Abstract
Background
Recently, non-vitamin K antagonist oral anticoagulants such as direct thrombin and direct factor Xa inhibitors have been prescribed for prevention of embolic stroke. While in Japan, argatroban, also a direct thrombin inhibitor, is available for the treatment of atherothrombotic stroke patients. This study aimed to explore whether there is any differences between direct thrombin and direct factor Xa inhibitors regarding the inhibiting effect against thrombogenesis in the clinical setting of acute ischemic stroke.
Methods
Acute ischemic stroke patients newly prescribed anti-thrombotic agents were consecutively screened, and 44 patients with single medicine were enrolled (median 72.0 years-old). Blood samples were obtained at 1 and 2 weeks after the medication started. The extent of anticoagulation activity, inflammatory markers and platelet aggregation were assessed. Patients with antiplatelets were used as control.
Results
Prescribed antithrombotics were dabigatran (group D: n = 12), apixaban (group A: n = 14) and antiplatelet agents (group P: n = 18). Prevalence of stroke risks and anticoagulation activity were not different between groups D and A. The alteration of inflammatory markers in a week in the group A showed similar trend to those in the group P. The group D presented relatively lower amount of high-sensitive C-reactive protein and higher amount of pentraxin-3 compared with groups A and P. While 88.9% of group P patients showed decreased platelet aggregation activity with adenosine diphosphate, 55.6% of group D and 40.0% of group A presented the inhibition of platelet aggregation activity.
Conclusions
Even in acute ischemic stroke patients, both apixaban and dabigatran equally showed the anticoagulation activity. The reduction of inflammatory response might be prominent in apixaban, whereas the inhibition of platelet aggregation activity might be evident in dabigatran.
Keywords: Thrombin, Factor Xa, Blood platelets, Inflammation, Stroke
Background
Recently non-vitamin K antagonist oral anticoagulants (NOACs), such as dabigatran, apixaban, rivaroxaban and edoxaban, have been used for preventing embolic stroke. The clinical trial data in NOACs reported the lower risk of hemorrhagic complication compared with warfarin [1–4]. Therefore, it can be said that it is preferable to use NOACs for preventing ischemic stroke in patients with high hemorrhagic risks [5]. Moreover, NOACs have recently been reported to show not only anticoagulation effect but also anti-inflammatory and antiplatelet effects [6–9]. In Japan, actually, argatroban which is also a direct thrombin inhibitor is available for treating acute atherothrombotic stroke patients [10, 11], supported by the evidence of decreasing micro thrombus from the fragile atheromatous plaques [12]. Meanwhile, other studies reported that NOACs did not present any effect against inflammatory response nor platelet activation [13–16]. It can be said that the pleiotropic effect of NOACs are still under debate. Therefore, if the anti-inflammatory effect and antiplatelet effect of NOACs will be revealed, it may influence on the decision making of prescription of antithrombotic agents including combination use of antiplatelet and anticoagulation medicines.
This study targeted acute ischemic stroke (AIS) patients, because the inflammatory response and platelet activity would be amplified in this condition. Then, we investigated whether there is any difference in these pleiotropic effects of NOACs along with anticoagulation effect or not, especially between direct thrombin inhibitor and direct factor Xa inhibitor.
