Abstract
Maternal depressive symptoms during pregnancy predict increased psychiatric problems in children. The underlying biological mechanisms remain unclear. Hence, we examined whether alterations in the morphology of 88 term placentas were associated with maternal depressive symptoms during pregnancy and psychiatric problems in 1.9–3.1-years old (Mean = 2.1 years) toddlers. Maternal depressive symptoms were rated biweekly during pregnancy with the Center of Epidemiological Studies Depression Scale (n = 86). Toddler psychiatric problems were mother-rated with the Child Behavior Checklist (n = 60). We found that higher maternal depressive symptoms throughout pregnancy [B = −0.24 Standard Deviation (SD) units: 95% Confidence Interval (CI) = −0.46; −0.03: P = 0.03; Mean difference = −0.66 SDs; 95% CI = −0.08; −1.23: P = 0.03; between those with and without clinically relevant depressive symptoms] were associated with lower variability in the placental villous barrier thickness of γ-smooth muscle actin-negative villi. This placental morphological change predicted higher total (B = −0.34 SDs: 95% CI = −0.60; −0.07: P = 0.01) and internalizing (B = −0.32 SDs: 95% CI = −0.56; −0.08: P = 0.01) psychiatric problems in toddlers. To conclude, our findings suggest that both maternal depressive symptoms during pregnancy and toddler psychiatric problems may be associated with lower variability in the villous membrane thickness of peripheral villi in term placentas. This lower heterogeneity may compromise materno-fetal exchange, suggesting a possible role for altered placental morphology in the fetal programming of mental disorders.
Introduction
Maternal clinically relevant depressive symptoms complicate up to 10–20% of pregnancies1–5. Maternal depressive symptoms not only disrupt the health of the pregnant woman4,6 but also have adverse consequences on offspring mental health2,3,7–9. Several studies have shown that maternal depressive symptoms during pregnancy predict increased psychiatric problems in offspring, and these effects are not explained by maternal depressive symptoms after pregnancy2,3,7–9. For example, we showed among 2296 Prediction and Prevention of Preeclampsia and Intrauterine Growth Restriction (PREDO)-study participants that maternal depressive symptoms during pregnancy predict increased psychiatric problems in children, independently of maternal depressive symptoms after pregnancy3. However, the biological mechanisms underlying these associations remain largely unknown.
Emerging evidence suggests that maternal depressive symptoms during pregnancy may alter maternal physiological homeostasis above and beyond alterations induced by the pregnancy itself, leading to changes in hypothalamic-pituitary-adrenocortical axis, inflammatory and autonomic nervous system functioning, and in oxidative stress and nutrition levels2,6,9–14. These depression-related physiological changes may alter the normal placental adaptation and remodelling which occurs to ensure that the requirements for oxygen, hormones, nutrients and waste removal of the growing fetus are met throughout gestation15.
Aberrations in placental development and maturation that adversely affect the capacity of the placenta to provide optimal materno-fetal exchange can influence fetal health and program the fetus for disease development later in life16,17. A mature placenta comprises an extensive villous tree containing a network of fetal capillaries that bathes in a pool of maternal blood18. The villous tree consists of large structural stem villi that arborize to form intermediate and terminal villi, with a γ-smooth muscle actin(SMA)-positive perivascular layer of myofibroblast-like cells that aborize in the (then myofibroblast-free and SMA-negative) peripheral part of the villous tree19. This peripheral part of the villous tree facilitates most materno-fetal exchange20. The whole villous tree is covered by a principally bi-layered epithelium with an at-term largely incomplete basal layer of cytotrophoblasts and an apical layer, called the syncytiotrophoblast. The layers from the apical aspect of the syncytiotrophoblast to the apical (luminal) surface of the fetal capillary endothelium constitute the placental villous membrane21, the villous materno-fetal barrier15. As pregnancy progresses and fetal demand for gaseous and nutrient exchange increases, the villous membrane thickness becomes irregular22. Some areas become extremely thin as locally dilated segments of fetal capillaries protrude into the trophoblast layer and some areas remain thicker, where syncytial organelles and nuclei accumulate outwith these thin barrier areas18,23. The areas of extremely thin villous membrane, as small as 1–2 microns, are known as vasculo-syncytial membranes (VSM) and are the primary materno-fetal exchange sites24. Thus, a healthy late gestation placenta has high variability in villous membrane thickness measures and hence diffusion distance evidenced by larger standard deviations (SD:s)25. Increased VSM thickness and a resulting decrease in villous membrane thickness variability has been shown in placentas from pregnancies complicated by preeclampsia26 and gestational diabetes27.
Previous studies have shown that maternal psychological distress during pregnancy may be associated with altered placental weight and fetoplacental circulation28,29, and alterations in placental weight and/or surface area may predict psychiatric disturbance in childhood and adolescence30, and certain personality disorders31 and traits32 in adulthood. Yet, no previous studies have tested associations between maternal depressive symptoms or child psychiatric problems and placental morphology. In this hypothesis-generating study, we tested whether maternal depressive symptoms during pregnancy are associated with alterations in the morphology of term placentas, and if term placental morphology predicts child psychiatric problems in toddlerhood.
