Abstract
The pelvis and the spine form a system balancing human skeleton. Within this system, the pelvis adapts to age-related changes in the spine. Previous studies were predominantly focused on changes of pelvic parameters in the sagittal plane. The aim of this study was to reveal age-related changes of lesser pelvic dimensions at different levels of the pelvic cavity in the sagittal and coronal planes and to explore sexual dimorphism in age-related tendencies. The computed tomography pelvimetry was performed on the three-dimensional workstation. The research sample included 211 females aged 18 to 84 years and 181 males aged 18 to 82 years, who underwent an examination at the Riga East University Hospital, Clinical Center “Gailezers,” Latvia. Three pelvic angles and transverse and sagittal diameters of the lesser pelvis were measured at four levels: the inlet, two axial planes in the mid-cavity, and the outlet. The results demonstrated that more pronounced age-related changes occurred in the inlet and the outlet of the lesser pelvis. The mid-cavity was less changing. The transverse diameter between acetabular centers and the sagittal diameter at the level of ischial spines were independent of age. In general, the common age-related trends were observed for pelvic parameters in females and males. A single exception was the proportion of diameters at the level of ischial spines, which decreased in males only. For parameters associated with pelvic floor diseases, age-related changes occurred in the direction of pathology.
Keywords: Lesser pelvis, Dimorphism, CT pelvimetry, Aging
Introduction
The pelvis and the spine constitute a system balancing human skeleton in its vertical posture, bipedal locomotion, and sitting position [1,2,3,4,5,6,7]. Aimed at maintaining a relatively fixed gravity line, the pelvis compensates age-related changes in the vertebral column. For example, progressing of thoracic kyhosis is compensated by a small retroversion of the pelvis [8]. At the same time, the sacrum takes more horizontal position with age in both sexes [9,10]. The study in a female sample confirms decreasing pelvic inclination [11]. In addition, motion in the sacroiliac joint becomes more limited because of morphological changes in the articular cartilage of the joint [12,13]. These findings demonstrate that clinical studies are predominantly focused on parameters of the spine and the pelvis in the sagittal plane. The aim of this study was to reveal age-related changes of pelvic dimensions at different levels of the pelvic cavity in the sagittal and coronal planes.
For such a description, sexual dimorphism of the human pelvis should be included into analysis. In males, the pelvic cavity is more triangular that provides effective bipedal locomotion [4]. The female pelvis represents a compromise between bipedal locomotion and obstetric requirements [14]. Additional evolutionary pressure results in higher sagittal and transverse diameters of the lesser pelvis in females than in males [15,16]. Empirical findings on sexual dimorphism of sacrolumbal balance angles are more inconsistent. For example, some studies [9,17] reveal higher sacral slope in females, while other studies [10,18] demonstrate no significant sexual dimorphism in sacrum orientation. As a result of sexual dimorphism, generalization of age-related tendencies observed in female samples [11] to male population is limited.
In addition, the assessment of age-related changes can provide information for a better understanding of pelvic floor diseases associated with aging. Clinical studies demonstrate that the risk of development of pelvic organ prolapse, urinary and fecal incontinences increases with age [19,20]. The main explanation for the development of these pathologies was related to changes in the soft tissues of the inferior wall of the pelvic cavity [21,22]. However, some characteristics of the bony pelvic shape can contribute to the development of the diseases [11,23,24,25,26]. The pelvic floor dysfunction and the pelvic organ prolapse are observed more often in females with a wider [23,24,25] and more horizontally oriented pelvic inlet [11], a wider distance between ischial spines [26], and a longer sagittal diameter of the outlet [11].
Taking into account incomplete data concerning age-related changes and sexual dimorphism of the lesser pelvis, this study tests a set of regression models. Within this set, age, sex, and their interaction were selected as independent variables predicting pelvic angles or pelvic dimensions in the sagittal or coronal plane.
Materials and Methods
In order to assess age-related changes, this study followed a cross-sectional design. It based on archive data of the Department of Radiology, Riga East University Hospital, Clinical Center “Gailezers,” Latvia, in the period from October 2009 till November 2010. Archive data were available according to legal requirements.
The research sample included 211 females aged 18 to 84 years (mean±SD, 48.3±18.3) and 181 males aged 18 to 82 years (mean±SD, 43.6±16.1). The age sub-groups 18–39, 40–59, and older than 59 involved 73 females and 80 males, 73 females and 66 males, and 65 females and 35 males, respectively. The percentage of females and males in the age sub-groups was close to the proportion of the Latvian population. Indications for computed tomography (CT) examination were abdominal pain and abdominal inflammatory processes. Exclusion criteria were bones' fractures, transitional vertebras, and polytrauma. The number of participants provided an acceptable ratio of cases to independent variables suggested for a multiple linear regression [27].