Methods
Patients
All procedures in this study was approved by the ethical committee of the Research Institute for Brain and Blood Vessels-Akita (#14-7). Between January 2015 and May 2016, AIS patients newly prescribed antithrombotic agents were consecutively screened, and 44 patients were enrolled (30 male and 14 female, median age 72.0 years-old). All patients were consented with written document. Patients who had multiple antithrombotic agents, consciousness disturbance, dysphagia and complications such as pneumonia or active malignancy were excluded (Fig. 1). Thus far, stroke severity became similar level among the enrolled patients. Blood sampling was performed at 1 and 2 weeks after the antithrombotic medication became a single kind. Evaluated laboratory data were activated partial thromboplastin time (APTT), prothrombin time (PT), the amounts of prothrombin fragment (F1 + 2), interleukin-6 (IL-6), high sensitivity C-reactive protein (hsCRP) and pentraxin-3 (PTX3). The activity of platelet aggregation elicited by adenosine diphosphate (ADP) or collagen was evaluated using an aggregation analyzer (PA-200, Kowa Co. Ltd., Tokyo, Japan). The anti-inflammatory and antiplatelet effects were compared between patients with dabigatran and apixaban. Patients with antiplatelets were used as control. The selection of prescribing antithrombotic medicines was basically followed by the guidelines [17, 18]. Especially for the choice of NOACs, the algorithm in our hospital was used (Fig. 2). The initiation of antithrombotic agents was at the appropriate days after stroke onset following the guideline [19]. The average day was at 9.6th day (range: 1–18 days) in dabigatran, at 12.8th day (range: 1–30 days) in apixaban and at 10.5th day (range: 1–20 days) in antiplatelets.
Fig. 1.
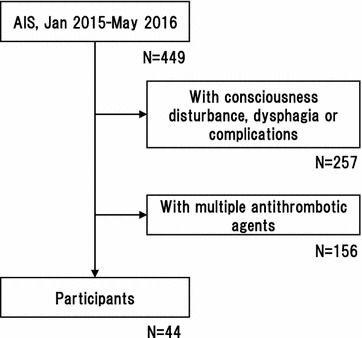
The inclusion and exclusion criteria with number of patients
Fig. 2.
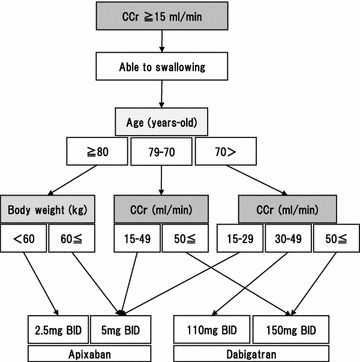
The algorithm of selection of non-vitamin K antagonist oral anticoagulant (NOAC). This arm is used when a patient prefer a medicine twice a day administration. A patient with poor kidney function (CCr < 15 ml/min) cannot apply this algorithm. If a patient shows dysphagia, a NOAC which can be pulverized is considered for prescription. CCr creatinine clearance, BID twice a day oral administration
Background characteristics of each patient were collected from patient’s clinical records. Stroke subtype was classified into (1) cardioembolic stroke, (2) large artery atherothrombotic infarction and (3) small vessel occlusion based on the criteria of the Trial of Org 10172 in the acute stroke treatment classification system [20]. Risk factors were defined as hypertension (HT), hyperlipidemia (HL), diabetes mellitus (DM) and smoking.
Statistical analysis
All data are presented as mean ± standard deviation (SD) for continuous variables and a number and percentage (%) for categorical variables. Patients’ characteristics were compared with Pearson’s χ2 test among different groups. Comparisons of the amount of inflammatory markers were performed with Kruskal–Wallis one-way analysis of variance. All statistical analysis was performed by JMP9 software (SAS Inst. Inc. Cary, NC).
Results
Patients’ background characteristics
Prescribed antithrombotics were dabigatran (group D: n = 12), apixaban (group A: n = 14) and antiplatelet agents (group P: clopidogrel n = 8 and cilostazol n = 10). There was no significant difference of age, sex distribution and atherosclerotic risk factors, such as hypertension, dyslipidemia and diabetes mellitus among three groups. As known that the anticoagulants are prescribed to embolic stroke patients, the frequency of atrial fibrillation was higher in the group D and A compared with the group P (Table 1).
Table 1.
