Abstract
Vibrio is a genus of Gram-negative bacteria, some of which can cause serious infectious diseases. Vibrio infections are associated with the consumption of contaminated food and classified in Vibrio cholera infections and non-cholera Vibrio infections. In the present study, we investigate whether bovine lactoferrin (bLF) and several synthetic peptides corresponding to bLF sequences, are able to inhibit the growth or have bactericidal effect against V. cholerae and other Vibrio species. The antibacterial activity of LF and LF-peptides was assessed by kinetics of growth or determination of colony forming unit in bacteria treated with the peptides and antibiotics. To get insight in the mode of action, the interaction between bLF and bLF-peptides (coupled to FITC) and V. cholera was evaluated. The damage of effector-induced bacterial membrane permeability was measured by inclusion of the fluorescent dye propidium iodide using flow cytometry, whereas the bacterial ultrastructural damage in bacteria treated was observed by transmission electron microscopy. The results showed that bLF and LFchimera inhibited the growth of the V. cholerae strains; LFchimera permeabilized the bacteria which membranes were seriously damaged. Assays with a multidrug-resistant strain of Vibrio species indicated that combination of sub-lethal doses of LFchimera with ampicillin or tetracycline strongly reduced the concentration of the antibiotics to reach 95% growth inhibition. Furthermore, LFchimera were effective to inhibit the V. cholerae counts and damage due to this bacterium in a model mice. These data suggest that LFchimera and bLF are potential candidates to combat the V. cholerae and other multidrug resistant Vibrio species.
Keywords: lactoferrin, lactoferrin peptides, LFchimera, bactericide, Vibrio cholerae
Introduction
The human innate-immune system is made up of a large variety of important components that attack or destroy any form of infection, in these components are included antibodies; white blood cells, antimicrobial proteins, and peptides, etc (Zaiou, 2007). This defense system is also found in other species of mammals, including bovines, sheep, and camels (Baveye et al., 1999). Although the development of new generation of antibiotics has rapidly progressed and gained popularity over the antimicrobial peptides, even the most powerful antibiotics have been unsuccessful to diminish morbidity and mortality due to the antimicrobial resistance showed by emergent multi-resistant strains of pathogens (Longworth, 2001; Spellberg et al., 2008). Antimicrobial peptides that are less prone to induction of resistance by bacteria are a class of substances that are now investigated to combat multi-drug resistant bacteria, with promising results (Ellison et al., 1990b; Garbacz et al., 2017; Greber and Dawgul, 2017). Such compounds include bovine lactoferrin (bLF) and LF-derived peptides (Ellison et al., 1990b). LF is an abundant iron-chelating protein present in colostrum and milk of most mammals, participating in the newborn protection against infections (Brock, 1980, 2002). LF is also present in mucosae and secreted bodily fluids such as bile, bronchioalveolar fluid, and intestinal and reproductive tract secretions, and it is produced and released by the polymorphonuclear neutrophils during inflammation (Brock, 2002). LF from bovine (bLF) and the LF derived peptides have been studied most extensively, due to exhibit antibacterial, antifungal, antiviral, and antiparasitic activities, in a direct way by a direct damage on pathogens and also by enhancing the mucosal immune function against pathogens (Brock, 2002; Orsi, 2004; Aguilar-Diaz et al., 2017; Juretic et al., 2017). bLF exerts its bactericidal action in two ways: indirectly, by limiting the amount of iron available for the growth and metabolism, and directly, by affecting the bacterial membrane (Ellison et al., 1988, 1990a; Ellison and Giehl, 1991; Orsi, 2004; Vogel, 2012). Other functions such as inhibition of bacterial adhesion or invasion to target cells, decrement of aggregation or biofilm development, have been also reported to LF and LF-peptides in bacteria (Singh et al., 2002; Orsi, 2004; Abbas et al., 2007; Juretic et al., 2017). The antimicrobial activity of LF is attributed to a region located at the N1-domain of the protein (Farnaud and Evans, 2003). In this sense, a peptide called lactoferricin B (LFcinB) is released from the N-terminus of bLF in during its passage through the intestine (Bellamy et al., 1992). Other antimicrobial peptides of the N1-domain have been identified and synthetically produced, for example, lactoferrampin (LFampin) (Van Der Kraan et al., 2004, 2005). Furthermore, a chimerical structure based in the active parts of the protein LF was designed and synthesized, this peptide contain the amino acids 17–30 of LFcinB and amino acids 265-284 of LFampin 265-284, the resulting peptides was called LF chimera (Bolscher et al., 2009). The bactericidal activity of LFchimera has been definitively stronger than that of the peptides (LFcin17-30 and LFampin265-284), as has been demonstrated in many experiments; due to lower concentrations, shorter incubation time, and salt concentrations present in the environment (needed for the growth of halophile bacteria) permit the bactericidal activity of LF chimera, compared with the peptides that conform this molecule which is not effective at these conditions (Bolscher et al., 2009; Haney et al., 2009; Leon-Sicairos et al., 2014). Otherwise, the microbicidal effect of LFchimera against Candida spp, Vibrio parahaemolyticus, Staphylococcus aureus, enterotoxigenic and enterohaemorragic Escherichia coli, or in the parasites Entamoeba histolytica, Burkholderia thailandensis, and Leishmania pifanoi has been established in vitro (Bolscher et al., 2009; Lopez-Soto et al., 2009, 2010; Flores-Villasenor et al., 2010, 2012; Kanthawong et al., 2014; Leon-Sicairos et al., 2014; Puknun et al., 2016).
Vibriosis is an infection caused by species of the Vibrio genus. V. cholerae, V. parahaemolyticus, and V. vulnificus are serious human pathogens (Thompson et al., 2004). Vibrio cholerae is the causative agent of cholera. It has been reported that the mortality rate of untreated cholera cases is about 50 to 60% (Frost, 1976; Faruque et al., 1998). Other Vibrios clinically significant for humans are V. algynoyticus, V. parahaemolyticus, and V. vulnificus. V. alginolyticus is medically important since it causes otitis and wound infection (Powell, 1999; Hernandez-Robles et al., 2016). The halophilic (salt-loving) V. parahaemolyticus has been identified as a leading cause of human gastroenteritis, associated to the consumption of raw or improperly cooked seafood (Ellison and Giehl, 1991; Su and Liu, 2007). Another halophilic Vibrio has recently been identified as V. vulnificus. This bacterium is an opportunistic pathogen that can cause infections of humans and other animals including fish. V. vulnificus is extremely harmful and is responsible for the devastating majority of reported seafood-related deceases in the United States (Warnock and Macmath, 1993; Powell, 1999; Jones and Oliver, 2009). The bacteria is located as a natural flora of coastal marine environments worldwide, for that it has been isolated from a seafood, shrimp, fish, oysters and clams, water, and sediments (Do Nascimento et al., 2001; Jones and Oliver, 2009; Jones et al., 2014).
It has been reported that the number of V. cholerae and V. non-cholerae cases has augmented increasingly in recent years. However; the major health problem is the emergence and spread of V. cholerae and Vibrio non-cholera strains antibiotics-resistant (Colwell, 1996; Faruque et al., 1998; Lipp et al., 2002; Sedas, 2007). For these reasons, the search for new compounds for Vibrio infections; treatment or prevention is needed. In previous work, it was demonstrated that LF had antibacterial effect against V. cholerae (Ellison and Giehl, 1991). Then; we reported that LF and the LFpeptides display antibacterial activity against a V. parahaemolyticus multidrug resistant strain, and also in V. cholerae O1 Inaba and non-O1 strains (Leon-Sicairos et al., 2009). In the present study; we continue the research of the bactericidal activity of bLF and bLF-derived peptides LFcin17-30, LFampin265-284 and LFchimera against Vibrio species resistant to antibiotics (including V. cholerae strains O1 and non-O1, in vitro and in vivo). In addition, we explore the mechanism of damage of these compounds into bacteria, and their synergism with antibiotics in the bactericidal effect.
Materials and methods
Lactoferrins, bacterial strains, and culture conditions
Bovine LF (bLF, 20% iron saturated) was kindly donated by Abial (Santander, Spain). The purity of bLF (>98%) was confirmed by SDS-PAGE gels using silver nitrate staining. LF concentration was measured by UV spectroscopy on the basis of an extinction coefficient of 15.1 (280 nm, 1% solution) (Valenti et al., 1999). The bLF iron saturation was about 20% as detected by optical spectroscopy at 468 nm on the basis of an extinction coefficient of 0.54 (100% iron saturation). LPS contamination of bLf, estimated by Limulus Amebocyte assay (LAL Pyrochrome kit, ThermoFicherScientific, Waltham, MA, USA), was equal to 0.7 ± 0.06 ng/mg of bLF. Synthetic peptides (LFcin17-30, LFampin265-284 and LFchimera) were obtained by solid phase peptide synthesis using Fmoc chemistry, as has been reported previously (Bolscher et al., 2009; Cutone et al., 2014).
The following Vibrio strains obtained by us were used: V. cholerae O1 Inaba, V. cholerae non-O1 (toxigenic), V. fluvialis, V. alginolyticus, V. vulnificus, and V. furnissii (Velazquez-Roman et al., 2012; De Jesus Hernandez-Diaz et al., 2015). Bacteria were incubated in Luria-Bertani medium (LB) (Difco, Becton Dickinson, USA) with 3% NaCl, incubated in agitation (5,000 rpm) and grown at 37°C for 16–18 h. In all the experiments in the presence of bLF or peptides, to avoid saturation with iron the ion was removed from the LB medium by incubation with Chelex-100 resin (5 g/l) in constant agitation at 4°C. After 16 h, the resin was taken off by filtration and finally the medium was sterilized (iron-depleted medium). The viability of bacterial cultures grown at these conditions was not affected. Additionally, all glass materials were treated with 6 M HCl to eliminate iron traces as previously reported (Leon-Sicairos et al., 2009).
