Abstract
Breast cancer (BC) is the most common cancer diagnosed in women and the second most common cancer overall, ranking as the fifth cause of death from cancer. The chronicity of the disease produces long-term physiological and psychological manifestations, which adversely affect the quality of life of the individual. The primary treatment while managing cancer presents with various debilitating side effects. With the recent advances in treatment techniques that have improved the survival rate, patients suffer from continuing posttreatment complications. Patients seem to cope well with the stress of treatment of BC and sustain a normal life; however, the deterioration in physical well-being makes the patient functionally inefficient. Exercise has been proven to be an effective, safe, and feasible tool in combating the adverse effects of treatment, prevents complications and decreases the risk of BC-specific mortality. This review briefly presents an overview of the burden of the disease and its management strategies. Owing to the heterogeneity of the population and the multitude of therapies they receive, the response of each patient to treatment is different and so is the magnitude of adverse effects. The review discusses the late sequelae following treatment and evidence supporting the role of physical activity in their management. In conclusion, there is a need for personalized physical activity plans to be developed to suit the individual and their circumstances.
Keywords: breast cancer complications, exercise, quality of life, aerobic training, resistance training
Introduction
Breast cancer (BC) is the second most common cancer overall with 1.7 million new cases reported worldwide. With 883,000 cases occurring in less developed and 794,000 cases in more developed regions, it is the most common cancer diagnosed in women and ranks as the fifth cause of death from cancer overall (522,000 deaths) (1). The peak age of onset is between 40 and 50 years in Asian countries compared with the West (60–70 years) (2). Family history, female sex, age, and changing reproductive trends, including first childbirth after age of 30 years, early age at menarche, later menopause, and nulliparity, are its major independent risk factors (3). BC is classified based on the presence of three receptors found on cancer cells: the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor 2-neu (HER2) receptor. Hormone receptor (HR) positive BCs include expression of ER and PR, accounting for approximately 60% of all BC cases (4). The oncogene HER2 is overexpressed in around 20% of all cases while the remaining 20% are negative for the expression of ER, PR, and HER2, also known as triple-negative breast cancer (TNBC) (5, 6).
The primary treatment of BC includes surgery, adjuvant cytotoxic chemotherapy, radiotherapy, adjuvant endocrine therapy, neoadjuvant anti-HER2 therapies, and personalized medicine. Principle factors in establishing treatment procedure include patient’s age, menopausal status, comorbidities, histologic grade, lymphovascular spread, HR status, and HER2 overexpression (7). Patients with HR positive tumors typically receive endocrine therapy [e.g., selective estrogen-receptor response modulators and aromatase inhibitors (AI)] as one of the treatment options, however, when the disease becomes metastatic, all patients eventually develop endocrine resistance and require cytotoxic chemotherapy (8, 9). Significant advances in the treatment of patients with HER2 overexpressing tumors include targeted therapies that have improved the clinical outcomes for patients with metastatic disease and enhanced survival (10). Patients with TNBCs tend to display an aggressive phenotype, currently do not have targeted therapy options as a standard of care, and have only a limited amount of cytotoxic agents available to treat their disease (11). In addition, according to the International Expert Consensus on the Primary Treatment of Early Breast Cancer, radiotherapy was indicated in patients with four or more positive nodes and should be avoided in elderly and those with substantial comorbidity following breast conserving surgery (7).
Depending on the primary treatment conferred, cancer management might present with various debilitating side effects in 72–96% of cancer patients (12). Treatment-specific changes, along with the morbidity associated with the disease, can lead to impairments in physiological as well as psychological and behavioral attributes, eventually leading to limitations in the ability to execute daily activities and participate in social events (13). BC survivors experience treatment-related distress, fear of recurrence, changes in body image and sexuality, as well as physical toxicities that result from adjuvant therapies (14). Post treatment symptoms such as pain and fatigue often persist, and interfere with functional capacity (15). This impairment is reflected in the fact that BC is responsible for the highest number of disability-adjusted life years in women (13.1 million) (16), imposing an economic burden of over $88 billion (17). Such functional limitation directly affects quality of life (QOL) and should not be left untreated (15). Exercise has been proven to be an effective, safe, and feasible tool to combat these adverse effects of treatment. It has further been shown to prevent complications in BC patients (18, 19), including the risk of postmenopausal BC which is decreased by 12–29% (20–23), and that of BC-specific mortality which is reduced by 15–67% (24). In addition, exercise-induced adaptations and better muscular performance may attenuate cancer toxicities, which in turn could augment the cure rate, improve the QOL for cancer survivors, and may even increase long-term survival (25–27). However, owing to the heterogeneity of this population and the multitude of therapies they receive, the response of each patient to treatment is different and so is the magnitude of adverse effects. Therefore, the purpose of the present review is to discuss the evidence regarding the late sequelae following primary treatment and the role of physical activity in their management. It includes recommendations for future physical activity interventions in brief.
Pain and Lymphedema
About 12–51% of patients complain of pain after treatment (28), which might be of the following two types: (i) musculoskeletal pain resulting from injuries to muscle and ligaments that usually heal and are more likely to be transient and (ii) neuropathic pain from damage to the nerve tissue, which may become a more persistent problem (29). The symptoms of pain tend to diminish with time, affecting 47% of patients 1–3 years following treatment and persisting in up to 30% of patients even after 5 years. In addition, pain in the arm and shoulder ranged between 9 and 68% and in the breast area between 15 and 72% post-surgery (30). Although the occurrence and severity are wide-ranging, the most significant predictor for pain was found to be age. Women aged less than 40 are 3.6 times more likely to report pain than women aged 60–69 years. Treatment was another key determinant with axillary dissection and radiotherapy resulting in significantly more pain, whereas the surgical procedure (breast conserving surgery vs. mastectomy) or use of chemotherapy showed no differences in pain outcomes (31).
Another sequel of BC treatment is lymphedema, affecting 6–43% of BC patients (32). It results from insufficient lymph transport caused by damage to the lymphatic vasculature by lymph node dissection and radiotherapy (33). The most significant risk factors are mastectomy, radiotherapy, axillary dissection, tumor positive lymph nodes (34), and young age (35). Lymphedema has been shown to cause considerable functional disability as a result of pain, swelling, heaviness, paresthesia, and overall reduced mobility of the affected limb (36–38). It has also been associated with physiological and psychological side effects, such as compromised immune function (39), anxiety, distress, and social inhibition (40).
Role of Physical Activity
The mainstay of treatment for cancer-related lymphedema includes complex decongestive therapy, exercise, and skin care (41). Ahmed et al. (42) proposed physiological changes in lymphatic function because of exercise, which include stimulation of lymph flow from skeletal muscle pumping and cardiopulmonary system (43). In addition, exercise has been shown to improve venous hemodynamics of the upper extremities causing a reduction in swelling (44). The American College of Sports Medicine (ACSM) in agreement with previous studies (45–47) now accepts resistance training as a safe and effective intervention (48) to reduce lymphedema in BC patients (49, 50). Schmidt et al. (47) concurred that resistance training decreased the incidence and intensity of arm and hand symptoms, and lymphedema exacerbations, and improved muscular strength.
Muscle Strength
An estimated 10–70% of BC patients reported considerable restrictions in arm and shoulder mobility, and 17–33% reported decreased muscle strength following primary treatment (including surgery, chemotherapy, radiotherapy, and endocrine therapy) (32). The wide variation in prevalence could be attributed to the differences in assessment methods (measured or self-reported), time since treatment and type of surgery, with mastectomy and radiotherapy showing greater impairments (30). Hand grip and lower extremity strength have been an established prognostic variable for disability and mortality in elderly populations (51, 52). In BC survivors, the association between handgrip strength and QOL has been reported (53). More than 10% deficit in handgrip strength, specifically, on the surgical side was observed in 40% of BC survivors post-mastectomy (54). A significant reduction in the tip to tip pinch, key pinch, and palmar pinch has also been observed before and after radiotherapy following modified radical mastectomy.
Reduced muscle strength has been attributed to the loss of lean mass, which (55) is associated with disability, deteriorated QOL, altered functional status, and increase in fatigue and falls (56, 57). Lower extremity strength is a significant predictor of cancer-related fatigue, and its reduction increases fracture risk in BC survivors (58, 59). Chemotherapy is correlated with the loss of lean mass, especially in the lower limbs (60), leading to impaired isometric and isokinetic strength capacity, as well as muscular fatigue (61) that deteriorates with time since the completion of treatment (62).
Role of Physical Activity
Exercise interventions have demonstrated significant improvements in lean body mass (LBM) and muscular strength (63). Specifically, resistance exercises have been shown to be effective for increasing lower-limb muscular strength and preventing the loss of LBM. These effects can be explained by increased motor unit recruitment and firing rate (64, 65), causing neural adaptations that result in increased force development (66). A recent review (67) also found improvements in upper and lower-limb muscular strength after resistance exercise program.