Patients’ characteristics
| Total | Group D | Group A | Group P | P value | |
|---|---|---|---|---|---|
| N | 44 | 12 | 14 | 18 | |
| Sex (m/f) | 30/14 | 9/3 | 9/5 | 12/6 | 0.829 |
| Age (mean ± SD) | 70.8 ± 11.8 | 69.6 ± 14.3 | 74.1 ± 12.1 | 69.1 ± 9.8 | 0.335 |
| (median) | 72.0 | 69.5 | 80.0 | 70.5 | |
| Hypertension | 68.2% | 66.7% | 50% | 83.3% | 0.132 |
| Dyslipidemia | 29.5% | 8.3% | 28.6% | 44.4% | 0.104 |
| Diabetes | 38.6% | 33.3% | 21.4% | 55.6% | 0.131 |
| Atrial fibrillation | 34.1% | 58.3% | 57.1% | 0% | < 0.001 |
| Smoking | 54.5% | 66.7% | 57.1% | 44.4% | 0.475 |
| Stroke subtype (n) | < 0.001 | ||||
| CE | 23 | 9 | 14 | 0 | |
| LAA | 10 | 2 | 0 | 8 | |
| SVO | 11 | 1 | 0 | 10 |
CE cardioembolism, LAA large artery atherothrombosis, SAO small vessel occlusion
Laboratory data was shown in Table 2. APTT was prolonged only in the group D. Whereas, the groups A and P showed the same amount of APTT as the normal range. Both groups D and A showed the extended PT and decreased F1 + 2 amount.
Table 2.
Anticoagulation indicators
| Group D | Group A | Group P | ||||
|---|---|---|---|---|---|---|
| 1 week | 2 week | 1 week | 2 week | 1 week | 2 week | |
| APTT (24–34 s) |
47.3 ± 9.7 | 50.1 ± 12.2 | 33.6 ± 4.9 | 31.8 ± 5.4 | 29.8 ± 2.3 | 30.0 ± 2.2 |
| PT (11–14 s) |
16.4 ± 1.9 | 16.3 ± 1.8 | 15.4 ± 2.6 | 15.0 ± 2.3 | 13.1 ± 0.5 | 13.0 ± 0.6 |
| F1 + 2 (69–229 pmol/l) |
150.4 ± 62.3 | 159.0 ± 62.1 | 130.9 ± 40.5 | 182.0 ± 116.6 | 230.7 ± 59.9 | 247.2 ± 76.9 |
| IL-6 (< 8 pg/ml) |
2.9 ± 4.2 | 2.3 ± 1.4 | 5.1 ± 4.8 | 3.1 ± 1.9 | 5.3 ± 6.7 | 2.4 ± 2.5 |
| hsCRP (< 0.30 mg/dl) |
0.10 ± 0.14 | 0.11 ± 0.13 | 0.22 ± 0.21 | 0.20 ± 0.19 | 0.19 ± 0.20 | 0.12 ± 0.16 |
| PTX3 (0.73–5.49 ng/ml) |
3.4 ± 3.8 | 2.9 ± 2.4 | 3.9 ± 2.8 | 3.5 ± 2.1 | 3.3 ± 2.5 | 3.0 ± 2.3 |
Normal range of each factor is indicated in parentheses. Data are presented as average ± standard deviation
APTT activated partial thromboplastin time, PT prothrombin time, F1 + 2 prothrombin fragment, IL-6 interleukin 6, hsCRP high sensitivity C-reactive protein, PTX3 pentraxin 3
The anti-inflammatory effect
As shown in Table 2, IL-6 was slightly lower in the group D compared with the groups A and P at the 1st week (2.9 ± 4.2, 5.1 ± 4.8 and 5.3 ± 6.7 ng/ml, respectively. Not significant), then the amounts became the same level among all three groups at the 2nd week (2.3 ± 1.4, 3.1 ± 1.9 and 2.4 ± 2.5 ng/ml, respectively). The amount of hsCRP was lower in the group D compared with groups A and P at the 1st week (0.10 ± 0.14, 0.22 ± 0.21 and 0.19 ± 0.20 mg/dl, respectively. Not significant). While the group P showed the decline of hsCRP in the 2nd week (0.12 ± 0.16 mg/dl), the groups D and A showed an equivocal level in the 2nd week (0.11 ± 0.13 and 0.20 ± 0.19 mg/dl, respectively). The amount of PTX3 exhibited similar level among all three groups both at the 1st and 2nd weeks, and there was no significance.