Growth inhibition in the presence of bLF and bLF-peptides
To determine the antibacterial activity of bLF and bLF peptides, ~1 × 107 CFU/ml of V. cholerae O1 Inaba or V. cholerae non-O1 in 96-well microplates (Corning) containing 200 μl iron-depleted LB media were incubated at 37°C with 5, 10, 20, 30, and 40 μM bLF, LFcin17-30, LFampin265-284, or LFchimera, for 1, 2, 4, and 6 h. In parallel bacterial suspensions were treated with 25 μg/ml of Gentamicin or without additions as growth control. Bacterial growth was followed by measuring the OD660 nm of cultures. Next, the percentage of viable cells was estimated in relation to untreated cultures (without peptides or antibiotics). Viable cells were also counted as colony forming units/ml (CFU/ml) from serial 10-fold dilutions incubated in Muller-Hinton-Broth (MH broth), and then plated on MH agar plates at time and conditions afore mentioned. An electronic counter (CountTM, Heathrow Scientific) was used to count colonies. All experiments were repeated at least twice in triplicate. The synergistic effect of bLf on the antibiotic MIC was evaluated using the fractional inhibitory concentration (FIC). The interpretation of results was based on the following scale: FIC > 2 indicated a synergistic effect (Luna-Castro et al., 2014). All experiments were repeated at least twice in triplicate. Statistical significance was determined using a Student's t-test (p < 0.05), or ANOVA (with Bonferroni correction).
Flow cytometry
To see if bLF and bLFpeptides cause membrane permeabilization, we used the staining with propidium iodide (PI), a fluorescent dye (this PI-assay is a quick assay that allowed us to compare the membrane damage by inclusion of PI, in a series of peptides under different conditions and incubation times). In brief, aliquots of 107 CFU/ml of V. cholerae O1 and non-O1-strains were cultured in LB broth, harvested by centrifugation (5,000 rpm/5 min), washed three times with LB broth, and incubated with 40 μM bLF or 20 μM of either LFcin17-30, LFampin265-284, or LFchimera at 37°C for 2 h. Next, bacteria were washed and incubated with 10 mg/ml PI during 10 min at 4°C, washed five times with PBS (pH 7.4), and after fixed with 4% paraformaldehyde. Samples were washed twice with PBS and finally analyzed with a FACScan (Fluorescence-Associated Cell Scanner; Becton Dickinson, USA). Control experiments were carried out with bacteria either without the addition of bLF or bLFpeptides (membranes integrity control), or with 0.5% Triton X-100 (which permeabilizes bacterial membranes). All experiments were done at least twice in duplicate.
Electron microscopy
V. cholerae O1 and non-O1 strains (108 CFU/ml) cultures were incubated in iron-depleted LB without additions (negative control of damage), with 0.5% SDS (positive control of damage), or with 40 μM of bLF, or with 20 μM LFcin17-30, LFampin265-284 or 5 μM LFchimera, at 37°C for 1.5 h. Cells were collected, placed in tubes with PBS (pH 7.4) and fixed with 4% paraformaldehyde plus 0.5% glutaraldehyde. Next, samples were washed with distilled water and deposited on bare 200-mesh copper grids. Next, phosphotungstic acid (1%, pH 5.5, 30 s) was added, replicas were then dehydrated and finally studied with a transmission electron microscope (IEM2000Ex), operated at 100 kV.
Confocal microscopy
The interaction of V. cholerae with bLF and the bLFpeptides was investigated by confocal microscopy. Briefly, 107 CFU/ml of V. cholerae O1 cells were incubated in iron-depleted LB containing 2 μM FITC-labeled peptides for 30 min. Bacteria were centrifuged (5 min, 10,000 × g), resuspended and fixed (4% paraformaldehyde, pH 7.4 during 30 min at 37°C), washed twice and prepared to be examined under confocal microscopy. To find out whether bLF and bLFpeptides are recognized by the bacterial membrane of dead bacteria, V. cholerae O1 cells were fixed, then washed twice with PBS and incubated with 2 μM of FITC-bLF or FITC-labeled peptides for 30 min. After, samples were washed with PBS and mounted on slides and processed. All samples were analyzed under confocal microscopy by using a confocal laser-scanning microscope (Leica, Heidelberg, Germany).
Effect of bLF and lfchimera on the antibacterial activity of classic antibiotics used against vibrio spp.
V. cholerae O1 Inaba, V. cholerae non-O1, V. vulnificus (resistant to tetracycline and ampicillin), V. fluvialis (resistant to ampicillin and cefotaxime), V. alginolyticus (resistant to ampicillin and tetracycline), and V. furnissii (resistant to ampicillin), were used to determine whether bLF and LFchimera potentialize the bactericidal activity of common antibiotics. First, to test the resistance level to common antibiotics, the bacterial strains were grown with or without gentamicin (2–25 μg/ml), tetracycline (2.5–20 μg/ml), chloramphenicol (2.5–30 μg/ml), or ampicillin (2.5–32 μg/ml); bactericidal activity of LFchimera (1, 5, 10, and 20 μM) was tested in parallel. Next the antibiotics ampicillin (2.5–32 μg/ml), chloramphenicol (2.5–30 μg/ml), and tetracycline (2.5–20 μg/ml) were tested in the presence of a sub-MIC concentration of bLF (10 μM) or LFchimera (1 μM). Percentage of viable cells was determined in relation to cultures without added peptides or antibiotics. All experiments were repeated at least twice in triplicate.
In vivo model
Bacterial strain and culture conditions
The Vibrio cholerae O1 serotype Inaba was maintained in TCBS agar (BD, USA) at 37°C during 24 h. Bacterial cultures (used for mice inoculations) were routinely grown on LB agar plates with 100 mg/ml streptomycin for 18 h and finally were grown with shaking in LB broth with antibiotic at 37°C to mid-log phase. The OD620 nm was adjusted to 1 and this inoculum was used in the assays.
Inoculation of vibrio cholerae O1 serotype inaba in mice and treatments
Six to eight-week-old female BALB/cAnNHsd mice (Harlan Laboratories, Inc., Mexico), were purchased and housed under specific-pathogen-free conditions as stipulated by the Ethical Committee for Laboratory Animals in Faculty of Medicine of UAS and were divided into five groups. Mice were given 0.1% (w/v) Streptomycin for 3 days to ablate normal flora. A day prior to inoculation, food was removed from cages to empty the stomach. Mice were injected intraperitoneally with 12.5 mg/kg xylazine. When mice were deeply sedated, 50 μl of 0.5 M NaHCO3 was administered intragastrically immediately followed by 500 μl of bacterial suspension (2.5 × 107 CFU). After inoculation, mice were kept with free access to food and sterile water without streptomycin. Then, after 4 h post-inoculation (after infection and symptoms were established) different treatments were administered into mice each 12 h for 3 days. Treatment doses administered were as follows; 65 mg/Kg of bLF, 5 μg/Kg of LFchimera, and 14 mg/Kg of Tetracycline (Sigma Inc. USA). Mice of the control group were administered 0.5 ml of PBS instead of antimicrobial agents. All of the mice were housed in groups consisting of 10 mice each and permitted food and water ad libitum.
Identification of vibrio cholerae O1 in infected mice
In order to evaluate the V. cholerae mice infection procedure and establishment, a disposable 1 μl plastic inoculation loop (diameter 2.0 mm) was introduced into the rectum. The loop was turned around to obtain V. cholerae O1 from the inner surface of the rectum. The tip of the loop was subsequently clipped into a 1.5 ml tube containing 500 μl of enriched alkaline peptone water and incubated for 18 h at 37°C. The bacteria from slopes were streaked onto TCBS agar and CHROMagarTM Vibrio to confirm the infection. Once the infection was demonstrated (after 4 h) the treatments were administered.
CFU enumeration of vibrio cholerae O1 in feces and intestines of mice
Briefly, fresh feces of mice were weighed and suspended in PBS. Then, samples were homogenized and serial dilutions were prepared and plated onto LB agar plates with 100 mg/ml streptomycin for 24 h at 37°C. For confirmation, developed colonies were counted and then plated onto CHROMagarTM Vibrio (CHROMagar; Paris, France). On the other hand, the intestines were dissected, homogenized in PBS and serial dilutions were prepared in PBS and plated in LB agar plates with 100 mg/ml streptomycin. Finally, colonies were counted and plated onto CHROMagarTM Vibrio.