Results
Participant Characteristics and Potential Confounders
Table 1 shows the sample characteristics. In our study sample, maternal depressive symptoms showed high inter-individual stability across pregnancy trimesters (r’s from =0.63 to =0.84, P < 0.001). Toddler internalizing, externalizing and total psychiatric problems also had high inter-correlations (r’s from =0.63 to =0.88, P < 0.001). Supplementary Table S1 shows the intercorrelations between the different placental morphology indicators.
Table 1.
Characteristics of the Study Sample.
| Maternal Characteristics | Data Available(N) | Mean(SDa)/N(%) |
|---|---|---|
| Age at Delivery (years) | 88 | 31.5 (4.9) |
| Education, Tertiary | 88 | 57 (64.8%) |
| Pre-Pregnancy Body Mass Index (kg/m2) | 88 | 24.0 (4.2) |
| Any Diabetic or Hypertensive Disorder in Pregnancy (type 1 or gestational diabetes, chronic or gestational hypertension, pre-eclampsia), Yes | 88 | 15 (17.0%) |
| History of Physician-Diagnosed Mental Disorders Before Pregnancy, Yes | 80 | 13 (16.3%) |
| Depressive Symptoms | ||
| Trimester-Weighted Mean Score of Center for Epidemiological Studies | 86 | 10.3 (6.0) |
| Depression Scale Depressive Symptoms during Pregnancy | ||
| Trimester-Weighted Mean Score of Center for Epidemiological Studies | 86 | 14 (16.3%) |
| Depression Scale Depressive Symptoms during Pregnancy ≥ 16 | ||
| 1st Pregnancy Trimester Center for Epidemiological Studies Depression | 81 | 10.2 (6.7) |
| Scale Depressive Symptom Score | ||
| Mean of 2nd Pregnancy Trimester Center for Epidemiological Studies | 86 | 10.4 (6.5) |
| Depression Scale Depressive Symptom Scores | ||
| Mean of 3rd Pregnancy Trimester Center for Epidemiological Studies | 86 | 10.3 (6.6) |
| Depression Scale Depressive Symptom Scores | ||
| Number of Pregnancy Trimesters with Center for Epidemiological Studies | 81 | |
| 0 | 53 (65.4%) | |
| 1 | 16 (19.8%) | |
| 2–3 | 12 (14.8%) | |
| Beck Depression Inventory-II Score Concurrently to Rating the Child | 60 | 5.9 (5.5) |
| Placental Morphology | ||
| Volume of SMAb-Positive Villi per Placenta (ml) | 88 | 95.1 (50.9) |
| Volume of Capillaries per SMA-Positive Villi per Placenta (ml) | 88 | 56.0 (35.6) |
| Volume of SMAb-Negative Villi per Placenta (ml) | 88 | 182.7 (63.9) |
| Volume of Capillaries per SMAb-Negative Villi per Placenta (ml) | 88 | 157.5 (51.8) |
| Ratio of the Distribution of SMAb-Positive Villi to SMA-Negative Villi (%) | 88 | 63.7 (56.8) |
| Villous Barrier Thickness of SMAb-Positive Villi (µm) | 88 | 7.5 (1.7) |
| Standard Deviation of Villous Barrier Thickness of SMAb-Positive Villi (Variability in Thickness) (µm) | 88 | 6.3 (2.3) |
| Villous Barrier Thickness of SMAb-Negative Villi (µm) | 88 | 11.9 (4.6) |
| Standard Deviation of Villous Barrier Thickness of SMAb-Negative Villi (µm) | 88 | 10.2 (5.5) |
| Placental Weight (grams) | 88 | 589.7 (107.4) |
| Toddler Characteristics | ||
| Gestation Length (weeks) | 88 | 40.3 (1.0) |
| Sex | 88 | |
| Boys | 41 (46.6%) | |
| Girls | 47 (53.4%) | |
| Age at Follow-up (months) | 60 | 25.5 (2.9) |
| Child Behavior Checklist/1½-5 Psychiatric Problems | ||
| Total Problems | 60 | 46.8 (9.2) |
| Internalizing Problems | 60 | 45.4 (8.5) |
| Externalizing Problems | 60 | 48.6 (9.4) |
aSMA = γ-smooth muscle actin. bSD = standard deviation.
Maternal diabetic and hypertensive disorders in pregnancy were associated with smaller capillary volumes per SMA-positive villi per placenta (Mean Difference (MD) = 0.56: 95% Confidence Interval (CI) = 0.00;1.12: P = 0.05). Girls had larger capillary volumes per SMA-negative villi per placenta than boys (MD = 0.41: 95%CI = 0.01;0.81: P = 0.05). Gestation length was positively associated with volumes of SMA-negative villi (r = 0.24; P = 0.02) and capillaries per SMA-negative villi (r = 0.27; P = 0.01) per placenta. Maternal age, pre-pregnancy body mass index (BMI), education, or history of mental disorders before pregnancy were not associated with placental morphology (P-values ≥ 0.10).