The antiplatelet effect
Figure 3 showed the bubble chart graph of platelet aggregation activity. Four of 12 patients in the group D and 4 of 14 patients in the group A were excluded from the analysis because of the insufficient data of platelet aggregation activity. As expected in the group P, 16 of 18 patients (88.9%) showed the reduced platelet aggregation activity with ADP at both 1st and 2nd weeks. Two patients remained in the equivocal level of platelet aggregation. Whereas, 7 of 15 patients (46.7%) at the 1st week and 7 of 13 patients (53.8%) at the 2nd week showed the reduced platelet aggregation activity with collagen. In the group D, 3 of 8 patients (37.5%) at the 1st week and 5 of 8 patients (55.6%) at the 2nd week showed the reduced platelet aggregation activity with ADP. Moreover, 1 of 6 patients (16.7%) and 3 of 7 patients (42.9%) presented the inhibition of platelet aggregation activity with collagen at the 1st and 2nd weeks, respectively. In the group A, 2 of 10 patients (20.0%) at the 1st week and 4 of 10 patients (40.0%) at the 2nd week showed the reduced platelet aggregation activity with ADP. While, 2 of 10 patients (20.0%) and 3 of 9 patients (33.3%) presented the reduced platelet aggregation activity with collagen at the 1st week and 2nd week, respectively.
Fig. 3.
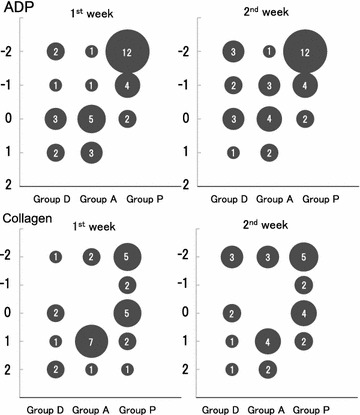
Platelet aggregation activity elicited by ADP or collagen. The number of patients in whom platelet aggregation activity is reduced are increasing in 1 week in both groups D and A. This finding is observed in the same tendency as for elicitation by ADP and collagen. Bubble size and the number indicate the number of patients. Y axis expresses the degree of platelet coagulation activity. Negative number indicates reduced activity and positive number indicates accelerated activity
During the 1 month observation period, no patients had stroke recurrence or hemorrhagic complication.
Discussion
This study clearly demonstrated that, even in the acute ischemic stroke patients, both dabigatran and apixaban could express not only anticoagulation effect but also some amount of anti-inflammatory and antiplatelet effects.
Regarding the indicator of anticoagulation effect, APTT was reported to be prolonged not by apixaban but by dabigatran [21, 22], supported our findings. Moreover, according to the observed data of PT and F1 + 2, it can be said that both dabigatran and apixaban might accurately be able to express the anticoagulation activity in the acute phase of ischemic stroke.
Along with the activation of fibrinogen, thrombin can effect on platelet membrane and cleave protease activated receptor (PAR)-1 and PAR-4, partly participating in the platelet aggregation [23, 24]. Moreover, in normal condition, thrombin combined with thrombomoduline can activate the protein C at the vascular endothelia. Then, the activated protein C (APC) provides not only anticoagulation effect but also anti-inflammatory effect [25, 26]. However at the advanced atherosclerotic lesion, the amount of APC might be declined, and the effect of thrombin may become dominant [27]. Therefore, among the patient of acute ischemic stroke or with advanced atherosclerotic lesion, thrombin might play an accelerative role in inflammation and coagulation.