Bone Health
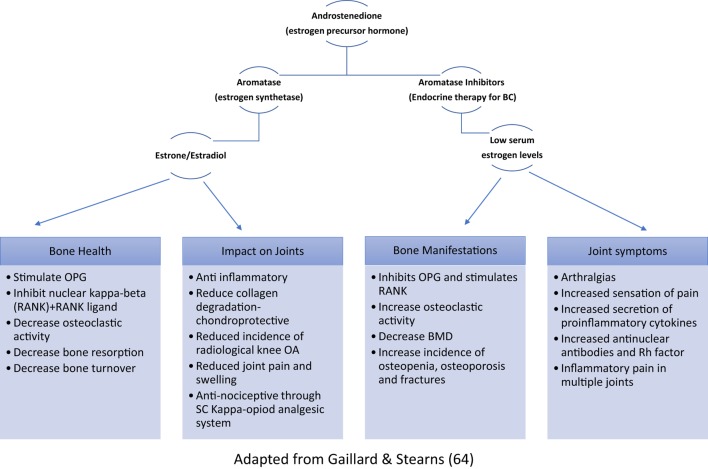
The rates of osteoporosis and osteopenia in cancer patients in remission were found to be 16 and 44%, respectively (68). Bone mineral homeostasis resulting from a balance between osteoblastic and osteoclastic activities has been found to be regulated by estrogen (69). Chemotherapy can inhibit bone proliferation directly and, along with ovarian suppression, indirectly reduce bone turnover via reduced estrogen (70) (Figure 1). Premature menopause is associated with an 11% reduction in bone mineral density (BMD), whereas chemotherapy and AI have shown to reduce BMD by around 4% in the lumbar spine (71–73). Therefore, these patients with increased risk of accelerated bone loss should have a baseline assessment of BMD (DXA-scan) within 3 months of ovarian suppression therapy and AI therapy, and 12 months post-chemotherapy amenorrhea. Furthermore, dietary supplementation with calcium and vitamin D, treatment with bisphosphonates and lifestyle advice should be incorporated into their management strategies (74).
Figure 1.
Role of estrogen in bone homeostasis and musculoskeletal symptoms (OPG, osteoprotegerin; RANK, receptor activator of nuclear kappa-beta; OA, osteoarthritis; SC, spinal cord; BMD, bone mineral density).
Role of Physical Activity
The potential negative side effects to body composition and bone loss from AIs may be diminished or eliminated through implementing regular physical activity and exercise (75). Aerobic exercise has shown to maintain total body BMD, and resistance training plus impact training preserved spinal BMD, posttreatment (76, 77). Upper-body resistance exercise recovers spinal density via tension produced by muscle insertion (78), whereas impact exercises activate hip and femur bone recovery through weight bearing by lower limbs (79).
Arthralgias and Associated Symptoms
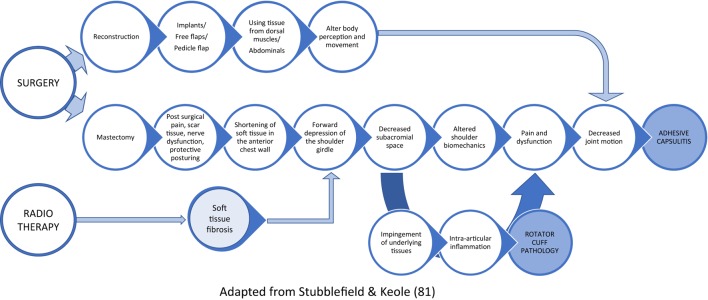
Around 10–60% of BC patients report at least one upper-body symptom after surgery (80), and up to 61% report new or worsening joint symptoms following AI-treatment (81, 82), mediated by estrogen deficiency (Figure 1). These symptoms have been reported to significantly limit the performance of activities of daily living as well as work-related tasks (82, 83). AI-induced arthralgias were found to be severe enough to cause therapy interruption in up to 20% of patients (81, 84). The most commonly reported symptoms include morning stiffness and joint pain in the wrist (70%), hand (63%), knee (70%), back (54%), ankle/foot (51.8%), and hip (42.5%) (85). Restricted shoulder range of motion (ROM) was found in up to 50% of patients after treatment, with rotator cuff dysfunction and adhesive capsulitis being the commonest underlying pathologies (86). Surgery, specifically mastectomies or lymph node dissections, is an evidential risk factor for these complications and may cause axillary paresthesia, muscle dysfunction, and pain affecting the intercostal brachial or thoracodorsal nerve (Figure 2). Other frequently reported symptoms were digital stiffness, trigger finger, and carpal tunnel syndrome (81, 83, 87). AI when compared with tamoxifen increased patients’ predisposition to undergo surgery for carpal tunnel syndrome by up to seven times. Ultrasound and MRI evaluations have revealed fluid in the joint space and tendon sheath surrounding the digital flexor tendons and thickening of the tendon sheath (87, 88).
Figure 2.
Shoulder dysfunction following breast cancer treatment.
Role of Physical Activity
Gentle articular movements and stretching, during and after treatments, promotes joint mobility and restores muscle flexibility (89, 90), prevents muscle contractures, and alters shoulder mechanics (91). Resistance exercise prevents musculoskeletal injury, improves muscular strength, improves ROM, and reduces body fat as well as systemic inflammation levels (75).
Body Composition
Approximately 65% of all BC survivors are overweight or obese (92) with up to 84% reporting weight gain following diagnosis ranging from 2.5 to 5.2 kg (93). Sedentary lifestyle, postmenopausal status, intake of supportive medication, particularly glucocorticoids, slow metabolism, and endocrine manipulation predispose the individual to weight gain (94). Obesity and sedentary lifestyle are not only the causative factors for 25 and 33% of all BC cases (95) but also associated with poorer outcomes after a diagnosis, such as increased recurrence and total mortality. High level of fat mass decreases the survival rates of postmenopausal patients (19). Breasts are the primary site for estrogen production and produce pro-inflammatory cytokines and pro-tumorigenesis proteins, which are related to a poor quality of survival (18, 95). A 35% higher risk of BC-related death and a 41% higher risk of death due to other causes (96) have been noted in patients with obesity. In addition, BC treatment is related to increases in body fat as well as decreases in LBM and BMD (97, 98).
Role of Physical Activity
A combination of aerobic and resistance training was most effective in reducing fat mass and raising LBM, as compared with aerobic exercise only (99, 100). In addition, performing resistance training twice a week for 6 months can increase LBM by 1–2 kg, a change that may prevent or reverse age-associated lean mass losses (101). It has been observed that changes in body composition and body weight take place only after 20 weeks of intervention (63). In postmenopausal women, exercise reduces body fat mass, which is associated with reduction in waist–hip ratio, serum estradiol, and inflammatory biomarkers levels (102, 103). Exercise and training increase muscle mass, which is correlated with a higher basal metabolism (104) promoting the transformation of white fat mass into brown fat mass (105).
Physical Fitness
Breast cancer patients report reduced physical capacity (approximately 30%) (106, 107) compared with age- and sex-matched sedentary individuals. Peak oxygen uptake (VO2peak) reduces to 5–10% with ongoing chemotherapy (26, 108) and remains, on average, 22% lower in BC survivors despite normal cardiac function (as indicated by left ventricular ejection fraction ≥50%) (109). This implies that the decline in cardiorespiratory fitness could be attributed to other components of oxygen transport (i.e., pulmonary, hematologic, vascular, and skeletal muscle function) (109). This chemotherapy-induced reduction in VO2peak, spanning over a period of 12–24 weeks, is equivalent to that reported with 30 years of normal aging (109, 110). The evidence of a relationship between VO2peak and risk of cancer-related death in females and with BC-specific death also exists (111, 112).
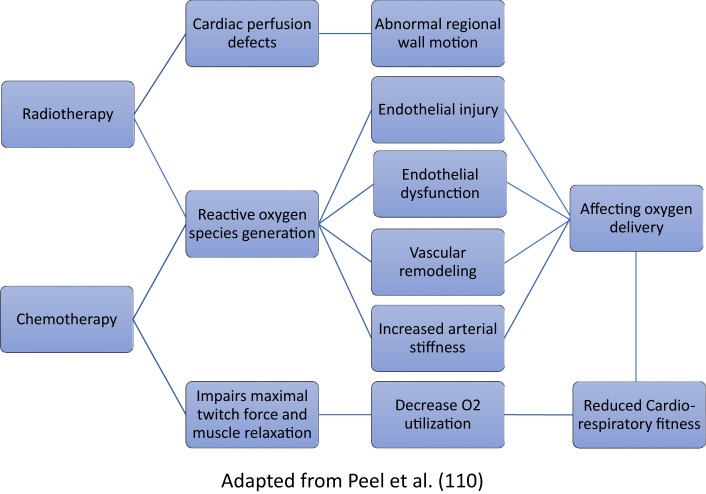
Radiotherapy can impair the pulmonary gas exchange, as incidental radiation to the lungs can cause fibrosis (113). Both radiotherapy and chemotherapy for BC, especially anthracycline-containing chemotherapy, have shown to hamper both oxygen delivery and oxygen utilization (114). The proposed mechanisms have been outlined in Figure 3. Anemia may develop while undergoing therapy (115) and reduce oxygen delivery to muscle cells (116). It is evidential that exercise training is an effective intervention to improve cardiorespiratory function as well as QOL, strength, body composition and symptoms of fatigue, and depression in BC patients (63).
Figure 3.
Proposed pathways for decline in cardiorespiratory fitness following breast cancer treatment.
Role of Physical Activity
Interventions that have improved VO2peak have typically lasted 8–24 weeks (117) and involve a prescription of 20–45 min of aerobic exercise two to three times per week at moderate intensity [65% maximum heart rate (MHR)] that may progress to vigorous intensity (>85% MHR) (117, 118). Many studies have also included resistance training. These varied prescriptions have resulted in improvements in VO2peak ranging from 2 to 32%, implying improved cardiorespiratory fitness of these patients (117, 118).