Maternal Depressive Symptoms during Pregnancy and Placental Morphology
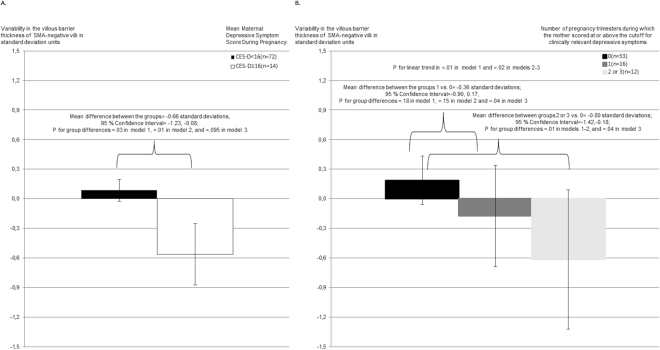
Higher maternal depressive symptoms throughout pregnancy (Table 2) and during each pregnancy trimester (Table 3) were associated with a significantly smaller SD of the villous barrier thickness of SMA-negative villi (indicating less villous barrier thickness variability) in unadjusted and/or adjusted regression analyses. Figure 1 shows that similar associations with a smaller SD of SMA-negative villi villous barrier thickness were found when CES-D depressive symptoms throughout pregnancy were above the clinical cutoff score (Panel A), and when these symptoms were more frequently above the cutoff during pregnancy trimesters (Panel B). The associations with this placental morphology indicator were most consistent across analytic models for maternal depressive symptoms during the first pregnancy trimester (Table 3).
Table 2.
Maternal Depressive Symptoms across Pregnancy and Placental Morphology. Unstandardized regression coefficients (B) and their 95% Confidence Intervals (CI) indicating standard deviation unit increase in placental morphology criteria per one standard deviation increase in maternal depressive symptoms across pregnancy from linear regression models where maternal depressive symptoms during pregnancy were used as predictors of placental morphology criteria.
| Placental Morphology Criteria | Maternal Depressive Symptoms during Pregnancy | |||||
|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 3c | ||||
| B(95% CI) | p | B(95% CI) | p | B(95% CI) | p | |
| Volume of SMA-positive villi per placenta | 0.06(−0.15;0.28) | 0.56 | 0.11(−0.11;0.33) | 0.34 | 0.11(−0.13;0.34) | 0.36 |
| Volume of capillaries per SMA-positive villi per placenta | 0.05(−0.17;0.27) | 0.65 | 0.09(−0.13;0.31) | 0.42 | 0.10(−0.14;0.34) | 0.41 |
| Volume of SMA-negative villi per placenta | −0.09(−0.33;0.14) | 0.43 | −0.08(−0.31;0.14) | 0.47 | −0.06(−0.30;0.18) | 0.64 |
| Volume of capillaries per SMA-negative villi per placenta | −0.13(−0.35;0.09) | 0.26 | −0.12(−0.34;0.10) | 0.28 | −0.10(−0.33;0.13) | 0.40 |
| Ratio of the distribution of stem villi to SMA-negative villi | 0.13(−0.09;0.34) | 0.26 | 0.16(−0.06;0.38) | 0.15 | 0.16(−0.07;0.40) | 0.18 |
| Villous barrier thickness of SMA-positive villi | −0.10(−0.32;0.12) | 0.36 | −0.12(−0.35;0.11) | 0.31 | −0.06(−0.30;0.18) | 0.60 |
| Standard deviation of villous barrier thickness of SMA-positive villi (variability in thickness) | −0.10(−0.31;0.13) | 0.42 | −0.08(−0.31;0.15) | 0.49 | −0.07(−0.32;0.18) | 0.57 |
| Villous barrier thickness of SMA-negative villi | −0.17(−0.38;0.04) | 0.12 | −0.21(−0.43;0.01) | 0.06 | −0.14(−0.37;0.09) | 0.22 |
| Standard deviation of villous barrier thickness of SMA-negative villi (variability in thickness) | −0.24(−0.46;−0.03) | 0.03 | −0.27(−0.50;−0.05) | 0.02 | −0.20(−0.43;0.04) | 0.096 |
SMA = γ-smooth muscle actin.
aModel 1 refers to unadjusted linear regression models.
bModel 2 refers to linear regression models adjusted for gestation length, maternal age at delivery, education, pre-pregnancy body mass index and diabetic and hypertensive disorders in pregnancy.
cModel 3 refers to linear regression models adjusted for model 2 covariates and maternal history of mental disorders before pregnancy.
Table 3.
