PHP.B-Mediated Targeting of the DREADD M4 Chemogenetic Inhibitory Receptor in PV+ Cortical Interneurons Sensitizes the Mice to Proepileptic Insults
(A) Schematic view depicting the chemogenetic DREADD M4 inhibitory receptor fused to mCherry cloned in a FLEX cassette and integrated in the AAV-PHP.B vector and injection of the viral particles into the tail vein of a PV-Cre adult mouse. (B and C) Parvalbumin (PV) (B) and mCherry (C) immunofluorescence on transduced forebrain coronal sections. (D) High-magnification images of cortical tissue co-stained for PV and mCherry. (E) Bar graph depicting the relative fractions of PV and mCherry++ cells (n = 4 PV-Cre mice). (F) Electrophysiological recordings on transduced PV-Cre brain slices showing that CNO perfusion strongly inhibits the membrane excitability of mCherry+ neurons (n = 6). (G) Representative EEG traces of 12-hr recordings after CNO injection into transduced PV-Cre mice (3 recordings in 4 mice). (H) Bar graph showing the number of mice succumbed after treatment with kainic acid (KA) between the two animal groups treated either with the GFP- or the DM4C-expressing viruses. Scale bars are 500 μm (B and C) and 100 μm (D).
Rapid Analysis of Tsc1 Gene Function in Adulthood by Systemic Injection of Cre-Expressing AAV-PHP.B
Global nervous system targeting by AAV-PHP.B might also be convenient to regulate gene activity in transgenic mice carrying floxed gene alleles. In fact, deleting genes with Cre-loxP technology to study their effects exclusively in adulthood requires a rather extended time to obtain the mutant mice for phenotypic analysis. Thus, we generated a constitutively Cre-expressing AAV-PHP.B and systemically injected it in adult mice carrying a floxed allele for Tsc1 (Figure 4A). Mutations in TSC1 in humans are responsible for tuberous sclerosis complex (TSC), a disorder characterized by severe intellectual disability and intractable seizures.21, 22 Inactivation of TSC1 or its homolog TSC2, with which it forms a multimeric complex, causes hyperactivation of mTOR complex 1 (mTORC1) and hyperphosphorylation of its downstream effectors including ribosomal protein S6.23, 24 TSC patients present with focal brain lesions, known as cortical tubers and subependymal nodules, characterized by general cellular disorganization and giant cells. It is believed that these structural brain alterations are the primary cause of the chronic epileptogenic state.25 Homozygous Tsc1 or Tsc2 mutant mice recapitulate the pathological milestones described in patients.26 In fact, Tsc1/2 gene deletion causes overt brain pathology associated with severe epileptic crises and consequent death soon after birth.26 Whether epilepsy is a result of the cortical tissue disorganization occurring during development or, conversely, is caused by a cell-autonomous dysfunction in the mutated neurons has remained controversial. Recently, full-body acute Tsc1 inactivation in adulthood by classical mouse transgenic breeding was found to lead to profound epileptic seizures in the absence of neurodevelopmental brain lesions.27 To extend this analysis, we infused AAV-PHP.B-Cre at a high dose (2 × 1012 vg) in adult Tsc1f/f and Ai9 reporter mice. Starting 1 week after systemic viral injection, mice developed severe epileptic seizures, with a minimum of 6 crises detected in a 12-hr continuous EEG recording (Figures 4B and 4C). About half of these animals died in the following 4 weeks (4 of 9). A similar dose of AAV-PHP.B-Cre robustly activated tdTomato in the brains of the Ai9 mice (Figure 4D). To assess mTOR activation at the cellular level, we performed immunohistochemistry for phospho-S6 (pS6). As expected, pS6 staining was strongly increased in transduced floxed Tsc1 brains but not in Ai9 control brains, with most of the neurons in the cerebral cortex and hippocampus presenting a strong positive cytoplasmatic signal (Figures 4E–4H). We then asked whether loss of Tsc1 in only a fraction of neurons would be sufficient to cause a disease state. Thus, we injected AAV-PHP.B-Cre at a low dose (1011 vg) in Tsc1f/f mice. This dose of virus transfused in the brain of Ai9 conditional mice activated the tdTomato reporter in approximately 35% of cells in the cerebral cortex (Figure 4K). Nonetheless, even with this dose of virus, all the animals developed severe seizures starting 3 weeks after treatment, although the number of crises was reduced to an average of 1–2 events in 12 hr (Figures 4I and 4J). Remarkably, strong pS6 staining was detectable only in a mosaic fashion in the cortex and hippocampus, accounting for only a 25% of neurons, in treated Tsc1 mice but not in Ai9 control mice (Figures 4K–4O). These results demonstrate that the Tsc1 gene has an indispensable cell-autonomous role in adult neurons and that its loss triggers severe epileptogenesis in mice, even when only a fraction of neurons carry mutant alleles for this gene.
Figure 4.
Complete or Partial Loss of Tsc1 in the Adult Brain Mediated by the Cre-Expressing AAV-PHP.B Leads to Severe Epileptic Seizures
(A) Experimental setup for the tail vein injection of the virus AAV-PHP.B-Cre in Tsc1flox/flox mice. (B) Representative traces of EEG recordings in baseline state (above) and during seizure (bottom) in treated Tsc1flox/flox mice injected with a viral dose of 2 × 1012 vg. (C) Quantification of epileptic events in 12 hr (3 recordings in 3 mice). (D and E) tdTomato direct signal (D) and pS6 immunofluorescence (E) on cortical tissue of transduced Ai9 tdTomato reporter mice. (F and G) Images at low (F) and high (G) magnification of transduced TSC1flox/flox cortical tissue stained for pS6. (H) Quantification of tdTomato- and pS6+ cells on the NeuN neuronal fraction in transduced Ai9 or TSC1flox/flox mice, respectively (n = 3 mice). (I) Representative traces of EEG recordings in baseline state (above) and during seizure (bottom) in treated Tsc1flox/flox mice injected with a viral dose of 5 × 1010 vg. (J) Quantification of epileptic events in 12 hr (3 recordings in 2 mice). (K and L) tdTomato direct signal (K) and pS6 immunofluorescence (L) on cortical tissue of transduced Ai9 tdTomato reporter mice. (M and N) Images at low (M) and high (N) magnifications of transduced TSC1flox/flox cortical tissue stained for pS6. (O) Quantification of tdTomato− and pS6+ cells on the NeuN neuronal fraction in transduced Ai9 or TSC1flox/flox mice, respectively (n = 3 mice). Scale bars are 100 μm (D–F and K–M) and 50 μm (G and N). LE, left emisphere; RE, right emisphere.
