Abstract
Articular cartilage does not heal spontaneously due to its limited healing capacity, and thus effective treatments for cartilage injuries has remained challenging. Since the first report by Brittberg et al. in 1994, autologous chondrocyte implantation (ACI) has been introduced into the clinic. Recently, as an alternative for chondrocyte-based therapy, mesenchymal stem cell (MSC)-based therapy has received considerable research attention because of the relative ease in handling for tissue harvest, and subsequent cell expansion and differentiation. In this review, we discuss the latest developments regarding stem cell-based therapies for cartilage repair, with special focus on recent scaffold-free approaches.
Keywords: Scaffold-free, Mesenchymal stem cell, Cartilage repair
Background
Dealing with articular cartilage injuries is quite common in the fields of orthopaedic surgery and musculoskeletal medicine. Due to its avascular and aneural surroundings, as well as its relatively unique matrix organization, articular cartilage does not heal in response to the currently available treatments, and subsequently, the injury may lead to the development of osteoarthritis. Therefore, a variety of approaches have been tested to improve cartilage healing over the past few decades (Huang et al. 2016; Vavken and Samartzis 2010; Krych et al. 2016).
Since the first report of autologous chondrocyte implantation (ACI) was published in 1994 by Brittberg et al. (Brittberg et al. 1994), chondrocyte-based therapies have been extensively studied, some of which have been successfully introduced into the clinic (Niemeyer et al. 2014; Goyal et al. 2013; Steinwachs and Kreuz 2007). On the other hand, these procedures have some limitations including the sacrifice of undamaged cartilage within the same joint, as well as potential cellular alterations associated with the in vitro expansion of the chondrocytes. Furthermore, due to the degenerative changes in cartilage that can accompany aging, the availability of cells may be limited in elderly individuals, both quantitatively and qualitatively (Hickery et al. 2003).
To overcome such potential problems, stem cell-based therapies have been the focus of attention to facilitate regenerative tissue repair. Mesenchymal stem cells (MSCs) have the capability to differentiate into a variety of connective tissue cells including bone, cartilage, tendon, muscle, and adipose tissue (Pittenger et al. 1999). These cells can be isolated from various tissues such as bone marrow, skeletal muscle, synovial membrane, adipose tissue, and umbilical cord blood (Pittenger et al. 1999; Jankowski et al. 2002; De Bari et al. 2001; Sakaguchi et al. 2005; Wickham et al. 2003; Lee et al. 2004), as well as synovial fluid (Ando et al. 2014). MSCs isolated from synovium may be well suited for cell-based therapies for cartilage because of the relative ease of harvest and their strong capability for chondrogenic differentiation (De Bari et al. 2001). Among mesenchymal tissue-derived cells, synovium-derived cells are reported to exhibit the greatest chondrogenic potential (Sakaguchi et al. 2005). As other options for a cell source, allogeneic synovial or bone marrow MSCs (Shimomura et al. 2010; Dashtdar et al. 2011) or induced pluripotent stem (iPS) cells (Takahashi and Yamanaka 2006; Tsumaki et al. 2015) have been assessed. However, not much conclusive evidence using these cells has yet been forth coming in terms of preclinical and clinical safety, and thus further studies with such cells are likely still required.
With the advancement of cell-based technologies, many of the recent tissue engineering approaches have taken advantage of scaffolds made of either synthetic (Vunjak-Novakovic et al. 1999; Andriano et al. 1999; Guo et al. 1989; Masuda et al. 2003) or natural polymers (Lee et al. 2001; Homminga et al. 1993; Brun et al. 1999; Lahiji et al. 2000; Shimomura et al. 2014) as the supporting biomaterials, providing an appropriate three-dimensional (3D) environment for cells to optimize their proliferation and chondrogenic differentiation (De Bari et al. 2004), and effectively delivering cells into cartilage defects (Bright and Hambly 2014; Gobbi et al. 2009). This technique is robust and easy for surgeons to handle, and was reported to significantly improve the healing of cartilage defects. Interestingly, a recent systematic review indicated that the scaffold-based ACI methodology using collagen scaffold (Matrix-induced ACI; MACI) provided better clinical results than did the use of chondrocytes alone, but the evidence is still not strong (Goyal et al. 2013). On the other hand, there are still several issues associated with the long-term safety and efficacy of these materials. Synthetic polymers may have potential problems regarding retention and degradation in situ (Daniels et al. 1994; van der Elst et al. 1999). Biological materials potentially carry the risk of transmission of infectious agents and initiating immunological reactions (Yang et al. 2004; Martin et al. 2005). Taken together, and in order to minimize unknown risk, such materials may ideally be excluded throughout the treatment procedure. However, there have been no direct comparative studies of scaffold-based and scaffold-free approaches reported and thus, it is still unknown which approache(s) would provide better long-term outcomes. In spite of this limitations, a scaffold-free cell delivery system may be an excellent alternative due to its simplicity in development and ease of subsequent implantation. In this review, we discuss the latest developments regarding cell-based therapies for cartilage repair, focusing specifically on recent scaffold-free approaches, in the first section. Subsequently, we focus the second half of the review on our experiences regarding development of a unique MSC-based scaffold-free approach, taking a bench to bedside for clinical application approach.
Review
Recent studies of scaffold-free cell-based therapies for cartilage repair
We searched MEDLINE for reports published in English up to November, 2017, using the terms “scaffold free”, “scaffold less” and “cartilage repair”, with the exception of studies using simple intra-articular cell injection. Additionally, we collected related researches at second hand as far as possible. We identified 48 publications, of which 26 were related to in-vitro experiments, 21 reported in-vivo tests, and one was a clinical study as listed in Table 1. Also, three review articles were identified (Shimomura et al. 2015; Yasui et al. 2016; DuRaine et al. 2015).
Table 1.
Summary of scaffold-free cell-based therapies for cartilage repair
| Authors | Journal | Year | Experimental Model | Scaffold-free Technique | Shape | Cell Source | Matrix | Amount of Cells | Defect Size (in vivo) | Graft fixation (in vivo) |
|---|---|---|---|---|---|---|---|---|---|---|
| Adkisson HD | Clin Orthop Relat Res | 2001 | in vitro | High-density in Monolayer | Disc | Chondrocyte | Catilage-like | 2 × 105/cm2 per plate | N/A | N/A |
| Mainil-Varlet P, et al. | Osteoarthritis Cartilage | 2001 | Minipig | Bioreactor | Spheroid | Chondrocyte | Catilage-like | 2 × 107 | 4 mm diameter(Full-thickness cartilage defect) | Press-fit |
| Anderer U, et al. | J Bone Miner Res | 2002 | in vitro | Self Aggregating | Spheroid | Chondrocyte | Catilage-like | 2 × 105 | N/A | N/A |
| Masuda K, et al. | J Orthop Res | 2003 | in vitro | Alginate Recovered Chondrocyte Method | Sheet | Chondrocyte | Catilage-like | 4 × 106per mL | N/A | N/A |
| Lu Y, et al. | J Knee Surg | 2005 | Sheep | High-density in Monolayer | Disc | Chondrocyte | Catilage-like | 2 × 105/cm2 per plate | 5.5 mm diameter 400–500 μm depth | Suture |
| Stoddart MJ, et al. | J Cell Mol Med | 2006 | in vitro | Cell Pellet After Culture in Alginate | Disc | Chondrocyte | Catilage-like | 4 × 106per mL | N/A | N/A |
| Stoddart MJ, et al. | Biotechnol Bioeng | 2006 | in vitro | Cell Pellet After Culture in Alginate | Spheroid | Chondrocyte | Catilage-like | 4 × 106per mL | N/A | N/A |