Cardiovascular Dysfunction
Breast cancer patients are more prone to develop cardiovascular diseases, including heart failure and myocardial infarction compared with women who do not have a BC diagnosis (119, 120). Primary treatment may lead to cardiovascular impairments such as acute reductions in red blood cells and cardiotoxicity after treatment with anthracyclines (more permanent) or trastuzumab (reversible) (26, 108). A total of 13–39% of BC patients treated with anthracyclines present with cardiac events at some point in their life (106, 114, 121) (Figure 3). Radiotherapy at the left side increases cardiovascular mortality by 25% 15 years after diagnosis compared with women irradiated in the right side (122) and can also lead to valvular disease and accelerated coronary artery disease (123). Furthermore, radiotherapy-induced cardiotoxicity is amplified using adjuvant systemic chemotherapy, particularly anthracycline-based regimens (124) and newer agents such as trastuzumab (more commonly known as Herceptin) (125).
Role of Physical Activity
Low-intensity aerobic exercise increases left ventricle volume, contractility, and elasticity of the cardiac muscle. Scott et al. inferred that exercise improves muscle cardiac irrigation (117) and reduces global low-grade inflammation, which is associated with cardiovascular diseases.
Cardiovascular Risk Factors
Commonly encountered modifiable risk factors prevalent in BC patients include hypertension, dyslipidemia, overweight, and obesity and raised blood glucose or diabetes. Hypertension occurs in 25–50% of survivors (126) and is twice as common than age-matched female controls (127). Physical activity during and after BC treatment have consistently shown drops in systolic and diastolic blood pressure by up to 4.6–4.4 mmHg (128, 129). Aerobic training spanning 8–16 weeks with sessions lasting at least 20 min performed two to three times per week at moderate to vigorous intensity played an instrumental role in reducing blood pressure in BC survivors (63, 128).
On an average, BC women are likely to have high total cholesterol, triglycerides, and low-density lipoprotein (LDL) levels and lower high-density lipoprotein levels even before primary treatment, as compared to healthy, age-matched women (130, 131). Chemotherapy treatment may also increase triglyceride levels (132), however, tamoxifen decreases total cholesterol and LDL levels (133). Patients with type II diabetes mellitus are twice more susceptible to develop BC compared with age-matched women (127) and this increased risk has been proposed to be associated with weight gain (134). Moreover, studies on multiple GDM (gestational diabetes mellitus) pregnancies and BC risk (135, 136) suggest that abnormalities in glucose metabolism result in increased bioactivity of insulin-like growth factors influencing breast tissue remodeling and contributing to the initiation and progression of BC (137, 138). A combination of aerobic and resistance training has reported significant improvements in fasting insulin and blood glucose levels (139).
Modes of Physical Activity
Previous studies examining the effect of physical activity are extremely varied in terms of (i) mode including aerobic, resistance, tai chi, and combined training (aerobic+resistance), (ii) frequency ranging from one to five times per week, (iii) length of a session between 15 and 90 min, and (iv) total duration of training spanning from 4 to 52 weeks.
Aerobic Training
The meta-analysis by Schmid and Leitzmann (140) indicated that the moderate physical activity of 150 min/week after diagnosis is associated with a 24% reduction in total mortality among BC survivors and a 28% decrease in the risk of total mortality. Similar studies have also found statistically significant association between higher levels of physical activity and reduced risk of BC mortality (141, 142).
Aerobic exercise training improves metabolic function, functional capacity, and immune system, thereby diminishing the side effects before, during, or after cancer treatment (103, 143, 144). Despite these positive effects, a significant reduction in the QOL of BC survivors has been noted. Therefore, ways to make the aerobic exercise training effective for a long period of time are warranted. Cancer events such as diagnosis and completion of primary treatment have been proposed as unique windows of opportunity or “teachable moments” that can be used to influence behavior (145). Drum et al. (146) advised that post-cancer treatment aerobic training may be implemented based on the ACSM aerobic exercise guidelines for sedentary healthy persons. Exercise prescription must be based on cardiopulmonary exercise testing to make the training parameters more precise.
Resistance Training
Most of the studies followed the ACSM recommendations focusing on large muscle groups (chest, back, shoulders, arms, buttock, hips, thighs, and calves) performing 1–3 sets of 8–10 repetitions, 2–3 days/week at an exercise intensity of 60–70% of one repetition maximum (RM) (48, 147). Owing to considerable variability in the implementation and responses to resistance training in different cancer populations and within patients of the same cancer type, a baseline assessment of strength is essential to individualize prescription. This mandates the highly individualized nature of exercise prescription in an oncology setting, and the need to investigate different doses, frequencies, duration, or load of resistance training (148).
The principle of specificity emphasizes that the training session is designed in view of the desired goals. The principle of overload mandates the exercise stimulus must be adequate to stress the system to a point where adaptations occur without disrupting the homeostasis and increasing the fatigue and risk of injury (149, 150). The incorporation of progression is critical because the body quickly adapts to a given exercise stress; the training stimulus must be gradually increased for continued development (151).
Complementary Therapies and Alternative Medicine (CAM)
NIH has classified CAM into four categories: (1) mind and body medicine (meditation, yoga, acupuncture, guided imagery, qi gong), (2) manipulative and body-based practices (spinal manipulation, massage therapy, chiropractic medicine), (3) alternative systems (traditional Chinese medicine, Native American healing systems, Reiki, homeopathic medicine, Ayurveda), and (4) natural products (herbal supplements, botanical supplements, single supplements, and combinations of vitamins or minerals) (152). In addition to these, electrotherapeutic modalities, such as LASER, electrical stimulation, microwave diathermy, and thermotherapy have demonstrated insufficient evidence to support their use (153). Interventions with some evidence of efficacy in lymphedema include compression bandaging (154), pneumatic compression pumps (155), and decongestive therapies (154). Furthermore, the non-pharmacological management of pain with the use of transcutaneous electrical neuromuscular stimulation (TENS), acupuncture, or acupressure has been documented (155–157). However, there is a dearth of high-quality RCT’s to conclusively establish their effectiveness and dosimetry.
Yoga
Yoga is a mind-body exercise program that provides physiological effects that are similar to aerobic exercise, including physical poses, breathing, and meditation (158, 159). It is widely accepted as an integrative form of therapy for BC (160). Its efficacy has been established both on and off treatment, and used as an adjunct to primary BC treatment (161). The main types of yoga used among cancer patients are Iyengar yoga, restorative yoga, and hatha yoga (162). 20–60 min of yoga per session for 4–24 weeks has been found to be an effective complementary modality of treatment in improving physical and psychosocial symptoms (160). Yoga practicing patients had better outcomes in terms of improved sleep quality, duration, latency, efficiency (163), improved overall QOL (164), reduced fatigue (165), menopausal symptoms (166), reduced body fat (167), and reduced depression scores (168).
It is usually the union of body and mind that is achieved in yoga (160). Mindfulness practices can address the cognitive and emotional components of pain and are associated with better coping skills, overall well-being, and spiritual development after chemotherapy, radiotherapy, and systemic treatment (164). Although there is no clear evidence how yoga induces relaxation, it has been postulated that yoga may lower stress induced arousal in BC patients in addition to increasing proprioception of somatic symptoms, building inner awareness, altering perceptions and mental responses to both internal and external stimuli (169). But there is a need to substantiate the genuinity, sustainability, and overall impact of yoga on BC survivors. A standardized intervention protocol which includes at least 18 h of yoga practice and is spread over at least 1 month, using a combination of selected asanas (physical postures adapted to the abilities of cancer patients), pranayamas (breathing exercises) and dhyanas (meditation) would be helpful for future recommendations (160). Yagli et al. found yoga with aerobic training to be more beneficial than aerobic exercise alone and recommended the incorporation of mind-body exercises in oncologic rehabilitation of patients who survive BC (164).
Research Gaps and Future Perspectives
The major limitation of the present article was that it did not systematically review the literature, which makes the findings susceptible to selection bias. However, to control for these threats, the authors assessed the quality of selected studies using the PEDRO score which is a reliable and valid measure of methodological quality of RCTs (170, 171) and included findings from good-excellent quality RCTs, i.e., PEDRO score ≥ 5/11 (Table 1). Furthermore, for the most prevalent comorbidities, we have tried to summarize the efficacy of various exercise interventions (Table 2). However, a more quantitative efficacy analysis of each intervention may be separately performed comparing their effect size and 95% confidence interval, which was beyond the scope of the present study. A systematic review would provide more robust findings and an objective understanding of the efficacy of physical activity in combating treatment complications following BC. Future high-quality studies should also seek to explore training characteristics other than the standard ACSM exercise prescription, investigate the dose–response relationship between exercise and outcome variables, and finally draw comparisons with the traditional guidelines to design an optimal training protocol. While designing these programs, it is necessary to ensure optimal timing, and that all alternative exercise modes offer sufficient training stimulus in accordance with specific cancer site.
Table 1.