Results
bLF and bLFpeptides inhibited the growth of V. cholerae O1 and non-O1 strains
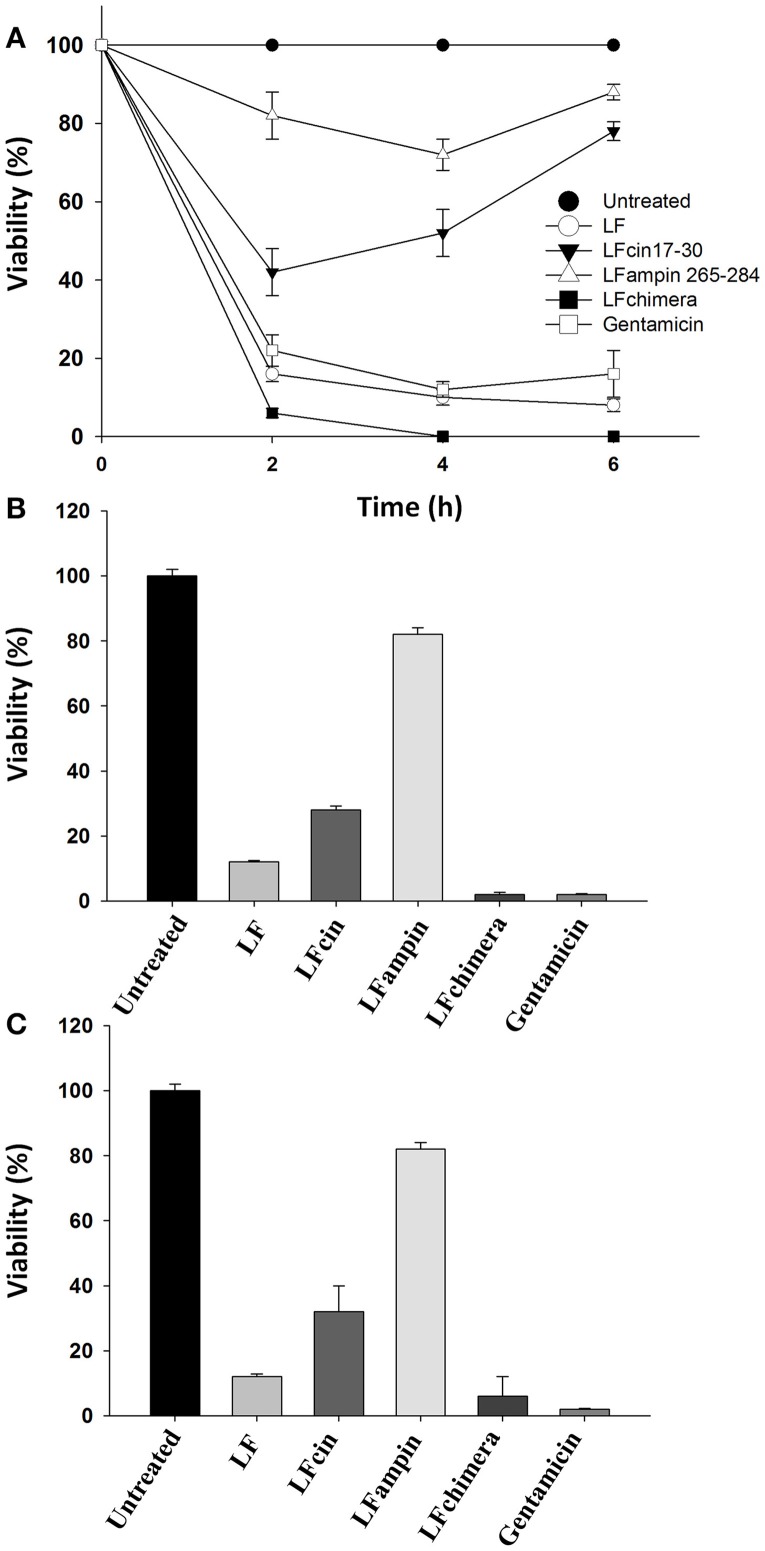
Historically, V. cholerae O1 and non-O1 strains have caused more problems to human health that other Vibrio species. So, we used these strains in order to test the antibacterial activity of bLF and LFpeptides. The ability of bLF and LFpeptides (LFcin17-30, LFampin265-284, and LFchimera) to inhibit the growth of V. cholerae O1 and non-O1 strains of V. cholerae was analyzed by measuring the growth in untreated and treated cells after several incubation times. Results shown that LFchimera had the best bactericidal effect, since 5 μM inhibited the growth until 6% at 2 h of incubation (with respect to the untreated bacteria). This inhibition was better than those exerted by 25 μg/ml of Gentamicin which inhibited the culture until 22% during the first 2 h of incubation. Percentage of growth inhibition in cultures treated with 40 μM bLF was 16, 42% with 20 μM LFcin17-30 and 82% with LFampin265-284 (Figure 1A). In addition, only LFchimera at a concentration of 5 μM showed bactericidal activity after 4 and 6 h incubation. Gentamicin at 25 μg/ml showed low percentage of viability without any increase within 6 h, whereas in the presence of peptides LFcin17-30 and LFampin265-284 a decrease in viability was only found after 2 h and followed by a recovery of the viability after 4 and 6 h (Figure 1A).
Figure 1.
Bactericidal effect of bLF and LF-peptides on Vibrio cholerae O1 and non-O1. Approximately 1 × 107 CFU/ml of V. cholerae O1 and non-O1 strains were incubated with bLF and LFpeptides solutions at final concentrations of 40 μM bLF or 20 μM of LFcin17-30, LFampin265-284, respectively; and 5 μM of LFchimera at 37°C with constant agitation for 1, 2, 4, or 6 h. Bacteria grown in LB broth were used as a control for optimal growth and 100 μM of Gentamicin was used as a control for growth inhibition. Bacterial growth was followed by measuring the OD660 nm of cultures. Percentage of viable cells was determined in relation to cultures Gentamicin without peptides or antibiotics (A). All Experiments were repeated at least twice in triplicates. V. cholerae O1 (B) and non-O1 (C) strains were incubated with bLF and LFpeptides solutions at final concentrations of 40 μM bLF and 20 μM LFcin17-30, LFampin265-284, and 5 μM LFchimera; respectively. Viability was monitored by enumerating colony forming units CFU/ml (viable cells) obtained from serial 10-fold dilutions plated onto MH agar (B,C). Percentage of viable cells was calculated relative to viable bacteria untreated grown in MH agar. Experiments were performed in triplicate; mean and standard deviation are indicated. Statistical significance was determined using a Student's t-test for P-values < 0.05, and ANOVA (with Bonferroni correction).
On the other hand, by CFU counts after 2 h of incubation, V. cholerae O1 cultures treated with 5 μM LFchimera and 40 μM bLF were significantly reduced (until 2 and 6% respectively, relative to untreated bacteria) and the effect were similar to the bactericidal activity of Gentamicin (6% growth inhibition relative to untreated bacteria) (Figure 1B). However, in V. cholerae non-O1 strain Gentamicin and LFchimera apparently inhibited the cultures with the same efficacy (Figure 1C). In all treatments and incubation times (longer than 2 h) LFchimera completely inhibited the growth of V. cholerae O1 and V. cholerae non-O1 strains (Figures 1B, C; respectively). The bactericidal activity of LFchimera was stronger than those of bLF, LFcin17-30, and LFampin265-284 (6, 22, and 82% of growth inhibition, relative to untreated bacteria).
Lactoferrin and lactoferrin-derived peptides showed combined effect with antibiotics and inhibited the growth of vibrio
V. cholerae O1 Inaba, V. cholerae non-O1, V. vulnificus (resistant to tetracycline and ampicillin), V. fluvialis (resistant to ampicillin and cefotaxime), V. alginolyticus (resistant to amplicillin and tetracycline), and V. furnissii (resistant to ampicillin) were used to determine whether bLF and LFchimera, each in combination with common antibiotics increase the bactericidal effect.
In the results, the combination of 1 μM LFchimera plus 2.5 μg/ml ampicillin were able to inhibit more than 95% of growth of V. vulnificus, V. fluvialis, V. alginolyticus, and V. furnissii. Similar effects on growth inhibition (more than 95%) were found with concentrations of 5 μM LFchimera (Table 1), or more than 32 μg/ml ampicillin, suggesting that the combination of LFchimera and ampicillin (1 and 2.5 μg/ml) can inhibit the growth of Vibrio spp. resistant to ampicillin (Table 1). On the other hand, by using a combination of LFchimera with tetracycline, the combination of 1 μM LFchimera plus 2.5 μg/ml of tetracycline was able to inhibit more than 95% of the growth of V. vulnificus; this inhibition growth is only reached with concentrations of 5 μM of LFchimera or more than 20 μg/ml of tetracycline (Table 1). A mixture of 1 μM LFchimera and 2.5 μg/ml chloramphenicol inhibited more than 95% of the growth of V. cholerae O1 and non-O1 strains, whereas this growth inhibition level was only reached by using concentrations of 5 μM LFchimera or 30 μg/ml chloramphenicol (Table 1). Ten microliters bLF also had synergism or combined effect with antibiotics in the strains above mentioned, whereas without antibiotics the concentration needed to inhibit more than 95% was 10 μM bLF. These data suggest that LFchimera and bLF combined with low concentrations of antibiotics have bactericidal effect in multidrug resistant strains of genus Vibrio (Table 1). According to the calculation of FICs, both bLF and LFchimera have synergistic effects when they were used with the antibiotics, suggestion that they could improve the management of Vibrio spp multidrug resistant strains to antibiotics in vivo.
Table 1.