Whole-Brain GBA1 Gene Transfer Significantly Prevents α-Syn Inclusion Formation in A53T-SCNA Transgenic Mice
The brain-penetrating AAV-PHP.B is an unprecedented platform to exploit gene therapy protocols to treat neurodegenerative disorders affecting the whole nervous system. In particular, this system can be explored to sustain diffuse expression of GBA1 in the brain to potentially counteract the gradual widespread accumulation of α-syn inclusions in the nervous system. To test this hypothesis, we employed A53T-SCNA transgenic mice that overexpress the SCNA mutation responsible for a genetic form of PD in humans. Starting from 6 months of age, these mice gradually accumulate insoluble α-syn deposits throughout the brain, with particular enrichment within the cerebral cortex, the midbrain, and the pons; at 10–12 months of age, most of them die after developing a severe and rapid loss of voluntary movements and fatal paresis.28 We focused particularly on the somatosensory and visual (vCx) cortical areas, where α-syn aggregates were particular evident and diffuse (Figures 5A and 5B, arrowheads), resembling the α-syn toxicity in the cerebral cortex of PD patients, which leads to cognitive disabilities and dementia. Initially, we asked whether the overexpressed GCase enzyme encoded by the GBA1 transgene could be properly targeted to the lysosome and acquire functionality. Thus, GBA1 was tagged with mCherry, a fluorescence tag, which maintains its activity in the acidic lysosomal environment, and transfected into HeLa cells. Co-staining for mCherry and Lamp2 revealed that the exogenous GCase protein was at least in part correctly localized in the lysosomes (Figure S5A). To determine whether the expressed GCase was functional, we assessed the overall enzymatic activity using a quantitative assay with a specific synthetic substrate. GBA1-overexpressing cells exhibited a significant increase in GCase catalytic activity compared with untransfected cells, demonstrating the complete functional maturation of the exogenous GCase (Figures S5B and S5C). Hence, we cloned the GBA1 cDNA upstream of a P2A-GFP cassette driven by the EF1α promoter in a shuttle vector and used it to generate AAV-PHP.B viral particles. Then, 5-month-old A53T-SCNA transgenic mice were infused with either the GFP- (control) or the GBA1-P2A-GFP-expressing virus. A group of animals was subsequently euthanized at 10 months of age, when control mice started to perish, and brain tissue was isolated for molecular and neuropathological inspection. Since GBA1 antibodies failed to give reliable immunohistochemical staining, we investigated the global pattern of brain transduction in A53T-SCNA mice by GFP reporter analysis. As shown in Figures 5D–5G, GBA1-P2A-GFP (hereinafter referred to as GBA1 only) gene transfer was efficient and diffuse in all the forebrain regions, infecting both neurons and glia. Accordingly, the immunoblotting profiling of cortical and hippocampal tissues from GBA1-transduced animals confirmed a robust increase in the overall amount of GCase protein (Figures 5H and 5I). We then evaluated the levels of GCase activity in control and treated animals. Interestingly, GCase enzymatic activity was strongly reduced in control A53T-SCNA transgenic mice in most of the neural regions tested, in line with previous data suggesting that α-syn pathology affects GCase protein processing and targeting to lysosomes.14 Conversely, GBA1-transduced animals exhibited a strong rescuing of GCase enzymatic levels, which were at least comparable to those detected in wild-type animals in all the CNS regions (Figure 5J). Then, α-syn pathology was specifically assessed by immunostaining for pS129-α-syn in proteinase K (PK)-treated brain sections to enable the accurate identification of insoluble intracellular α-syn deposits. In the visual cortex, α-syn aggregates were mainly detected within the somata of neurons, as revealed by both immunohistochemistry and immunofluorescence imaging (Figures 6A–6D). Remarkably, GBA1 gene transfer elicited a strong reduction of α-syn pathology in the visual cortical areas (Figures 6A–6E). To extend this analysis, we performed stereological semi-automatic counting of α-syn inclusions within the visual (anteroposterior position from −3 mm to −4 mm, centered on bregma), cingulate (cCx), motor (mCx), and somatosensory cortical areas as well as the striatum (anteroposterior position from +1 to −0.5 mm, centered on bregma). Accordingly, the overall quantity of PK-resistant deposits was significantly diminished in all these brain domains to a comparable extent, confirming efficacious and widespread protection from α-syn pathology (Figure 6E). To confirm the effects of the exogenous GCase activity on α-syn protein processing, the various forms of α-syn were resolved and analyzed by western blotting of Tris-buffered saline (TBS)-soluble and TBS-insoluble fractions of forebrain lysates (Figure 6F). Indeed, a significant decrease in both monomeric and oligomeric forms of α-syn (including low- [LMW] and high-molecular-weight [HMW] aggregates) was observed in GBA1- compared with GFP-transduced tissues (Figure 6F).
Figure 5.
AAV-PHP.B Intravenous Delivery Enables a Global Stimulation of GCase Activity in Adult A53T-SCNA Mice
(A–C) Immunostaining for phospho-S129-α-syn shows the diffuse accumulation of PK-resistant α-syn deposits in the somatosensory cortex (SCx) of 8-month-old A53T-SCNA transgenic mice mainly localized in the neuronal soma (arrowheads in B and arrows in C). (D–G) Representative pictures showing the amount and distribution of GFP-transduced brain cells in the cortex (D), dentate gyrus (E), striatum (F), and thalamus (G) in 10-month-old A53T-SCNA mice infused with the AAV-PHP.B-GBA1-P2A-GFP virus. (H) Immunoblotting analysis showing the total amount of GCase protein in cortical and hippocampal tissues of wild-type (WT) and A53T-SCNA transgenic mice treated with the GFP- or GBA1-expressing AAV-PHP.B. (I) Bar graph illustrating the quantification of the GCase the immunoblotting signal (n = 3, A53T-SCNA plus GFP; n = 3, A53T-SCNA plus GBA1; n = 3, WT tested at 3 months after infection; p < 0.05). (J) Direct quantification of total GCase catalytic activity showing a significant recovery of the enzymatic activity in spinal cord (Sc), cerebral cortex (Sc), hippocampus (Hp), striatum (Str), midbrain (Mb), and cerebellum (Cb) of the treated A53T-SCNA transgenic mice (yellow bars). The GCase-selective inhibitor conduritol-B-epoxide (CBE) was included to evaluate the specificity of the reaction (n = 3, WT; n = 3, A53T-SCNA plus GFP; n = 3, A53T-SCNA plus GBA1 tested at 3 months after infection). Data are expressed as means ± SEM and were analyzed with the unpaired Student’s t test (*p < 0.05, **p < 0.01, ***p < 0.001). Scale bars are 100 μm (B–G) and 10 μm (C).
Figure 6.
Global Brain GCase Gene Transfer Ensures a Diffuse Protection from α-Syn Deposits throughout All the Forebrain Regions in Adult A53T-SCNA Mice
(A–D) Immunohistochemistry (A and B) and immunofluorescence (C and D) analysis for pS129-α-syn on PK-treated visual cortical tissue from 10-month-old A53T-SCNA transgenic mice treated with GFP (control) or GCase expressing AAV-PHP.B. Insets in (A) and (B) are high-power enlargements showing the α-syn deposits concentrated in the cytoplasm of the cortical neurons (n = 6, A53T-SCNA plus GFP; n = 5, A53T-SCNA plus GBA1). (E) Total number of insoluble α-syn inclusions in different forebrain regions as quantified by semi-automatic stereology counting in selected forebrain areas. Counting was automatically performed in a selected patterning within the brain tissue as highlighted in the drawing. (F) Immunoblotting with TBS- and SDS-soluble tissue lysates from GFP- and GBA1-transduced brains detecting the monomeric (m) and high- (HMW) and low-molecular weight (LMW) α-syn aggregates. Asterisks indicate an unspecific band. Quantitative analysis showed a significant reduction of α-syn monomeric and aggregated species protein after GCase treatment (n = 3, A53T-SCNA plus GFP; n = 3, A53T-SCNA). Data are expressed as means ± SEM and were analyzed with the unpaired Student’s t test (*p < 0.05, **p < 0.01, ***p < 0.001). Scale bars are 50 μm (A–D) and 10 μm (insets in A and B). cCx, cingulate cortex; mCx, motor cortex; sCx, somatosensory cortex; vCx, ventral cortex.
Next, we wondered whether the acute reduction of α-syn pathology in adulthood correlated with any behavioral amelioration. GBA1-transduced animals showed a consistent increase in median survival compared with control treated mice, with a consistent fraction of animals surviving when all control mice had expired (Figure S6A). In addition, GBA1-treated but not control mice exhibited a strong recovery in learning and cognitive performance, as revealed by a significant improvement in the novel object recognition test both at 3 and 5 months after treatment (Figure S6B). Overall, GBA1-transduced mice showed a robust reduction of α-syn pathology in the whole forebrain, suggesting that the exogenous GCase provided sufficient supplemental activity to limit and counteract the widespread development and accumulation of α-syn deposits. Hence, these data strongly indicate that AAV-PHP.B-mediated gene transfer in the adult brain is an outstanding system to express a therapeutic gene throughout the brain tissue in order to curb pervasive pathological manifestations often associated with the progression of neurodegenerative diseases.