| Kaneshiro N, et al. | Biochem Biophys Res Commun | 2006 | in vitro | Layered Chondrocyte Sheet | Sheet | Chondrocyte | Catilage-like | 1 × 104per cm2 | N/A | N/A |
| Hu JC, et al. | Tissue Eng | 2006 | in vitro | High-density in Monolayer | Disc | Chondrocyte | Catilage-like | 5.5 × 106 | N/A | N/A |
| Park K, et al. | Artificial Organs | 2006 | Pig | High-density in Monolayer | Disc | Chondrocyte | Catilage-like | 1.9 × 105/cm2per plate | 6 mm diameter 3–4 mm depth | Bovine collagen gel |
| Brehm W, et al. | Osteoarthritis Cartilage | 2006 | Goat | Bioreactor | Disc | Chondrocyte | Catilage-like | 2 × 107 | 6 mm diameter 0.8 mm depth | Periosteal falp, PRP, Fibrin |
| Hayes AJ, et al. | J Histochem Cytochem | 2007 | in vitro | High-density in Monolayer | Sheet | Chondrocyte | Catilage-like | 6 × 106 | N/A | N/A |
| aAndo W, et al. | Biomaterials | 2007 | Pig | High-density in Monolayer | Sheet | Synovial MSC | Collagen I, III-rich | 4 × 105/cm2per plate | 8.5 mm diameter2 mm depth | Nether glue nor suture |
| Murdoch AD, et al. | Stem Cells | 2007 | in vitro | Transwell Culture | Disc | Bone Marrow MSC | Catilage-like | 5 × 105 | N/A | N/A |
| aAndo W, et al. | Tissue Eng Part A | 2008 | in vitro | High-density in Monolayer | Sheet | Synovial MSC | Catilage-like | 4 × 105/cm2per plate | N/A | N/A |
| Ofek G, et al. | ProS One | 2008 | in vitro | High-density in Monolayer | Spheroid | Chondrocyte | Catilage-like | 5.5 × 106 | N/A | N/A |
| Nagai T, et al. | Tissue Eng Part A | 2008 | Rabbit | High-density in Monolayer | Disc | Chondrocyte | Catilage-like | 6 × 106 | 5 mm diameter3 mm depth | Nether glue nor suture |
| Jubel A, et al. | Am J Sports Med | 2008 | Sheep | Cell Pellet After Culture in Alginate | Disc | Chondrocyte | Catilage-like | 4 × 106 per mL | 4 mm diameter(Full-thickness cartilage defect) | Periosteal flap |
| Schubert T, et al. | Int J Mol Med | 2009 | Mouse | Self Aggregating | Spheroid | Chondrocyte | Catilage-like | 2 × 105 | 5 mm diameter3-4 mm depth(Human cartilageco-implant model) | Nether glue nor suture |
| Lewis PB, et al. | J Knee Surg | 2009 | Goat | High-density in Monolayer | Disc | Chondrocyte | Catilage-like | 2 × 105/cm2per plate | 4 or 6 mm diameter | Fibrin glue |
| aShimomura K, et al. | Biomaterials | 2010 | Pig | High-density in Monolayer | Sheet | Synovial MSC | Collagen I, III-rich | 4 × 105/cm2per plate | 8.5 mm diameter2 mm depth | Nether glue nor suture |
| Kraft JJ, et al. | Cartilage | 2011 | in vitro | Self Aggregating | Disc | Chondrocyte | Catilage-like | 2 × 107per mL | N/A | N/A |
| Zhang B, et al. | Tissue Eng Part C | 2011 | in vitro | Self Aggregating | Round or Oval | Bone MarrowMSC | Catilage-like | 2.5 × 107per mL | N/A | N/A |
| Maeda S, et al. | J Biosci Bioeng | 2011 | in vitro | Culture on Membrane (polyethylene terephthalate) | Disc | Bone MarrowMSC | Catilage-like | 3.1 × 106 | N/A | N/A |
| Cheuk YC, et al. | J Orthop Res | 2011 | Rabbit | Pellet Culture | Spheroid | AllogenicChondrocyte | Not assessed | 5 × 105 | 3 mm diameter3 mm depth | Press-fit |
| Yoshioka T, et al. | J Tissue Eng Regen Med | 2011 | Rabbit | Rotatory Culture(Bioreactor) | Spheroid | Bone MarrowMSC | Mainly hyaline-like appearance | 1.5 - 3.0 × 107 | 5 mm diameter4 mm depth | Nether glue nor suture |
| Qu C, et al. | Cell Tissue Res | 2012 | in vitro | Transwell Culture | Disc | Chondrocyte | Catilage-like | 6 × 106 | N/A | N/A |
| aAndo W, et al. | Eur Cell Mater | 2012 | Pig | High-density in Monolayer | Sheet | Synovial MSC | Collagen I, III-rich | 4 × 105/cm2per plate | 8.5 mm diameter2 mm depth | Nether glue nor suture |
| Ebihara G, et al. | Biomaterials | 2012 | Minipig | Layered Chondrocyte Sheet | Sheet | Chondrocyte | Catilage-like | 5 × 104per cm2 | 6 mm diameter5 mm depth | Suture |
| Sato Y, et al. | J Biosci Bioeng | 2013 | in vitro | Culture on Membrane (polyethylene terephthalate) | Disc | Bone MarrowMSC | Catilage-like | 1.86 × 106 | N/A | N/A |
| Brenner JM, et al. | Biotechnol Prog | 2013 | in vitro | Bioreactor | Sheet | Chondrocyte | Catilage-like | 2 × 104 | N/A | N/A |
| Giardini-Rosa R, et al. | Tissue Eng Part A | 2014 | in vitro | Bioreactor | Sheet | Chondrocyte | Catilage-like | 6.5 or 13 × 103per cm2 | N/A | N/A |
| Mohanraj B, et al. | J Biomech | 2014 | in vitro | High-density in Monolayer | Disc | Chondrocyte | Catilage-like | 1 × 106 | N/A | N/A |
| Liu T, et al. | Tissue Eng Part A | 2014 | in vitro | Self Assembling | Disc | Fat Pad-derivedStem Cell | Catilage-like | 3.8 × 106 | N/A | N/A |
| Ylärinne JH, et al. | Cell Tissue Res | 2014 | in vitro | Transwell Culture | Disc | Chondrocyte | Catilage-like | 6 × 106 | N/A | N/A |
| Oda K, et al. | J Orthop Sci | 2014 | Rabbit | High-density in Monolayer | Disc | Chondrocyte | Catilage-like | 6 × 106per cm2 | 4 mm diameter(Osteochondral defect) | Not addressed |
| Ishihara K, et al. | J Orthop Surg Res | 2014 | Rabbit | Spheroid Formation | Cylinder | Bone MarrowMSC | Not stained with Safranion O | 4 × 104per well | 4.8 mm diameter4-5 mm depth | Nether glue nor suture |
| Brenner JM, et al. | Artificial Organs | 2014 | Rabbit | Bioreactor | Sheet | Chondrocyte | Catilage-like | 2 × 104 | 4 mm diameter(Chondral only defect) | Fibrin glueand Suture |
| aFujie H, et al. | J Biomech | 2015 | Pig | High-density in Monolayer | Sheet | Synovial MSC | Collagen I, III-rich | 4 × 105/cm2per plate | 8.5 mm diameter2 mm depth | Nether glue nor suture |
| Yamashita A, et al. | Stem Cell Reports | 2015 | Minipig | Staged Differentiation Media | Spheroid | iPSC | Catilage-like | 1-2 × 105 | Not addressed | Fibrin glue |
| He P, et al. | Acta Biomater | 2016 | in vitro | Culture on Alginate-based Micro-cavity Hydrogel | Disc | iPSC | Catilage-like | 1 × 107per mL | N/A | N/A |
| Islam A, et al. | Eur Cell Mater | 2016 | in vitro | Pellet Culture | Spheroid | Umbilical Cord MSC | Catilage-like | 5 × 104 | N/A | N/A |
| aYasui Y, et al. | Tissue Eng Part A | 2016 | in vitro | High-density in Monolayer | Sheet | Synovial MSC | Catilage-like | 4 × 105/cm2per plate | N/A | N/A |
| Kimura T, et al. | Tissue Eng Part A | 2016 | in vitro | Staged Differentiation Media | Spheroid | iPSC | Catilage-like | 1-2 × 105 | N/A | N/A |
| Bartz C, et all. | J Transl Med | 2016 | ex vivo | Self Aggregating | Spheroid | Chondrocyte | Catilage-like | 2 × 105 | 4 mm diameter | Nether glue nor suture |
| aKoizumi K, et al. | OsteoarthritisCartilage | 2016 | Rat | High-density in Monolayer | Sheet | Synovial MSC | Collagen I, III-rich | 4 × 105/cm2per plate | 1.5 mm diameter1 mm depth | Nether glue nor suture |
| Itokazu M, et al. | Cartilage | 2016 | Rat | Culture on Membrane (polyethylene terephthalate) | Disc | Bone MarrowMSC | Catilage-like | 1.86 × 106 | 2 mm diameter1 mm depth | Fibrin glue |
| Becher C, et al. | J Orthop Surg Res | 2017 | Clinical | Self Aggregating | Spheroid | Chondrocyte | Catilage-like | 2 × 105 | 4 - 10 cm2ICRS grade III or IV | Not addressed |
MSC mesencymal stem cell, PRP platelet rich plasma, iPSC induced pluripotent stem cell
a:Our study
To date, there have been several scaffold-free techniques developed. DuRaine et al. defined such techniques to divide two categories, self-organization and self-assembly, according to the fabrication method (DuRaine et al. 2015). Self-organization describes a process in which order appears when external energy or forces are input into the system, including bioprinting and cell-sheet engineering. Also, cell aggregates are commonly formed in culture by applying a rotational force to cells in suspension or other non-adherent culture conditions, and thus that is categorized as a self-organization. On the other hand, a self-assembling process is a tissue engineering technique that does not employ external forces to form tissues. The process of tissue maturation follows a course similar to that of native cartilage development, in which cellular interactions and coalescence (e.g. high-density cell culture) are driven by spontaneous minimization of free energy, and then tissue-specific extracellular matrix (ECM) is produced to form functional tissue via maturation process.