Effects of LFchimera and bLF combined with antibiotics on Vibrio spp growth.
| Concentrations (μg/ml) of antibiotics for causing more than 95% growth inhibition | Concentrations (μg/ml) of antibiotics for causing more than 95% growth inhibition | ||||
|---|---|---|---|---|---|
| Strain | Ampicillin without LFchimera | Ampicillin with 1 μM LFchimera | Strain | Ampicillin without bLF | Ampicillin with 10 μM bLF |
| V. vulnificus | 32 | 2.5 | V. vulnificus | 32 | 2.5 |
| V. fluvialis | 32 | 2.5 | V. fluvialis | 32 | 2.5 |
| V. alginolyticus | 32 | 2.5 | V. alginolyticus | 32 | 2.5 |
| V. furnissii | 32 | 2.5 | V. furnissii | 32 | 2.5 |
| Strain | Tetracycline without LFChimera | Tetracycline with 1 μM LFChimera | Strain | Tetracycline without LF | Tetracycline with 10 μM LF |
| V. vulnificus | 20 | 2.5 | V. vulnificus | 20 | 2.5 |
| Strain | Cloramphenicol without LFChimera | Cloramphenicol with 1 μM LFChimera | Strain | Cloramphenicol without LF | Cloramphenicol with 10 μM LF |
| V. cholerae O1 | 30 | 2.5 | V. cholerae O1 | 20 | 2.5 |
| V. cholerae non O1 | 30 | 2.5 | V. cholerae non O1 | 20 | 2.5 |
An inoculum (OD 0.005 at 660 nm) was used in each experiment. Viability relative to the 100% growth in untreated bacteria cultures was determined by measuring the OD every 30 min during 1.5 h. Data are mean values of two experiments performed in triplicate. Standard deviations were less that 6.2% in each experiment.
bLF and bLFpeptides cause damage on vibrio cholerae O1 and non-O1 strains
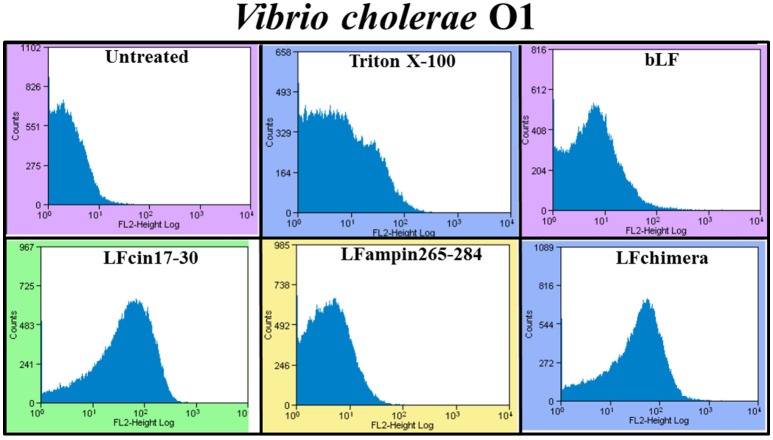
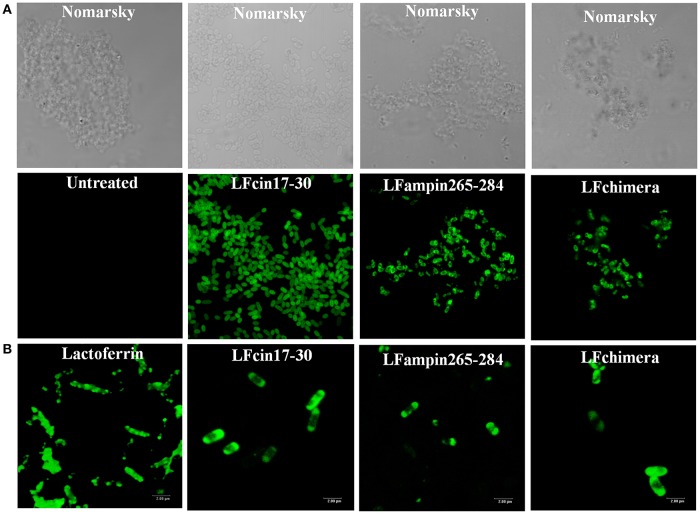
The effect of bLF and bLFpeptides on the bacterial membrane integrity was investigated using the fluorescent dye PI (it only enters in permeabilized cells). PI was measured under flow cytometry. After the treatment, almost all V. cholera cells had taken up the dye fluorescence upon incubation with 5 μM LFchimera, 20 μM bLF LFcin17-30, and LFampin265-284; indicating that the bacterial membrane was permeabilized by the peptides (Figure 2). The treatment with Triton X-100 used as a positive control of bacterial permeabilization also stained bacterial cells, corroborating that this treatment damaged the bacteria (Figure 2).
Figure 2.
Determination of membrane permeabilization in Vibrio cholerae O1 treated with bLF and LF peptides. V. cholerae O1 was incubated with 40 μM LF, or with 20 μM LFcin17-30, or LFampin265-284, respectively; or 5 μM LFchimera, at 37°C with constant agitation for 2 h. Then, samples were processed and stained with the fluorescent dye Propidium iodide. Untreated bacteria were used as control of membrane integrity and 0.5% Triton X-100 treated bacteria were used as control of permeabilized membranes. Experiments were performed at least twice in duplicate. Samples were processed to be analyzed by Flow Cytometry.
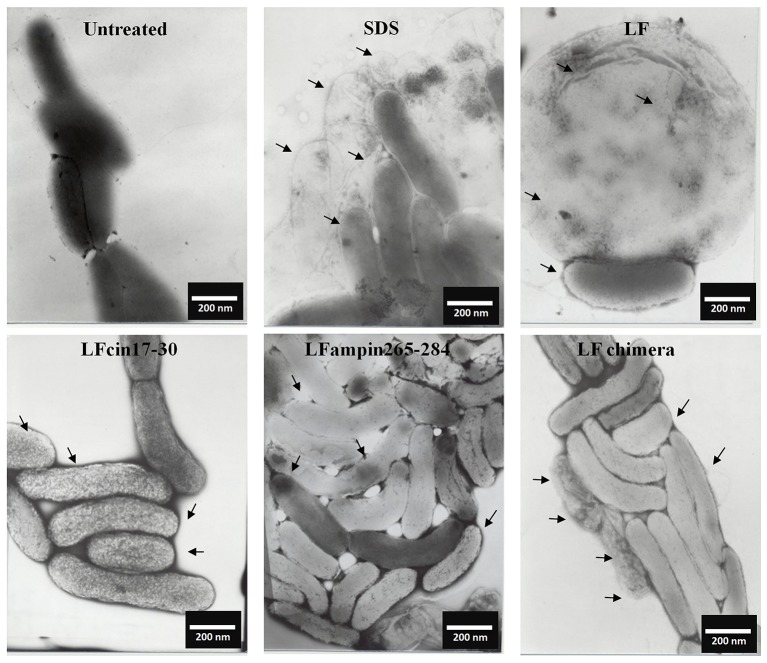
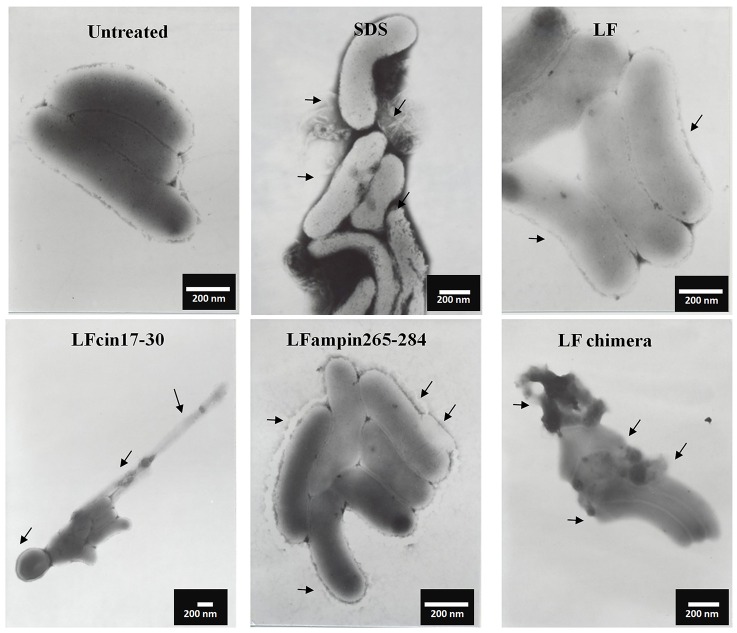
Similar incubations of V. cholerae O1 analyzed by SEM after negative staining showed severe membrane damage such as vesicularization, the occurrence of protrusions and filamentation (Figure 3, arrows). The same damage was found in V. cholerae non-O1 cells treated with bLF and LFpeptides (Figure 4). These results demonstrate that LFchimera and peptides destabilize the bacterial membrane integrity and also that LFchimera has a higher activity than bLF and LF peptides.
Figure 3.
LF and LFpeptides cause ultrastructural damage to Vibrio cholerae O1 cells. V. cholerae O1 cells (1.0 × 108 cells/ml) were incubated in LB alone (negative control for damage) or with 0.5% SDS (positive control for damage), or with 40 μM of bLF, 20 μM LFcin 17-30 or LFampin265-284 respectively, or 5 μM LFchimera for 1.5 h at 37°C. Cells were harvested, resuspended in PBS and fixed with 4% para-formaldehyde plus 0.5% glutaraldehyde. Next, bacterial samples were placed on 200-mesh Formvar-coated copper grids (3%), post-stained with phosphotungstic acid and examined with a JEOL electron microscope JEM1400 at 40 kv.
Figure 4.
LF and LFpeptides cause ultrastructural damage to Vibrio cholerae non-O1 cells. V. cholerae non-O1 cells (1.0 × 108 cells/ml) were incubated in LB alone (negative control for damage) or with 0.5% SDS (positive control for damage) or with 40 μM of bLF, or 20 μM LFcin 17-30 and LFampin265-284 respectively, or 5 μM LFchimera, for 1.5 h at 37°C. Cells were harvested, resuspended in PBS and fixed with 4% para-formaldehyde plus 0.5% glutaraldehyde. Next, bacterial samples were placed on 200-mesh Formvar-coated copper grids (3%), post-stained with phosphotungstic acid and examined with a JEOL electron microscope JEM1400 at 40 kv.
bLF and bLFpeptides interact with vibrio cholerae strains
The interaction of bLF and LFpeptides was investigated by confocal microscopy. In the results, we observed that the peptides LFcin17-30, LFampin265-284, and LFchimera interact with V. cholerae bacteria (Figure 5A). On the other hand, in fixed bacteria the fluorescent compounds were found interacting with the bacteria, indicating that the membrane of V. cholerae contains components which are recognized by the peptides (Figure 5B).