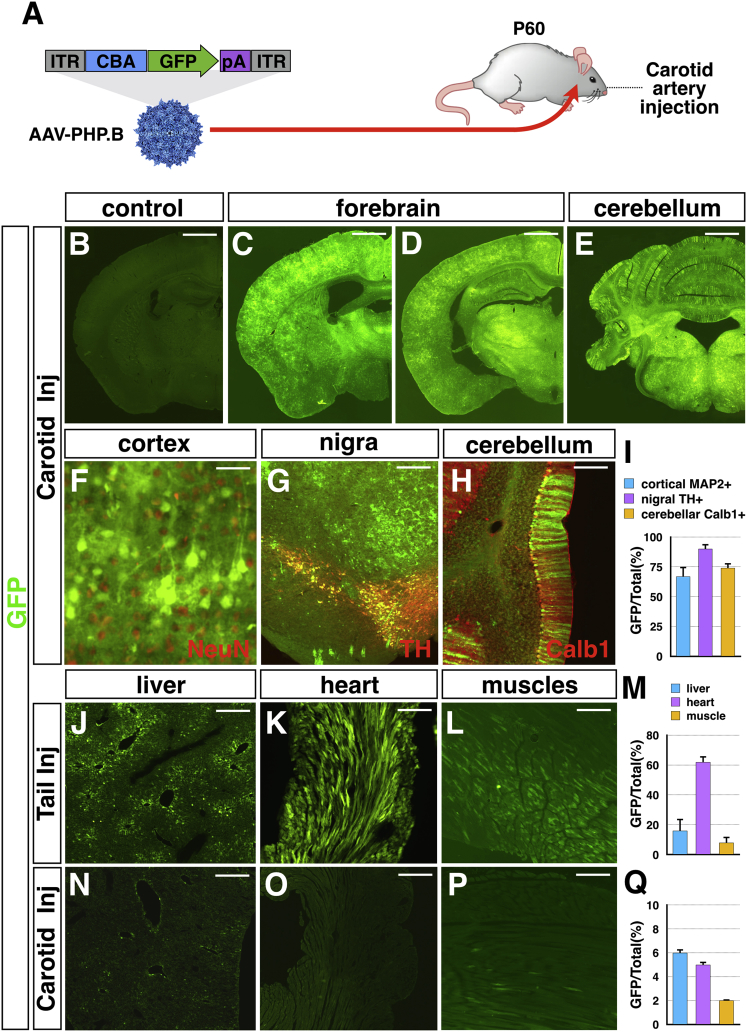
AAV-PHP.B Viral Brain Transduction through the Carotid Artery Route Limited Viral Diffusion in Peripheral Organs
Systemic i.v. delivery enables the effective spreading of the virus throughout the brain vasculature and subsequently in the neural parenchyma. However, the peripheral venous route diffuses the virus to the whole body and its peripheral organs. Thus, a single i.v. injection of AAV-PHP.B is sufficient to transduce, beyond the nervous system, a non-marginal fraction of cells in all the peripheral organs.10 This undesired viral spreading might result in a serious drawback for many potential applications of this system. To restrict the viral delivery to the nervous system, we sought to inject the virus directly into the brain circulation. For this, 2 × 1012 vg AAV-PHP.B-GFP was directly infused into the internal carotid artery via a microcatheter (Figure 7A). Brains and peripheral organs were retrieved 3 weeks after injection for immunofluorescence analysis. Notably, GFP transgene expression was detected diffusely throughout the brain with high transduction efficiency (Figures 7B–7D). Co-labeling between GFP and either NeuN, TH, or calbindin-1 showed that a high fraction of cortical and mesencephalic nigral neurons as well as cerebellar Purkinje cells were effectively transduced (Figures 7F–7I). We then compared the viral GFP gene transfer in peripheral organs after tail vein and carotid artery injections. Analysis of GFP expression in the liver, heart, and muscles showed that carotid infusion substantially reduced the viral distribution in all these peripheral organs (Figures 7J–7Q). In particular, the viral transduction in the liver and heart was decreased by more than 5- and 10-fold, respectively (Figure 7Q). These data indicate that the carotid artery route is advantageous since the nervous system targeting is coupled to a reduction in peripheral spread.
Figure 7.
AAV-PHP.B Carotid Artery Delivery Efficiently Targets the Neuraxis while Sparing Peripheral Organ Viral Transduction
(A) Schematic view depicting the transgenic cassette integrated in the AAV-PHP.B vector and injection of the viral particles into the carotid artery of an adult mouse. (B) GFP immunofluorescence in a brain transduced with an empty AAV-PHP.B (negative control). (C–E) GFP localization on coronal hemi-sections at different rostro-caudal coordinates, including the anterior forebrain (C) and posterior forebrain (D) and the cerebellum (E), of a brain transduced with the AAV-PHP.B-GFP virus. (F–H) High-magnification images of the cerebral cortex (F), mesencephalic nigral tissue (G), and cerebellum (H) showing the double staining for GFP and the neuronal marker NeuN, TH, and Calb1, respectively. (I) Bar graph showing the fraction of cells positive for a specific neuronal marker (NeuN, TH, and Calb1) and expressing the viral GFP transgene. (J–Q) GFP localization in the peripheral organs liver (J and N), heart (K and O), and muscles (L and P) after injection into either the tail vein (J–M) or the carotid artery (N–Q). (M and Q) Bar graphs showing the percentage of GFP+ cells on total cells. Note that the artery route substantially reduces viral targeting in peripheral organs (n = 3 mice). Scale bars are 500 μm (B–E), 200 μm (J–L and N–P), 100 μm (G), 50 μm (H), and 20 μm (F).
AAV-PHP.B Brain Targeting Does Not Impair BBB Integrity or Selectivity
Considering the extremely efficient brain diffusion of the AAV-PHP.B after i.v. injection, we wondered whether it could alter BBB properties. We therefore analyzed BBB permeability and inflammation after AAV-PHP.B transduction in vivo. For this aim, mice were i.v. injected with fluorescent-conjugated cadaverine dye, a small (640 Da) BBB permeability marker, together with the virus AAV-PHP.B-GFP (Figure 8A). Staining for viral capsids with the AAV-VP3-specific antibody (B1) confirmed the localization of the viral particles within the brain endothelium 24 hr after viral delivery (Figure 8B). However, the transduced brain tissue did not show any evident diffusion of the cadaverine dye (Figure 8C). In addition, no signs of astrocytosis were revealed by GFAP staining in the targeted tissue 2 days after viral transduction (Figure 8C). As a positive control, diffuse cadaverine staining and astrocyte activation were detected in the brain parenchyma of kainic acid-treated mice that developed seizure-induced BBB permeability and severe inflammation (Figures 8E–8G). To further assess BBB integrity upon AAV-PHP.B transduction, we employed a simplified in vitro BBB model obtained by isolating and culturing primary mouse brain microvascular endothelial cells (BMVECs). Acutely dissociated BMVECs were cultured to confluence to form an organized epithelial layer and then either infected with AAV-PHP.B-GFP or left untreated for 5 days (Figure 8H). In these conditions, untreated cells maintained cell-cell contacts positive for the tight junction markers ZO-1 and claudin-5 (Figures 8I and 8J). Similarly, virally transduced cells, identified by GFP expression, displayed comparable ZO-1 and claudin-5 protein localization at cell junctions (Figures 8K and 8L). Finally, we asked whether the viral infection could perturb the transendothelial electrical resistance (TEER), a key measurement of tight junction resistance in endothelial cells. Notably, there was no significant difference in TEER values between untreated and infected cells as measured up to 5 days from viral loading (Figure 8M). Conversely, TEER signal was strongly abolished when EDTA was added to the culture, causing a loss of calcium-dependent cell junctions (Figure 8M).29 Altogether, these data indicate that AAV-PHP.B targeting to the BBB does not alter the basic properties of the brain endothelium, maintaining unaltered its barrier selectivity in vivo and morphological integrity in vitro.