Abdominal or pelvic examination was performed by a scanner (General Electric Medical Systems Light Speed, General Electric Healthcare, Waukesha, WI, USA) with scanning parameters established at 120 kV, 150–500 mA with slice thickness at 1.25 mm. The CT pelvimetry was performed on a three-dimensional workstation (Adwantage Workstation for Diagnostic Imaging, General Electric Healthcare) using multiplanar reconstruction and volume-rendered images. The CT pelvimetry was performed on pelvic images in the coronal and sagittal views. All measures were obtained by one investigator. Assessed in a subsample of 23 participants, the intra-observer reliability varied from 0.92 to 0.99 (Table 1).
Table 1. Intra-observer reliability and descriptive statistics for lesser pelvic measures in both sexes.
| Factor | Intra-observer reliability Pearson correlation (n=23) | Sex | |
|---|---|---|---|
| Female (n=211) | Male (n=181) | ||
| Transverse diameter (mm) | |||
| Inlet | 0.99*** | 135.0±8.5 | 126.8±7.0 |
| Midplane 1 (biacetabular) | 0.96*** | 122.1±8.3 | 113.8±7.2 |
| Midplane 2 (bispinous) | 0.96*** | 112.2±9.2 | 93.6±8.4 |
| Outlet (bituberous) | 0.98*** | 123.9±10.4 | 103.5±9.3 |
| Sagittal diameter (mm) | |||
| Inlet | 0.95*** | 124.6±10.3 | 119.2±10.2 |
| Midplane 1 | 0.92*** | 131.5±9.9 | 127.8±9.0 |
| Midplane 2 | 0.99*** | 122.8±8.7 | 116.5±7.5 |
| Outlet | 0.95*** | 99.9±9.8 | 96.0±7.4 |
| Pelvic angles (°) | |||
| Sacral slope | 0.93*** | 39±7 | 39±6 |
| Pelvic inclination | 0.97*** | 64±7 | 62±6 |
| Subpubic angle | 0.99*** | 132±12 | 94±12 |
| Pelvic indexes | |||
| Inlet | – | 0.93±0.10 | 0.94±0.08 |
| Midplane 1 | – | 1.08±0.10 | 1.13±0.10 |
| Midplane 2 | – | 1.10±0.10 | 1.25±0.14 |
| Outlet | – | 0.81±0.11 | 0.94±0.11 |
***P<0.001.
Sagittal and transverse diameters of the lesser pelvis were evaluated at the following levels: the level of the inlet (inlet plane); the level of acetabular centers (midplane 1); the level of ischial spines (midplane 2); the level of the outlet (outlet plane).
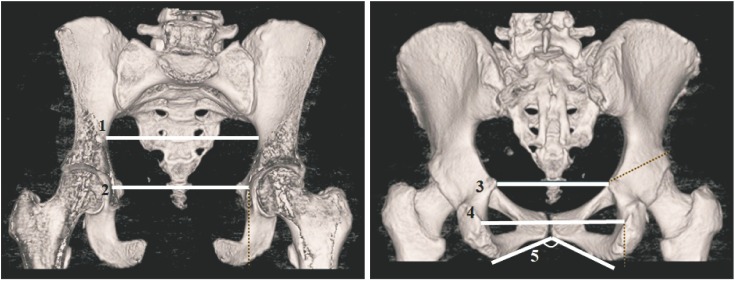
Transverse parameters of the lesser pelvis and one pelvic angle were measured in the coronal plane (Fig. 1): (1) transverse diameter of inlet, the widest distance between the arcuate lines; (2) transverse diameter of midplane 1 (biacetabular diameter), the distance between the centers of acetabulums; (3) transverse diameter of midplane 2 (bispinous diameter), the narrowest distance between two ischial spines; (4) transverse diameter of outlet (bituberous diameter), the widest distance between inner margins of the ischial tuberosities; and (5) subpubic angle, the angle formed by two inferior pubic ramus.
Fig. 1. 3D reformatted computed tomography images: 1, transverse diameter of inlet; 2, transverse diameter of midplane 1 (biacetabular diameter); 3, transverse diameter of midplane 2 (bispinous diameter); 4, transverse diameter of outlet (bituberous diameter); 5, subpubic angle.