Regarding cell selection in a scaffold-free approach, chondrocytes have been mostly employed to generate neocartilage (Table 1). These cells readily produce their tissue-specific ECM, especially in 3D culture environment (Huang et al. 2016). On the other hand, the limited cell availability and dedifferentiation potential during cell expansion might be a concern as mentioned above, although chondrocyte-based neocartilage is reported to achieve biochemical and biomechanical values within the range for native cartilage (Mainil-Varlet et al. 2001; Mohanraj et al. 2014; Ebihara et al. 2012; Jubel et al. 2008). In addition, the implantation of such a neocartilage usually needs to be fixed with fibrin glue, suture, or a periosteal covering, since these tissues might not exhibit adhesive properties required to integrate with host cartilage (Brehm et al. 2006; Jubel et al. 2008; Lewis et al. 2009; Ebihara et al. 2012). As an alternative, MSCs and iPS cells have been recently tested (Table 1), and an engineered tissue generated from these cells showed feasibility for cartilage repair comparable to chondrocyte-based tissues (Murdoch et al. 2007; Ishihara et al. 2014; Yamashita et al. 2015).
Interestingly, some research has progressed to the stage of preclinical studies using a large animal model and clinical study, and we introduce such recent scaffold-free approaches with high potential clinical impact. Mainil-Varlet et al. developed a cartilage-like implant in chondrocyte high density culture supported by a bioreactor, and implanted the materials onto minipig cartilage defects by press-fit fixation (Mainil-Varlet et al. 2001). Histological analysis showed such an implant yielded consistent cartilage repair with a matrix predominantly composed of type II collagen. Lu et al. produced a neocartilage allograft under defined serum-free conditions, and transplanted such graft, which were cultured to produce these constructs between 107 and 130 days, onto sheep chondral defects with suturing (Lu et al. 2005). Eight-week histology showed the reparative tissue appeared to be hyaline-like with weak Safranin O staining, and no inflammatory cells were observed around the grafted area. Park et al. produced chondrocyte/ECM membranes in high density culture, peeled the membrane from the culture dishes, consolidated them by centrifuge, and then generated an engineered cartilage construct by an additional culture period (Park et al. 2006). Jubel et al. reported that chondrocytes cultured in alginate beads for 21 days, then collected by centrifuge after the beads were dissolved, and then additionally cultured for 7 days in a cylinder mold formed cartilage-like de novo tissue (Jubel et al. 2008). These two authors further demonstrated the feasibility of their cartilage-like constructs for cartilage repair in vivo, but the implanted constructs needed to be fixed with collagen gel and periosteal flap, respectively. Ebihara et al. used layered chondrocyte sheets prepared on a temperature-responsive culture dish, and demonstrated these constructs facilitated cartilage repair in a minipig model (Ebihara et al. 2012). With this technique, cultured cells could be harvested noninvasively from the dishes by reducing only temperature (Kushida et al. 2000). Moreover, since the harvest did not need enzymatic digestion, differentiated cell phenotypes were retained.
As another scaffold-free approach, chondrocytes were cultured at high density by cell aggregation to fabricate engineered cartilage constructs (Huang et al. 2016). Cells were aggregated, adhered each other as spheroids in a few hours, and, after an additional few weeks culture, cartilage-specific matrices were secreted to subsequently form solid neotissues (Anderer and Libera 2002). Such a neotissue contributed to the regeneration of full thickness cartilage defects in a pig study (Libera et al. 2009). Additionally, Becher et al. treated 73 patients with chondral lesion (ICRS grade III or IV) using these neotissues, and showed well tolerated clinical results without any serious adverse events (Becher et al. 2017). On the other hand, with this method, the size of the tissue was limited due to its diffusion limit (DuRaine et al. 2015), and thus a large number of small aggregates may be required to cover a large cartilage defect.
More recently, Yamashita et al. developed a scaffold-less hyaline cartilaginous tissue (particle) from human iPS cells (Yamashita et al. 2015). These iPS cell-derived cartilaginous particles were feasible for use in hyaline cartilage regeneration based on the results of a mini-pig study, although these constructs required fixation with fibrin glue due to their non-adhesive properties.
In summary, many promising scaffold-free approaches have been developed until now, and such technologies could become a next generation vehicle for cartilage repair, with regard to a high level of safety by avoiding extrinsic materials (Huey et al. 2012). On the other hand, there are still several issues that need to be solved prior to future clinical applications such as complicated fabrication methods, long culture periods, and the nonadhesive properties of generated tissues needed to be overcome for integration with host cartilage. Moreover, a large number of cells may be required to cover large chondral lesions since a scaffold-free approach lacks exogenous materials and must promote ECM production by the cells themselves.
To address several of these issues mentioned above, we have developed a novel scaffold-free 3D tissue engineered construct (TEC) that is comprised of either human or porcine MSCs derived from synovium and an ECMs synthesized by the cells (Shimomura et al. 2015). The safety and effectiveness of this TEC methodology for cartilage repair and regeneration will be focused on hereafter, as it moves from bench to bedside for clinical application.
In vitro development of a scaffold-free tissue engineered construct (TEC) derived from MSCs
Synovial membrane harvested from either porcine or human knee joints was enzymatically digested, and synovial MSCs were isolated, and then expanded in growth media containing virus- and prion-free fetal bovine serum. The isolated cells showed characteristics of MSCs with regard to morphology, growth characteristics, and multipotent differentiation capacity (to osteogenic, chondrogenic, and adipogenic lineages) (Ando et al. 2007; Ando et al. 2008). When synovial MSCs were cultured to confluence in a basic growth medium, they did not synthesize an abundant collagenous matrix. However, in the presence of >0.1 mM ascorbic acid-2 phosphate (Asc-2P), collagen synthesis significantly increased with time in culture (Ando et al. 2008). Subsequently, the monolayer cell-matrix complex cultured in Asc-2P became a stiff sheet-like structure. After detachment from culture dishes by mild shear stress, the monolayer sheet immediately began to actively contract and form a thick 3D tissue (Fig. 1a). Histology of this 3D tissue showed that the cells and the corresponding ECM were three dimensionally integrated together at high cell density without evidence for the appearance of central necrosis (Fig. 1b). Immunohistochemical analysis showed that such TEC were rich in collagen I and III, but with no detectable expression of collagen II (Fig. 1c). Interestingly, adhesive molecules such as fibronectin and vitronectin were diffusely observed throughout the matrix of such TEC (Fig. 1c). These characteristics likely contribute to the expression of the highly adhesive properties of the TEC. As the TEC develops when the matrix becomes folded and contracted, it was thus apparent that the layers were integrated into each other, leading to development of a spherical body several millimeters thick. The contraction feature of the TEC is likely due to the presence of alpha-smooth muscle actin positive cells (Ando et al. 2008). The TEC generated were of a sufficient size to cover a large cartilage defect. This contracted tissue was termed a tissue engineered construct (TEC) derived from MSCs.