Maternal Trimester-Specific Prenatal Depressive Symptoms and Placental Morphology.
| Depressive Symptoms during | 1st Pregnancy Trimester (n = 81) | 2nd Pregnancy Trimester (n = 86) | 3rd Pregnancy Trimester (n = 86) | |||
|---|---|---|---|---|---|---|
| Placental Criteria | B(95% CI) | p | B(95% CI) | p | B(95% CI) | p |
| Volume of SMA-positive villi per placenta | ||||||
| Model 1 | 0.01(−0.22; 0.23) | 0.97 | 0.05(−0.16; 0.26) | 0.61 | 0.12(−0.09; 0.33) | 0.27 |
| Model 2 | 0.06(−0.18; 0.31) | 0.61 | 0.10(−0.11; 0.32) | 0.33 | 0.14(−0.07; 0.36) | 0.19 |
| Model 3 | 0.07(−0.18; 0.32) | 0.59 | 0.10 (−0.12; 0.32) | 0.37 | 0.15 (−0.08; 0.38) | 0.21 |
| Volume of capillaries per SMA-positive villi per placenta | ||||||
| Model 1 | 0.00(−0.23; 0.23) | 0.99 | 0.04(−0.17; 0.25) | 0.68 | 0.11(−0.11; 0.32) | 0.33 |
| Model 2 | 0.07(−0.17; 0.32) | 0.55 | 0.09(−0.13; 0.30) | 0.43 | 0.12(−0.10; 0.34) | 0.27 |
| Model 3 | 0.09(−0.17; 0.34) | 0.49 | 0.09(−0.14; 0.32) | 0.43 | 0.14(−0.10; 0.37) | 0.25 |
| Volume of SMA-negative villi per placenta | ||||||
| Model 1 | 0.05(−0.19; 0.29) | 0.70 | −0.07(−0.29; 0.14) | 0.48 | −0.16(−0.37; 0.06) | 0.15 |
| Model 2 | −0.01(−0.26; 0.25) | 0.97 | −0.05(−0.27; 0.17) | 0.66 | −0.13(−0.35; 0.09) | 0.23 |
| Model 3 | 0.01(−0.26; 0.28) | 0.94 | 0.02(−0.25; 0.21) | 0.86 | −0.11(−0.35; 0.13) | 0.36 |
| Volume of capillaries per SMA-negative villi per placenta | ||||||
| Model 1 | −0.02(−0.22; 0.26) | 0.88 | −0.12(−0.33; 0.09) | 0.27 | −0.18(−0.40; 0.03) | 0.09 |
| Model 2 | −0.07(−0.32; 0.18) | 0.59 | −0.08(−0.29; 0.13) | 0.46 | −0.15(−0.36; 0.07) | 0.17 |
| Model 3 | −0.06(−0.32; 0.20) | 0.66 | −0.06(−0.28; 0.17) | 0.61 | −0.13(−0.37; 0.10) | 0.26 |
| Ratio of the distribution of stem villi to SMA-negative villi | ||||||
| Model 1 | 0.01(−0.23; 0.25) | 0.92 | 0.11(−0.10; 0.32) | 0.30 | 0.20(−0.01; 0.41) | 0.07 |
| Model 2 | 0.09(−0.16; 0.35) | 0.47 | 0.14(−0.07; 0.36) | 0.19 | 0.21(−0.01; 0.42) | 0.06 |
| Model 3 | 0.09(−0.17; 0.36) | 0.48 | 0.14(−0.09; 0.36) | 0.23 | 0.22(−0.02; 0.45) | 0.07 |
| Villous barrier thickness of SMA-positive villi | ||||||
| Model 1 | −0.09(−0.33; 0.15) | 0.45 | −0.06(−0.27; 0.15) | 0.58 | −0.14(−0.35; 0.08) | 0.20 |
| Model 2 | −0.13(−0.39; 0.13) | 0.33 | −0.07(−0.29; 0.15) | 0.52 | −0.15(−0.38; 0.07) | 0.18 |
| Model 3 | −0.08(−0.34; 0.18) | 0.55 | −0.03(−0.25; 0.20) | 0.82 | −0.10(−0.34; 0.14) | 0.40 |
| Standard deviation of villous barrier thickness of SMA-positive villi (variability in thickness) | ||||||
| Model 1 | −0.06(−0.30; 0.19) | 0.65 | −0.07(−0.28; 0.14) | 0.49 | −0.10(−0.32; 0.11) | 0.34 |
| Model 2 | −0.06(−0.32; 0.21) | 0.67 | −0.07(−0.29; 0.16) | 0.56 | −0.10(−0.33; 0.13) | 0.38 |
| Model 3 | −0.04(−0.32; 0.23) | 0.76 | −0.06(−0.29; 0.18) | 0.63 | −0.10(−0.34; 0.15) | 0.45 |
| Villous barrier thickness of SMA-negative villi | ||||||
| Model 1 | −0.16(−0.40; 0.08) | 0.18 | −0.16(−0.36; 0.04) | 0.12 | −0.17(−0.38; 0.04) | 0.11 |
| Model 2 | −0.25(−0.50; 0.00) | 0.052 | −0.19(−0.40; 0.03) | 0.08 | −0.19(−0.40; 0.02) | 0.08 |
| Model 3 | −0.19(−0.44; 0.06) | 0.14 | −0.12(−0.34; 0.09) | 0.26 | −0.11(−0.34; 0.12) | 0.35 |
| Standard deviation of villous barrier thickness of SMA-negative villi (variability in thickness) | ||||||
| Model 1 | −0.24(−0.48; −0.01) | 0.04 | −0.20(−0.40; 0.01) | 0.06 | −0.22(−0.43; −0.01) | 0.04 |
| Model 2 | −0.33(−0.58; −0.08) | 0.01 | −0.23(−0.44; −0.01) | 0.05 | −0.23(−0.45; −0.01) | 0.04 |
| Model 3 | −0.27(−0.53; −0.02) | 0.04 | −0.15(−0.37; 0.07) | 0.18 | −0.14(−0.37; 0.10) | 0.24 |
SMA = γ-smooth muscle actin.