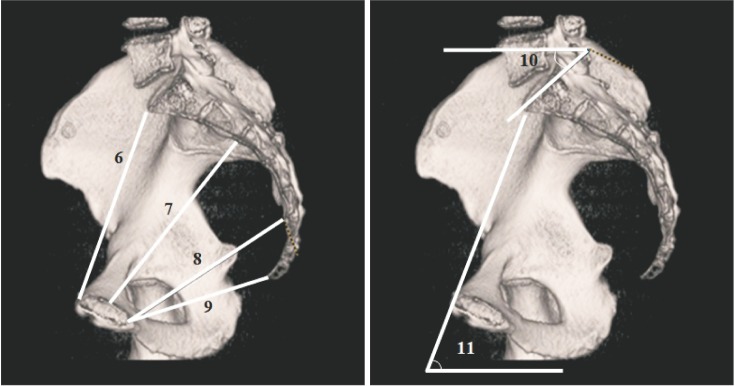
Sagittal pelvic measures and two pelvic angles were measured in the sagittal plane (Fig. 2): (6) sagittal diameter of inlet, the distance between anterosuperior border of the pubic symphysis and the promontory of the sacrum; (7) sagittal diameter of midplane 1, the distance between the posterior midpoint of pubic symphysis and the anterior point between the second and the third sacral vertebrae; (8) sagittal diameter of midplane 2, the distance between the inferior border of the pubic symphysis and the anterior point between the fourth and the fifth sacral vertebrae; (9) sagittal diameter of outlet, the distance between the inferior border of the pubic symphysis and the tip of the coccyx; (10) sacral slope, the angle between the superior surface of the first sacral vertebra and a horizontal plane; and (11) pelvic inclination, the angle between the pelvic inlet and a horizontal plane.
Fig. 2. 3D reformatted computed tomography images: 6, sagittal diameter of inlet; 7, sagittal diameter of midplane 1; 8, sagittal diameter of midplane 2; 9, sagittal diameter of outlet; 10, sacral slope; 11, pelvic inclination.
In accordance with Young and Ince [28], four indexes of the pelvic planes were calculated in order to assess proportions of the lesser pelvis as relatively independent of linear measures of the body.
| Index=(Sagittal diameter)/(Transverse diameter) |
Statistical analysis of age-related changes in both sexes was performed through a standard multiple linear regression between participants' age, sex, and their interaction as independent variables and each pelvic measure as a dependent one. In order to avoid multicollinearity among independent variables [27], the interaction was represented by the product of age (centered) by sex (centered). All computations were performed using the IBM SPSS Statistics program version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Table 1 demonstrates descriptive statistics for female and male subsamples. The Kolmogorov-Smirnov test with the Lilliefors significance correction indicated normality for all pelvic measures in females and males.
The regression model was tested for each dependent variable separately (Table 2). The variance inflation factor values varied from 1.02 to 1.06 that indicated acceptable level of multicollinearity among independent variables.