Fig. 1.
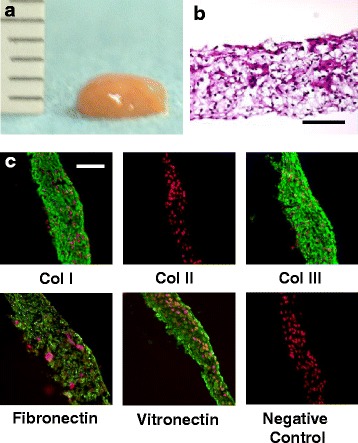
Development of the tissue-engineered construct (TEC). a Macroscopic view of the TEC that was integrated to one spherical body. b Hematoxylin and eosin staining of TEC. c Immunohistochemical analysis of the TEC stained with type I collagen (Col I), type II collagen (Col II), type III collagen (Col III), fibronectin, vitronectin, and negative IgG (control). Red are nuclei and green is target antibody. Adhesion molecules such as fibronectin and vitronectin are diffusely distributed within the TEC. Bar = 100 μm. Quoted and modified from Ando et al., Biomaterials 2007 and Shimomura et al. Cartilage 2015
Chondrogenic differentiation capacity of the TEC
TEC derived from human MSCs and then cultured in a chondrogenic medium containing BMP-2 showed increased glycosaminoglycan (GAG) synthesis and deposition as evidenced by intense Alcian Blue staining (Fig. 2a). The quantification of GAGs indicated that GAG synthesis was significantly higher in the TEC exposed to the chondrogenic medium compared to those generated in the absence of such components (Fig. 2b). Similarly, semiquantitative RT-PCR analyses showed elevations in expression of cartilage-specific markers including collagen II (Col2a1), aggrecan, and sox9 messenger RNA (mRNA) following exposure of the TEC to the chondrogenic differentiation medium (Fig. 2c). In contrast, TEC not exposed to this medium showed only weak expression of these cartilage-specific markers.
Fig. 2.
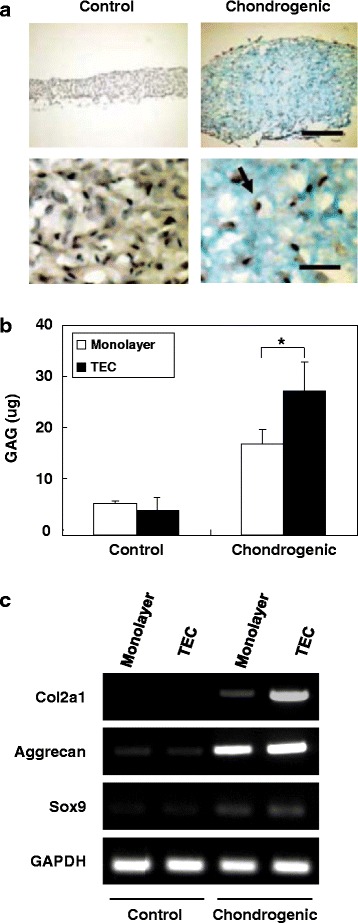
Chondrogenic differentiation capacity of TECs. a Alcian blue staining of a TEC in control medium or in chondrogenic differentiation medium. Bar = 300 um (upper). Bar = 50 um (lower). Arrow showing the cell nuclei are in lacuna. b The quantification of GAG content of synovial MSC monolayer culture or TEC in the control medium or in the chondrogenic medium. GAG synthesis is significantly higher in the TEC exposed to the chondrogenic medium (N = 8). *; p < 0.05. c Semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) analysis of synovial MSC monolayer culture or TEC for chondrogenic marker genes, type II collagen (Col2a1), aggrecan, SOX9, and GAPDH. Quoted and modified from Ando et al., Tissue Eng Part A 2008
Selection of a preclinical large animal model
One of the crucial factors that may affect the results of cell-based therapies is the age of the donors and the recipients. Regarding the cell proliferation and differentiation capacities of MSCs, it is controversial as to whether they are age-dependent (Murphy et al. 2002; Quarto et al. 1995; Bergman et al. 1996; Kretlow et al. 2008) or not (De Bari et al. 2001; Oreffo et al. 1998; Leskela et al. 2003; De Bari et al. 2001; Scharstuhl et al. 2007). In terms of the host tissue reaction, natural healing responses of osteochondral defects has been compared between immature and mature animals using rabbit models, and in this species, the studies demonstrated better healing responses in immature animals (Rudert 2002; Bos et al. 2006; Yamamoto et al. 2004; Wei et al. 1997). On the other hand, there have been no studies reported which directly compared the results of cell-based repair of chondral defects between immature and mature animal models.
Regarding the use of a clinically relevant animal model for cartilage repair, it is difficult to create a chondral injury which does not breach the subchondral bone in small animals such as rabbits, rats, and mice due to the limited thickness of their articular cartilage, and therefore, these conditions may not be as clinically relevant as would be obtained with the use of a larger animal. Thus, in consideration of clinical relevance, it is preferable to utilize a large animal model to investigate the influence of maturity on the results of cell-based therapies to repair chondral lesions. Therefore, in order to assess the efficacy of the TEC in an in vivo model, a porcine model was chosen as the physiology of the pig is similar to that of humans in many respects (Vodicka et al. 2005), and porcine articular cartilage of the knee is sufficiently thick as to allow creation of a chondral defect without damaging the subchondral bone.
Cartilage repair using TECs in a preclinical large animal study
Prior to performing a large animal study, we compared the in vitro characteristics of cell proliferation and chondrogenic capacity of porcine MSCs isolated from skeletally immature animals (3–4 month old) with those from mature animals (12 month old). We demonstrated that there were no significant differences in either the proliferation or chondrogenic capacity of porcine synovial MSCs derived from immature or mature animals (Shimomura et al. 2010).
To test the feasibility of using the porcine TEC approach for a wide range of recipient ages without chondrogenic manipulation to repair a chondral injury, immature (TEC implantation for eight knees and untreated control for four knees) as well as mature (TEC implantation for six knees and untreated control for six knees) porcine chondral injury models were utilized in experimental studies. After implantation, the TEC firmly adhered to the injured joint surface without suturing or any glues. At 6 months post-implantation, and regardless of starting age, untreated lesions exhibited no evidence for repair or only partial tissue coverage, while the defects treated with a TEC were totally or primarily covered with repair tissue (Fig. 3a, b). Histologically, the chondral lesions in the non-treatment control groups showed evidence of osteoarthritic changes, with loss of cartilage and destruction of subchondral bone in both skeletally immature and mature animals (Fig. 4a). In contrast, when treated with a TEC, the defects were filled with repair tissue exhibiting good integration to the adjacent cartilage and restoration of a smooth surface, regardless of age at the time of implantation (Fig. 4a). The repair tissue exhibited predominantly spindle-shaped fibroblast-like cells in the superficial zone of the repair tissue, while the majority of the remaining repair matrix contained round-shaped cells in lacuna (Fig. 4a). Following implantation, no histological findings were obtained that suggested either central necrosis of the implanted TEC, or that an abnormal inflammatory macrophage and lymphocyte response consistent with some form of immunological rejection had occurred in this allogenic situation, regardless of the age of the pigs. Histological scoring showed that the TEC groups exhibited significantly higher scores than did the untreated control group, regardless of skeletal maturity (Fig. 4b). Comparing the repair tissues developing following TEC implantation in immature and mature animals, no significant detectable differences were detected at the histological level (Fig. 4b).
Fig. 3.
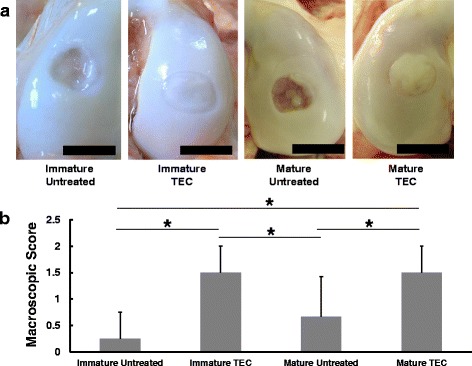
a Macroscopic view of a chondral lesion treated with or without TEC at 6 months post-implantation. Bar = 10 mm. b Macroscopic score of the repair tissue at 6 months. Regardless of skeletal maturity, the TEC group shows significantly higher scores than does the untreated group. *; p < 0.05. Quoted and modified from Shimomura et al., Biomaterials 2010
Fig. 4.