Unstandardized regression coefficients (B) and their 95% Confidence Intervals (CI) from linear regression models, indicating standard deviation unit increase in placental morphology criteria per one standard deviation increase in mean maternal depressive symptoms during each pregnancy trimester.
Regression Model 1 is unadjusted. Model 2 is adjusted for maternal age and education level, pre-pregnancy body mass index, hypertensive and diabetic disorders in pregnancy and gestation length. Model 3 is adjusted for Model 2 covariates and maternal history of mental disorders before pregnancy.
Figure 1.
(A) Maternal clinically significant antenatal depressive symptoms and placental SMA-negative villi villous barrier thickness variability. The figure shows the unadjusted mean values and 95% Confidence Intervals of the standard deviation of placental SMA-negative villi villous barrier thickness (in standard deviation units) for mothers with trimester-weighted mean antenatal depressive symptoms below or above the clinical cutoff of ≥16 points. P-values refer to group difference significances from unadjusted linear regression models (model 1), models adjusted for maternal age, education, pre-pregnancy BMI, hypertensive and diabetic disorders in pregnancy and gestation length (model 2), and models adjusted also for maternal history of physician-diagnosed mental disorders before pregnancy (model 3). (B) Maternal accumulative depressive symptoms during pregnancy and SMA-negative villi villous barrier thickness variability. The figure shows the unadjusted mean values and 95% Confidence Intervals of the standard deviation of SMA-negative villi villous barrier thickness in standard deviation units, according to the number of pregnancy trimesters [0, 1, 2–3] maternal depressive symptom scores during pregnancy were above the clinical cutoff of ≥16, when calculated from the value at the first trimester and mean values across second and third trimesters. P-values refer to the significances of group differences and linear trends from unadjusted linear regression models (model 1), models adjusted for maternal age, education, pre-pregnancy BMI, hypertensive and diabetic disorders in pregnancy and gestation length (model 2), and models adjusted also for maternal history of physician-diagnosed mental disorders before pregnancy (model 3).
Placental Morphology and Child Psychiatric Problems
A smaller SD of the villous barrier thickness of SMA-negative villi predicted significantly higher toddler total and internalising psychiatric problems in unadjusted and adjusted regression models (Table 4). A smaller SD of SMA-negative villi villous barrier thickness also predicted higher toddler sleep, affective, anxiety, anxious/depressed, emotionally reactive, pervasive developmental and oppositional defiant problems in unadjusted and/or adjusted regression models (Supplementary Table S2).
Table 4.
Placental Morphology and Toddler Psychiatric Problems. Unstandardized regression coefficients (B) and their 95% Confidence Intervals (CI) indicating standard deviation unit increase in toddler psychiatric problems per standard deviation increase in placental morphology criteria from linear regression models where placental morphology criteria were used to predict toddler psychiatric problems.
| Toddler Psychiatric Problem Scale | Total Problems | Internalizing Problems | Externalizing Problems | |||
|---|---|---|---|---|---|---|
| Placental Morphology Structure | B(95% CI) | p | B(95% CI) | p | B(95% CI) | p |
| Volume of SMA-positive villi per placenta | ||||||
| Model 1 | 0.02(−0.23; 0.27) | 0.88 | −0.03(−0.27; 0.19) | 0.77 | −0.05(−0.31; 0.21) | 0.70 |
| Model 2 | −0.00(−0.27; 0.27) | 0.98 | −0.05(−0.29; 0.19) | 0.69 | −0.08(−0.36; 0.20) | 0.58 |
| Model 3 | −0.04(−0.20; 0.27) | 0.76 | −0.02(−0.23; 0.20) | 0.88 | −0.04(−0.29; 0.22) | 0.78 |
| Volume of capillaries per SMA-positive villi per placenta | ||||||
| Model 1 | 0.02(−0.23; 0.27) | 0.90 | −0.03(−0.25; 0.20) | 0.81 | −0.05(−0.31; 0.21) | 0.68 |