Quality of studies assessing efficacy of exercise interventions.
| Reference | Intervention groups | Outcomes | Quality of evidencea |
|---|---|---|---|
| Kilbreath et al. (89) | Stretching+PRE (n = 81) Control group (n = 97) 8 weeks |
Sh. ROM ↑, Sh. strength ↑, lymphedema↔, QOL↔. | 9/11 |
| Lee et al. (91) | Scapula-oriented exercises (n = 16) General exercises (n = 16) Control group (n = 18) 8 weeks |
Sh. strength↔, ROM ↑, Sh. disability↔, pain (VAS↔, BPI ↑), depression↔, QOL↑. | 8/11 |
| Fairey et al. (103) | RT (n = 25) Control group (n = 28) 15 weeks |
CRP↔, RHR↔, HRR↑, SBP↔, DBP↔, HDL↔, LDL↔, TG↓, TC↔, TC: HDL↔ | 9/11 |
| Schmitz et al. (104) | Immediate resistance (n = 42) Delayed resistance (n = 43) 12 months |
BW↔, BMI↔, WC↔, FG↔, IGF II↓, insulin resistance↔, BF%↓, FFM↑, 1RM↑, insulin↔, LB 1RM↑. | 8/11 |
| Scott et al. (117) | AE+RT (n = 47) Control group (n = 43) 6 months |
BW↓, BMI↓, WC↓, WHR↓, BF%↔, VO2Max↑, RHR↔, SBP↔, DBP↓, QOL↑, CRP↔, TC↓, estrone↔, estradiol↔, IGF↔, leptin↓, IGBP1, 3↔, testosterone↔, SHPG↔, insulin resistance↔. | 8/11 |
| Nuri et al. (139) | AE+RT (n = 14) Control group (n = 15) 15 weeks |
FI↓, FG↓, insulin resistance↔, HDL↑, TG↓, VO2Peak↑, RHR↓, SBP↓, BW↓ BMI↓, WHR↓ | 6/11 |
| Hughes et al. (167) | Yoga exercises (n = 31) Comprehensive exercises(n = 31) Control group (n = 32) 6 months |
BW↔, BMI↔, RHR↔, SBP↔, DBP↔, VO2Max↔, arm strength↔, torso strength↔, arm volume, BF%↓, leg strength↑, flexibility↑, ROM↑. | 5/11 |
| Ahmed et al. (42) | RT (n = 42) Control group (n = 43) 6 months |
Lymphedema↔, UL 1RM↑, LL 1RM↑ | 5/11 |
| Vardar Yag˘lı et al. (164) | AE (n = 28) Y+AE (n = 32) 6 weeks |
6 MWT↑(FC), strength↑, fatigue↓, QOL↑ | 6/11 |
| Courneya et al. (26) | RT (n = 82) AE (n = 82) Control group (n = 78) 9–24 weeks |
QOL↑ (R > A), fatigue↔, depression↔, VO2Max↑, (A > R), body fat% ↓(A > R), strength ↑(R > A), LBM ↑(R > A) | 8/11 |
| Friedenreich et al. (102) | AE (n = 160) Control group (n = 160) 12 weeks |
Estrone↔, estradiol↓, androstenedione↔, testosterone ↔, SHBG ↑ | 8/11 |
| Irwin et al. (75) | AE+RT (n = 61) Control group (n = 60) 12 months |
Arthralgia↓, pain↓, disability↓, VO2Max↑, grip strength↑, BW↓, physical activity↑ | 7/11 |
| Kim et al. (45) | PT+RT (n = 20) PT (n = 20) 8 weeks |
Lymphedema ↓, QOL ↑ | 6/11 |
| Nelson (101) | RT (n = 20) Control group (n = 19) 12 months |
BMD↑, strength↑, muscle mass↑, balance↑ | 7/11 |
| Winters-Stone et al. (77) | RT+Impact training (Power) (n = 52) Control group (n = 54) 12 months |
Spine BMD ↑, osteocalcin↔, deoxypyrodinoline ↓ | 9/11 |
| Winters-Stone et al. (78) | Power (n = 35) Control group (n = 36) 12 months |
Hip and spine BMD ↔, Body fat% ↓, bone turnover ↔, upper-body strength ↑, LBM ↔, Strength↔ | 8/11 |
| Sagen et al. (46) | RT (n = 104) Control group (n = 100) 6 months |
Arm volume ↔, pain ↓ | 8/11 |
| Schmitz et al. (47) | RT (n = 71) Control group (n = 70) |
Bench press ↑, leg press ↑, BW↔, BMI↔, BF%↔, FM↔, LBM↔, lymphedema symptoms↓, lymphedema symptoms severity ↓ | 8/11 |
PRE, progressive resistance exercise; QOL, quality of life; VAS, visual analog scale; BPI, Brief Pain Inventory; CRP, C-reactive protein; RHR, resting heart rate; HRR, heart rate recovery; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipids; LDL, low-density lipids; TG, triglycerides; TC, total cholesterol; BW, body weight; BMI, body mass index; WC, waist circumference; FM, fat mass; LBM, lean body mass; Sh., shoulder; RT, resistance training; AE, aerobic exercise; FG, fasting glucose; IGF II, insulin-like growth factor; BF%, body fat%; FFM, fat-free mass; RM, repetition maximum; LB, lower body; WHR, waist hip ratio; IGBP, immunoglobulin-binding protein; SHBG, sex hormone binding globulin; FI, fasting insulin; FG, fasting glucose; ROM, range of motion UL, upper limb; LL, lower limb; BMD, bone mineral density. ↑ sig. increase; ↓ sig. decrease; ↔ no sig. change.
aMethodological Quality of RCTs was assessed using the PEDRO score.
Table 2.
Strength of evidence of various exercise interventions for Breast cancer-related morbidities.
| Outcome measure | Intervention | PEDRO scorea | Strength of evidence |
|---|---|---|---|
| Pain and arthralgia | Scapular exercises | 8 | Good |
| RT | 8 | Good | |
| AE+RT | 7 | Good | |
| Lymphedema | RT | 8 | Good |
| PT+RT | 6 | Good | |
| Strength | Stretching+PRE | 9 | Excellent |
| RT | 5–8 | Fair-Good | |
| POWER | 8 | Good | |
| AE+Y | 6 | Good | |
| AE+RT | 7 | Good | |
| ROM | Stretching+PRE | 9 | Excellent |
| Scapular | 8 | Good | |
| Y | 5 | Fair | |
| Body composition | |||
| BW | AE+RT | 6–7 | Good |
| BMI | AE+RT | 6 | Good |
| POWER | 8 | Good | |
| BF | AE+RT | 6 | Good |
| Y | 5 | Fair | |
| LBM | RT | 7/8 | Good |
| BMD | RT | 7 | Good |
| POWER | 9 | Excellent | |
| Lipid | |||
| TC | AE+RT | 6 | Good |
| HDL | AE+RT | 8 | Good |
| TG | RT | 9 | Excellent |
| AE+RT | 6 | Good | |
| Cardiovascular fitness | |||
| VO2 max | AE | 8 | Good |
| AE+RT | 6–8 | Good | |
| RHR | AE+RT | 6/8 | Good |
| DBP | AE+RT | 8 | Good |
| SBP | AE+RT | 6 | Good |
| FC | AE+Y | 8 | Good |
| Hormones | |||
| Estradiol | AE | 8 | Good |
| SHBG | AE | 8 | Good |
| Leptin | AE+RT | 8 | Good |
| FBG | AE+RT | 6 | Good |
| FATIGUE | AE+Y | 6 | Good |
| QOL | Scapular Ex | 8 | Good |
| AE+RT | 8 | Good | |
| AE+Y | 6 | Good | |
| RT | 8 | Good | |
| PT+RT | 6 | Good | |
Y, yoga; PT, physiotherapy; FBG, fasting blood glucose; FC, functional capacity; QOL, quality of life; RHR, resting heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high-density lipids; TG, triglycerides; TC, total cholesterol; BW, body weight; BMI: body mass index; LBM: lean body mass; BMD: bone mineral density; RT: resistance training; AE, aerobic exercise; BF, body fat; SHBG, sex hormone binding globulin; ROM: range of motion.
This review briefly outlines the ongoing primary treatment options available for BC population, prevalent musculoskeletal complications, and appropriate physical activity measures that can be adopted to combat the same, consequently, emphasizing the role of exercise post-primary treatment in BC survivors to improve overall QOL and reduce mortality. The intent is to encourage BC survivors to adopt a physically active lifestyle as part of the path to recovery. The survivors mainly face the challenge of initiating, reinitiating, and maintaining the activity levels, owing to confounding factors, such as personal, physiological, psychosocial, immunological, and endocrinological. To remedy this, tailor made exercise programs should be designed that embrace the interests, needs, capabilities, and preferences of patients. These personalized programs should offer a variety of alternative exercise modes in order to cater to specific outcome measures (strength, physical fitness, fatigue), help combat treatment-related adverse effects (decline in muscle mass, bone density, physical function, and psychological well-being), and maximize adherence to treatment, thus enhancing their efficacy.
To conclude, it is imperative to mention that the need of the hour is to explore the possibilities of above therapies to combat the deadly disease and most importantly the complications associated with cancer to destigmatize the social taboos associated with it. A detailed, focused and evidence-based research should be carried out to establish the efficacy of individual therapy to benefit the patient population. In addition to this, there should be awareness about Human papilloma virus vaccination, cancer websites, one to one interaction, government schemes, and government sponsored treatment modalities to improve its acceptability and affordability.