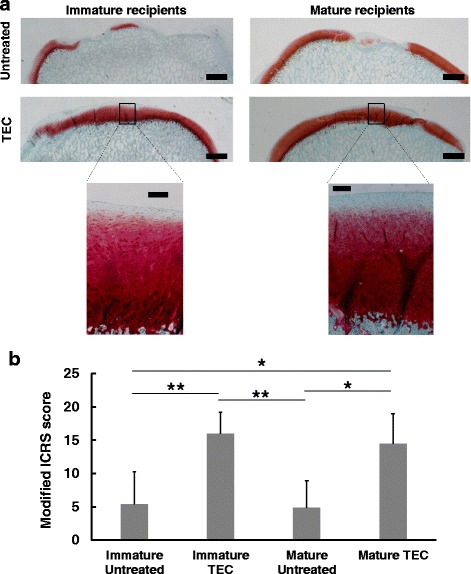
a Safranin O staining of chondral lesions treated with or without TEC. Bar = 1 mm (upper). Bar = 200 um (lower). Regardless of skeletal maturity, the defects treated with TEC are completely filled with Safranin O positive repair tissue with good tissue integration. b Modified ICRS score for repair cartilage in skeletally immature and mature recipients. The TEC group exhibits significantly higher scores than does the untreated control group, regardless of maturity. *; p < 0.05, **; p < 0.01. Quoted and modified from Shimomura et al., Biomaterials 2010
To assess the viscoelasticity of the repair cartilage (Huang et al. 2003), we performed in vitro compression testing at both fast and slow speeds. In the tissue localized to the defects of the untreated control group, the tangent modulus (defined as the slope of the curve at 5% strain) was significantly lower than that for normal cartilage at a compression rate of either 4 μm/s (Fig. 5a) or 100 μm/s (Fig. 5b), regardless of skeletal maturity of the pigs. In contrast, there were no significant differences detected between the tangent modulus for the repair tissue resulting from implantation of a TEC and that for normal cartilage at either 4 μm/s (Fig. 5a) or 100 μm/s (Fig. 5b) in either the immature or mature animals. These results suggest that the viscoelastic properties of the repair tissue in defects receiving TEC implants are likely very similar to those of normal cartilage, regardless of host skeletal maturity at the time of implantation.
Fig. 5.
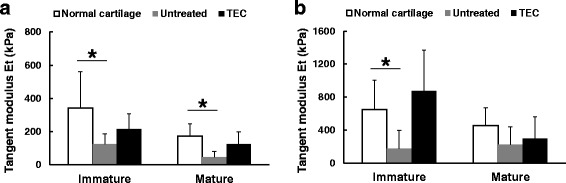
The results of compression tests at a slow compression speed (4 um/s) (a) and at a fast compression speed (100 um/s) (b). Regardless of skeletal maturity, no significant differences were detected in the tangent modulus of the TEC-mediated repair tissue and that of normal cartilage at either compression speed. Conversely, untreated defects, whether in immature or mature recipients, showed significantly lower tangent modulus than did normal cartilage at either compression speed. *; p < 0.05. Quoted and modified from Shimomura et al., Biomaterials 2010
Based on the encouraging results of the preclinical studies discussed above, we have now proceeded to clinical studies under the auspices of an approved first in man protocol by the institutional review board of Osaka University Graduate School of Medicine (Nakamura et al. 2014). This pilot clinical study is currently in progress, and the data are now under analysis (Shimomura et al. 2015).
Implications of the findings from the preclinical studies on TEC-use for cartilage repair in vivo
Based on the findings to date, the exogenous scaffold-free TEC approach offers a number of advantages for hyaline cartilage repair. Firstly, since the TEC develops without any exogenous scaffold, implantation of the TEC would likely have minimal risk of potential side effects induced by artificial or extrinsically added biological materials contained in a scaffold. On the other hand, the MSCs were exposed to certified virus- and prion-free fetal bovine serum (FBS) during cell culture in the present studies. Thus, there are some concerns regarding this exposure of the TECs to FBS-origin proteins during the development process. Although the TEC were washed extensively in vitro with sterile phosphate buffered saline, it cannot be concluded that no FBS proteins were retained in the constructs. To address this issue, we have confirmed that human serum is no less effective than bovine serum in promoting proliferation of synovium-derived MSCs and their chondrogenic capacities (Tateishi et al. 2008). Accordingly, with the use of autologous human serum, it is technically possible to develop the TEC in a totally xeno-free system for cartilage repair, a set of circumstances which would minimize the risk of infectious agents, as well as potential immune reactivity developing after implantation of the TEC (Martin et al. 2005).
Secondly, a further structural advantage of the TEC is that the MSCs and the ECM synthesized by the cells are integrated together into a 3D structure with a uniform cellular distribution. Thus, there is no need to modify or adjust the cellular distribution within the TEC. It is also notable that these TEC possess sufficiently self-supporting mechanical properties in spite of the fact that they do not contain an exogenous artificial scaffold. The tensile strength of the TEC, which develops in the presence of Asc-2P for 14 or 21 days, is comparable with that of healing ligament tissue at 1–2 weeks after injury (Provenzano et al. 2002). Therefore, such TEC can be readily handled without causing overt damage to the matrix-cell complex during implantation procedures.
Thirdly, an important biological characteristic of the TEC described is its tissue adhesiveness. This property contributes to the rapid and secure adhesion of the TEC to a natural cartilage matrix and thus, simple implantation procedures for the placement of the TEC into chondral lesions or defects could be expected to proceed without augmentation of the initial fixation. Moreover, such adhesiveness also enables rapid self-association internally with its own matrix, a factor which likely contributes to the tissue plasticity of the TEC. In reality, it is thus possible to develop a spherical-shaped tissue several millimeter thick by allowing the released monolayers from several dishes to fold in series. With such “plasticity”, it is possible to develop a TEC that matches the needed size and shape to repair a chondral defect more than several millimeters in depth. Although we have not yet identified the crucial factor(s) which determine the tissue adhesiveness of the TEC, immunohistochemical analysis has shown that fibronectin and vitronectin are localized at the interface between the TEC and the base of the chondral lesions. Therefore, fibronectin and vitronectin may likely be, at least partially, involved in the adhesive properties of these in vitro generated TEC.
Fourthly, it is notable that implantation of TEC without any pretreatment to promote a specific differentiation pathway resulted in tissue repair associated with an active chondrogenic differentiation response. Thus, the implantation of a basic TEC lead to the in vivo differentiation of such TEC in response to the in vivo biological and biomechanical cues following differentiation. Therefore, in vitro differentiation may not offer additional value to the success of the repair. On the other hand, it is still controversial whether implanted MSCs directly contribute to cartilage repair and are retained within the hyaline-like tissue that develops post-implantation, or they interact with the surrounding environment through the release of anti-inflammatory and trophic mediators and facilitate involvement of endogenous cells (Caplan and Hariri 2015). Therefore, further studies are required to determine whether the implanted TEC contributed to cartilage repair directly by local differentiation to chondrocytes or indirectly via release of mediators which enhance repair by activation of endogenous chondrocytes or activation and differentiation of other cells in the intra-articular environment.
However, based on the outcomes from the porcine preclinical studies, the in vivo repair was not perfect, in that the repair tissue still contained some fibrous tissue, mainly at the surface or in the superficial zone. In the detailed biomechanical studies discussed above, the TEC-mediated repair cartilage still exhibited some compromised mechanical properties at the upper level of the superficial zone (lamina splendens), a deficiency that will likely need improvement in the future for maintenance of long term repair cartilage integrity (Ando et al. 2012). However, as the lamina splendens apparently develops during the post-natal period in some species (Takada et al. 1999; Fujioka et al. 2013; Gannon et al. 2015), further understanding of this process could provide a solution to the observed structural deficiency following in vivo TEC implantation.