Table 2. Standard multiple regression of lesser pelvic measures on age, sex, and age-sex interaction.
| Variable | B | SE B | β | t |
|---|---|---|---|---|
| Transverse diameter | ||||
| Inlet: F(3,388)=42.011, P<0.001, R2=0.245 | ||||
| Age | 0.084 | 0.023 | 0.165 | 3.631*** |
| Sex | −7.795 | 0.788 | −0.441 | −9.891*** |
| Age×Sex | −0.042 | 0.046 | −0.041 | −0.913 |
| Midplane 1: F(3,388)=35.707, P<0.001, R2=0.217 | ||||
| Age | −0.003 | 0.024 | −0.007 | −0.145 |
| Sex | −8.271 | 0.806 | −0.467 | −10.263*** |
| Age×Sex | 0.010 | 0.047 | 0.010 | 0.216 |
| Midplane 2: F(3,388)=145.881, P<0.001, R2=0.530 | ||||
| Age | 0.067 | 0.026 | 0.091 | 2.541* |
| Sex | −18.232 | 0.903 | −0.710 | −20.185*** |
| Age×Sex | 0.057 | 0.053 | 0.039 | 1.087 |
| Outlet: F(3,388)=144.056, P<0.001, R2=0.527 | ||||
| Age | −0.086 | 0.029 | −0.106 | −2.946** |
| Sex | −20.877 | 1.005 | −0.733 | −20.771*** |
| Age×Sex | 0.002 | 0.059 | 0.001 | 0.034 |
| Sagittal diameter | ||||
| Inlet: F(3,388)=46.808, P<0.001, R2=0.266 | ||||
| Age | −0.264 | 0.027 | −0.436 | −9.724*** |
| Sex | −6.584 | 0.929 | −0.312 | −7.090*** |
| Age×Sex | 0.078 | 0.054 | 0.064 | 1.445 |
| Midplane 1: F(3,388)=6.903, P<0.001, R2=0.051 | ||||
| Age | −0.061 | 0.028 | −0.110 | −2.157* |
| Sex | −4.026 | 0.966 | −0.208 | −4.169*** |
| Age×Sex | 0.023 | 0.056 | 0.021 | 0.411 |
| Midplane 2: F(3,388)=19.302, P<0.001, R2=0.133 | ||||
| Age | −0.014 | 0.025 | −0.029 | −0.581 |
| Sex | −6.381 | 0.845 | −0.365 | −7.548*** |
| Age×Sex | −0.051 | 0.049 | −0.050 | −1.031 |
| Outlet: F(3,388)=17.466, P<0.001, R2=0.119 | ||||
| Age | 0.126 | 0.025 | 0.245 | 4.990*** |
| Sex | −3.364 | 0.863 | −0.188 | −3.900*** |
| Age×Sex | −0.075 | 0.050 | −0.072 | −1.489 |
| Pelvic indexes | ||||
| Index of inlet: F(3,388)=46.607, P<0.001, R2=0.265 | ||||
| Age | −0.003 | 0.000 | −0.493 | −10.992*** |
| Sex | 0.003 | 0.008 | 0.016 | 0.362 |
| Age×Sex | 0.001 | 0.000 | 0.073 | 1.639 |
| Index of midplane 1: F(3,388)=7.631, P<0.001, R2=0.056 | ||||
| Age | 0.000 | 0.000 | −0.085 | −1.669 |
| Sex | 0.042 | 0.010 | 0.209 | 4.179*** |
| Age×Sex | 0.000 | 0.001 | 0.013 | 0.251 |
| Index of midplane 2: F(3,388)=55.967, P<0.001, R2=0.307 | ||||
| Age | −0.001 | 0.000 | −0.130 | −2.946** |
| Sex | 0.147 | 0.012 | 0.515 | 11.898*** |
| Age×Sex | −0.002 | 0.001 | −0.095 | −2.174* |
| Index of outlet: F(3,388)=53.705, P<0.001, R2=0.294 | ||||
| Age | 0.002 | 0.000 | 0.242 | 5.498*** |