Figure 8.
AAV-PHP.B Brain Transduction Does Not Affect BBB Permeability In Vivo and Endothelial Integrity In Vitro
(A) Experimental setup for the tail vein injection of the AAV-PHP.B-GFP in wild-type adult mice. (B) Viral capsid staining using the B1 anti-AAV VP3 antibody reveals a robust targeting of the AAV-PHP.B-GFP in the brain endothelium 4 hr after infection. (C) Alexa Fluor-555 conjugated with cadaverine is undetectable in brain parenchyma 24 hr after AAV-PHP.B injection. (D) No evident sign of astrogliosis (GFAP staining) is present 2 days after AAV-PHP.B injection. (E) Cadaverine staining in the brain parenchyma in kainic acid (KA)-treated animals. (F) High magnification of cadaverine staining in the cortical tissue. (G) Strong GFAP+ astrogliosis in KA-injected animals. (H) Schematic view depicting the infection of brain microvascular endothelial cells (BMVECs) with the AAV-PHP.B-GFP. (I–L) Immunofluorescence for the cell-cell junction markers ZO1 and Claudin-5 (Cld5) in confluent BMVECs that were either untreated (I and J) or infected with the AAV-PHP.B-GFP (K and L). Transduced cells are visible for GFP expression in (K) and (L). (M) Transendothelial electrical resistance (TEER) analysis of confluent BMVEC cultures infected with AAV-PHP.B-GFP at time 0 (purple line). Cultures never exposed to virus were used as stable baseline controls (blue line). EDTA in the culture medium leads to a strong loss of the TEER signal (yellow line). All treatments were performed in triplicate. Scale bars are 50 μm (B–G) and 10 μm (I–L).
Discussion
AAV9 is the only AAV serotype able to cross the BBB when delivered through the vascular system. However, this ability is considerably diminished in adulthood, raising significant hurdles for pervasive targeting of the adult brain through a peripheral route. Remarkably, the AAV-PHP.B variant maintains efficient penetration even in the mature BBB and widely diffuses in the brain parenchyma in adult mice.10 Herein, we confirmed and extended these data, showing that a single i.v. injection of AAV-PHP.B can globally transduce both the central and the peripheral nervous system. Interestingly, we showed that DRGs and SGs are both efficiently targeted by AAV-PHP.B, which mostly infects the sensory neurons. Given that satellite glial cells and interneurons were poorly transduced by the virus, it is likely that the infection mainly followed a retrograde route, with initial uptake at the periphery followed by retrograde transport to the neuronal soma. Our results and those of previous studies have shown that the systemic i.v. route is intrinsically associated with widespread transduction of peripheral organs.30, 31
In this work, we employed single-stranded AAVs (ssAAVs) exclusively. Recent studies have shown that self-complementary AAV9 (scAAV9) can transduce the adult brain parenchyma.9 However, in all cases, the efficiency of transduction was far from the level necessary for supporting the technical approaches presented herein. In addition, scAAV9 facilitates viral transduction by circumventing the limiting step of the synthesis of the viral DNA complementary strand, but it also causes a loss of half of the coding packaging, reducing it to only 2.2 kb for the entire expression cassette including the promoter, coding gene, and poly(A) sequences.32 Thus, AAV-PHP.B supports the global spread of the virus in the nervous system while maintaining the full packaging space for AAV, providing convenient flexibility in designing the transgene cassette.
Herein, we established straightforward approaches to control gene expression and neuronal activity in the adult mouse brain with a single-step protocol. These procedures will have a strong impact by accelerating functional studies of genes and molecular labeling of neuronal cell types for anatomical tracing. Furthermore, the AAV-PHP.B whole-brain delivery of the chemogenetic DREADD system opens the opportunity to manipulate the activity of selected neurons in large brain areas and eventually in the entire brain and subsequently evaluate the resulting behavioral response.
We provided a strong proof of concept of this strategy by showing that whole-forebrain inactivation of PV+ GABAergic interneurons, while not sufficient per se to elicit spontaneous epileptic seizures, creates a strong predisposition to them after a proepileptic insult. However, PV is also expressed in some caudal brain areas and in Purkinje cerebellar neurons. Overall, we could not exclude the possibility that other cell populations might have influenced this phenotype. Thus, the choice of selective genetic tracing, when available, is a crucial prerequisite to subsequently retrieve conclusive functional data. Wide-scale access to the adult mouse nervous system makes it feasible to misexpress genes and evaluate their direct impact on brain functions and consequent behavior.
Taken together, these results provide solid evidence that the brain-penetrant AAV-PHP.B is an ideal platform for transducing therapeutic genes to treat neurodegenerative disorders that globally affect the brain tissue. Herein, we focused on α-syn inclusions that spread over time throughout large brain areas in PD, LBD, and MSA and are responsible for cortical functional decline leading to severe dementia.33, 34 Approximately 5%–8% of PD patients are carriers of a heterozygous GBA1 mutation, causing a detectable reduction in GCase global activity.11, 12 PD patients carrying GBA1 mutations often develop more severe symptoms then GBA1 non-carrier patients, including an accelerated cognitive decline associated with increased α-syn accumulation.35, 36 Therefore, stimulating GCase activity in these patients represents a direct and valuable therapeutic approach. Furthermore, GCase activity gradually declines with aging in healthy individuals; in addition, sporadic patients showed a further reduction in GCase functionality.37, 38 Given this, increasing GCase levels can also be an effective therapeutic strategy for age-related and sporadic forms of PD. Herein, we showed that AAV-PHP.B-mediated global expression of GCase is sufficient to provide robust and long-lasting protection from α-syn deposits in a mouse model of synucleinopathy. Exogenous virally delivered GCase is targeted to the lysosome and acquires functionality, which resulted in significantly diminished accumulation of insoluble α-syn species in all the forebrain regions. Previous studies have shown that α-syn accumulation is promoted by diminished GCase activity in vitro and in vivo, which leads to an abnormal accumulation of its glycolipid substrates in the lysosomes.39, 40 Conversely, α-syn inhibits the lysosomal activity of GCase, thereby causing a loss of its catalytic function upon progressive accumulation of α-syn.14 Hence, pathological conditions establish a vicious cycle between α-syn and GCase that can sustain and progressively worsen the disease.14, 15 Along these lines, stimulating GCase activity has been shown to counteract α-syn pathology in mouse and human neurons in vitro.16, 17, 41 Our results support this view, showing that GCase is a strong determinant of α-syn accumulation and that increasing its enzymatic levels significantly protects against α-syn pathology and toxicity. Intriguingly, although GBA1 viral transduction did not target the entire neuronal population, we nonetheless observed a general and even reduction of α-syn inclusions throughout the neural parenchyma. These results are plausible considering that the GCase enzyme has non-cell-autonomous action, reducing α-syn deposits even in cells in which it is not directly stimulated as long as they are placed close enough to virally transduced cells. In support of this hypothesis, classical cell biology experiments have shown that lysosomal enzymes can be released from producing cells, endocytosed by their neighbors, and correctly trafficked to their lysosomes both in vitro and in vivo.42 Therefore, our findings imply that it is not necessary to transduce the entire brain neuronal population; GCase overproduction in a partial subset of neural cells (either neurons, glia, or both) is sufficient to achieve widespread protection from α-syn inclusions.