Future perspectives for the scaffold-free MSC-based TEC approach to repair cartilage
Building on the results of the preclinical studies, the present review has provided the current evidence for the safety and feasibility of using a unique scaffold-free TEC generated from synovial MSCs for effective cell-based cartilage repair in a clinical setting. Additionally, this technique is simple and should be easy for surgeons to handle for implantation into cartilage defects. Thus, such a new MSC-based technique could be considered as the next generation vehicle for cartilage repair.
Cartilage injuries might become curable with currently available cell-based therapies. On the other hand, the bigger clinical problem is related to the higher incidence of osteoarthritis (OA), an incidence that is much higher than that for isolated chondral injuries (Hjelle et al. 2002; Aroen et al. 2004; Dawson et al. 2004; Peat et al. 2001). Therefore, development of novel therapeutic methods for osteochondral repair are also urgently needed, considering the large population of patients with early and advanced osteoarthritis. As such lesions very often involve subchondral bone damage, it is important to also consider subchondral bone regeneration in addition to cartilage. Recently, we have combined the scaffold-free MSC-based TECs with an artificial bone block to fabricate a biphasic osteochondral implant, and demonstrated the feasibility of using such constructs for osteochondral repair in a rabbit study (Shimomura et al. 2014; Shimomura et al. 2017). Therefore, the combined TEC-artificial bone construct as another viable option for TEC application, could also be considered a promising MSC-based bio-implant to repair osteochondral lesions in the near future.
Conclusion
In this review, we have focused on recent advancements in scaffold-free approaches for cartilage repair. Many promising techniques have been developed and some of them have demonstrated their feasibility to repair cartilage lesions in preclinical and clinical studies. Therefore, the clinical application of such new technologies could be expected in near future. On the other hand, the optimization of and selection of cell sources and their fabrication methods have not been fully investigated, without any direct comparative studies. Thus, the ideal scaffold-free approaches that lead to repair of cartilage lesions have not been elucidated in detail. Further studies are needed and should be conducted in a methodologically rigorous fashion.
Notably, we have elucidated many of the characteristics of a scaffold-free 3D synthetic tissue (TEC) derived from cultured synovium-derived MSCs as a unique and promising clinically relevant implant for cartilage repair. This was demonstrated in vivo using a preclinical model and a range of ages (Shimomura et al. 2010; Ando et al. 2007; Ando et al. 2008). Due to the scaffold-free nature of their in vitro generated structure, implantation of TEC could potentially yield more long-term safety and efficacy than other options derived from scaffold-based cell therapies. Being a collagen I rich matrix, the basic TEC construct could also be potentially suitable for augmenting repair of compromised skin, or enhancing the repair of ligaments or tendons, which are also collagen I rich. Since TEC also have osteogenic and adipogenic differentiation capacity (data not shown) in addition to chondrogenic potential, basic TEC could likely also be used for other applications. Moreover, TEC could also be developed from MSCs derived from other tissues, such as adipose tissue which is an abundant source of MSC and readily obtained without entering the damaged joint. Therefore, tissue engineering using the TEC technology discussed could potentially provide a variety of therapeutic interventions for regenerative medicine in a number of tissue applications using MSC from different sources. However, as MSC populations from a given source are very heterogeneous, and such heterogeneity can vary between tissue sources (Ando et al. 2014; Hart 2014), this potential of the TEC technology will have to be rigorously characterized. Whether clonally-derived MSC versus specific MSC populations will be more efficacious for particular applications, remains to be determined.
Acknowledgments
This work is supported by a grant from the New Energy and Industrial Technology Development Organization, Japan, and a Grant-in-Aid for Scientific Research, Japan Society for the Promotion of Science. DAH was supported by the Alberta Innovates Health Solutions Osteoarthritis Team Grant and the Bone & Joint Health Strategic Clinical Network Program of Alberta Health Services.
Authors’ contribution
KS carried out the in vitro studies and histological analyses, participated in the design of the study, and drafted the manuscript. WA carried out the in vitro studies, animal surgeries and histological analyses, and participated in the design of the study. HF carried out the biomecnanical studies. DH drafted the manuscript. HY participated in the design of the study. NN participated in the design of the study and drafted the manuscript. All authors read and approved the final manuscript.
Abbreviations
- 3D
Three dimensional
- ACI
Autologous chondrocyte implantation
- Asc-2P
Ascorbic acid-2 phosphate
- ECM
Extracellular matrix
- GAG
Glycosaminoglycan
- iPS cells
Induced pluripotent stem cells
- KOOS
Knee injury and osteoarthritis outcome score
- mRNA
messenger RNA
- MSC
Mesenchymal stem cell
- RT-PCR
Reverse transcription–polymerase chain reaction
- TEC
Tissue engineered construct
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kazunori Shimomura, Email: kazunori-shimomura@umin.net.
Wataru Ando, Email: w-ando@umin.ac.jp.
Hiromichi Fujie, Email: fujie@sd.tmu.ac.jp.
David A. Hart, Email: hartd@ucalgary.ca
Hideki Yoshikawa, Email: yhideki@ort.med.osaka-u.ac.jp.
Norimasa Nakamura, Phone: +81-6-6352-0093, Email: norimasa.nakamura@ohsu.ac.jp.
References
- Anderer U, Libera J. In vitro engineering of human autogenous cartilage. J Bone Miner Res. 2002;17(8):1420–1429. doi: 10.1359/jbmr.2002.17.8.1420. [DOI] [PubMed] [Google Scholar]
- Ando W, Fujie H, Moriguchi Y, Nansai R, Shimomura K, Hart DA, Yoshikawa H, Nakamura N. Detection of abnormalities in the superficial zone of cartilage repaired using a tissue engineered construct derived from synovial stem cells. Eur Cell Mater. 2012;24:292–307. doi: 10.22203/eCM.v024a21. [DOI] [PubMed] [Google Scholar]
- Ando W, Kutcher JJ, Krawetz R, Sen A, Nakamura N, Frank CB, Hart DA. Clonal analysis of synovial fluid stem cells to characterize and identify stable mesenchymal stromal cell/mesenchymal progenitor cell phenotypes in a porcine model: a cell source with enhanced commitment to the chondrogenic lineage. Cytotherapy. 2014;16(6):776–788. doi: 10.1016/j.jcyt.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Ando W, Tateishi K, Hart DA, Katakai D, Tanaka Y, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N. Cartilage repair using an in vitro generated scaffold-free tissue-engineered construct derived from porcine synovial mesenchymal stem cells. Biomaterials. 2007;28(36):5462–5470. doi: 10.1016/j.biomaterials.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Ando W, Tateishi K, Katakai D, Hart DA, Higuchi C, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N. In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A. 2008;14(12):2041–2049. doi: 10.1089/ten.tea.2008.0015. [DOI] [PubMed] [Google Scholar]
- Andriano KP, Tabata Y, Ikada Y, Heller J. In vitro and in vivo comparison of bulk and surface hydrolysis in absorbable polymer scaffolds for tissue engineering. J Biomed Mater Res. 1999;48(5):602–612. doi: 10.1002/(SICI)1097-4636(1999)48:5<602::AID-JBM3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Aroen A, Loken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211–215. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]
- Becher C, Laute V, Fickert S, Zinser W, Niemeyer P, John T, Diehl P, Kolombe T, Siebold R, Fay J. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J Orthop Surg Res. 2017;12(1):71. doi: 10.1186/s13018-017-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11(5):568–577. doi: 10.1002/jbmr.5650110504. [DOI] [PubMed] [Google Scholar]