| Sex | 0.131 | 0.011 | 0.517 | 11.976*** |
| Age×Sex | 0.000 | 0.001 | −0.015 | −0.350 |
| Pelvic angles | ||||
| Sacral slope: F(3,388)=11.044, P<0.001, R2=0.079 | ||||
| Age | 0.105 | 0.019 | 0.276 | 5.487*** |
| Sex | 0.581 | 0.656 | 0.044 | 0.886 |
| Age×Sex | −0.023 | 0.038 | −0.030 | −0.596 |
| Pelvic inclination: F(3,388)=21.907, P<0.001, R2=0.148 | ||||
| Age | −0.129 | 0.019 | −0.338 | −6.915*** |
| Sex | −3.085 | 0.639 | −0.232 | −4.826*** |
| Age×Sex | 0.010 | 0.037 | 0.013 | 0.272 |
| Subpubic angle: F(3,388)=391.263, P<0.001, R2=0.753 | ||||
| Age | −0.249 | 0.034 | −0.194 | −7.435*** |
| Sex | −39.077 | 1.146 | −0.872 | −34.089*** |
| Age×Sex | 0.029 | 0.067 | 0.011 | 0.435 |
*P<0.05, **P<0.01, ***P<0.001.
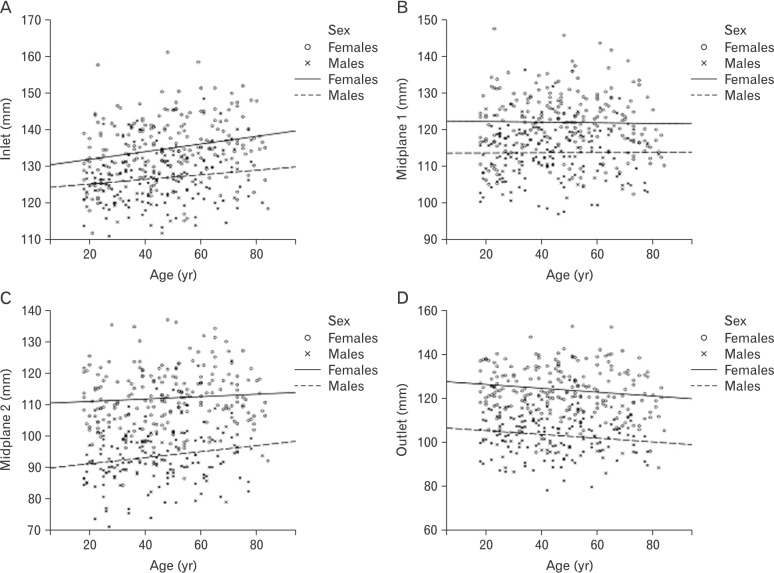
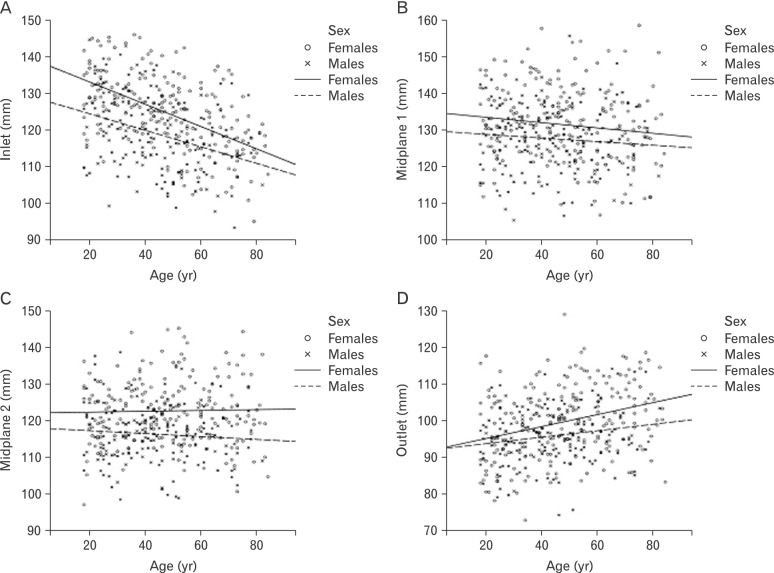
Females were higher on transverse (Fig. 3) and sagittal (Fig. 4) diameters of the lesser pelvis than males, but age-related trends were similar in both sexes. Transverse diameter of inlet and transverse diameter of midplane 2 (bispinous diameter) increased with age. Transverse diameter of outlet (bituberous diameter) decreased with age. Transverse diameter of midplane 1 (biacetabular diameter) demonstrated no age-related changes. Sagittal diameter of inlet and sagittal diameter of midplane 1 decreased with age. Sagittal diameter of outlet increased with age. Sagittal diameter of midplane 2 demonstrated no age-related changes.
Fig. 3. Age-related trend lines for transverse diameters of the lesser pelvis in females and males. (A) Transverse diameter of inlet. (B) Transverse diameter of midplane 1. (C) Transverse diameter of midplane 2. (D) Transverse diameter of outlet.
Fig. 4. Age-related trend lines for sagittal diameters of the lesser pelvis in females and males. (A) Sagittal diameter of inlet. (B) Sagittal diameter of midplane 1. (C) Sagittal diameter of midplane 2. (D) Sagittal diameter of outlet.
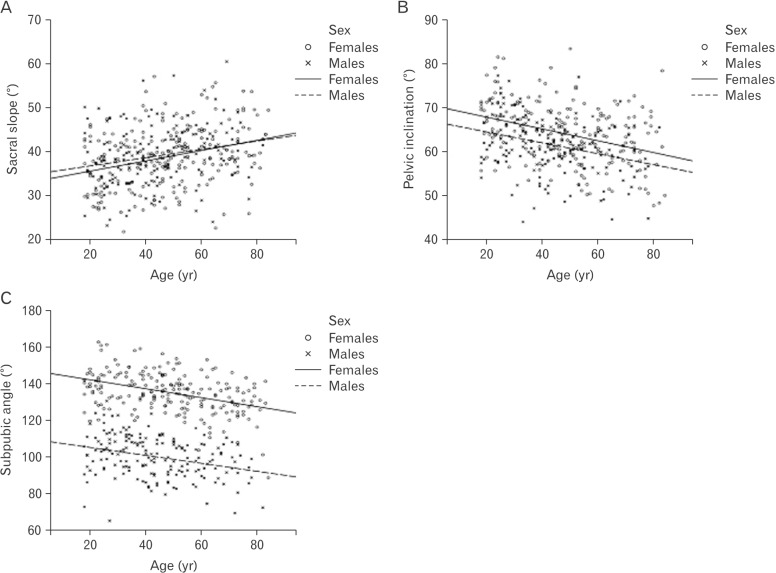
Similarly, age-related trends were common in pelvic angles (Fig. 5). Sacral slope increased with age, while subpubic angle and pelvic inclination decreased with age. Females were higher on subpubic angle and pelvic inclination. There were no significant differences between males and females on the sacral slope.
Fig. 5. Age-related trend lines for pelvic angles in females and males. (A) Sacral slope. (B) Pelvic inclination. (C) Subpubic angle.