Strategies to reduce α-syn toxicity by active immunization or by stimulating GCase activity through small-molecule non-inhibitory chaperones are currently being explored to establish therapeutic approaches for these diseases.43, 44, 45 However, limited crossing of the BBB, toxic side effects, and restricted efficacy to only some disease forms are some of the important hurdles that remain to be fully cleared to translate these treatments to patients. The present results strongly imply that gene therapy should be considered as a further therapeutic opportunity for synucleinopathies with the potential advantage of providing a long-lasting beneficial effect with a single treatment. The introduction of the AAV-PHP.B viral platform, which sustains effective BBB crossing and global spreading of the therapeutic GBA1 gene in the adult brain tissue, fulfills the necessary conditions for the development of an effective and non-invasive gene therapy approach for synucleinopathies. Further studies on wild-type animals will be necessary to address whether the chronic stimulation of the GCase activity might cause any long-term adverse effect on neuronal homeostasis and function, regardless of whether this stimulation will be achieved by a small-molecule or gene-based approach. However, this might not be a serious hurdle in our approach, since the GBA1 viral transduction elicited a strong rescuing of GCase activity in most brain regions of A53T-SCNA transgenic mice but without causing evident supraphysiological activity compared with wild-type conditions.
The massive penetration of this virus into the brain upon acute delivery in the circulation raises some concerns about altering normal BBB physiology. Thus, we searched for any sign of acute derangement of BBB integrity after virus administration. Importantly, no evidence of loss of functional selectivity was found; our work showed that a small (640 Da) fluorescent dye was continuously excluded from entering the brain space immediately or soon after viral infection. This is in line with the absence in transduced brain tissues of any overt sign of inflammation, which is closely associated with abnormal BBB permeability. Thus, the acute targeting of AAV-PHP.B to the brain appears to be substantially harmless, at least in regard to major BBB functionality.
To concentrate the viral transduction to the brain, we administered the virus through the carotid artery, thereby maintaining high transduction efficiency within the neural parenchyma while strongly diminishing the spread of the virus in the peripheral organs. Although viral targeting in the liver and heart was strongly restrained, some remaining infected cells were still detectable, indicating that even direct arterial brain delivery can diffuse some viral particles into the systemic circulation. However, this delivery route has important advantages, especially from a therapeutic prospective. In fact, this viral administration is feasible in large apes and human clinical practice, limiting unnecessary viral spreading to peripheral organs while concentrating the viral particles to the therapeutic target, as represented by the adult nervous system.
The diffuse penetration of the AAV-PHP.B in the adult mouse brain parenchyma is a unique property among all the recombinant viral strains in current use. Future studies will address whether this capacity will remain intact and equally efficient when tested in large animals. If so, this viral strain might become the system of choice to deliver therapeutic genes such as GBA1 to the brain, enabling the development of effective and non-invasive gene therapy approaches for synucleinopathies.
Materials and Methods
Generation of the AAV Transfer Vectors
The pAAV-CBA-EGFP construct was kindly donated by Dr. G. Gonzalez (CIMA, Pamplona, Spain). This plasmid was further modified to express under the control of the CBA promoter: (1) the CreNLS cassette, (2) the GBA1-GFP multicistronic sequence, (3) and the FLEX EGFP cassette. In FLEX cassettes, the transgene is antisense with respect to promoter-driven transcription and is flanked by two consecutive but different pairs of flox sequences. In the presence of Cre recombination, the transgene is reverted and its gene expression is activated. The GBA1 coding region was purchased from Origene (RG216061). Subsequently, GBA1 was cloned in frame with a P2A sequence followed by the EGFP coding region. Finally, for the GBA1-mCherry expression vector, the GBA1 and mCherry coding regions were cloned in frame but separated by a Gly-Ser-Gly linker and inserted downstream to the EF1α promoter. The pAAV-hSyn-DIO-hM4D(Gi)-MCherry plasmid was obtained by Prof. B.L. Roth (Chapel Hill, NC, USA) through Addgene (no. 44362).
Cell Cultures
293T cells were cultured in Iscove’s modified Eagle’s medium (Sigma-Aldrich) containing 10% fetal bovine serum (Sigma-Aldrich), 1% non-essential amino acids (Gibco), 1% sodium pyruvate (Sigma-Aldrich), 1% glutamine (Sigma-Aldrich), and 1% penicillin/streptomycin (Sigma-Aldrich). Cells were split every 3–4 days using 0.25% trypsin (Sigma-Aldrich). Isolation of mouse BMVECs was performed as previously reported by Liebner et al.46 BMVECs were grown in the EGM-2 Plus BulletKit (Lonza). For immunostaining, 2 × 105 cells were seeded on a 24-well plate coated with rat tail collagen type-1 (150 μg/mL; Sigma). The day after, fresh culture medium was added supplemented with LiCl (10 mM; Sigma) and the cells were infected 48 hr after seeding in 300 μl of total volume. The medium was changed every 2–3 days. HeLa cells were cultured in DMEM high glucose (Sigma-Aldrich) containing 10% fetal bovine serum (Sigma-Aldrich), 1% non-essential amino acids (Gibco), 1% sodium pyruvate (Sigma-Aldrich), 1% glutamine (Sigma-Aldrich), and 1% penicillin/streptomycin (Sigma-Aldrich). Cells were split every 2–3 days using 0.25% trypsin (Sigma-Aldrich). For transfection, Lipofectamine LTX (Thermo Fisher Scientific) was used according to the manufacturer’s protocol.
Virus Production and Purification
Replication-incompetent, recombinant viral particles were produced in 293T cells by polyethylenimine (PEI) (Polyscience) co-transfection of three different plasmids: transgene-containing plasmid, packaging plasmid for rep and cap genes, and pAdDeltaF6 for the three adenoviral helper genes, The cells and supernatant were harvested at 120 hr. Cells were lysed in Tris buffer (50 mM Tris, pH 8.5, and 150 mM NaCl; Sigma-Aldrich) by repetitive freeze-thawing cycles (3 times), whereas the viral particles present in the supernatant were concentrated by precipitation with 8% PEG8000 (polyethylene glycol 8000; Sigma-Aldrich), lysed in Tris buffer, and combined with correspondent cell lysates. To clarify the lysate, benzonase treatment was performed (250 U/mL, 37°C for 30 min; Sigma-Aldrich) in the presence of 1 mM MglCl2 (Sigma-Aldrich) and cellular debris separated by centrifugation (2,000 × g, 30 min). The viral phase was isolated by an iodixanol step gradient (15%, 25%, 40%, 60% Optiprep; Sigma-Aldrich) in the 40% fraction and concentrated in PBS with a 100,000 molecular weight cut-off concentrator (Vivaspin 20; Sartorius Stedim). Virus titers were determined by measuring the number of DNase I-resistant viral particles, using qPCR with linearized genome plasmid as a standard.
Animals
Mice were maintained at the San Raffaele Scientific Institute institutional mouse facility (Milan, Italy) in micro-isolators under sterile conditions and supplied with autoclaved food and water. The following transgenic mouse strains were used: NeuroD6-Cre,47 DAT-Cre,48 Ai9,18 PV-Cre,49 Olig2-Cre,50 and Tsc1 conditional mutants.51 All procedures were performed according to protocols approved by the internal institutional animal care and use committee (IACUC) and reported to the Italian Ministry of Health according to the European Commission Council Directive 2010/63/EU.