| Model 2 | −0.02(−0.29; 0.25) | 0.87 | −0.07(−0.30; 0.17) | 0.59 | −0.08(−0.36; 0.20) | 0.58 |
| Model 3 | −0.01(−0.25; 0.23) | 0.92 | −0.05(−0.27; 0.16) | 0.63 | −0.08(−0.33; 0.17) | 0.54 |
| Volume of SMA-negative villi per placenta | ||||||
| Model 1 | −0.05(−0.32; 0.23) | 0.72 | 0.04(−0.21; 0.29) | 0.75 | −0.11(−0.39; 0.18) | 0.46 |
| Model 2 | −0.12(−0.44; 0.19) | 0.44 | −0.02(−0.30; 0.26) | 0.88 | −0.20(−0.52; 0.13) | 0.22 |
| Model 3 | 0.05(−0.22; 0.32) | 0.71 | 0.13(−0.11; 0.37) | 0.28 | −0.03(−0.31; 0.26) | 0.84 |
| Volume of capillaries per SMA-negative villi per placenta | ||||||
| Model 1 | −0.09(−0.35; 0.18) | 0.51 | −0.00(−0.24; 0.24) | 0.99 | −0.12(−0.40; 0.15) | 0.36 |
| Model 2 | −0.20(−0.51; 0.11) | 0.21 | −0.09(−0.37; 0.19) | 0.51 | −0.25(−0.57; 0.07) | 0.13 |
| Model 3 | 0.00(−0.26; 0.25) | 0.98 | 0.08(−0.15; 0.31) | 0.49 | −0.06(−0.33; 0.21) | 0.67 |
| Ratio of the distribution of SMA-positive villi to SMA-negative villi | ||||||
| Model 1 | 0.02(−0.23; 0.27) | 0.88 | −0.07(−0.30; 0.16) | 0.54 | 0.00(−0.26; 0.26) | 0.99 |
| Model 2 | 0.03(−0.25; 0.31) | 0.82 | −0.06(−0.30; 0.19) | 0.64 | 0.01 (−0.27; 0.30) | 0.92 |
| Model 3 | 0.01(−0.23; 0.25) | 0.92 | −0.08(−0.29; 0.14) | 0.48 | −0.00(−0.26; 0.25) | 0.97 |
| Villous barrier thickness of SMA-positive villi | ||||||
| Model 1 | −0.08(−0.36; 0.21) | 0.60 | −0.12(−0.37; 0.14) | 0.36 | 0.02(−0.27; 0.31) | 0.89 |
| Model 2 | −0.11(−0.44; 0.21) | 0.48 | −0.19(−0.47; 0.09) | 0.18 | 0.01(−0.33; 0.35) | 0.95 |
| Model 3 | 0.02(−0.26; 0.29) | 0.91 | −0.04(−0.29; 0.21) | 0.75 | 0.10(−0.19; 0.39) | 0.49 |
| Standard deviation of villous barrier thickness of SMA positive villi (variability in thickness) | ||||||
| Model 1 | −0.02(−0.28; 0.23) | 0.86 | 0.02(−0.21; 0.25) | 0.87 | −0.05(−0.31; 0.21) | 0.70 |
| Model 2 | −0.05(−0.32; 0.22) | 0.71 | −0.01(−0.25; 0.23) | 0.93 | −0.07(−0.35; 0.21) | 0.62 |
| Model 3 | 0.06(−0.19; 0.31) | 0.63 | 0.09(−0.13; 0.31) | 0.40 | 0.02(−0.24; 0.27) | 0.91 |
| Villous barrier thickness of SMA-negative villi | ||||||
| Model 1 | −0.14(−0.42; 0.13) | 0.31 | −0.18(−0.42; 0.07) | 0.16 | −0.00(−0.29; 0.28) | 0.98 |
| Model 2 | −0.16(−0.45; 0.12) | 0.26 | −0.20(−0.45; 0.05) | 0.12 | −0.01(−0.31; 0.29) | 0.93 |
| Model 3 | −0.08(−0.34; 0.19) | 0.56 | −0.12(−0.36; 0.12) | 0.31 | 0.05(−0.23; 0.33) | 0.71 |
| Standard deviation of villous barrier thickness of SMA-negative villi (variability in thickness) | ||||||
| Model 1 | −0.34(−0.60; −0.07) | 0.01 | −0.32(−0.56; −0.08) | 0.01 | −0.20(−0.48; 0.09) | 0.17 |
| Model 2 | −0.35(−0.63; −0.07) | 0.01 | −0.34(−0.59; −0.10) | 0.01 | −0.21(−0.51; 0.09) | 0.16 |
| Model 3 | −0.29(−0.54; −0.03) | 0.03 | −0.28(−0.51; −0.05) | 0.02 | −0.15(−0.43; 0.13) | 0.28 |
SMA = γ-smooth muscle actin.
Model 1 is unadjusted. Model 2 is adjusted for toddler’s age and sex, gestation length, maternal age at childbirth, education, pre-pregnancy BMI and maternal diabetic and hypertensive disorders in pregnancy. Model 3 is adjusted for maternal depressive symptoms concurrently to assessing her child’s psychiatric problems.
Discussion
This explorative, hypothesis-generating study examined whether maternal depressive symptoms during pregnancy and subsequent toddler psychiatric problems were associated with morphology alterations of term placentas. We found that both maternal depressive symptoms across pregnancy and toddler total and internalizing psychiatric problems were associated with less variation in SMA-negative villi villous barrier thickness, suggesting an association with placental maturation. These associations were independent of several maternal and toddler sociodemographic and perinatal characteristics. The associations of maternal depressive symptoms with placental morphology were the most consistent for depressive symptoms during the first pregnancy trimester, and they were also independent of maternal history of physician-diagnosed mental disorders before pregnancy. The associations of placental morphology with toddler psychiatric problems were also independent of maternal depressive symptoms concurrently to child assessment.