Figure 5.
Interaction of Lactoferrin derived peptides with Vibrio cholerae O1 and non-O1 strains. Vibrio cholerae O1 cells (107 CFU/ml) were incubated with 2 μM FITC-labeled peptides for 30 min. Bacteria were centrifuged (5 min, 10,000 × g), resuspended and incubated with 2 μM of FITC-bLF or FITC-labeled peptides for 30 min (A), or fixed (4% paraformaldehyde, pH 7.4 during 30 min at 37°C) (B), washed and then incubated with 2 μM bLF and LFpeptides as before was described. In both cases samples were washed twice with PBS mounted on slides and processed. All samples were analyzed under confocal microscopy by using a confocal laser-scanning microscope (Leica, Heidelberg, Germany). Bar 20 nm.
Bovine lactoferrin and lactoferrin chimera reduce damage on intestine and cecum of mice infected with vibrio cholerae
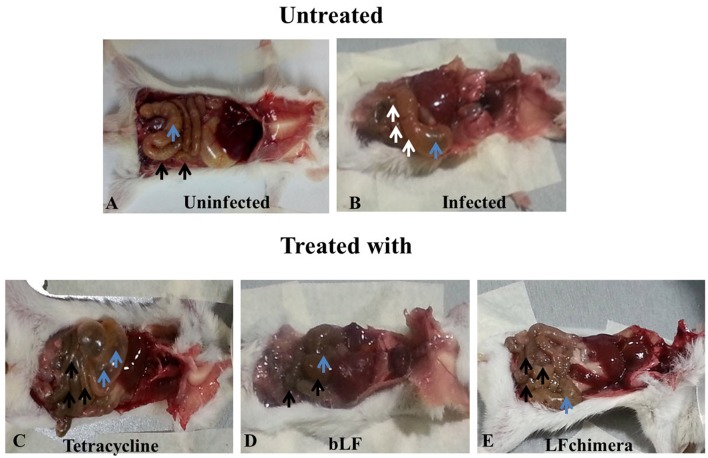
Mice infected with V. cholerae developed symptoms such as diarrhea, weakness, and abdominal tremor after 4 h of infection. Additionally; the infection was confirmed by counting V. cholerae obtained from rectal swabs (data nor shown), Once infection was confirmed, mice were treated with bLF, LFchimera, and tetracycline. Twenty-four post-infection three mice were sacrificed in order to see the effectiveness of the treatments. In the results, representative macroscopic images are shown for the gross morphological alterations of the small intestine and caecum from mice at 24 h of treatment (Figure 6). In a mouse of the uninfected group black arrows indicate the typical appearance of a normal small intestine, and blue arrowheads indicate normal caecum (Figure 6A). In a mouse from the infected and untreated group, white arrows point to injury in small intestine and blue arrow shows caecum is swelling (Figure 6B). A mouse from the group treated with tetracycline black arrows indicate injury in small intestine and blue arrows shows caecum is swelling and enlarged (Figure 6C). Interestingly, a mouse from the group treated with bLF Black arrows indicate normal small intestine as in Figure 6A and blue arrows indicates normal caecum (similar to macroscopic findings of uninfected mice). A mouse treated with LFchimera shows black arrows indicating a normal small intestine and blue arrow indicates a normal caecum. Similar results were observed in other mice sacrificed. These results indicated that bLF and LFchimera have the capacity to diminishing macroscopic damage induced by V. cholerae in intestines and cecum in Mice.
Figure 6.
Alterations in gut morphology of mice. Representative macroscopic images for the gross morphological alterations of the small intestine and caecum from mice at 24 h of treatment. (A) Small intestine Black arrows indicate normal small intestine in the uninfected group (blue arrowhead) indicate normal caecum. (B) White arrows point to injury in small intestine in the infected and untreated group (blue arrow) shows caecum is swelling. (C) Black arrows indicate injury in small intestine (blue arrows) caecum is swelling and enlarged in the tetracycline group. (D) Black arrows indicate normal small intestine as in A (blue arrowhead) indicates normal caecum. (E) Black arrows indicate normal small intestine in the LFchimera group as in D (blue arrow) indicate caecum.
Bovine lactoferrin and lactoferrin chimera reduce vibrio cholerae counts in feces and intestines
V. cholerae counts diminished in feces (Figure 7A) and intestines (Figure 7B) from mice infected and treated with bLF and LFchimera after the first dose administered (Figure 7), compared with infected and untreated animals (Positive control of infection). In the group treated with LFchimera, V. cholerae was undetected in feces and intestines after 12 h of treatment, in the group treated with bLF V. cholerae was undetected until 24 h of treatment and with tetracycline the minimal bacterial count was done during 12–16 h of treatment, and then the bacteria recovered its growth in feces and intestines. The results show that LFchimera and bLF kill V. cholerae in in vivo model.
Figure 7.
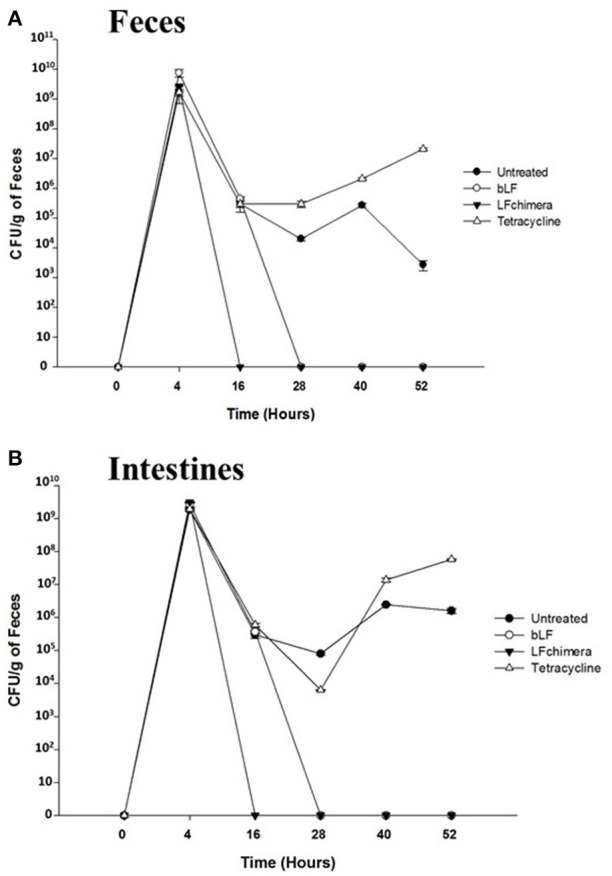
LF chimera and bLF diminish Vibrio cholerae O1 counts in (A) feces and (B) intestine. Recovery feces and intestines from mice infected and treated were homogenized and serial dilutions were prepared and plated onto LB agar plates with 100 mg/ml streptomycin for 24 h at 37°C. For confirmation, developed colonies were counted and then plated onto CHROMagar™ Vibrio (CHROMagar; Paris, France). On the other hand, the intestines were dissected, homogenized in PBS and serial dilutions were prepared in PBS and plated in LB agar plates with 100 mg/ml streptomycin. Finally, colonies were counted and plated onto CHROMagar™ Vibrio.
Discussion
Antimicrobial resistance is a global health concern because the infections can be more severe and difficult to treat (Bonomo, 2000). This is a consequence in part by overuse and misuse of antibiotics and represents a serious health concern throughout the world (Longworth, 2001). In this sense, in this problem is included the development of antibiotic resistance by Vibrio species (Rahmani et al., 2012; Sperling et al., 2015). The genus Vibrio includes at least 12 species pathogenic to humans. In these species are included V. cholerae, V. parahaemolyticus, V. vulnificus, V. alginolyticus, V. furnissii, V. fluvialis, V. damsela, V. hollisae, V. metschnikovii, and V. mimicus (Altekruse et al., 2000). Pathogenic Vibrio can cause both intestinal and extra-intestinal illnesses. Vibrio infections potentially requiring antimicrobial therapy fall into three distinct syndromes; (1) cholera; caused by either V. cholerae O1 and other serogroups such as non-o1 or other species (V. parahaemolyticus), (2) Soft tissue infections; due to V. vulnificus, and (3) sepsis due to V. vulnificus and other Vibrio (Powell, 1999). Acquired multidrug resistant in V. cholerae O1 and other pathogenic Vibrio is now common and firmly established wherever infections occur (Dhar et al., 1996; Elmahdi et al., 2016).
In this work, we demonstrated that bLF and LFchimera have bactericidal activity against V. cholerae O1 Inaba, V. cholerae non-O1 (toxigenic), V. vulnificus (resistant to tetracycline and ampicillin), V. fluvialis (resistant to ampicillin and cefotaxime), V. alginolyticus (resistant to amplicillin and tetracycline), and V. furnissii (resistant to ampicillin). Previous works have reported the bactericidal activity of bLF in V. cholerae (Arnold et al., 1980; Ellison and Giehl, 1991); however, the mechanism of action or damage was not investigated in detail. In regards with the bactericidal activity of bLF and LFpeptides on V. cholerae, the peptide LFchimera and the protein bLF had the best bactericidal activity (Figure 1). LFchimera was more effective to inhibit the growth of V. cholerae strains and other Vibrio spp., compared with its peptides of origin (LFcin17-30 and LFampin265-284). V. cholerae strains were also susceptible to both peptides; however, this antibacterial activity remained curiously lower when was compared to the antibacterial action of Gentamicin (drug used as a negative control for growth), or when was compared with the action of bLF and LFchimera (Figure 1). Nonetheless, LFcin17-30 and LFampin265-284 may be antibacterial, due they were able to damage membranes and cause disruption on V. cholerae cells (Figures 2–4). The more effective antibacterial ability of LFchimera compared to the effect reached with native bLF, LFcin17-30, and LFampin265-284 peptides has been reported for other bacteria, as well as parasites or fungi (Bolscher et al., 2009; Kanthawong et al., 2014; Leon-Sicairos et al., 2014). These differences on the effect could be due to the LFchimera structure (Haney et al., 2012a,b). An obvious question is why human or bovine LF doesn't prevent the infection by Vibrio species? We speculate that human LF present in mucosae and fluids, or released by neutrophils, or bLF ingested from dairy milk products is not enough to combat Vibrio spp infections.