Author Contributions
SZ and SV were involved in the primary writing of the manuscript. SH conceptualized the paper and guided the writing of the manuscript. SH also contributed to regular manuscript corrections and revisions of content. SN, NS, AK, AS, GR, ZV, JM, PT, and RM were involved in critical revision of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136(5):E359–86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg (2010) 34(10):2308–24. 10.1007/s00268-010-0683-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol (2012) 13(11):1141–51. 10.1016/S1470-2045(12)70425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzdar AU. Role of biologic therapy and chemotherapy in hormone receptor- and HER2-positive breast cancer. Ann Oncol (2009) 20(6):993–9. 10.1093/annonc/mdn739 [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med (2001) 344(11):783–92. 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 6.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer (2009) 9(Suppl 2):S73–81. 10.3816/CBC.2009.s.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol (2013) 24(9):2206–23. 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez-Aya LF, Chavez-Macgregor M, Lei X, Meric-Bernstam F, Buchholz TA, Hsu L, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol (2011) 29(19):2628–34. 10.1200/JCO.2010.32.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail-Khan R, Bui MM. A review of triple-negative breast cancer. Cancer Control (2010) 17(3):173–6. 10.1177/107327481001700305 [DOI] [PubMed] [Google Scholar]
- 10.Figueroa-Magalhães MC, Jelovac D, Connolly R, Wolff AC. Treatment of HER2-positive breast cancer. Breast (2014) 23(2):128–36. 10.1016/j.breast.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med (2010) 363(20):1938–48. 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 12.Kaya T, Karatepe AG, Günaydn R, Yetiş H, Uslu A. Disability and health-related quality of life after breast cancer surgery: relation to impairments. South Med J (2010) 103(1):37–41. 10.1097/SMJ.0b013e3181c38c41 [DOI] [PubMed] [Google Scholar]
- 13.Campbell KL, Pusic AL, Zucker DS, McNeely ML, Binkley JM, Cheville AL, et al. A prospective model of care for breast cancer rehabilitation: function. Cancer (2012) 118(8 Suppl):2300–11. 10.1002/cncr.27464 [DOI] [PubMed] [Google Scholar]
- 14.Ganz PA. Psychological and social aspects of breast cancer. Oncology (Williston Park) (2008) 22(6):642–6, 650; discussion 650, 653. [PubMed] [Google Scholar]
- 15.Costa WA, Eleutério J, Jr, Giraldo PC, Gonçalves AK. Quality of life in breast cancer survivors. Revista da Associação Médica Brasileira (2017) 63:583–9. 10.1590/1806-9282.63.07.583 [DOI] [PubMed] [Google Scholar]
- 16.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M. The global burden of cancer 2013. JAMA Oncol (2015) 1(4):505–27. 10.1001/jamaoncol.2015.0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John R, Ross H. The global economic cost of cancer. Atlanta, GA: American Cancer Society and LIVESTRONG; (2010). [Google Scholar]
- 18.Galanti G, Stefani L, Gensini G. Exercise as a prescription therapy for breast and colon cancer survivors. Int J Gen Med (2013) 6:245–51. 10.2147/IJGM.S42720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnsson A, Johnsson A, Johansson K. Physical activity during and after adjuvant chemotherapy in patients with breast cancer. Physiotherapy (2013) 99(3):221–7. 10.1016/j.physio.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 20.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res (2011) 188:125–39. 10.1007/978-3-642-10858-7_11 [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat (2013) 137(3):869–82. 10.1007/s10549-012-2396-7 [DOI] [PubMed] [Google Scholar]
- 22.Zhong S, Jiang T, Ma T, Zhang X, Tang J, Chen W, et al. Association between physical activity and mortality in breast cancer: a meta-analysis of cohort studies. Eur J Epidemiol (2014) 29(6):391–404. 10.1007/s10654-014-9916-1 [DOI] [PubMed] [Google Scholar]
- 23.Bellocco R, Marrone G, Ye W, Nyrén O, Adami HO, Mariosa D, et al. A prospective cohort study of the combined effects of physical activity and anthropometric measures on the risk of post-menopausal breast cancer. Eur J Epidemiol (2016) 31(4):395–404. 10.1007/s10654-015-0064-z [DOI] [PubMed] [Google Scholar]
- 24.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun (2013) 30(Suppl):S75–87. 10.1016/j.bbi.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Cancer treatment-induced alterations in muscular fitness and quality of life: the role of exercise training. Ann Oncol (2007) 18(12):1957–62. 10.1093/annonc/mdm364 [DOI] [PubMed] [Google Scholar]
- 26.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol (2007) 25(28):4396–404. 10.1200/JCO.2006.08.2024 [DOI] [PubMed] [Google Scholar]
- 27.Courneya KS, Segal RJ, McKenzie DC, Dong H, Gelmon K, Friedenreich CM, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med Sci Sports Exerc (2014) 46(9):1744–51. 10.1249/MSS.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 28.Rietman JS, Dijkstra PU, Hoekstra HJ, Eisma WH, Szabo BG, Groothoff JW, et al. Late morbidity after treatment of breast cancer in relation to daily activities and quality of life: a systematic review. Eur J Surg Oncol (2003) 29(3):229–38. 10.1053/ejso.2002.1403 [DOI] [PubMed] [Google Scholar]
- 29.Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain (2003) 104(1–2):1–13. 10.1016/S0304-3959(03)00241-0 [DOI] [PubMed] [Google Scholar]
- 30.Lee TS, Kilbreath SL, Refshauge KM, Herbert RD, Beith JM. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res Treat (2008) 110(1):19–37. 10.1186/bcr1865 [DOI] [PubMed] [Google Scholar]
- 31.Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol (2011) 50(2):187–93. 10.3109/0284186X.2010.533190 [DOI] [PubMed] [Google Scholar]
- 32.Casla S, Hojman P, Márquez-Rodas I, López-Tarruella S, Jerez Y, Barakat R, et al. Running away from side effects: physical exercise as a complementary intervention for breast cancer patients. Clin Transl Oncol (2015) 17(3):180–96. 10.1007/s12094-014-1184-8 [DOI] [PubMed] [Google Scholar]
- 33.Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol (2008) 52(10):799–806. 10.1016/j.jacc.2008.06.005 [DOI] [PubMed] [Google Scholar]
- 34.Tsai RJ, Dennis LK, Lynch CF, Snetselaar LG, Zamba GK, Scott-Conner C. The risk of developing arm lymphedema among breast cancer survivors: a meta-analysis of treatment factors. Ann Surg Oncol (2009) 16(7):1959–72. 10.1245/s10434-009-0452-2 [DOI] [PubMed] [Google Scholar]
- 35.Gärtner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment – a nationwide study of prevalence and associated factors. Breast (2010) 19(6):506–15. 10.1016/j.breast.2010.05.015 [DOI] [PubMed] [Google Scholar]
- 36.Velanovich V, Szymanski W. Quality of life of breast cancer patients with lymphedema. Am J Surg (1999) 177(3):184–7; discussion 188. 10.1016/S0002-9610(99)00008-2 [DOI] [PubMed] [Google Scholar]
- 37.Shih YC, Xu Y, Cormier JN, Giordano S, Ridner SH, Buchholz TA, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: a 2-year follow-up study. J Clin Oncol (2009) 27(12):2007–14. 10.1200/JCO.2008.18.3517 [DOI] [PubMed] [Google Scholar]
- 38.Norman SA, Localio AR, Potashnik SL, Simoes Torpey HA, Kallan MJ, Weber AL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol (2009) 27(3):390–7. 10.1200/JCO.2008.17.9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes SC, Speck RM, Reimet E, Stark A, Schmitz KH. Does the effect of weight lifting on lymphedema following breast cancer differ by diagnostic method: results from a randomized controlled trial. Breast Cancer Res Treat (2011) 130(1):227–34. 10.1007/s10549-011-1547-6 [DOI] [PubMed] [Google Scholar]
- 40.McWayne J, Heiney SP. Psychologic and social sequelae of secondary lymphedema: a review. Cancer (2005) 104(3):457–66. 10.1002/cncr.21195 [DOI] [PubMed] [Google Scholar]
- 41.Liebl ME, Preiß S, Pögel S, Pinnow J, Schwedtke C, Taufmann I, et al. Elastisches Tape als therapeutische Intervention in der Erhaltungsphase der Komplexen Physikalischen Entstauungstherapie (KPE Phase II) von Lymphödemen. Phys Med Rehab Kuror (2014) 24(01):34–41. 10.1055/s-0033-1357157 [DOI] [Google Scholar]
- 42.Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol (2006) 24(18):2765–72. 10.1200/JCO.2005.03.6749 [DOI] [PubMed] [Google Scholar]
- 43.Havas E, Parviainen T, Vuorela J, Toivanen J, Nikula T, Vihko V. Lymph flow dynamics in exercising human skeletal muscle as detected by scintography. J Physiol (1997) 504(Pt 1):233–9. 10.1111/j.1469-7793.1997.233bf.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keilani M, Hasenoehrl T, Neubauer M, Crevenna R. Resistance exercise and secondary lymphedema in breast cancer survivors – a systematic review. Support Care Cancer (2016) 24(4):1907–16. 10.1007/s00520-015-3068-z [DOI] [PubMed] [Google Scholar]