This study showed that dabigatran might decrease the inflammatory response by means of reducing IL-6 and hsCRP within 1 week under the stimulated condition. In fact, dabigatran was reported to reduce the formation of atheromatous thrombus by decreasing the inflammatory response in ApoE knock-out mice [28]. Apixaban was also reported it’s anti-inflammatory effect by reducing the production of free radicals in in vitro ischemic stress model [8]. In this study, the amount of IL-6 and PTX3 in apixaban presented the same trend as those in antiplatelets. Since PTX3 is reported to relate to vascular inflammation [29], it can be said that apixaban might show anti-inflammatory effect like antiplatelet agents [30, 31]. Very recent study reported that antithrombotic therapy with dabigatran plus antiplatelet medicine showed significantly lower risk of hemorrhagic complication compared with triple therapy (warfarin plus dual antiplatelet medicines), along with no difference of the risk of thromboembolic events between two therapies [32]. Considering the findings of our study, if dabigatran will be prescribed as anticoagulation agent, it may replace warfarin plus single antiplatelet agent, and we might be able to avoid triple therapy (anticoagulation plus dual antiplatelets).
According to previous reports, the platelet aggregation was normally observed in healthy volunteer taking dabigatran or rivaroxaban [16]. The platelet aggregation induced by ADP was reported not to be changed in blood samples obtained from chronic atrial fibrillation patients by taking dabigatran and warfarin [14]. While, apixaban was reported to show the inhibitory effect of platelet aggregation in vitro [15]. Herein, this study focused on acute ischemic stroke patients in whom the platelet aggregation might be elicited. Then, both dabigatran and apixaban were observed to express some amount of antiplatelet effect under the stimulated condition. Actually, our findings can be supported by other studies in which argatroban, another direct thrombin antagonist, could inhibit the platelet aggregation [33] and decrease the amount of microemboli from unstable atherothrombotic plaque [12, 34].
There are some limitations in this study. First, the number of sample cases was small, and the statistical analysis was not able to evaluate enough. Even though, we screened patients who took the only one antithrombotic agent during the study period, so that the data should not be contaminated by any other medicines. Second, this study adopted patients with antiplatelet medicines as control. Since prescribed antithrombotic agents were various depend on stroke subtype, it is not enough to compare the anti-inflammatory and antiplatelet effects among patients prescribed NOACs or antiplatelets. Moreover, it was quite difficult to set control group in which no antithrombotic agent was prescribed, since this study was conducted under standard stroke treatments. Nevertheless, this is the first report in which the pleiotropic effects of dabigatran and apixaban were assessed in the acute ischemic stroke patients. A large scale prospective study will be needed to confirm our current findings.
Conclusions
Even in the setting of acute ischemic stroke, the effect of anticoagulation activity was equally expressed in dabigatran and apixaban. The anti-inflammatory effect might be observed in apixaban as the same trend as that in antiplatelet agents. Whereas, the antiplatelet aggregation effect might be stronger in dabigatran compared with that in apixaban.
Authors’ contributions
TN conducted the study protocol and data analysis. JM involved in the screening of patients. TI performed statistical analysis and gave a design of this study. All authors read and approved the final manuscript.
Acknowledgements
We thank Ms. Tobisawa for excellent support in statistical analysis and the members of the Department of Stroke Science for clinical works.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data supporting our findings was presented in tables and text in this article.
Consent to publish
Consent for publication was obtained from all authors.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Research Institute for Brain and Blood Vessels-Akita. Written consent was obtained from all participants.
Funding
None.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AIS
acute ischemic stroke
- APTT
activated partial thromboplastin time
- PT
prothrombin time
- F1 + 2
the amounts of prothrombin fragment
- IL-6
interleukin-6
- hsCRP
high sensitivity C-reactive protein
- PTX3
pentraxin-3
- ADP
adenosine diphosphate
- NOAC
non-vitamin K antagonist oral anticoagulant
- HT
hypertension
- HL
hyperlipidemia
- DM
diabetes mellitus
- SD
standard deviation
- APC
activated protein C
- PAR
protease activated receptor
Contributor Information
Taizen Nakase, Phone: +81-18-833-0115, Email: nakase@akita-noken.jp.
Junta Moroi, Email: moroi@akita-noken.jp.
Tatsuya Ishikawa, Email: teddyish@akita-noken.jp.