As we found that LFchimera and bLF had the best bactericidal activity, we investigate the effects of them combined with low concentrations of antibiotics on multidrug resistant Vibrio strains. In the results, apparently a combined effect was found when antibiotics were mixed with bLF and LFchimera (Table 1). It is interesting that LFchimera mixed with ampicillin inhibited the growth of V. vulnificus, V. fluvialis, V. alginolyticus, and V. furnissii and also the growth of some Vibrio spp resistant to ampicillin (Table 1). On the other hand, the combination of LFchimera tetracycline inhibited the growth of V. vulnificus (Table 1). A mixture of LFchimera and chloramphenicol inhibited the growth of V. cholerae O1 and non-O1 strains, whereas this growth inhibition level was only reached by using higher concentrations of LFchimera or chloramphenicol (Table 1). bLF also had synergism or combined effect with antibiotics in the strains above mentioned, whereas without antibiotics the concentration needed to inhibit more than 95% was higher. These data suggest that LFchimera and bLF combined with low concentrations of antibiotics have bactericidal effect in multidrug resistant strains of genus Vibrio (Table 1). According to the calculation of FICs, both bLF and LFchimera had synergistic effects when they were used with the antibiotics, suggesting that they could improve the management of Vibrio spp multidrug resistant strains in vivo. In regards with this data, we speculate that the magnified effect of LFchimera plus antibiotics in Vibrio resistant strains could be due to the damage exerted by bLF and LFchimera on outer membrane of Vibrio spp plus the effect of the antibiotics.
The marked effect of LFchimera could be explained by its composition. LFchimera is formed by the peptides LFcin17-30 and LFampin265-284 (linked by a lysine). In consequence, this new peptide presents the following characteristics; an artificial conformation that mimics the spatial arrangement of the LF native, and a net charge of 12+ at neutral pH (compared with 6+ from LFcin17-30 and 4+ from LFampin265-84; respectively) (Bolscher et al., 2009). In this sense, it has been reported that the negatively charged membrane molecules present in pathogens are the main target of cationic antimicrobial peptides, so we speculate that Fchimera can act with the negatively charged microbial membrane components and destabilizes it, causing antimicrobial effect. Additionally, it has been reported that the bactericidal effect of LFchimera was not hampered by salt concentrations present in the media of bacterial growth.
Until the best of our knowledge this is the first report of the bactericidal activity of bLF and synthetic LFpeptides (LFin17-30, LFampin265-285, and LFchimera) on Vibrio species resistant to antibiotics.
In this study, we sought to get further insight into this mechanism by focusing our studies on V. cholerae O1 and non-O1 strains due to V. cholera is the most pathogenic specie of genus Vibrio, for this reason we made experiments to assess the mechanism of action. We found that bLF and LFpeptides caused membrane perturbation in both V. cholera strains (Figure 2) as well as damage on structural level (Figures 3, 4, respectively). Certainly, the measurement of FITC-labeling peptides by flow cytometry and microscopy indicated interaction of them with the bacteria (Figure 5), this interaction could then permits the damage of V. cholerae membranes. The interaction was visible by confocal microscopy (Figure 5) and was quantified by flow cytometry (data not shown). In additional experiments, we pre-incubated bacteria with high amounts of unlabeled bLF and then the FICT-peptides were added. In all cases bLF did not avoid the binding of peptides to the outer membrane of V. cholera, however we found a significant decrease in the fluorescent exhibited by bacteria, indicating that bLF and LFpeptides uses the same sites of recognition present in V. cholerae, and maybe specific sites (data not shown). Together this data shown that V. cholera contains sites on its membrane that bind bLF and LF peptides.
It has been reported that bLF interacts in a direct manner with negative charged components present in microbial membranes; inducing alterations in its permeability through dispersion of them. For example; bLF interacts with lipopolysaccharides (LPS) from Gram-negative bacteria, or with Lipoteichoic acid (LTA) from Gram-positive bacteria. After this interaction it has been postulated that there is an alteration on membranes, leading the death of the pathogens (Ellison et al., 1988; Orsi, 2004; Leon-Sicairos et al., 2014). We speculate that lipid A from LPS is also one of the targets form LFchimera and LFpeptides. In our precious work LFchimera produced damage on V. parahaemolyticus, the appearance of the bacteria shown typical perturbations of a bacteria undergoing programmed cell death type II (Leon-Sicairos et al., 2009), as these kind of damage was not found in all Vibrio tested we think LFchimera exerts different type of damage.
Treatment for V. cholerae infection involves antibiotics and oral hydration for cholera since 1964. Hydration includes the drinking of fluid with electrolytes, such as sodium, potassium, calcium ions to restore the high amount of electrolytes lost due watery diarrhea (Seas et al., 1996). Regarding drugs, tetracycline has been and effective treatment for cholera with better effect compared with others antibiotics such as furazolidone, chloramphenicol, and sulfaguanidine in reducing cholera morbidity (Lewis and Sanyal, 1965; Gharagozloo et al., 1970; Finkelstein, 1996; Escobar et al., 2015). However, it has been demonstrated the resistance to the antibiotic tetracycline and others (used for V. cholerae) in both; endemic and epidemic cholera settings.
Concerning our model in vivo, in was clear that LFchimera and bLF were effective to resolve V. cholerae infection in mice. LFchimera and bLF had bactericidal activity against the bacteria, and this was confirmed by the resolution of macroscopic damage (Figures 6D,E) and by the diminution of V. cholerae counts in feces and intestines of mice infected and treated, compared whit those infected and untreated. It seems to be that the bactericidal effect of LFchimera and bLF was better in comparison with tetracycline. So, In our model LFchimera and bLF were effective against V. cholerae infection.
Antibiotic resistance can be acquired by the acquisition of selected mutations, plasmids, introns, or conjugative elements, which could confer rapid spread of resistance (Towner et al., 1980; Hassan and Teh, 1993; Weber et al., 1994; Bhattacharya et al., 2011). Furthermore, it has been demonstrated that in cholera; the mass supply of antibiotics for prophylaxis in asymptomatic persons and household contacts of cholera patients during previous epidemics, represented a risk factor for the acquisition of resistance of V. cholera to antibiotics employed (Kitaoka et al., 2011; Marin et al., 2014). Treatment of infections due to Vibrio non cholerae also has been difficult in recent times, due to the spread of multidrug resistant Vibrio spp strains.
These facts indicated that is necessary searching for new products and interventions that can combat V. cholerae and other Vibrio spp., because of the increasing resistance against antibiotics. LFchimera and bLF at low concentrations were antibacterial against Vibrio spp.; we speculate that both compounds present potential to prevent or combat infections caused by Vibrio spp. On the other hand, antibiotics combined with LFchimera could act together, this also represent potential of new option against infections caused by the multidrug resistant Vibrio species. In addition, LFchimera could be used as an antibacterial in seafood or in humans, but first its efficacy as food preservative and in in vivo must to be determined.
Conclusions
We performed a study in order to see if bLF and LFpeptides (LFcin17-30, LFampin 265-284, and LFchimera) are effective as bactericides in V. cholera O1 and non-O1 strains and other Vibrio spp resistant to antibiotics. Data reported here demonstrated that LFchimera and the native bLF are bactericide peptides that damage Vibrio spp after a direct interaction. On the other hand, LFchimera and bLF combined with antibiotics could have a combinatory effect against Vibrio spp., for this reason they have potential as bactericidal agents against infections caused by Vibrio spp.
Ethics statement
Mice were purchased and housed under specific-pathogen-free conditions, treated and finally killed as stipulated and approved by the Ethical Committee for Laboratory Animals in School of Medicine, University of Sinaloa.
Author contributions
EA-S, KV-J, AC-R, MR-L, JB, KN, HF-V, GA-C, MdlG, JM-G, and JV-R: Substantial contributions to the conception of the work; acquisition of data and analysis; Drafting the work; Final approval of the version to be published. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. NL-S: designed the work; analyzed and interpreted the data for the work; Revised the work for important intellectual; Final approval of the version. She is in Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank to BS Lourdes Rojas-Morales and BS Sirenia González-Pozos from LANSE- CINVESTAV-IPN, México for their valuable technical assistance. Authors also thank Francisco Pacheco-Astorga from Departamento de Investigación, Hospital Pediatrico de Sinaloa, for his technical support. This work was supported by grants from CONACYT (CB-2014-236546) (NL-S), UAS PROFAPI-2014/105 (NL-S), and PROMEP/2014 DSA/103.5/14/11063 (Secretaría de Educación Pública), FOLIO UAS NPTC-121- (JV-R).