- Bos PK, Verhaar JA, van Osch GJ. Age-related differences in articular cartilage wound healing: a potential role for transforming growth factor beta1 in adult cartilage repair. Adv Exp Med Biol. 2006;585:297–309. doi: 10.1007/978-0-387-34133-0_20. [DOI] [PubMed] [Google Scholar]
- Brehm W, Aklin B, Yamashita T, Rieser F, Trub T, Jakob RP, Mainil-Varlet P. Repair of superficial osteochondral defects with an autologous scaffold-free cartilage construct in a caprine model: implantation method and short-term results. Osteoarthr Cartil. 2006;14(12):1214–1226. doi: 10.1016/j.joca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Bright P, Hambly K. A systematic review of reporting of rehabilitation in articular-cartilage-repair studies of third-generation autologous chondrocyte implantation in the knee. J Sport Rehabil. 2014;23(3):182–191. doi: 10.1123/JSR.2013-0045. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Brun P, Cortivo R, Zavan B, Vecchiato N, Abatangelo G. In vitro reconstructed tissues on hyaluronan-based temporary scaffolding. J Mater Sci Mater Med. 1999;10(10/11):683–688. doi: 10.1023/A:1008960413362. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Hariri R. Body management: Mesenchymal stem cells control the internal regenerator. Stem Cells Transl Med. 2015;4(7):695–701. doi: 10.5966/sctm.2014-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels AU, Andriano KP, Smutz WP, Chang MK, Heller J. Evaluation of absorbable poly(ortho esters) for use in surgical implants. J Appl Biomater. 1994;5(1):51–64. doi: 10.1002/jab.770050108. [DOI] [PubMed] [Google Scholar]
- Dashtdar H, Rothan HA, Tay T, Ahmad RE, Ali R, Tay LX, Chong PP, Kamarul T. A preliminary study comparing the use of allogenic chondrogenic pre-differentiated and undifferentiated mesenchymal stem cells for the repair of full thickness articular cartilage defects in rabbits. J Orthop Res. 2011;29(9):1336–1342. doi: 10.1002/jor.21413. [DOI] [PubMed] [Google Scholar]
- Dawson J, Linsell L, Zondervan K, Rose P, Randall T, Carr A, Fitzpatrick R. Epidemiology of hip and knee pain and its impact on overall health status in older adults. Rheumatology (Oxford) 2004;43(4):497–504. doi: 10.1093/rheumatology/keh086. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44(1):85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Luyten FP. Failure of in vitro-differentiated mesenchymal stem cells from the synovial membrane to form ectopic stable cartilage in vivo. Arthritis Rheum. 2004;50(1):142–150. doi: 10.1002/art.11450. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- DuRaine GD, Brown WE, Hu JC, Athanasiou KA. Emergence of scaffold-free approaches for tissue engineering musculoskeletal cartilages. Ann Biomed Eng. 2015;43(3):543–554. doi: 10.1007/s10439-014-1161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara G, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, Ito S, Ukai T, Kobayashi M, Kokubo M, Okano T, Mochida J. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials. 2012;33(15):3846–3851. doi: 10.1016/j.biomaterials.2012.01.056. [DOI] [PubMed] [Google Scholar]
- Fujioka R, Aoyama T, Takakuwa T. The layered structure of the articular surface. Osteoarthr Cartil. 2013;21(8):1092–1098. doi: 10.1016/j.joca.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Gannon AR, Nagel T, Bell AP, Avery NC, Kelly DJ. The changing role of the superficial region in determining the dynamic compressive properties of articular cartilage during postnatal development. Osteoarthr Cartil. 2015;23(6):975–984. doi: 10.1016/j.joca.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, Bathan L, Marcacci M. Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med. 2009;37(6):1083–1092. doi: 10.1177/0363546509331419. [DOI] [PubMed] [Google Scholar]
- Goyal D, Goyal A, Keyhani S, Lee EH, Hui JH. Evidence-based status of second- and third-generation autologous chondrocyte implantation over first generation: a systematic review of level I and II studies. Arthroscopy. 2013;29(11):1872–1878. doi: 10.1016/j.arthro.2013.07.271. [DOI] [PubMed] [Google Scholar]
- Guo JF, Jourdian GW, MacCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19(2–4):277–297. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- Hart DA. Why Mesenchymal stem/progenitor cell heterogeneity in specific environments? Implications for tissue engineering applications following injury or degeneration of connective tissues. J Biomed Sci Eng. 2014;7(8):526–532. doi: 10.4236/jbise.2014.78054. [DOI] [Google Scholar]
- Hickery MS, Bayliss MT, Dudhia J, Lewthwaite JC, Edwards JC, Pitsillides AA. Age-related changes in the response of human articular cartilage to IL-1alpha and transforming growth factor-beta (TGF-beta): chondrocytes exhibit a diminished sensitivity to TGF-beta. J Biol Chem. 2003;278(52):53063–53071. doi: 10.1074/jbc.M209632200. [DOI] [PubMed] [Google Scholar]
- Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- Homminga GN, Buma P, Koot HW, van der Kraan PM, van den Berg WB. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand. 1993;64(4):441–445. doi: 10.3109/17453679308993663. [DOI] [PubMed] [Google Scholar]
- Huang BJ, Hu JC, Athanasiou KA. Cell-based tissue engineering strategies used in the clinical repair of articular cartilage. Biomaterials. 2016;98:1–22. doi: 10.1016/j.biomaterials.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Soltz MA, Kopacz M, Mow VC, Ateshian GA. Experimental verification of the roles of intrinsic matrix viscoelasticity and tension-compression nonlinearity in the biphasic response of cartilage. J Biomech Eng. 2003;125(1):84–93. doi: 10.1115/1.1531656. [DOI] [PubMed] [Google Scholar]
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Nakayama K, Akieda S, Matsuda S, Iwamoto Y. Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells. J Orthop Surg Res. 2014;9:98. doi: 10.1186/s13018-014-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski RJ, Deasy BM, Huard J. Muscle-derived stem cells. Gene Ther. 2002;9(10):642–647. doi: 10.1038/sj.gt.3301719. [DOI] [PubMed] [Google Scholar]
- Jubel A, Andermahr J, Schiffer G, Fischer J, Rehm KE, Stoddart MJ, Hauselmann HJ. Transplantation of de novo scaffold-free cartilage implants into sheep knee chondral defects. Am J Sports Med. 2008;36(8):1555–1564. doi: 10.1177/0363546508321474. [DOI] [PubMed] [Google Scholar]
- Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krych AJ, Gobbi A, Lattermann C, Nakamura N (2016) Articular cartilage solutions for the knee: present challenges and future direction. J ISAKOS. 10.1136/jisakos-2015-000037
- Kushida A, Yamato M, Konno C, Kikuchi A, Sakurai Y, Okano T. Temperature-responsive culture dishes allow nonenzymatic harvest of differentiated Madin-Darby canine kidney (MDCK) cell sheets. J Biomed Mater Res. 2000;51(2):216–223. doi: 10.1002/(SICI)1097-4636(200008)51:2<216::AID-JBM10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Lahiji A, Sohrabi A, Hungerford DS, Frondoza CG. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J Biomed Mater Res. 2000;51(4):586–595. doi: 10.1002/1097-4636(20000915)51:4<586::AID-JBM6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221(1–2):1–22. doi: 10.1016/S0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103(5):1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- Leskela HV, Risteli J, Niskanen S, Koivunen J, Ivaska KK, Lehenkari P. Osteoblast recruitment from stem cells does not decrease by age at late adulthood. Biochem Biophys Res Commun. 2003;311(4):1008–1013. doi: 10.1016/j.bbrc.2003.10.095. [DOI] [PubMed] [Google Scholar]
- Lewis PB, McCarty LP, 3rd, Yao JQ, Williams JM, Kang R, Cole BJ. Fixation of tissue-engineered human neocartilage constructs with human fibrin in a caprine model. The journal of knee surgery. 2009;22(3):196–204. doi: 10.1055/s-0030-1247749. [DOI] [PubMed] [Google Scholar]
- Libera J, Ruhnau K, Baum P, Lüthi U, Schreyer T, Meyer U, Wiesmann HP, Herrmann A, Korte T, Pullig O, Siodla V. Cartilage engineering. Fundamentals of tissue engineering and regenerative medicine (Springer) 2009. pp. 233–242. [Google Scholar]
- Lu Y, Adkisson HD, Bogdanske J, Kalscheur V, Maloney W, Cheung R, Grodzinsky AJ, Hruska KA, Markel MD. In vivo transplantation of neonatal ovine neocartilage allografts: determining the effectiveness of tissue transglutaminase. The journal of knee surgery. 2005;18(1):31–42. doi: 10.1055/s-0030-1248155. [DOI] [PubMed] [Google Scholar]