- 45.Sim YJ, Jeong HJ, Kim GC. Effect of active resistive exercise on breast cancer-related lymphedema: a randomized controlled trial. Arch Phys Med Rehabil (2010) 91(12):1844–8. 10.1016/j.apmr.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 46.Sagen A, Karesen R, Risberg MA. Physical activity for the affected limb and arm lymphedema after breast cancer surgery. A prospective, randomized controlled trial with two years follow-up. Acta Oncol (2009) 48(8):1102–10. 10.3109/02841860903061683 [DOI] [PubMed] [Google Scholar]
- 47.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med (2009) 361(7):664–73. 10.1056/NEJMoa0810118 [DOI] [PubMed] [Google Scholar]
- 48.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc (2010) 42(7):1409–26. 10.1249/MSS.0b013e3181e0c112 [DOI] [PubMed] [Google Scholar]
- 49.Torres Lacomba M, Yuste Sánchez MJ, Zapico Goñi A, Prieto Merino D, Mayoral del Moral O, Cerezo Téllez E, et al. Effectiveness of early physiotherapy to prevent lymphoedema after surgery for breast cancer: randomised, single blinded, clinical trial. BMJ (2010) 340:b5396. 10.1136/bmj.b5396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Todd J, Scally A, Dodwell D, Horgan K, Topping A. A randomised controlled trial of two programmes of shoulder exercise following axillary node dissection for invasive breast cancer. Physiotherapy (2008) 94(4):265–73. 10.1016/j.physio.2008.09.005 [DOI] [Google Scholar]
- 51.Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports (2003) 13(1):3–8. 10.1034/j.1600-0838.2003.00298.x [DOI] [PubMed] [Google Scholar]
- 52.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci (2006) 61(1):72–7. 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 53.Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodríguez L, Fernández-de-Las-Peñas C, Ruiz JR, Arroyo-Morales M. The handgrip strength test as a measure of function in breast cancer survivors: relationship to cancer-related symptoms and physical and physiologic parameters. Am J Phys Med Rehabil (2012) 91(9):774–82. 10.1097/PHM.0b013e31825f1538 [DOI] [PubMed] [Google Scholar]
- 54.Rietman JS, Dijkstra PU, Debreczeni R, Geertzen JH, Robinson DP, De Vries J. Impairments, disabilities and health related quality of life after treatment for breast cancer: a follow-up study 2.7 years after surgery. Disabil Rehabil (2004) 26(2):78–84. 10.1080/09638280310001629642 [DOI] [PubMed] [Google Scholar]
- 55.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci (2006) 61(10):1059–64. 10.1093/gerona/61.10.1059 [DOI] [PubMed] [Google Scholar]
- 56.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc (2002) 50(5):889–96. 10.1046/j.1532-5415.2002.50216.x [DOI] [PubMed] [Google Scholar]
- 57.Beaudart C, Gillain S, Petermans J, Reginster JY, Bruyère O. [Sarcopenia: what’s new in 2014]. Rev Med Liege (2014) 69(5–6):251–7. [PubMed] [Google Scholar]
- 58.Winters-Stone KM, Bennett JA, Nail L, Schwartz A. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol Nurs Forum (2008) 35(5):815–21. 10.1188/08.ONF.815-821 [DOI] [PubMed] [Google Scholar]
- 59.Winters-Stone KM, Nail L, Bennett JA, Schwartz A. Bone health and falls: fracture risk in breast cancer survivors with chemotherapy-induced amenorrhea. Oncol Nurs Forum (2009) 36(3):315–25. 10.1188/09.ONF.315-325 [DOI] [PubMed] [Google Scholar]
- 60.Kutynec CL, McCargar L, Barr SI, Hislop TG. Energy balance in women with breast cancer during adjuvant treatment. J Am Diet Assoc (1999) 99(10):1222–7. 10.1016/S0002-8223(99)00301-6 [DOI] [PubMed] [Google Scholar]
- 61.Klassen O, Schmidt ME, Ulrich CM, Schneeweiss A, Potthoff K, Steindorf K, et al. Muscle strength in breast cancer patients receiving different treatment regimes. (2017) 8(2):305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harvie MN, Campbell IT, Baildam A, Howell A. Energy balance in early breast cancer patients receiving adjuvant chemotherapy. Breast Cancer Res Treat (2004) 83(3):201–10. 10.1023/B:BREA.0000014037.48744.fa [DOI] [PubMed] [Google Scholar]
- 63.Battaglini CL, Mills RC, Phillips BL, Lee JT, Story CE, Nascimento MG, et al. Twenty-five years of research on the effects of exercise training in breast cancer survivors: a systematic review of the literature. World J Clin Oncol (2014) 5(2):177–90. 10.5306/wjco.v5.i2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sale DG. Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev (1987) 15:95–151. 10.1249/00003677-198700150-00008 [DOI] [PubMed] [Google Scholar]
- 65.Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc (1988) 20(5 Suppl):S135–45. 10.1249/00005768-198810001-00009 [DOI] [PubMed] [Google Scholar]
- 66.Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Neural adaptation to resistance training: changes in evoked V-wave and H-reflex responses. J Appl Physiol (1985) (2002) 92(6):2309–18. 10.1152/japplphysiol.01185.2001 [DOI] [PubMed] [Google Scholar]
- 67.Hasenoehrl T, Keilani M, Sedghi Komanadj T, Mickel M, Margreiter M, Marhold M, et al. The effects of resistance exercise on physical performance and health-related quality of life in prostate cancer patients: a systematic review. Support Care Cancer (2015) 23(8):2479–97. 10.1007/s00520-015-2782-x [DOI] [PubMed] [Google Scholar]
- 68.Reuss-Borst M, Hartmann U, Scheede C, Weiß J. Prevalence of osteoporosis among cancer patients in Germany: prospective data from an oncological rehabilitation clinic. Osteoporos Int (2012) 23(4):1437–44. 10.1007/s00198-011-1724-9 [DOI] [PubMed] [Google Scholar]
- 69.Gaillard S, Stearns V. Aromatase inhibitor-associated bone and musculoskeletal effects: new evidence defining etiology and strategies for management. Breast Cancer Res (2011) 13(2):205. 10.1186/bcr2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lester J, Dodwell D, McCloskey E, Coleman R. The causes and treatment of bone loss associated with carcinoma of the breast. Cancer Treat Rev (2005) 31(2):115–42. 10.1016/j.ctrv.2005.01.008 [DOI] [PubMed] [Google Scholar]
- 71.Riggs BL, Khosla S, Melton LJ, III. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res (1998) 13(5):763–73. 10.1359/jbmr.1998.13.5.763 [DOI] [PubMed] [Google Scholar]
- 72.Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol (2005) 23(3):619–29. 10.1200/JCO.2005.09.121 [DOI] [PubMed] [Google Scholar]
- 73.Lester J, Coleman R. Bone loss and the aromatase inhibitors. Br J Cancer (2005) 93(S1):S16–22. 10.1038/sj.bjc.6602691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, et al. Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK Expert Group. Cancer Treat Rev (2008) 34(Suppl 1):S3–18. 10.1016/j.ctrv.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 75.Irwin ML, Cartmel B, Gross CP, Ercolano E, Li F, Yao X, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol (2015) 33(10):1104–11. 10.1200/JCO.2014.57.1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winters-Stone KM, Schwartz A, Nail LM. A review of exercise interventions to improve bone health in adult cancer survivors. J Cancer Surviv (2010) 4(3):187–201. 10.1007/s11764-010-0122-1 [DOI] [PubMed] [Google Scholar]
- 77.Winters-Stone KM, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat (2011) 127(2):447–56. 10.1007/s10549-011-1444-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winters-Stone KM, Dobek J, Nail LM, Bennett JA, Leo MC, Torgrimson-Ojerio B, et al. Impact + resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteoporos Int (2013) 24(5):1637–46. 10.1007/s00198-012-2143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med (2009) 43(12):898–908. 10.1136/bjsm.2008.052704 [DOI] [PubMed] [Google Scholar]
- 80.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol (2013) 14(6):500–15. 10.1016/S1470-2045(13)70076-7 [DOI] [PubMed] [Google Scholar]
- 81.Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat (2008) 111(2):365–72. 10.1007/s10549-007-9774-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moxley G. Rheumatic disorders and functional disability with aromatase inhibitor therapy. Clin Breast Cancer (2010) 10(2):144–7. 10.3816/CBC.2010.n.019 [DOI] [PubMed] [Google Scholar]
- 83.Morales L, Pans S, Paridaens R, Westhovens R, Timmerman D, Verhaeghe J, et al. Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res Treat (2007) 104(1):87–91. 10.1007/s10549-006-9394-6 [DOI] [PubMed] [Google Scholar]
- 84.Donnellan PP, Douglas SL, Cameron DA, Leonard RC. Aromatase inhibitors and arthralgia. J Clin Oncol (2001) 19(10):2767. [PubMed] [Google Scholar]
- 85.Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer (2009) 115(16):3631–9. 10.1002/cncr.24419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stubblefield MD, Keole N. Upper body pain and functional disorders in patients with breast cancer. PM R (2014) 6(2):170–83. 10.1016/j.pmrj.2013.08.605 [DOI] [PubMed] [Google Scholar]
- 87.Arimidex, Tamoxifen, Alone or in Combination Trialists’ Group. Buzdar A, Howell A, Cuzick J, Wale C, Distler W, et al. Comprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trial. Lancet Oncol (2006) 7(8):633–43. 10.1016/S1470-2045(06)70767-7 [DOI] [PubMed] [Google Scholar]
- 88.Coombes RC, Paridaens R, Jassem J, Van De Velde CJ, Delozier T, Jones S, et al. First mature analysis of the Intergroup Exemestane Study. J Clin Oncol (2006) 24(18_suppl):LBA527–527. 10.1200/jco.2006.24.18_suppl.lba527 [DOI] [Google Scholar]