References
- Abbas A., Adams C., Scully N., Glennon J., O'gara F. (2007). A role for TonB1 in biofilm formation and quorum sensing in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 274, 269–278. 10.1111/j.1574-6968.2007.00845.x [DOI] [PubMed] [Google Scholar]
- Aguilar-Diaz H., Canizalez-Roman A., Nepomuceno-Mejia T., Gallardo-Vera F., Hornelas-Orozco Y., Nazmi K., et al. (2017). Parasiticidal effect of synthetic bovine lactoferrin peptides on the enteric parasite Giardia intestinalis. Biochem. Cell Biol. 95, 82–90. 10.1139/bcb-2016-0079 [DOI] [PubMed] [Google Scholar]
- Altekruse S. F., Bishop R. D., Baldy L. M., Thompson S. G., Wilson S. A., Ray B. J., et al. (2000). Vibrio gastroenteritis in the US gulf of Mexico region: the role of raw oysters. Epidemiol. Infect. 124, 489–495. 10.1017/S0950268899003714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Brewer M., Gauthier J. J. (1980). Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 28, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baveye S., Elass E., Mazurier J., Spik G., Legrand D. (1999). Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin. Chem. Lab. Med. 37, 281–286. 10.1515/CCLM.1999.049 [DOI] [PubMed] [Google Scholar]
- Bellamy W., Takase M., Wakabayashi H., Kawase K., Tomita M. (1992). Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 73, 472–479. 10.1111/j.1365-2672.1992.tb05007.x [DOI] [PubMed] [Google Scholar]
- Bhattacharya K., Kanungo S., Sur D., Lal Sarkar B., Manna B., Lopez A. L., et al. (2011). Tetracycline-resistant Vibrio cholerae O1, Kolkata, India. Emerg. Infect. Dis. 17, 568–569. 10.3201/eid1703.101176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolscher J. G., Adão R., Nazmi K., Van Den Keybus P. A., Van 'T Hof W., Nieuw Amerongen A. V., et al. (2009). Bactericidal activity of LFchimera is stronger and less sensitive to ionic strength than its constituent lactoferricin and lactoferrampin peptides. Biochimie 91, 123–132. 10.1016/j.biochi.2008.05.019 [DOI] [PubMed] [Google Scholar]
- Bonomo R. A. (2000). Multiple antibiotic-resistant bacteria in long-term-care facilities: an emerging problem in the practice of infectious diseases. Clin. Infect. Dis. 31, 1414–1422. 10.1086/317489 [DOI] [PubMed] [Google Scholar]
- Brock J. H. (1980). Lactoferrin in human milk: its role in iron absorption and protection against enteric infection in the newborn infant. Arch. Dis. Child 55, 417–421. 10.1136/adc.55.6.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. H. (2002). The physiology of lactoferrin. Biochem. Cell Biol. 80, 1–6. 10.1139/o01-212 [DOI] [PubMed] [Google Scholar]
- Colwell R. R. (1996). Global climate and infectious disease: the cholera paradigm. Science 274, 2025–2031. 10.1126/science.274.5295.2025 [DOI] [PubMed] [Google Scholar]
- Cutone A., Frioni A., Berlutti F., Valenti P., Musci G., Bonaccorsi Di Patti M. C. (2014). Lactoferrin prevents LPS-induced decrease of the iron exporter ferroportin in human monocytes/macrophages. Biometals 27, 807–813. 10.1007/s10534-014-9742-7 [DOI] [PubMed] [Google Scholar]
- De Jesús Hernández-Díaz L., Leon-Sicairos N., Velazquez-Roman J., Flores-Villasenor H., Guadron-Llanos A. M., Martinez-Garcia J. J., et al. (2015). A pandemic Vibrio parahaemolyticus O3:K6 clone causing most associated diarrhea cases in the Pacific Northwest coast of Mexico. Front. Microbiol. 6:221. 10.3389/fmicb.2015.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar U., Bennish M. L., Khan W. A., Seas C., Huq Khan E., Albert M. J., et al. (1996). Clinical features, antimicrobial susceptibility and toxin production in Vibrio cholerae O139 infection: comparison with V. cholerae O1 infection. Trans. R. Soc. Trop. Med. Hyg. 90, 402–405. 10.1016/S0035-9203(96)90522-2 [DOI] [PubMed] [Google Scholar]
- Do Nascimento S. M., Dos Fernandes Vieira R. H., Theophilo G. N., Dos Prazeres Rodrigues D., Vieira G. H. (2001). Vibrio vulnificus as a health hazard for shrimp consumers. Rev. Inst. Med. Trop. Sao Paulo 43, 263–266. 10.1590/S0036-46652001000500005 [DOI] [PubMed] [Google Scholar]
- Ellison R. T., III., Giehl T. J. (1991). Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Invest. 88, 1080–1091. 10.1172/JCI115407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., III., Giehl T. J., Laforce F. M. (1988). Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 56, 2774–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., III., Laforce F. M., Giehl T. J., Boose D. S., Dunn B. E. (1990a). Lactoferrin and transferrin damage of the gram-negative outer membrane is modulated by Ca2+ and Mg2+. J. Gen. Microbiol. 136, 1437–1446. [DOI] [PubMed] [Google Scholar]
- Ellison R. T., III., Luo Q., Reller L. B. (1990b). Enhancement of the activity of cefotaxime by iron-binding proteins. J. Antimicrob. Chemother. 25, 479–481. [DOI] [PubMed] [Google Scholar]
- Elmahdi S., Dasilva L. V., Parveen S. (2016). Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol. 57, 128–134. 10.1016/j.fm.2016.02.008 [DOI] [PubMed] [Google Scholar]
- Escobar L. E., Ryan S. J., Stewart-Ibarra A. M., Finkelstein J. L., King C. A., Qiao H., et al. (2015). A global map of suitability for coastal Vibrio cholerae under current and future climate conditions. Acta Trop. 149, 202–211. 10.1016/j.actatropica.2015.05.028 [DOI] [PubMed] [Google Scholar]
- Farnaud S., Evans R. W. (2003). Lactoferrin–a multifunctional protein with antimicrobial properties. Mol. Immunol. 40, 395–405. 10.1016/S0161-5890(03)00152-4 [DOI] [PubMed] [Google Scholar]
- Faruque S. M., Albert M. J., Mekalanos J. J. (1998). Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62, 1301–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A. (1996). Cholera, Vibrio cholerae O1 and O139, and other pathogenic vibrios, in Medical Microbiology, 4th Edn, ed Baron S. (Galveston, TX: United States; ). [PubMed] [Google Scholar]
- Flores-Villaseñor H., Canizalez-Román A., De La Garza M., Nazmi K., Bolscher J. G., Leon-Sicairos N. (2012). Lactoferrin and lactoferrin chimera inhibit damage caused by enteropathogenic Escherichia coli in HEp-2 cells. Biochimie 94, 1935–1942. 10.1016/j.biochi.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Flores-Villaseñor H., Canizalez-Román A., Reyes-Lopez M., Nazmi K., De La Garza M., Zazueta-Beltrán J., et al. (2010). Bactericidal effect of bovine lactoferrin, LFcin, LFampin and LFchimera on antibiotic-resistant Staphylococcus aureus and Escherichia coli. Biometals 23, 569–578. 10.1007/s10534-010-9306-4 [DOI] [PubMed] [Google Scholar]
- Frost W. H. (1976). Cholera: synopsis of clinical aspects and principles of treatment. Can. Med. Assoc. J. 115, 401–403. [PMC free article] [PubMed] [Google Scholar]
- Garbacz K., Kamysz W., Piechowicz L. (2017). Activity of antimicrobial peptides, alone or combined with conventional antibiotics, against Staphylococcus aureus isolated from the airways of cystic fibrosis patients. Virulence 8, 94–100. 10.1080/21505594.2016.1213475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharagozloo R. A., Naficy K., Mouin M., Nassirzadeh M. H., Yalda R. (1970). Comparative trial of tetracycline, chloramphenicol, and trimethoprim-sulphamethoxazole in eradication of Vibrio cholerae El Tor. Br. Med. J. 4, 281–282. 10.1136/bmj.4.5730.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber K. E., Dawgul M. (2017). Antimicrobial peptides under clinical trials. Curr. Top Med. Chem. 17, 620–628. 10.2174/1568026616666160713143331 [DOI] [PubMed] [Google Scholar]
- Haney E. F., Nazmi K., Bolscher J. G., Vogel H. J. (2012a). Influence of specific amino acid side-chains on the antimicrobial activity and structure of bovine lactoferrampin. Biochem. Cell Biol. 90, 362–377. [DOI] [PubMed] [Google Scholar]
- Haney E. F., Nazmi K., Bolscher J. G., Vogel H. J. (2012b). Structural and biophysical characterization of an antimicrobial peptide chimera comprised of lactoferricin and lactoferrampin. Biochim. Biophys. Acta 1818, 762–775. 10.1016/j.bbamem.2011.11.023 [DOI] [PubMed] [Google Scholar]
- Haney E. F., Nazmi K., Lau F., Bolscher J. G., Vogel H. J. (2009). Novel lactoferrampin antimicrobial peptides derived from human lactoferrin. Biochimie 91, 141–154. 10.1016/j.biochi.2008.04.013 [DOI] [PubMed] [Google Scholar]
- Hassan H., Teh A. (1993). Tetracycline-resistant Vibrio cholerae El Tor. Med. J. Malaysia 48, 95–96. [PubMed] [Google Scholar]