Viral Injections
For tail vein injection, 2- to 6-month-old mice were previously warmed under a heat lamp for 10 min and then placed into a restrainer for further manipulation. Mice were injected with variable viral concentrations depending on the experimental setup in a total volume of 200 μL PBS (from 1 × 109 to 2 × 1012 vg/each mouse). For injections into mouse neonates, 1-day-old pups were rested on a bed of ice for anesthetization. 50 μL AAV viral suspension (1.5 × 1011 vg) was manually injected into the facial vein using a 29-gauge insulin syringe. After injection, pups were rubbed with bedding to prevent rejection before reintroducing the mother into the cage. For carotid artery injections, 8- to 10-week-old C57Bl6/J mice were anesthetized with a mixture of ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively) and the temperature was maintained during the procedure between 36 and 36.5°C using a feedback-controlled heating system. Using a stereomicroscope, a midline neck incision was made, the common carotid artery was exposed, and the external carotid artery was ligated. A microcatheter was placed inside the common carotid artery and advanced up to the internal carotid artery. Under a sterile hood, the 50-μL viral suspension (2 × 1012 vg) in PBS was infused for 5–6 min with an infusion pump (World Precision Instruments).52 At the end of the procedure, the microcatheter was withdrawn, the incisions were sutured, and animals were allowed to recover.
Immunohistochemistry
Immunohistochemical analysis of the tissue sections was conducted essentially as previously described.53 Briefly, mice were anesthetized with ketamine/xylazine and transcardially perfused with 0.1 M phosphate buffer (PB) at room temperature (RT) at pH 7.4 with freshly prepared, ice-cold 4% paraformaldehyde (PFA) in PB. Tissues were post-fixed in 4% PFA overnight and then soaked in cryoprotective solution (30% sucrose in PBS). Tissues were sectioned using cryostat after optimal cutting temperature (OCT) compound embedding in dry ice. For immunofluorescence, free-floating 30-μm-thick coronal sections were rinsed in PBS and were incubated for 10 min with 3% H2O2 and 10% methanol and then for 20 min with Triton X-100 2%. 3% BSA for 1 hr was used to saturate the unspecific binding site before the overnight incubation with the primary antibody (diluted in a solution containing 1% BSA and Triton X-100 at RT). Following incubation, sections were rinsed three times in PBS and incubated for 1 hr with the secondary antibody. For immunohistochemistry, free-floating slices were rinsed in PBS and treated for 1 hr with a blocking solution containing 3% BSA and 0.3% Triton X-100 in PBS. After blocking, samples were incubated with the primary antibody diluted with a solution containing 1% BSA and 0.3% Triton X-100 overnight at RT. The slices were then incubated with the secondary antibody, followed by Vectastain ABC enhancing reaction, and finally the staining was revealed in 3,3'-diaminobenzidine (DAB) solution. After mounting, the slices were dehydrated in xylene and the coverslip was sealed with Eukitt mounting medium. The slices treated with PK were incubated for 10 min in a solution with 1 μg/mL PK, and the tissue was processed for the immunostaining.
Primary antibodies for the following epitopes were used: GFP (1:500; Molecular Probes), TH (1:1,000; Immunological Sciences), calbindin (1:200; Swant), PV (1:500; Sigma), beta-III-tubulin (1:1,000; Covance), NeuN (1:1,000; Immunological Sciences), human α-Syn (1:200, Syn211; BD Biosciences), phosphoS129-α-syn (1:100; Abcam). Slices were mounted with fluorescent mounting medium (Dako). For BBB integrity evaluation, cadaverine (0.2 mg/animal) conjugated to Alexa Fluor-555 (Life Technologies) was injected i.v. into the tail vein 2 hr before euthanizing the animal. Images were captured with a Nikon Eclipse 600 fluorescent microscope. Images were then imported and processed with the Adobe Creative Suite applications.
Stereological Counting
Unbiased semi-automatic stereological sampling and counting was performed with a Leica DM4000B microscope equipped with a MAC 6000 system and Stereo Investigator 9 software (MFB Bioscience, Williston, VT, USA). After structure boundaries delimitation, cortical phosphoS129-α-syn+ cells were automatically counted at ×40 magnification. Slices were collected every 180 μm, encompassing about 1.5 mm of the cortex (antero-posterior +1 to −0.5 from bregma). The optical fractionator stereological probe (40 × 40 sized, 240 × 240 spaced) was then used to estimate the total number of phosphoS129-α-syn+ neurons.
GCase Enzymatic Assay
Dissected brain regions were lysates and mechanical homogenized in GCase assay buffer, pH 5.4 [1× citrate buffer, 0.25% Triton X-100 (w/w), 0.25% taurocholic acid (w/w), and H20], supplemented with 1% protease inhibitor mixture (Roche Diagnostics). After 30 min of lysis on ice, samples were then centrifuged at 13,000 × g for 15 min at 4°C. The supernatant was collected and used for activity assays and western blotting analysis. GCase activity in tissues was measured using 10 μg protein/well, with protein quantification assessed by the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). The substrate 4-methylumbelliferyl β-D-glucopyranoside (4MUG) (M3633; Sigma) was then assembled with the tissue lysates to a final concentration of 5 mM for 1 hr at 37°C. After blocking the reaction with 1 M glycine solution, the signal was detected with the Victor plate reader (Perkin Elmer) with excitation and emission wavelengths at 360 nm and 440 nm, respectively. The standard used for this assay was the fluorescent product 4-methylumbelliferone (4MU) (M1381; Sigma). The specific activity was calculated with the 4MU standard curve by converting the relative fluorescence units (RFUs) to the concentration of the fluorescent cleaved product (GraphPad Prism 5.1; GraphPad Software). This interpolated value was then used to calculate the GCase enzymatic activity in the lysed tissue, which was expressed in nanomoles per hour per milligram. Specificity of the enzymatic activity was assessed by adding the specific GCase inhibitor conduritol-B-epoxide (CBE) at 16 mM (Sigma).
Immunoblotting
Brain lysate samples (∼30 μg protein lysates) were separated using 10% or 15% polyacrylamide gel and then transferred to PVDF membranes. Membranes were incubated overnight at 4°C with the following primary antibodies: C-terminal GCase antibody (G4171, 1:1,000; Sigma), anti-actin (A3853, 1:10,000; Sigma), or anti-calnexin (catalog no. C4731, 1:5,000; Sigma). Subsequently, membranes were incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies (1:5,000; Dako). The signal was then revealed with a chemiluminescence solution (ECL reagent, RPN2232; GE Healthcare) and detected with the ChemiDoc imaging system (Bio-Rad). For α-syn immunoblotting, brain homogenates were processed in order to collect the TBS- and SDS-soluble fraction of α-syn as described.54 Briefly, brains were homogenated with TBS (pH 7.4), clarified from non-homogenated residue and submitted to 100,000 × g centrifugation at 4°C for 1 hr. The resulting supernatant represents the TBS-soluble fraction. Then pellets were solubilized in TBS-SDS [5% SDS (w/v)] by sonication and centrifuged at 100,000 × g for 30 min at 25°C. Supernatants were collected and referred to as the SDS-soluble fractions. Sampled (15 μg/μL total proteins) were loaded in a gradient gel (4%–12% Bis-tris gel, NP0322BOX; Invitrogen) with 3-(N-morpholino)propanesulfonic acid (MOPS) as the running buffer (NuPAGE MOPS SDS, NP0001; Invitrogen) at 200 V. The transfer was performed for 2 hr at 40 V on nitrocellulose membrane (0.45 μm nitrocellulose membrane, 1060003; GE Healthcare). Membranes were then blocked in 5% BSA for 1 hr and the primary antibody (Syn211) was incubated overnight at 4°C. After incubation with the appropriate HRP-conjugated secondary antibody for 30 min, the signal was then revealed and processed as previously described.
EEG Recordings
At least 3 days before recording, epidural stainless-steel screw electrodes (0.9-mm diameter, 3-mm long) were surgically implanted under ketamine/xylazine anesthesia and secured using dental cement (Ketac Cem; ESPE Dental AG, Seefeld, Germany). Two active electrodes were placed on the right and left parietal areas (2 mm lateral to midline, 1 mm posterior to bregma) and one was placed over the occipital area (1 mm posterior to lambda) as a common reference. Freely moving 12-hr sessions of digital EEG monitoring were performed via a flexible cable connected to the amplifier (Micromed, Mogliano Veneto, Italy) in a Faraday cage, with food and water available ad libitum. EEG traces were filtered between 0.53 and 60 Hz and sampled at 256 Hz (16 bits). EEG recordings were visually inspected to detect epileptiform discharges and/or seizures, defined as high-amplitude (at least 2 times the baseline) rhythmic discharges lasting at least 5 s.