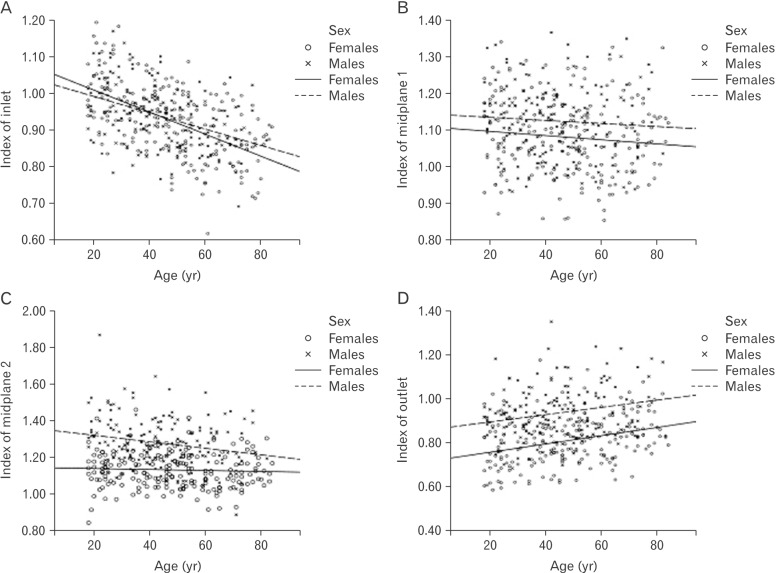
Indexes of inlet and of midplane 2 decreased with age (Fig. 6). Index of midplane 1 was not related to age. Index of outlet increased with age. Index of inlet demonstrated no sexual dimorphism, while indexes of midplane 1, midplane 2, and outlet were higher in males.
Fig. 6. Age-related trend lines for pelvic indexes. (A) Index of inlet. (B) Index of midplane 1. (C) Index of midplane 2. (D) Index of outlet.
In addition to the main effects, index of midplane 2 had a significant age-sex interaction indicating more pronounced decrease with age in males than in females. An estimation through a simple linear regression indicated no significant changes of the index with age in the female sample (β=−0.049, t=−0.709, P=0.479) and its decrease in the male sample (β=−0.214, t=−2.892, P=0.004). For other pelvic dimensions, an absence of the interaction confirmed similar slopes of regression lines in both sexes.
Discussion
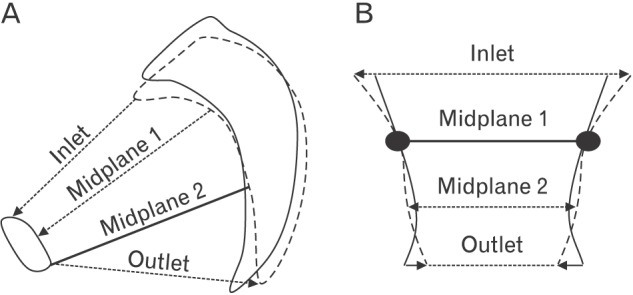
Our findings demonstrated that age-related changes (Fig. 7) were similar to changes of pelvic parameters during a regular nutation of the sacrum. As the regular nutation was described in details [2,3,7], the sagittal diameter of the inlet decreased, the sagittal diameter of the outlet increased, the transverse diameter of the inlet increased, and ischial spines diverged because of anterior tilting of the sacral base into the pelvic cavity. Observed age-related tendencies in linear parameters of the pelvic cavity confirmed anterior tilting of the sacral base and fixing of the sacrum in a more horizontal position with aging. As it was found previously [10,13], age-related ankylotic processes decreased mobility of the sacroiliac joint and facilitated these changes. In our study, increase of the sacral slope confirmed more horizontal orientation of the sacrum with aging and supported the results of other studies [9,10].
Fig. 7. Age-related changes of pelvic measures in the sagittal (A) and coronal (B) planes (dashed lines show a changing position of pelvic bones with age, while dotted lines with arrows indicate changing diameters).
A significant tendency was found in the transverse diameter of the outlet. Our results demonstrated that the distance between ischial tuberosities (bituberous diameter) shortened with age. These changes can be related to life-long adaptation of the pelvic bone system for a sitting position associated with an increasing load on ischial tuberosities [1,6]. Age-related decrease of the subpubic angle was in line with narrowing of the bituberous diameter.
The observed increase of the transverse diameter of the inlet and decrease of the transverse diameter of the outlet provided an evidence for more triangular lesser pelvic shape in the coronal plane, which was described as an advantage for efficient bipedal locomotion [4]. Therefore, age-related changes can be associated with both an adaptation to the sitting position and a compensation of changes in spino-pelvic balance impacted gait stability in elderly people [5].