The villous barrier is the physical barrier through which maternal-fetal exchange of gases, nutrients and waste occurs. Its thickness directly correlates with exchange efficiency, thus it is a highly relevant physiological measurement. As gestation progresses the exchange demands placed on this barrier increase and the villous barrier of the SMA-negative villi becomes more heterogeneous in thickness. Fetal capillaries, especially their sinusoidal vessels in the terminal segments of the villous tree23, dilate into anuclear areas of the syncytium leading to areas of the villous barrier that are extremely thin to maximize exchange efficiency24. However, to accommodate these areas of thinning, syncytiotrophoblastic organelles accumulate in thicker areas outwith the VSMs, called syncytial knots33. Thus, an irregularity in villous barrier thickness develops and its appearance indicates optimal placental maturation. According to previous findings the development of VSMs and syncytial knots decrease diffusion resistance by 26%, compared with that of a barrier with uniform thickness34 and there is an inverse relationship between the number of VSMs and fetal hypoxia26. Analysis of uncomplicated full-term placentas showed that although absolute villous barrier thickness measurements varied between different placentas, the uniformity index (a ratio between the thick and thin villous barrier areas) was relatively constant from one placenta to another34. This indicates that there is likely an optimal irregularity of the villous barrier to maximally and optimally serve the growing fetus. Our data showing that less heterogeneity in placental villous barrier thickness is associated with maternal depressive symptoms could point to a mechanism whereby maternal depression-related changes cause subtle alterations in placental maturation, especially in the dynamic villous trophoblast layers. This may compromise the efficiency and robustness of materno-fetal exchange (diffusion controlled transport in thinned membrane areas and actively supported transport in thicker areas), which then affects fetal neurodevelopment, leading to psychiatric problems in toddlers. Among villous trophoblast layers, the most important for this finding could be the syncytiotrophoblast, which is the epithelium at the materno-fetal border and can be considered a steady state structure in a vulnerable sandwich position between cytotrophoblast proliferation and syncytial shedding35.
While maternal diabetes or hypertension in pregnancy did not explain the associations of placental morphology with maternal or toddler psychopathology, placentas from diabetic or hypertensive pregnancies had smaller vessel volumes in SMA-positive villi. The vessels within the SMA-positive villi are the fundamental conduits of blood supply from fetus to the SMA-negative peripheral part of the villous tree, which are the primary sites of materno-fetal exchange15. Any reduction in vessel volume within the more centrally located and larger SMA-positive villi would result in suboptimal blood supply to peripheral villi and hence compromised fetal nutrient and oxygen supply and waste removal15. Although the villous barrier thickness and hence the diffusing capacity of the barrier is unaltered in pregnancies with diabetic or hypertensive disorders36,37, if the carrying capacity of stem villi capillaries is compromised due to their scarceness, a deficient exchange may ensue due to blood supply from the fetus being limited by the suboptimal stem villi vessel volume.
The limitations of our study include the small sample size and use of only maternal reports of maternal depressive symptoms and toddler psychiatric problems. The sample size was 86 for the analyses on maternal depressive symptoms during pregnancy and placental morphology, and only 60 for the analyses on placental morphology and toddler psychiatric problems. Hence, there is a possibility for chance findings, and our findings need to be replicated in larger study samples.
Furthermore, attrition analyses suggested that some key characteristics of the study sample may be associated with study attrition, which may limit the generalizability of our findings. Since the women participating in the current study scored lower on antenatal depressive symptoms than the other women in the PREDO, and since birth register data suggests that none of the participating mothers had been diagnosed with mental disorders during pregnancy, the generalizability of the findings to more severe levels of maternal depression is questionable. However, we did detect associations with the same indicator of placental morphology for both continuously assessed antenatal depressive symptoms and for antenatal depressive symptoms exceeding a cutoff score for clinically relevant symptoms. Also the toddlers in the current study sample were younger than the other PREDO children at the time of completion of the CBCL, reflecting that sampling of placentas were added late to the PREDO study protocol, and some psychiatric problems assessed in the CBCL may not yet have developed to their full range of variation by 2–3 years of toddler age when psychiatric problems were assessed. Previous studies show that the level of internalizing problems increases between 1.5 and 5 years of age3,38. However, the age range of the current study sample is within the intended age range for the use of CBCL questionnaire (1.5–5 years), the scale has been validated for this whole age range38–40, and toddler age was not associated with toddler internalizing, externalizing, or total psychiatric problems in our study sample (r = 0.00, = −0.12, = −0.07, respectively, P-values ≥ 0.37).Yet, our findings need to be replicated also among older children.
Also, even though our explorative study indicated associations of the biological markers of placental morphology with maternal antenatal depressive symptoms and toddler psychiatric problems, we do not know which physiological factors underlie these associations. As stated, maternal depression during pregnancy is associated with physiological changes in the functioning of the neurobiological stress system, oxidative stress and maternal nutrition6,9–14. As such changes are also associated with placental morphology41–45 and/or with psychiatric problems in the offspring46,47, these factors may have contributed to the associations found. Furthermore, we cannot rule out or confirm genetic explanations for our findings, with some genetic vulnerabilities contributing both to maternal depressive symptoms, aberrations in placental development and increased psychiatric problems in toddlers. Further studies are needed to elucidate the contributory effects of genetic and environmental factors to our findings.