References
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 2.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 3.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 5.Okada T, Nakase T, Sasaki M, Ishikawa T. Do the antithrombotic therapy at the time of intracerebral hemorrhage influence clinical outcome? Analysis between the difference of antiplatelet and anticoagulant agents and clinical course. J Stroke Cerebrovasc Dis. 2014;23(7):1781–1788. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Ellinghaus P, Perzborn E, Hauenschild P, Gerdes C, Heitmeier S, Visser M, Summer H, Laux V. Expression of pro-inflammatory genes in human endothelial cells: comparison of rivaroxaban and dabigatran. Thromb Res. 2016;142:44–51. doi: 10.1016/j.thromres.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Esmon CT. Targeting factor Xa and thrombin: impact on coagulation and beyond. Thromb Haemost. 2014;111(4):625–633. doi: 10.1160/TH13-09-0730. [DOI] [PubMed] [Google Scholar]
- 8.Ishibashi Y, Matsui T, Yamagishi S. Apixaban exerts anti-inflammatory effects in mesangial cells by blocking thrombin/protease-activated receptor-1 system. Thromb Res. 2014;134(6):1365–1367. doi: 10.1016/j.thromres.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Perzborn E, Heitmeier S, Laux V. Effects of rivaroxaban on platelet activation and platelet–coagulation pathway interaction. In vitro and in vivo studies. J Cardiovasc Pharmacol Ther. 2015;20(6):554–562. doi: 10.1177/1074248415578172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosomi N, Naya T, Kohno M, Kobayashi S, Koziol JA, Japan Standard Stroke Registry Study G Efficacy of anti-coagulant treatment with argatroban on cardioembolic stroke. J Neurol. 2007;254(5):605–612. doi: 10.1007/s00415-006-0365-y. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Tazaki Y. Effect of the thrombin inhibitor argatroban in acute cerebral thrombosis. Semin Thromb Hemost. 1997;23(6):531–534. doi: 10.1055/s-2007-996131. [DOI] [PubMed] [Google Scholar]
- 12.Hanzawa KOH, Furui E, Hayashi J, Naritomi H. Anticoagulant agents reduce high intensity transient signals without associated sound during VA ECMO. Neurosonology. 1999;12(3):120–124. doi: 10.2301/neurosonology.12.120. [DOI] [Google Scholar]
- 13.Hartter S, Sennewald R, Schepers C, Baumann S, Fritsch H, Friedman J. Pharmacokinetic and pharmacodynamic effects of comedication of clopidogrel and dabigatran etexilate in healthy male volunteers. Eur J Clin Pharmacol. 2013;69(3):327–339. doi: 10.1007/s00228-012-1304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martischnig AM, Mehilli J, Pollak J, Petzold T, Fiedler AK, Mayer K, Schulz-Schupke S, Sibbing D, Massberg S, Kastrati A, et al. Impact of dabigatran versus phenprocoumon on ADP induced platelet aggregation in patients with atrial fibrillation with or without concomitant clopidogrel therapy (the Dabi-ADP-1 and Dabi-ADP-2 trials) Biomed Res Int. 2015;2015:798486. doi: 10.1155/2015/798486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong PC, Jiang X. Apixaban, a direct factor Xa inhibitor, inhibits tissue-factor induced human platelet aggregation in vitro: comparison with direct inhibitors of factor VIIa, XIa and thrombin. Thromb Haemost. 2010;104(2):302–310. doi: 10.1160/TH10-02-0097. [DOI] [PubMed] [Google Scholar]
- 16.Zemer-Wassercug N, Haim M, Leshem-Lev D, Orvin KL, Vaduganathan M, Gutstein A, Kadmon E, Mager A, Kornowski R, Lev EI. The effect of dabigatran and rivaroxaban on platelet reactivity and inflammatory markers. J Thromb Thrombolysis. 2015;40(3):340–346. doi: 10.1007/s11239-015-1245-z. [DOI] [PubMed] [Google Scholar]
- 17.Japanese Circulation Society Joint Working Group Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013) Circ J. 2014;78(8):1997–2021. doi: 10.1253/circj.CJ-66-0092. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara Y. The joint committee on guidelines for the management of stroke. Jpn Guidel Manag Stroke. 2009;2009:103–109. [Google Scholar]