- 89.Kilbreath SL, Refshauge KM, Beith JM, Ward LC, Lee M, Simpson JM, et al. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: a randomized controlled trial. Breast Cancer Res Treat (2012) 133(2):667–76. 10.1007/s10549-012-1964-1 [DOI] [PubMed] [Google Scholar]
- 90.Stegink-Jansen CW, Buford WL, Jr, Patterson RM, Gould LJ. Computer simulation of pectoralis major muscle strain to guide exercise protocols for patients after breast cancer surgery. J Orthop Sports Phys Ther (2011) 41(6):417–26. 10.2519/jospt.2011.3358 [DOI] [PubMed] [Google Scholar]
- 91.Lee SA, Kang JY, Kim YD, An AR, Kim SW, Kim YS, et al. Effects of a scapula-oriented shoulder exercise programme on upper limb dysfunction in breast cancer survivors: a randomized controlled pilot trial. Clin Rehabil (2010) 24(7):600–13. 10.1177/0269215510362324 [DOI] [PubMed] [Google Scholar]
- 92.Irwin ML, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Gilliland FD, et al. Changes in body fat and weight after a breast cancer diagnosis: influence of demographic, prognostic, and lifestyle factors. J Clin Oncol (2005) 23(4):774–82. 10.1200/JCO.2005.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghose A, Kundu R, Toumeh A, Hornbeck C, Mohamed I. A review of obesity, insulin resistance, and the role of exercise in breast cancer patients. Nutr Cancer (2015) 67(2):197–202. 10.1080/01635581.2015.990569 [DOI] [PubMed] [Google Scholar]
- 94.Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol (1999) 17(1):120–9. 10.1200/JCO.1999.17.1.120 [DOI] [PubMed] [Google Scholar]
- 95.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. JAMA (2003) 290(10):1331–6. 10.1001/jama.290.10.1331 [DOI] [PubMed] [Google Scholar]
- 96.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol (2014) 25(10):1901–14. 10.1093/annonc/mdu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab (2004) 89(5):2248–53. 10.1210/jc.2003-031874 [DOI] [PubMed] [Google Scholar]
- 98.Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol (2008) 26(33):5465–76. 10.1200/JCO.2008.18.4184 [DOI] [PubMed] [Google Scholar]
- 99.Schwingshackl L, Dias S, Strasser B, Hoffmann G. Impact of different training modalities on anthropometric and metabolic characteristics in overweight/obese subjects: a systematic review and network meta-analysis. PLoS One (2013) 8(12):e82853. 10.1371/journal.pone.0082853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Giangregorio LM, McGill S, Wark JD, Laprade J, Heinonen A, Ashe MC, et al. Too Fit To Fracture: outcomes of a Delphi consensus process on physical activity and exercise recommendations for adults with osteoporosis with or without vertebral fractures. Osteoporos Int (2015) 26(3):891–910. 10.1007/s00198-014-2881-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A randomized controlled trial. JAMA (1994) 272(24):1909–14. 10.1001/jama.272.24.1909 [DOI] [PubMed] [Google Scholar]
- 102.Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol (2010) 28(9):1458–66. 10.1200/JCO.2009.24.9557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Martin BS, et al. Effect of exercise training on C-reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain Behav Immun (2005) 19(5):381–8. 10.1016/j.bbi.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 104.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev (2005) 14(7):1672–80. 10.1158/1055-9965.EPI-04-0736 [DOI] [PubMed] [Google Scholar]
- 105.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol (2012) 8(8):457–65. 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 106.Jones LW, Douglas PS, Eves ND, Marcom PK, Kraus WE, Herndon JE, 2nd, et al. Rationale and design of the Exercise Intensity Trial (EXCITE): a randomized trial comparing the effects of moderate versus moderate to high-intensity aerobic training in women with operable breast cancer. BMC Cancer (2010) 10:531. 10.1186/1471-2407-10-531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haykowsky MJ, Mackey JR, Thompson RB, Jones LW, Paterson DI. Adjuvant trastuzumab induces ventricular remodeling despite aerobic exercise training. Clin Cancer Res (2009) 15(15):4963–7. 10.1158/1078-0432.CCR-09-0628 [DOI] [PubMed] [Google Scholar]
- 108.Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol (2014) 53(1):65–74. 10.3109/0284186X.2013.781673 [DOI] [PubMed] [Google Scholar]
- 109.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol (2012) 30(20):2530–7. 10.1200/JCO.2011.39.9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McGuire DK, Levine BD, Williamson JW, Snell PG, Blomqvist CG, Saltin B, et al. A 30-year follow-up of the Dallas Bedrest and Training Study: I. effect of age on the cardiovascular response to exercise. Circulation (2001) 104(12):1358–66. 10.1161/hc3701.096099 [DOI] [PubMed] [Google Scholar]
- 111.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA (1989) 262(17):2395–401. 10.1001/jama.1989.03430170057028 [DOI] [PubMed] [Google Scholar]
- 112.Peel JB, Sui X, Adams SA, Hébert JR, Hardin JW, Blair SN. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med Sci Sports Exerc (2009) 41(4):742–8. 10.1249/MSS.0b013e31818edac7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Marks LB, Munley MT, Bentel GC, Zhou SM, Hollis D, Scarfone C, et al. Physical and biological predictors of changes in whole-lung function following thoracic irradiation. Int J Radiat Oncol Biol Phys (1997) 39(3):563–70. 10.1016/S0360-3016(97)00343-X [DOI] [PubMed] [Google Scholar]
- 114.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc (2014) 3(1):e000432. 10.1161/JAHA.113.000432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grotto HZ. Anaemia of cancer: an overview of mechanisms involved in its pathogenesis. Med Oncol (2008) 25(1):12–21. 10.1007/s12032-007-9000-8 [DOI] [PubMed] [Google Scholar]
- 116.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol (2012) 9(5):288–96. 10.1038/nrclinonc.2012.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scott E, Daley AJ, Doll H, Woodroofe N, Coleman RE, Mutrie N, et al. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: a randomized controlled trial. Cancer Causes Control (2013) 24(1):181–91. 10.1007/s10552-012-0104-x [DOI] [PubMed] [Google Scholar]
- 118.Herrero F, San Juan AF, Fleck SJ, Foster C, Lucia A. Effects of detraining on the functional capacity of previously trained breast cancer survivors. Int J Sports Med (2007) 28(3):257–64. 10.1055/s-2006-924348 [DOI] [PubMed] [Google Scholar]
- 119.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst (2007) 99(5):365–75. 10.1093/jnci/djk064 [DOI] [PubMed] [Google Scholar]
- 120.Riihimäki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. Death causes in breast cancer patients. Ann Oncol (2012) 23(3):604–10. 10.1093/annonc/mdr160 [DOI] [PubMed] [Google Scholar]
- 121.Marks LB, Yu X, Prosnitz RG, Zhou SM, Hardenbergh PH, Blazing M, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys (2005) 63(1):214–23. 10.1016/j.ijrobp.2005.01.029 [DOI] [PubMed] [Google Scholar]
- 122.Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Møller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer (2007) 7:9. 10.1186/1471-2407-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol (2007) 50(15):1435–41. 10.1016/j.jacc.2007.06.037 [DOI] [PubMed] [Google Scholar]
- 124.Farolfi A, Melegari E, Aquilina M, Scarpi E, Ibrahim T, Maltoni R, et al. Trastuzumab-induced cardiotoxicity in early breast cancer patients: a retrospective study of possible risk and protective factors. Heart (2013) 99(9):634–9. 10.1136/heartjnl-2012-303151 [DOI] [PubMed] [Google Scholar]
- 125.Billingham ME, Bristow MR, Glatstein E, Mason JW, Masek MA, Daniels JR. Adriamycin cardiotoxicity: endomyocardial biopsy evidence of enhancement by irradiation. Am J Surg Pathol (1977) 1(1):17–23. 10.1097/00000478-197701010-00002 [DOI] [PubMed] [Google Scholar]
- 126.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA (2001) 285(7):885–92. 10.1001/jama.285.7.885 [DOI] [PubMed] [Google Scholar]
- 127.Yancik R, Havlik RJ, Wesley MN, Ries L, Long S, Rossi WK, et al. Cancer and comorbidity in older patients: a descriptive profile. Ann Epidemiol (1996) 6(5):399–412. 10.1016/S1047-2797(96)00063-4 [DOI] [PubMed] [Google Scholar]
- 128.Sturgeon KM, Ky B, Libonati JR, Schmitz KH. The effects of exercise on cardiovascular outcomes before, during, and after treatment for breast cancer. Breast Cancer Res Treat (2014) 143(2):219–26. 10.1007/s10549-013-2808-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kirkham AA, Davis MK. Exercise prevention of cardiovascular disease in breast cancer survivors. J Oncol (2015) 2015:13. 10.1155/2015/917606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Laisupasin P, Thompat W, Sukarayodhin S, Sornprom A, Sudjaroen Y. Comparison of serum lipid profiles between normal controls and breast cancer patients. J Lab Physicians (2013) 5(1):38–41. 10.4103/0974-2727.115934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yadav NK, Poudel B, Thanpari C, Chandra Koner B. Assessment of biochemical profiles in premenopausal and postmenopausal women with breast cancer. Asian Pac J Cancer Prev (2012) 13(7):3385–8. 10.7314/APJCP.2012.13.7.3385 [DOI] [PubMed] [Google Scholar]