Ex Vivo Electrophysiological Recordings
Mice (60–90 days of age) were anesthetized with an intraperitoneal injection of a mixture of ketamine/xylazine and transcardially perfused with ice-cold artificial cerebrospinal fluid (ACSF) containing the following: 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, 25 mM NaHCO3, 1 mM MgCl2, and 11 mM D-glucose saturated with 95% O2 and 5% CO2 (pH 7.3). After decapitation, brains were removed from the skull and mounted in a VT1000S vibratome chamber (Leica Microsystems, Wetzlar, Germany) filled with ACSF at 4°C. Sagittal brain slices were cut at 300-μm thickness. Individual slices were submerged in a recording chamber mounted on the stage of an upright BX51WI microscope (Olympus) equipped with differential interference contrast (DIC) optics and an optical filter set for the detection of mCherry red fluorescence (Semrock, Rochester, NY). The slices were continuously perfused with ACSF (3–5 mL/min) at RT. Fast-spiking interneurons expressing DREADD were visually identified by tdTomato fluorescence. Whole-cell patch-clamp recordings were performed using pipettes filled with a solution containing the following: 124 mM KH2PO4, 2 mM MgCl2, 10 mM NaCl, 10 mM HEPES, 0.5 mM EGTA, 2 mM Na2-ATP, and 0.02 mM Na-GTP (pH 7.2, adjusted with KOH; tip resistance: 6–8 MΩ). CNO (10 μM) was added through extracellular perfusion. All recordings were performed using a MultiClamp 700B amplifier interfaced with a PC through a Digidata 1440A (Molecular Devices, Sunnyvale, CA, USA). Data were acquired using pClamp10 software (Molecular Devices) and analyzed with Prism 5 (GraphPad Software Inc., La Jolla, CA). Current-clamp traces were sampled at a frequency of 10 kHz and low-pass filtered at 2 kHz.
Behavioral Studies
The novel object recognition test was performed in a square arena of 40 × 40 cm. On day 1, mice were habituated to the open-field apparatus in a 5-min session. On day 2, animals underwent the training phase (10 min), in which two identical objects were introduced into the arena before the mouse was allowed to explore. The amount of time that the rodents spent exploring each object was scored. Finally, on day 3, mice were tested for their memory (10 min). The discrimination index (DI) is defined as the difference between the exploration time for the novel object and the one for the familiar object, divided by total exploration time.
Statistical Analyses
The results were analyzed with GraphPad Prism version 6.0c for Macintosh. The unpaired Student’s t test or two-way ANOVA followed by Bonferroni’s post-tests was used in the datasets analyzed.
Author Contributions
G.M., S.G.G., and G.O. performed the experiments and analyzed data; S.B. carried out western blotting analysis; M.I. analyzed and performed mouse behavioral testing; C.C. contributed to AAV particle production and cell culture manipulation; M.L. contributed to gene cloning; A.S. performed neonatal viral injections; V.C. and L.L. performed and analyzed mouse EEG recordings; S.C. and S.T. performed and elaborated ex vivo electrophysiological recordings; T.C. contributed to tissue sectioning and histological analysis; M.B. performed carotid artery injections; J.L.L. contributed to morphometric tissue analysis; and V.B. conceived the experiments, supervised and supported the project, and wrote the paper with G.M., S.G.G., and S.B.
Conflicts of Interest
No conflict of interest is linked directly or indirectly to this study.
Acknowledgments
We thank Drs. E. Dejana, J. Wijnholds, R. Melki, L. Muzio, and M. Simonato for sharing of reagents; A. Bergamaschi for instructions on tail vein injections; B.E. Deverman and V. Gradinaru for providing the AAV-PHP.B capsid plasmid and instructions for virions production; and T. Pizzorusso, P. Striano, D. Bonanomi, A. Menegon, M. Cursi, and all members of the Broccoli laboratory for helpful discussion. We acknowledge the FRACTAL core facility for expert supervision in flow cytometry. This work was supported by the European Research Council (AdERC no. 340527).
Footnotes
Supplemental Information includes six figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.08.004.
Supplemental Information
References
- 1.Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:75–80. doi: 10.1016/j.coviro.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samulski R.J., Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 2014;1:427–451. doi: 10.1146/annurev-virology-031413-085355. [DOI] [PubMed] [Google Scholar]
- 3.Mingozzi F., High K.A. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 4.Murlidharan G., Samulski R.J., Asokan A. Biology of adeno-associated viral vectors in the central nervous system. Front. Mol. Neurosci. 2014;7:76. doi: 10.3389/fnmol.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foust K.D., Wang X., McGovern V.L., Braun L., Bevan A.K., Haidet A.M., Le T.T., Morales P.R., Rich M.M., Burghes A.H., Kaspar B.K. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Saraiva J., Nobre R.J., Pereira de Almeida L. Gene therapy for the CNS using AAVs: the impact of systemic delivery by AAV9. J. Control. Release. 2016;241:94–109. doi: 10.1016/j.jconrel.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Karda R., Buckley S.M.K., Mattar C.N., Ng J., Massaro G., Hughes M.P., Kurian M.A., Baruteau J., Gissen P., Chan J.K. Perinatal systemic gene delivery using adeno-associated viral vectors. Front. Mol. Neurosci. 2014;7:89. doi: 10.3389/fnmol.2014.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray S.J., Matagne V., Bachaboina L., Yadav S., Ojeda S.R., Samulski R.J. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol. Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deverman B.E., Pravdo P.L., Simpson B.P., Kumar S.R., Chan K.Y., Banerjee A., Wu W.L., Yang B., Huber N., Pasca S.P., Gradinaru V. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 2016;34:204–209. doi: 10.1038/nbt.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidransky E., Nalls M.A., Aasly J.O., Aharon-Peretz J., Annesi G., Barbosa E.R., Bar-Shira A., Berg D., Bras J., Brice A. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neumann J., Bras J., Deas E., O’Sullivan S.S., Parkkinen L., Lachmann R.H., Li A., Holton J., Guerreiro R., Paudel R. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sardi S.P., Clarke J., Kinnecom C., Tamsett T.J., Li L., Stanek L.M., Passini M.A., Grabowski G.A., Schlossmacher M.G., Sidman R.L. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc. Natl. Acad. Sci. USA. 2011;108:12101–12106. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzulli J.R., Xu Y.-H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., Krainc D. Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magalhaes J., Gegg M.E., Migdalska-Richards A., Doherty M.K., Whitfield P.D., Schapira A.H.V. Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum. Mol. Genet. 2016;25:3432–3445. doi: 10.1093/hmg/ddw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sardi S.P., Clarke J., Viel C., Chan M., Tamsett T.J., Treleaven C.M., Bu J., Sweet L., Passini M.A., Dodge J.C. Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc. Natl. Acad. Sci. USA. 2013;110:3537–3542. doi: 10.1073/pnas.1220464110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocha E.M., Smith G.A., Park E., Cao H., Brown E., Hayes M.A., Beagan J., McLean J.R., Izen S.C., Perez-Torres E. Glucocerebrosidase gene therapy prevents α-synucleinopathy of midbrain dopamine neurons. Neurobiol. Dis. 2015;82:495–503. doi: 10.1016/j.nbd.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajasethupathy P., Ferenczi E., Deisseroth K. Targeting neural circuits. Cell. 2016;165:524–534. doi: 10.1016/j.cell.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roth B.L. DREADDs for neuroscientists. Neuron. 2016;89:683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa-Mattioli M., Monteggia L.M. mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat. Neurosci. 2013;16:1537–1543. doi: 10.1038/nn.3546. [DOI] [PubMed] [Google Scholar]