The transverse diameter of midplane 1 (biacetabular diameter) was a special case in the analysis of measures in the coronal plane. The regression analysis confirmed independence of this diameter on age and its dependence on gender (higher in females than in males). Therefore, the distance between pelvic rotation centers in the coronal plane remained unchanged despite revealed age-related changes of the pelvic cavity at other levels. The previous works showed significance of midplane 1 in bipedal locomotion [1,4,7]: the body weight distributed evenly on two extremities through the acetabular centers [1]; flexion and extension of the torso occurred around the axis, which passed between both hip joints [1,7]. Therefore, the cavity width at this level was affected by adapting to the vertical posture in order to ensure a better balance and effective movement based on two extremities [4]. As a result, we have assumed that the age-independent distance at the level of centers of hip joints formed a stable element of the biomechanical system of bipedal locomotion in both females and males.
The analysis of the pelvic indexes confirmed that the pelvic cavity slightly flattened in the transverse direction at the inlet, while it lengthened in the sagittal direction at the level of the outlet. The shape of the pelvic cavity at the level of acetabular centers did not change with age. The shape of the inlet and outlet changed in a concordant way in females and males. The common changes of pelvic measures in females and males can be related to genetic similarity of homological structures [29], which is expressed during aging of the pelvic bone system.
The shape of the cavity at the level of ischial spines flattened in the transverse direction in males only. The observed age-sex interaction was a single exception of the common age-related trends revealed for both sexes. Unchanging cavity proportions at the level of ischial spines in females can be explained by more intensive evolutionary adaptation in females, which affected their pelvic cavity at the level of its narrowest plane [14,16]. Probably, this additional adaptation resulted in a higher genetic determination preserving the proportion of the narrowest plane in the pelvic cavity as independent of age.
From a practical perspective, our study demonstrated that age-related changes of the pelvic inlet, distance between ischial spines, and sagittal diameter of the outlet occurred in a direction associated with pelvic floor pathology [11,23,24,25,26]. The further clinical studies are needed in order to assess an independent contribution of changes in the pelvic bone system.
It should be noted that a cross-sectional design limited exploration of individual trajectories of change. However, this design was considered as a typical limitation in anthropological studies [18]. Implementation of a longitudinal design was limited by ethical issues concerning life-long ionizing radiation doses without medical indications. Another limitation of our study was related to different clinical status of participants undergoing the CT investigation in the hospital. Regulations for personal data protection limited accessibility of information on possible pelvic floor diseases, the number and outcome of childbirth, or pain in the lumbar and sacral regions. Possible degenerative processes in the spine or pelvic floor disorders should be taken into account for the further study focusing on differentiation of changes in clinical and asymptomatic groups.
In sum, common aging trends in the pelvic architecture were observed in females and males. In females, unchanging proportions of the lesser pelvis at the level of ischial spines provided an evidence for additional evolutionary pressure. The most part of linear pelvic measures was dependent on age, except for the distance between acetabular centers and the sagittal diameter at the level of ischial spines. These changes can be considered as the result of influence of vertical posture, sitting, and reduced mobility in the sacroiliac joint.
References
- 1.Pauwels F. Beitrag zur Klärung der Beanspruchung des Beckens, insbesondere der Beckenfugen. Z Anat Entwicklungsgesch. 1948;114:167–180. [PubMed] [Google Scholar]
- 2.Weisl H. The movements of the sacroiliac joint. Acta Anat (Basel) 1955;23:80–91. [PubMed] [Google Scholar]
- 3.Russell JG. Moulding of the pelvic outlet. J Obstet Gynaecol Br Commonw. 1969;76:817–820. doi: 10.1111/j.1471-0528.1969.tb06185.x. [DOI] [PubMed] [Google Scholar]
- 4.Lovejoy CO. Evolution of human walking. Sci Am. 1988;259:118–125. doi: 10.1038/scientificamerican1188-118. [DOI] [PubMed] [Google Scholar]