- 132.Alexopoulos CG, Pournaras S, Vaslamatzis M, Avgerinos A, Raptis S. Changes in serum lipids and lipoproteins in cancer patients during chemotherapy. Cancer Chemother Pharmacol (1992) 30(5):412–6. 10.1007/BF00689971 [DOI] [PubMed] [Google Scholar]
- 133.Love RR, Wiebe DA, Newcomb PA, Cameron L, Leventhal H, Jordan VC, et al. EFfects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med (1991) 115(11):860–4. 10.7326/0003-4819-115-11-860 [DOI] [PubMed] [Google Scholar]
- 134.Trédan O, Bajard A, Meunier A, Roux P, Fiorletta I, Gargi T, et al. Body weight change in women receiving adjuvant chemotherapy for breast cancer: a French prospective study. Clin Nutr (2010) 29(2):187–91. 10.1016/j.clnu.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 135.Bottalico JN. Recurrent gestational diabetes: risk factors, diagnosis, management, and implications. Semin Perinatol (2007) 31(3):176–84. 10.1053/j.semperi.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 136.Schwartz N, Nachum Z, Green MS. Risk factors of gestational diabetes mellitus recurrence: a meta-analysis. Endocrine (2016) 53(3):662–71. 10.1007/s12020-016-0922-9 [DOI] [PubMed] [Google Scholar]
- 137.Wolf I, Sadetzki R, Catane R, Karasik A, Kaufman D. Diabetes mellitus and breast cancer. Lancet Oncol (2005) 6(2):103–11. 10.1016/S1470-2045(05)01736-5 [DOI] [PubMed] [Google Scholar]
- 138.Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J (2014) 38(5):330–6. 10.4093/dmj.2014.38.5.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nuri R, Kordi MR, Moghaddasi M, Rahnama N, Damirchi A, Rahmani-Nia F, et al. Effect of combination exercise training on metabolic syndrome parameters in postmenopausal women with breast cancer. J Cancer Res Ther (2012) 8(2):238–42. 10.4103/0973-1482.98977 [DOI] [PubMed] [Google Scholar]
- 140.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol (2014) 25(7):1293–311. 10.1093/annonc/mdu012 [DOI] [PubMed] [Google Scholar]
- 141.West-Wright CN, Henderson KD, Sullivan-Halley J, Ursin G, Deapen D, Neuhausen S, et al. Long-term and recent recreational physical activity and survival after breast cancer: the California Teachers Study. Cancer Epidemiol Biomarkers Prev (2009) 18(11):2851–9. 10.1158/1055-9965.EPI-09-0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the women’s health initiative. Cancer Prev Res (Phila) (2011) 4(4):522–9. 10.1158/1940-6207.CAPR-10-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Newton RU, Galvao DA. Exercise in prevention and management of cancer. Curr Treat Options Oncol (2008) 9(2–3):135–46. 10.1007/s11864-008-0065-1 [DOI] [PubMed] [Google Scholar]
- 144.Schmitz KH, Speck RM. Risks and benefits of physical activity among breast cancer survivors who have completed treatment. Womens Health (Lond) (2010) 6(2):221–38. 10.2217/WHE.10.1 [DOI] [PubMed] [Google Scholar]
- 145.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol (2005) 23(24):5814–30. 10.1200/JCO.2005.01.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Drum SN, Klika RJ, Carter SD, Sprod LK, Donath L. A feasibility study related to inactive cancer survivors compared with non-cancer controls during aerobic exercise training. J Sports Sci Med (2016) 15(4):592–600. [PMC free article] [PubMed] [Google Scholar]
- 147.Campbell A, Stevinson C, Crank H. The BASES expert statement on exercise and cancer survivorship. J Sports Sci (2012) 30(9):949–52. 10.1080/02640414.2012.671953 [DOI] [PubMed] [Google Scholar]
- 148.Fairman CM, Hyde PN, Focht BC. Resistance training interventions across the cancer control continuum: a systematic review of the implementation of resistance training principles. Br J Sports Med (2017) 51(8):677–85. 10.1136/bjsports-2016-096537 [DOI] [PubMed] [Google Scholar]
- 149.Carfagno DG, Hendrix JC, III. Overtraining syndrome in the athlete: current clinical practice. Curr Sports Med Rep (2014) 13(1):45–51. 10.1249/JSR.0000000000000027 [DOI] [PubMed] [Google Scholar]
- 150.Gonzalez AM, Hoffman JR, Stout JR, Fukuda DH, Willoughby DS. Intramuscular anabolic signaling and endocrine response following resistance exercise: implications for muscle hypertrophy. Sports Med (2016) 46(5):671–85. 10.1007/s40279-015-0450-4 [DOI] [PubMed] [Google Scholar]
- 151.Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: a review of exercise studies for survivors of cancers other than breast. Br J Sports Med (2014) 48(12):987–95. 10.1136/bjsports-2012-091732 [DOI] [PubMed] [Google Scholar]
- 152.Neuhouser ML, Smith AW, George SM, Gibson JT, Baumgartner KB, Baumgartner R, et al. Use of complementary and alternative medicine and breast cancer survival in the Health, Eating, Activity, and Lifestyle Study. Breast Cancer Res Treat (2016) 160(3):539–46. 10.1007/s10549-016-4010-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harris SR, Schmitz KH, Campbell KL, McNeely ML. Clinical practice guidelines for breast cancer rehabilitation: syntheses of guideline recommendations and qualitative appraisals. Cancer (2012) 118(8 Suppl):2312–24. 10.1002/cncr.27461 [DOI] [PubMed] [Google Scholar]
- 154.Poage E, Singer M, Armer J, Poundall M, Shellabarger MJ. Demystifying lymphedema: development of the lymphedema putting evidence into practice card. Clin J Oncol Nurs (2008) 12(6):951–64. 10.1188/08.CJON.951-964 [DOI] [PubMed] [Google Scholar]
- 155.Harris SR, Hugi MR, Olivotto IA, Levine M. Clinical practice guidelines for the care and treatment of breast cancer: 11. Lymphedema. CMAJ (2001) 164(2):191–9. [PMC free article] [PubMed] [Google Scholar]
- 156.Swarm R, Abernethy AP, Anghelescu DL, Benedetti C, Blinderman CD, Boston B, et al. Adult cancer pain. J Natl Compr Cancer Netw (2010) 8(9):1046–86. 10.6004/jnccn.2010.0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Green E, Zwaal C, Beals C, Fitzgerald B, Harle I, Jones J, et al. Cancer-related pain management: a report of evidence-based recommendations to guide practice. Clin J Pain (2010) 26(6):449–62. 10.1097/AJP.0b013e3181dacd62 [DOI] [PubMed] [Google Scholar]
- 158.Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer (2009) 17(10):1301–9. 10.1007/s00520-009-0587-5 [DOI] [PubMed] [Google Scholar]
- 159.van Uden-Kraan CF, Chinapaw MJ, Drossaert CH, Verdonck-de Leeuw IM, Buffart LM. Cancer patients’ experiences with and perceived outcomes of yoga: results from focus groups. Support Care Cancer (2013) 21(7):1861–70. 10.1007/s00520-013-1728-4 [DOI] [PubMed] [Google Scholar]
- 160.Sharma M, Lingam VC, Nahar VK. A systematic review of yoga interventions as integrative treatment in breast cancer. J Cancer Res Clin Oncol (2016) 142(12):2523–40. 10.1007/s00432-016-2269-2 [DOI] [PubMed] [Google Scholar]
- 161.Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer (2012) 12:412. 10.1186/1471-2407-12-412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology (2009) 18(4):360–8. 10.1002/pon.1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mustian KM, Sprod LK, Janelsins M, Peppone LJ, Palesh OG, Chandwani K, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol (2013) 31(26):3233–41. 10.1200/JCO.2012.43.7707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vardar Yağlı N, Şener G, Arıkan H, Sağlam M, İnal İnce D, Savcı S, et al. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther (2015) 14(2):125–32. 10.1177/1534735414565699 [DOI] [PubMed] [Google Scholar]
- 165.Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol (2014) 32(10):1040–9. 10.1200/JCO.2013.51.8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cramer H, Rabsilber S, Lauche R, Kümmel S, Dobos G. Yoga and meditation for menopausal symptoms in breast cancer survivors – a randomized controlled trial. Cancer (2015) 121(13):2175–84. 10.1002/cncr.29330 [DOI] [PubMed] [Google Scholar]
- 167.Hughes DC, Darby N, Gonzalez K, Boggess T, Morris RM, Ramirez AG. Effect of a six-month yoga exercise intervention on fitness outcomes for breast cancer survivors. Physiother Theory Pract (2015) 31(7):451–60. 10.3109/09593985.2015.1037409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rao RM, Raghuram N, Nagendra HR, Usharani MR, Gopinath KS, Diwakar RB, et al. Effects of an integrated yoga program on self-reported depression scores in breast cancer patients undergoing conventional treatment: a randomized controlled trial. Indian J Palliat Care (2015) 21(2):174–81. 10.4103/0973-1075.156486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pan Y, Yang K, Wang Y, Zhang L, Liang H. Could yoga practice improve treatment-related side effects and quality of life for women with breast cancer? A systematic review and meta-analysis. Asia Pac J Clin Oncol (2017) 13(2):e79–95. 10.1111/ajco.12329 [DOI] [PubMed] [Google Scholar]
- 170.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother (2009) 55(2):129–33. 10.1016/S0004-9514(09)70043-1 [DOI] [PubMed] [Google Scholar]
- 171.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther (2003) 83(8):713–21. 10.1093/ptj/83.8.713 [DOI] [PubMed] [Google Scholar]