- Mainil-Varlet P, Rieser F, Grogan S, Mueller W, Saager C, Jakob RP. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthr Cartil. 2001;9 Suppl A:S6–15. doi: 10.1053/joca.2001.0438. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11(2):228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- Masuda K, Takegami K, An H, Kumano F, Chiba K, Andersson GB, Schmid T, Thonar E. Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosus and nucleus pulposus cells cultured in alginate beads. J Orthop Res. 2003;21(5):922–930. doi: 10.1016/S0736-0266(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Mohanraj B, Farran AJ, Mauck RL, Dodge GR. Time-dependent functional maturation of scaffold-free cartilage tissue analogs. J Biomech. 2014;47(9):2137–2142. doi: 10.1016/j.jbiomech.2013.10.022. [DOI] [PubMed] [Google Scholar]
- Murdoch AD, Grady LM, Ablett MP, Katopodi T, Meadows RS, Hardingham TE. Chondrogenic differentiation of human bone marrow stem cells in transwell cultures: generation of scaffold-free cartilage. Stem Cells. 2007;25(11):2786–2796. doi: 10.1634/stemcells.2007-0374. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46(3):704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Hui J, Koizumi K, Yasui Y, Nishii T, Lad D, Karnatzikos G, Gobbi A. Stem cell therapy in cartilage repair—culture-free and cell culture–based methods. Oper Tech Orthop. 2014;24(1):54–60. doi: 10.1053/j.oto.2014.02.006. [DOI] [Google Scholar]
- Niemeyer P, Salzmann G, Feucht M, Pestka J, Porichis S, Ogon P, Sudkamp N, Schmal H. First-generation versus second-generation autologous chondrocyte implantation for treatment of cartilage defects of the knee: a matched-pair analysis on long-term clinical outcome. Int Orthop. 2014;38(10):2065–2070. doi: 10.1007/s00264-014-2368-0. [DOI] [PubMed] [Google Scholar]
- Oreffo RO, Bennett A, Carr AJ, Triffitt JT. Patients with primary osteoarthritis show no change with ageing in the number of osteogenic precursors. Scand J Rheumatol. 1998;27(6):415–424. doi: 10.1080/030097498442235. [DOI] [PubMed] [Google Scholar]
- Park K, Huang J, Azar F, Jin RL, Min BH, Han DK, Hasty K. Scaffold-free, engineered porcine cartilage construct for cartilage defect repair--in vitro and in vivo study. Artif Organs. 2006;30(8):586–596. doi: 10.1111/j.1525-1594.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Provenzano PP, Hayashi K, Kunz DN, Markel MD, Vanderby R., Jr Healing of subfailure ligament injury: comparison between immature and mature ligaments in a rat model. J Orthop Res. 2002;20(5):975–983. doi: 10.1016/S0736-0266(02)00036-0. [DOI] [PubMed] [Google Scholar]
- Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int. 1995;56(2):123–129. doi: 10.1007/BF00296343. [DOI] [PubMed] [Google Scholar]
- Rudert M. Histological evaluation of osteochondral defects: consideration of animal models with emphasis on the rabbit, experimental setup, follow-up and applied methods. Cells Tissues Organs. 2002;171(4):229–240. doi: 10.1159/000063125. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- Scharstuhl A, Schewe B, Benz K, Gaissmaier C, Buhring HJ, Stoop R. Chondrogenic potential of human adult mesenchymal stem cells is independent of age or osteoarthritis etiology. Stem Cells. 2007;25(12):3244–3251. doi: 10.1634/stemcells.2007-0300. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Ando W, Moriguchi Y, Sugita N, Yasui Y, Koizumi K, Fujie H, Hart DA, Yoshikawa H, Nakamura N. Next generation Mesenchymal stem cell (MSC)–based cartilage repair using scaffold-free tissue engineered constructs generated with synovial Mesenchymal stem cells. Cartilage. 2015;6(Suppl 2):13S–29S. doi: 10.1177/1947603515571002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura K, Ando W, Tateishi K, Nansai R, Fujie H, Hart DA, Kohda H, Kita K, Kanamoto T, Mae T, Nakata K, Shino K, Yoshikawa H, Nakamura N. The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials. 2010;31(31):8004–8011. doi: 10.1016/j.biomaterials.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Moriguchi Y, Ando W, Nansai R, Fujie H, Hart DA, Gobbi A, Kita K, Horibe S, Shino K, Yoshikawa H, Nakamura N. Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial Mesenchymal stem cells and a Hydroxyapatite-based artificial bone. Tissue Eng Part A. 2014;20(17–18):2291–2304. doi: 10.1089/ten.tea.2013.0414. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Moriguchi Y, Murawski CD, Yoshikawa H, Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng Part B Rev. 2014;20(5):468–476. doi: 10.1089/ten.teb.2013.0543. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Moriguchi Y, Nansai R, Fujie H, Ando W, Horibe S, Hart DA, Gobbi A, Yoshikawa H, Nakamura N. Comparison of 2 different formulations of artificial bone for a hybrid implant with a tissue-engineered construct derived from synovial Mesenchymal stem cells. Am J Sports Med. 2017;45(3):666–675. doi: 10.1177/0363546516668835. [DOI] [PubMed] [Google Scholar]
- Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23(4):381–387. doi: 10.1016/j.arthro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Takada N, Wada I, Sugimura I, Sakuma E, Maruyama H, Matsui N. A possible barrier function of the articular surface. Kaibogaku zasshi Journal of anatomy. 1999;74(6):631–637. [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tateishi K, Ando W, Higuchi C, Hart DA, Hashimoto J, Nakata K, Yoshikawa H, Nakamura N. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: potential feasibility for clinical applications. Cell Transplant. 2008;17(5):549–557. doi: 10.3727/096368908785096024. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Okada M, Yamashita A. iPS cell technologies and cartilage regeneration. Bone. 2015;70:48–54. doi: 10.1016/j.bone.2014.07.011. [DOI] [PubMed] [Google Scholar]
- van der Elst M, Klein CP, de Blieck-Hogervorst JM, Patka P, Haarman HJ. Bone tissue response to biodegradable polymers used for intra medullary fracture fixation: a long-term in vivo study in sheep femora. Biomaterials. 1999;20(2):121–128. doi: 10.1016/S0142-9612(98)00117-3. [DOI] [PubMed] [Google Scholar]
- Vavken P, Samartzis D. Effectiveness of autologous chondrocyte implantation in cartilage repair of the knee: a systematic review of controlled trials. Osteoarthr Cartil. 2010;18(6):857–863. doi: 10.1016/j.joca.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Vodicka P, Smetana K, Jr, Dvorankova B, Emerick T, Xu YZ, Ourednik J, Ourednik V, Motlik J. The miniature pig as an animal model in biomedical research. Ann N Y Acad Sci. 2005;1049:161–171. doi: 10.1196/annals.1334.015. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17(1):130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- Wei X, Gao J, Messner K. Maturation-dependent repair of untreated osteochondral defects in the rabbit knee joint. J Biomed Mater Res. 1997;34(1):63–72. doi: 10.1002/(SICI)1097-4636(199701)34:1<63::AID-JBM9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wickham MQ, Erickson GR, Gimble JM, Vail TP, Guilak F (2003) Multipotent stromal cells derived from the infrapatellar fat pad of the knee. Clin Orthop Relat Res (412):196–212. 10.1097/01.blo.0000072467.53786.ca [DOI] [PubMed]
- Yamamoto T, Wakitani S, Imoto K, Hattori T, Nakaya H, Saito M, Yonenobu K (2004) Fibroblast growth factor-2 promotes the repair of partial thickness defects of articular cartilage in immature rabbits but not in mature rabbits, 12. Osteoarthr Cartil (8):636–641. 10.1016/j.joca.2004.04.007 [DOI] [PubMed]
- Yamashita A, Morioka M, Yahara Y, Okada M, Kobayashi T, Kuriyama S, Matsuda S, Tsumaki N. Generation of Scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem Cell Reports. 2015;4(3):404–418. doi: 10.1016/j.stemcr.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hillas PJ, Baez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW. The application of recombinant human collagen in tissue engineering. BioDrugs. 2004;18(2):103–119. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Ando W, Shimomura K, Koizumi K, Ryota C, Hamamoto S, Kobayashi M, Yoshikawa H, Nakamura N. Scaffold-free, stem cell-based cartilage repair. Journal of clinical orthopaedics and trauma. 2016;7(3):157–163. doi: 10.1016/j.jcot.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