- 5.Van Emmerik RE, McDermott WJ, Haddad JM, Van Wegen EE. Age-related changes in upper body adaptation to walking speed in human locomotion. Gait Posture. 2005;22:233–239. doi: 10.1016/j.gaitpost.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Moes NC. Variation in sitting pressure distribution and location of the points of maximum pressure with rotation of the pelvis, gender and body characteristics. Ergonomics. 2007;50:536–561. doi: 10.1080/00140130601138585. [DOI] [PubMed] [Google Scholar]
- 7.Kapandji A. The physiology of the joints. 6th ed. Vol. 3. Edinburg: Elsevier Limited; 2008. pp. 60–63. The spinal column, pelvic girdle and head. [Google Scholar]
- 8.Schwab F, Lafage V, Boyce R, Skalli W, Farcy JP. Gravity line analysis in adult volunteers: age-related correlation with spinal parameters, pelvic parameters, and foot position. Spine (Phila Pa 1976) 2006;31:E959–E967. doi: 10.1097/01.brs.0000248126.96737.0f. [DOI] [PubMed] [Google Scholar]
- 9.Amonoo-Kuofi HS. Changes in the lumbosacral angle, sacral inclination and the curvature of the lumbar spine during aging. Acta Anat (Basel) 1992;145:373–377. doi: 10.1159/000147392. [DOI] [PubMed] [Google Scholar]
- 10.Peleg S, Dar G, Medlej B, Steinberg N, Masharawi Y, Latimer B, Jellema L, Peled N, Arensburg B, Hershkovitz I. Orientation of the human sacrum: anthropological perspectives and methodological approaches. Am J Phys Anthropol. 2007;133:967–977. doi: 10.1002/ajpa.20599. [DOI] [PubMed] [Google Scholar]
- 11.Lazarevski MB. Pelvic bone system changes and pathogenesis of genital prolapse: a radiopelvimetric study. Gynaecol Perinatol. 2004;13:1–12. [Google Scholar]
- 12.Kampen WU, Tillmann B. Age-related changes in the articular cartilage of human sacroiliac joint. Anat Embryol (Berl) 1998;198:505–513. doi: 10.1007/s004290050200. [DOI] [PubMed] [Google Scholar]
- 13.Dar G, Peleg S, Masharawi Y, Steinberg N, Rothschild BM, Peled N, Hershkovitz I. Sacroiliac joint bridging: demographical and anatomical aspects. Spine (Phila Pa 1976) 2005;30:E429–E432. doi: 10.1097/01.brs.0000172232.32082.e0. [DOI] [PubMed] [Google Scholar]
- 14.Abitbol MM. Obstetrics and posture in pelvic anatomy. J Hum Evol. 1987;16:243–255. [Google Scholar]
- 15.Schultz AH. Sex differences in the pelves of primates. Am J Phys Anthropol. 1949;7:401–423. doi: 10.1002/ajpa.1330070307. [DOI] [PubMed] [Google Scholar]
- 16.Tague RG. Variation in pelvic size between males and females. Am J Phys Anthropol. 1989;80:59–71. doi: 10.1002/ajpa.1330800108. [DOI] [PubMed] [Google Scholar]
- 17.Damasceno LH, Catarin SR, Campos AD, Defino HL. Lumbar lordosis: a study of angle values and of vertebral bodies and intervertebral discs role. Acta Ortop Bras. 2006;14:193–198. [Google Scholar]
- 18.Mac-Thiong JM, Roussouly P, Berthonnaud E, Guigui P. Age- and sex-related variations in sagittal sacropelvic morphology and balance in asymptomatic adults. Eur Spine J. 2011;20(Suppl 5):572–577. doi: 10.1007/s00586-011-1923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG. 2000;107:1460–1470. doi: 10.1111/j.1471-0528.2000.tb11669.x. [DOI] [PubMed] [Google Scholar]
- 20.Stav K, Alcalay M, Peleg S, Lindner A, Gayer G, Hershkovitz I. Pelvis architecture and urinary incontinence in women. Eur Urol. 2007;52:239–244. doi: 10.1016/j.eururo.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Mallett VT, Bump RC. The epidemiology of female pelvic floor dysfunction. Curr Opin Obstet Gynecol. 1994;6:308–312. [PubMed] [Google Scholar]
- 22.DeLancey JO, Kearney R, Chou Q, Speights S, Binno S. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101:46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. Computed tomography comparison of bony pelvis dimensions between women with and without genital prolapse. Obstet Gynecol. 1999;93:229–232. doi: 10.1016/s0029-7844(98)00376-7. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen JK, Lind LR, Choe JY, McKindsey F, Sinow R, Bhatia NN. Lumbosacral spine and pelvic inlet changes associated with pelvic organ prolapse. Obstet Gynecol. 2000;95:332–336. doi: 10.1016/s0029-7844(99)00561-x. [DOI] [PubMed] [Google Scholar]
- 25.Handa VL, Pannu HK, Siddique S, Gutman R, VanRooyen J, Cundiff G. Architectural differences in the bony pelvis of women with and without pelvic floor disorders. Obstet Gynecol. 2003;102:1283–1290. doi: 10.1016/j.obstetgynecol.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Xu HN, Xia ZJ, Li BX, Yin YT, Wang F, Hu Q, Zhao Y. Investigation of correlation between diameters of pelvic inlet and outlet planes and female pelvic floor dysfunction. Eur J Obstet Gynecol Reprod Biol. 2011;159:461–464. doi: 10.1016/j.ejogrb.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- 28.Young M, Ince JG. A radiographic comparison of the male and female pelvis. J Anat. 1940;74(Pt 3):374–385. [PMC free article] [PubMed] [Google Scholar]
- 29.Tague RG. Do big females have big pelves? Am J Phys Anthropol. 2000;112:377–393. doi: 10.1002/1096-8644(200007)112:3<377::AID-AJPA8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]