- 19.European Stroke Organisation (ESO) Executive Committee, and ESO Writing Committee Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25(5):457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 20.Adams HP, Jr, Woolson RF, Biller J, Clarke W. Studies of ORG 10172 in patients with acute ischemic stroke. TOAST Study Group. Haemostasis. 1992;22(2):99–103. doi: 10.1159/000216301. [DOI] [PubMed] [Google Scholar]
- 21.Lippi G, Favaloro EJ. Recent guidelines and recommendations for laboratory assessment of the direct oral anticoagulants (DOACs): is there consensus? Clin Chem Lab Med. 2015;53(2):185–197. doi: 10.1515/cclm-2014-0767. [DOI] [PubMed] [Google Scholar]
- 22.Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;151(1):127–138. doi: 10.1016/j.chest.2016.08.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connolly TM, Condra C, Feng DM, Cook JJ, Stranieri MT, Reilly CF, Nutt RF, Gould RJ. Species variability in platelet and other cellular responsiveness to thrombin receptor-derived peptides. Thromb Haemost. 1994;72(4):627–633. [PubMed] [Google Scholar]
- 24.Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, Gilbert T, Davie EW, Foster DC. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci USA. 1998;95(12):6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esmon CT. Protein C anticoagulant system—anti-inflammatory effects. Semin Immunopathol. 2012;34(1):127–132. doi: 10.1007/s00281-011-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feistritzer C, Lenta R, Riewald M. Protease-activated receptors-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J Thromb Haemost. 2005;3(12):2798–2805. doi: 10.1111/j.1538-7836.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 27.Borissoff JI, Otten JJ, Heeneman S, Leenders P, van Oerle R, Soehnlein O, Loubele ST, Hamulyak K, Hackeng TM, Daemen MJ, et al. Genetic and pharmacological modifications of thrombin formation in apolipoprotein e-deficient mice determine atherosclerosis severity and atherothrombosis onset in a neutrophil-dependent manner. PLoS ONE. 2013;8(2):e55784. doi: 10.1371/journal.pone.0055784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pingel S, Tiyerili V, Mueller J, Werner N, Nickenig G, Mueller C. Thrombin inhibition by dabigatran attenuates atherosclerosis in ApoE deficient mice. Arch Med Sci. 2014;10(1):154–160. doi: 10.5114/aoms.2014.40742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornai F, Carrizzo A, Forte M, Ambrosio M, Damato A, Ferrucci M, Biagioni F, Busceti C, Puca AA, Vecchione C. The inflammatory protein Pentraxin 3 in cardiovascular disease. Immun Ageing. 2016;13(1):25. doi: 10.1186/s12979-016-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antonino MJ, Mahla E, Bliden KP, Tantry US, Gurbel PA. Effect of long-term clopidogrel treatment on platelet function and inflammation in patients undergoing coronary arterial stenting. Am J Cardiol. 2009;103(11):1546–1550. doi: 10.1016/j.amjcard.2009.01.367. [DOI] [PubMed] [Google Scholar]
- 31.Muhlestein JB. Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thromb Haemost. 2010;103(1):71–82. doi: 10.1160/TH09-03-0177. [DOI] [PubMed] [Google Scholar]
- 32.Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017 doi: 10.1056/NEJMoa1708454. [DOI] [PubMed] [Google Scholar]
- 33.Imiya M, Matsuo T. Inhibition of collagen-induced platelet aggregation by argatroban in patients with acute cerebral infarction. Thromb Res. 1997;88(2):245–250. doi: 10.1016/S0049-3848(97)00235-1. [DOI] [PubMed] [Google Scholar]
- 34.Kawai H, Yuki S, Sugimoto J, Tamao Y. Effects of a thrombin inhibitor, argatroban, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J Pharmacol Exp Ther. 1996;278(2):780–785. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting our findings was presented in tables and text in this article.