- 22.Saxton R.A., Sabatini D.M. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan J.A., Zhang H., Roberts P.S., Jozwiak S., Wieslawa G., Lewin-Kowalik J., Kotulska K., Kwiatkowski D.J. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J. Neuropathol. Exp. Neurol. 2004;63:1236–1242. doi: 10.1093/jnen/63.12.1236. [DOI] [PubMed] [Google Scholar]
- 24.Tavazoie S.F., Alvarez V.A., Ridenour D.A., Kwiatkowski D.J., Sabatini B.L. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- 25.Crino P.B. The mTOR signalling cascade: paving new roads to cure neurological disease. Nat. Rev. Neurol. 2016;12:379–392. doi: 10.1038/nrneurol.2016.81. [DOI] [PubMed] [Google Scholar]
- 26.Way S.W., McKenna J., 3rd, Mietzsch U., Reith R.M., Wu H.C.-J., Gambello M.J. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum. Mol. Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abs E., Goorden S.M.I., Schreiber J., Overwater I.E., Hoogeveen-Westerveld M., Bruinsma C.F., Aganović E., Borgesius N.Z., Nellist M., Elgersma Y. TORC1-dependent epilepsy caused by acute biallelic Tsc1 deletion in adult mice. Ann. Neurol. 2013;74:569–579. doi: 10.1002/ana.23943. [DOI] [PubMed] [Google Scholar]
- 28.Lee M.K., Stirling W., Xu Y., Xu X., Qui D., Mandir A.S., Dawson T.M., Copeland N.G., Jenkins N.A., Price D.L. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53--> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. USA. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy Z., Goehlert U.G., Wolfe L.S., Hüttner I. Ca2+ depletion-induced disconnection of tight junctions in isolated rat brain microvessels. Acta Neuropathol. 1985;68:48–52. doi: 10.1007/BF00688955. [DOI] [PubMed] [Google Scholar]
- 30.Bevan A.K., Duque S., Foust K.D., Morales P.R., Braun L., Schmelzer L., Chan C.M., McCrate M., Chicoine L.G., Coley B.D. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol. Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dashkoff J., Lerner E.P., Truong N., Klickstein J.A., Fan Z., Mu D., Maguire C.A., Hyman B.T., Hudry E. Tailored transgene expression to specific cell types in the central nervous system after peripheral injection with AAV9. Mol. Ther. Methods Clin. Dev. 2016;3:16081–16089. doi: 10.1038/mtm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCarty D.M. Self-complementary AAV vectors; advances and applications. Mol. Ther. 2008;16:1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 33.Wong Y.C., Krainc D. α-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat. Med. 2017;23:1–13. doi: 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin D.J., Lee V.M.Y., Trojanowski J.Q. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci. 2013;14:626–636. doi: 10.1038/nrn3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mata I.F., Leverenz J.B., Weintraub D., Trojanowski J.Q., Chen-Plotkin A., Van Deerlin V.M., Ritz B., Rausch R., Factor S.A., Wood-Siverio C. GBA variants are associated with a distinct pattern of cognitive deficits in Parkinson’s disease. Mov. Disord. 2016;31:95–102. doi: 10.1002/mds.26359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brockmann K., Srulijes K., Pflederer S., Hauser A.-K., Schulte C., Maetzler W., Gasser T., Berg D. GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov. Disord. 2015;30:407–411. doi: 10.1002/mds.26071. [DOI] [PubMed] [Google Scholar]
- 37.Gegg M.E., Burke D., Heales S.J.R., Cooper J.M., Hardy J., Wood N.W., Schapira A.H. Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy K.E., Gysbers A.M., Abbott S.K., Tayebi N., Kim W.S., Sidransky E., Cooper A., Garner B., Halliday G.M. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain. 2014;137:834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abeliovich A., Gitler A.D. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- 40.Schapira A.H.V. Glucocerebrosidase and Parkinson disease: recent advances. Mol. Cell. Neurosci. 2015;66(Pt A):37–42. doi: 10.1016/j.mcn.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzulli J.R., Zunke F., Tsunemi T., Toker N.J., Jeon S., Burbulla L.F., Patnaik S., Sidransky E., Marugan J.J., Sue C.M., Krainc D. Activation of β-glucocerebrosidase reduces pathological α-synuclein and restores lysosomal function in Parkinson’s patient midbrain neurons. J. Neurosci. 2016;36:7693–7706. doi: 10.1523/JNEUROSCI.0628-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz M.L., Tecedor L., Chang M., Davidson B.L. Clarifying lysosomal storage diseases. Trends Neurosci. 2011;34:401–410. doi: 10.1016/j.tins.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergström A.L., Kallunki P., Fog K. Development of passive immunotherapies for synucleinopathies. Mov. Disord. 2016;31:203–213. doi: 10.1002/mds.26481. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Martinez A., Beavan M., Gegg M.E., Chau K.-Y., Whitworth A.J., Schapira A.H.V. Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models. Sci. Rep. 2016;6:31380. doi: 10.1038/srep31380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aflaki E., Borger D.K., Moaven N., Stubblefield B.K., Rogers S.A., Patnaik S., Schoenen F.J., Westbroek W., Zheng W., Sullivan P. A new glucocerebrosidase chaperone reduces α-synuclein and glycolipid levels in iPSC-derived dopaminergic neurons from patients with Gaucher disease and parkinsonism. J. Neurosci. 2016;36:7441–7452. doi: 10.1523/JNEUROSCI.0636-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liebner S., Corada M., Bangsow T., Babbage J., Taddei A., Czupalla C.J., Reis M., Felici A., Wolburg H., Fruttiger M. Wnt/β-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwab M.H., Bartholomae A., Heimrich B., Feldmeyer D., Druffel-Augustin S., Goebbels S., Naya F.J., Zhao S., Frotscher M., Tsai M.J., Nave K.A. Neuronal basic helix-loop-helix proteins (NEX and BETA2/Neuro D) regulate terminal granule cell differentiation in the hippocampus. J. Neurosci. 2000;20:3714–3724. doi: 10.1523/JNEUROSCI.20-10-03714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bäckman C.M., Malik N., Zhang Y., Shan L., Grinberg A., Hoffer B.J., Westphal H., Tomac A.C. Characterization of a mouse strain expressing Cre recombinase from the 3′ untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- 49.Hippenmeyer S., Vrieseling E., Sigrist M., Portmann T., Laengle C., Ladle D.R., Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zawadzka M., Rivers L.E., Fancy S.P.J., Zhao C., Tripathi R., Jamen F., Young K., Goncharevich A., Pohl H., Rizzi M. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwiatkowski D.J., Zhang H., Bandura J.L., Heiberger K.M., Glogauer M., el-Hashemite N., Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum. Mol. Genet. 2002;11:525–534. doi: 10.1093/hmg/11.5.525. [DOI] [PubMed] [Google Scholar]
- 52.Janowski M., Lyczek A., Engels C., Xu J., Lukomska B., Bulte J.W.M., Walczak P. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J. Cereb. Blood Flow Metab. 2013;33:921–927. doi: 10.1038/jcbfm.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ricciardi S., Ungaro F., Hambrock M., Rademacher N., Stefanelli G., Brambilla D., Sessa A., Magagnotti C., Bachi A., Giarda E. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat. Cell Biol. 2012;14:911–923. doi: 10.1038/ncb2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bandopadhyay R. Sequential extraction of soluble and insoluble alpha-synuclein from parkinsonian brains. J. Vis. Exp. 2016;107:e53415. doi: 10.3791/53415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.