- Hernández-Robles M. F., Álvarez-Contreras A. K., Juárez-García P., Natividad-Bonifacio I., Curiel-Quesada E., Vázquez-Salinas C., et al. (2016). Virulence factors and antimicrobial resistance in environmental strains of Vibrio alginolyticus. Int. Microbiol. 19, 191–198. 10.2436/20.1501.01.277 [DOI] [PubMed] [Google Scholar]
- Jones J. L., Ludeke C. H., Bowers J. C., Derosia-Banick K., Carey D. H., Hastback W. (2014). Abundance of Vibrio cholerae, V. vulnificus, and V. parahaemolyticus in oysters (Crassostrea virginica) and clams (Mercenaria mercenaria) from Long Island sound. Appl. Environ. Microbiol. 80, 7667–7672. 10.1128/AEM.02820-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. K., Oliver J. D. (2009). Vibrio vulnificus: disease and pathogenesis. Infect. Immun. 77, 1723–1733. 10.1128/IAI.01046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juretić D., Vukicević D., Tossi A. (2017). Tools for designing amphipathic helical antimicrobial peptides. Methods Mol. Biol. 1548, 23–34. 10.1007/978-1-4939-6737-7_2 [DOI] [PubMed] [Google Scholar]
- Kanthawong S., Puknun A., Bolscher J. G., Nazmi K., Van Marle J., De Soet J. J., et al. (2014). Membrane-active mechanism of LFchimera against Burkholderia pseudomallei and Burkholderia thailandensis. Biometals 27, 949–956. 10.1007/s10534-014-9760-5 [DOI] [PubMed] [Google Scholar]
- Kitaoka M., Miyata S. T., Unterweger D., Pukatzki S. (2011). Antibiotic resistance mechanisms of Vibrio cholerae. J. Med. Microbiol. 60, 397–407. 10.1099/jmm.0.023051-0 [DOI] [PubMed] [Google Scholar]
- León-Sicairos N., Angulo-Zamudio U. A., Vidal J. E., López-Torres C. A., Bolscher J. G., Nazmi K., et al. (2014). Bactericidal effect of bovine lactoferrin and synthetic peptide lactoferrin chimera in Streptococcus pneumoniae and the decrease in luxS gene expression by lactoferrin. Biometals 27, 969–980. 10.1007/s10534-014-9775-y [DOI] [PubMed] [Google Scholar]
- Leon-Sicairos N., Canizalez-Roman A., De La Garza M., Reyes-Lopez M., Zazueta-Beltran J., Nazmi K., et al. (2009). Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus. Biochimie 91, 133–140. 10.1016/j.biochi.2008.06.009 [DOI] [PubMed] [Google Scholar]
- Lewis G., Sanyal S. (1965). Tetracycline sensitivity of Vibrio cholerae in Calcutta. Bull. Calcutta Sch. Trop. Med. 13, 40–42. [PubMed] [Google Scholar]
- Lipp E. K., Huq A., Colwell R. R. (2002). Effects of global climate on infectious disease: the cholera model. Clin. Microbiol. Rev. 15, 757–770. 10.1128/CMR.15.4.757-770.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth D. L. (2001). Microbial drug resistance and the roles of the new antibiotics. Cleve Clin. J. Med. 68, 496–497, 501–492, 504. 10.3949/ccjm.68.6.496 [DOI] [PubMed] [Google Scholar]
- López-Soto F., González-Robles A., Salazar-Villatoro L., León-Sicairos N., Pña-Vázquez C., Salazar E. P., et al. (2009). Entamoeba histolytica uses ferritin as an iron source and internalises this protein by means of clathrin-coated vesicles. Int. J. Parasitol. 39, 417–426. 10.1016/j.ijpara.2008.08.010 [DOI] [PubMed] [Google Scholar]
- Lopez-Soto F., Leon-Sicairos N., Nazmi K., Bolscher J. G., De La Garza M. (2010). Microbicidal effect of the lactoferrin peptides lactoferricin17-30, lactoferrampin265-284, and lactoferrin chimera on the parasite Entamoeba histolytica. Biometals 23, 563–568. 10.1007/s10534-010-9295-3 [DOI] [PubMed] [Google Scholar]
- Luna-Castro S., Aguilar-Romero F., Samaniego-Barron L., Godinez-Vargas D., De La Garza M. (2014). Effect of bovine apo-lactoferrin on the growth and virulence of Actinobacillus pleuropneumoniae. Biometals 27, 891–903. 10.1007/s10534-014-9752-5 [DOI] [PubMed] [Google Scholar]
- Marin M. A., Fonseca E. L., Andrade B. N., Cabral A. C., Vicente A. C. (2014). Worldwide occurrence of integrative conjugative element encoding multidrug resistance determinants in epidemic Vibrio cholerae O1. PLoS ONE 9:e108728. 10.1371/journal.pone.0108728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi N. (2004). The antimicrobial activity of lactoferrin: current status and perspectives. Biometals 17, 189–196. 10.1023/B:BIOM.0000027691.86757.e2 [DOI] [PubMed] [Google Scholar]
- Powell J. L. (1999). Vibrio species. Clin. Lab. Med. 19, 537–552, vi. [PubMed] [Google Scholar]
- Puknun A., Kanthawong S., Anutrakunchai C., Nazmi K., Tigchelaar W., Hoeben K. A., et al. (2016). Ultrastructural effects and antibiofilm activity of LFchimera against Burkholderia pseudomallei. World J. Microbiol. Biotechnol. 32, 33. 10.1007/s11274-015-1988-x [DOI] [PubMed] [Google Scholar]
- Rahmani F., Fooladi A. A., Marashi S. M., Nourani M. R. (2012). Drug resistance in Vibrio cholerae strains isolated from clinical specimens. Acta Microbiol. Immunol. Hung 59, 77–84. 10.1556/AMicr.59.2012.1.8 [DOI] [PubMed] [Google Scholar]
- Seas C., Dupont H. L., Valdez L. M., Gotuzzo E. (1996). Practical guidelines for the treatment of cholera. Drugs 51, 966–973. 10.2165/00003495-199651060-00005 [DOI] [PubMed] [Google Scholar]
- Sedas V. T. (2007). Influence of environmental factors on the presence of Vibrio cholerae in the marine environment: a climate link. J. Infect. Dev. Ctries 1, 224–241. [PubMed] [Google Scholar]
- Singh P. K., Parsek M. R., Greenberg E. P., Welsh M. J. (2002). A component of innate immunity prevents bacterial biofilm development. Nature 417, 552–555. 10.1038/417552a [DOI] [PubMed] [Google Scholar]
- Spellberg B., Guidos R., Gilbert D., Bradley J., Boucher H. W., Scheld W. M., et al. (2008). The epidemic of antibiotic-resistant infections: a call to action for the medical community from the infectious diseases society of America. Clin. Infect. Dis. 46, 155–164. 10.1086/524891 [DOI] [PubMed] [Google Scholar]
- Sperling L., Alter T., Huehn S. (2015). Prevalence and antimicrobial resistance of vibrio spp. in retail and farm shrimps in ecuador. J. Food Prot. 78, 2089–2092. 10.4315/0362-028X.JFP-15-160 [DOI] [PubMed] [Google Scholar]
- Su Y. C., Liu C. (2007). Vibrio parahaemolyticus: a concern of seafood safety. Food Microbiol. 24, 549–558. 10.1016/j.fm.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Thompson F. L., Iida T., Swings J. (2004). Biodiversity of vibrios. Microbiol. Mol. Biol. Rev. 68, 403–431. 10.1128/MMBR.68.3.403-431.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towner K. J., Pearson N. J., Mhalu F. S., O'grady F. (1980). Resistance to antimicrobial agents of Vibrio cholerae E1 Tor strains isolated during the fourth cholera epidemic in the united republic of Tanzania. Bull. World Health Organ. 58, 747–751. [PMC free article] [PubMed] [Google Scholar]
- Valenti P., Greco R., Pitari G., Rossi P., Ajello M., Melino G., et al. (1999). Apoptosis of Caco-2 intestinal cells invaded by Listeria monocytogenes: protective effect of lactoferrin. Exp. Cell Res. 250, 197–202. 10.1006/excr.1999.4500 [DOI] [PubMed] [Google Scholar]
- Van Der Kraan M. I., Groenink J., Nazmi K., Veerman E. C., Bolscher J. G., Nieuw Amerongen A. V. (2004). Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 25, 177–183. 10.1016/j.peptides.2003.12.006 [DOI] [PubMed] [Google Scholar]
- Van Der Kraan M. I., Nazmi K., Teeken A., Groenink J., Van 'T Hof W., Veerman E. C., et al. (2005). Lactoferrampin, an antimicrobial peptide of bovine lactoferrin, exerts its candidacidal activity by a cluster of positively charged residues at the C-terminus in combination with a helix-facilitating N-terminal part. Biol. Chem. 386, 137–142. 10.1515/BC.2005.017 [DOI] [PubMed] [Google Scholar]
- Velazquez-Roman J., Leon-Sicairos N., Flores-Villasenor H., Villafana-Rauda S., Canizalez-Roman A. (2012). Association of pandemic Vibrio parahaemolyticus O3:K6 present in the coastal environment of Northwest Mexico with cases of recurrent diarrhea between 2004 and 2010. Appl. Environ. Microbiol. 78, 1794–1803. 10.1128/AEM.06953-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H. J. (2012). Lactoferrin, a bird's eye view. Biochem. Cell Biol. 90, 233–244. 10.1139/o2012-016 [DOI] [PubMed] [Google Scholar]
- Warnock E. W., III., Macmath T. L. (1993). Primary Vibrio vulnificus septicemia. J. Emerg. Med. 11, 153–156. 10.1016/0736-4679(93)90510-E [DOI] [PubMed] [Google Scholar]
- Weber J. T., Mintz E. D., Canizares R., Semiglia A., Gomez I., Sempertegui R., et al. (1994). Epidemic cholera in Ecuador: multidrug-resistance and transmission by water and seafood. Epidemiol. Infect. 112, 1–11. 10.1017/S0950268800057368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiou M. (2007). Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J. Mol. Med. 85, 317–329. 10.1007/s00109-006-0143-4 [DOI] [PubMed] [Google Scholar]