On the other hand, we circumvented the weakness of conventional histologic villous classifications (differentiation in stem, intermediate, and terminal villi) in the recognition of peripheral villous arborizations48–50 by detecting SMA as a criterion of more central (stem villus like, SMA-positive) or peripheral (SMA-negative) villi. However, care should be taken since many SMA-positive villi did not have a larger calibre than many SMA-negative villi, making them histologically indiscernible. The term stem villus is thus not a synonym of term SMA-positive villus. By 2D section histology, recognizing differences in arborization topology is impossible; nodes cannot be recognized. The recently introduced 3D microscopy of peripheral villous trees48–50 can potentially provide this information in further studies. Yet, our study has a novel focus, and its strengths include the longitudinal design, the use of validated questionnaires on maternal and toddler psychopathology39,51–54, and the repeated, fortnightly assessments of maternal antenatal depressive symptoms.
In summary, our novel, explorative, hypothesis-generating study showed that maternal depressive symptoms during pregnancy were associated with lower variability in the thickness of the villous membrane of the peripheral villi in term placentas. This lower variability, indicating a reduction in the variability in the villous barrier thickness of SMA-negative villi, was also associated with subsequent toddler psychiatric problems. We propose that this lower heterogeneity in villous barrier thickness may compromise materno-fetal exchange, suggesting a possible role for altered placental structure in the fetal programming of mental disorders16.
Methods
Participants were from the prospective PREDO cohort55, which comprises 4777 pregnant women who gave birth to singleton live-born children in 2006–2010. These women were recruited to the study in early pregnancy at first ultrasound measurements at the antenatal clinics of ten study hospitals in Finland. All participating women signed informed consents. The PREDO study protocol was approved by Helsinki and Uusimaa Hospital District ethical committees55. The PREDO study has been conducted in accordance with the declaration of Helsinki.
Placental biopsy collection was introduced to the study protocol in 2009. Samples were collected at two maternity clinics in Helsinki, Finland. Placental morphology data were available from 96 mother-child dyads with infants born at term in 2009–2010. Eight had neither maternal nor child psychiatric data and were excluded from this study.
Our final study sample thus comprises 88 participants. Of them, 86 had data on maternal depressive symptoms during pregnancy and 60 on toddler psychiatric problems. Compared to other PREDO mothers, the mothers participating in this study had less depressive symptoms during the third pregnancy trimester (MD = −0.24 SD units: 95% CI = −0.45; −0.02: P = 0.03). Compared to non-participating PREDO children, the participating toddlers were younger at childhood follow-up (MD = −1.37 years: 95% CI = −1.30; −1.44: P < 0.001). There were no differences between participating and non-participating mothers or toddlers in other assessed characteristics (maternal age, education level, BMI, diabetes and hypertension in pregnancy, history of physician-diagnosed mental disorders before pregnancy, depressive symptoms at other time points, gestation length or toddler sex or psychiatric problems; P-values ≥ 0.08).
Placental Morphology
Two placenta samples with an edge length of ~1 cm and full depth of the placenta were taken from each placenta after birth without a preference for specific placental sites from macroscopically unsuspicious areas, then routinely fixed in formaldehyde. After fixation lasting at least 24 hours, the samples were routinely dehydrated in an alcohol step gradient and embedded in paraffin. For morphometric analysis, these paraffin blocks were transferred to LMU Munich, Germany.
Paraffin samples were sectioned as 4–6 micrometres thick sections and placed on super frost object slides (SuperFrost plus, Thermofisher, Munich, Germany). All slides were deparaffinized through xylene and a stepwise ethanol gradient. Immunohistochemical double labelling was performed, comprising an antibody to CD34 (labelling of fetal endothelium) and to SMA (labelling of perivascular myofibroblast-like cells)19. Peroxidase with DAB as substrate (anti-CD34, brown reaction product) and β-galactosidase with X-Gal as substrate (anti-SMA, indigo-blue reaction product) were used for differential visualization in brightfield microscopy. Nuclei were counterstained with haematoxylin. The sequence of steps was empirically optimized such that the anti-CD34 detection was always carried out first and completely. Both immunohistochemical sequences used streptavidin-enzyme conjugates. Cross reactivity due to streptavidin use in both sequences was excluded by controls included in the second immunohistochemical sequence (anti-SMA). SMA-detection was used for classification of villi based on recommendations in the literature49. After peroxidase detection, anti-SMA detection was performed. Supplementary Tables S3–S4 give full details of the immunohistochemical reaction steps. Finally, the nuclei were counterstained with haematoxylin and the slides mounted with coverslips (Kaiser’s glycerol jelly, Merck, Darmstadt, Germany). All object slides were stored at 4 °C prior to microscopic evaluations.
We examined nine placental morphology criteria: the volumes of SMA-positive and SMA-negative (peripheral) villi per placenta, the volume of capillaries per SMA-positive and SMA-negative villi per placenta, the ratio of volumes of SMA-positive to SMA-negative villi, the villous barrier thicknesses (distance from the outer trophoblast to the endothelium of the fetal vessels inside the villous tree) and the SDs of villous barrier thicknesses stratified by SMA-positive and SMA-negative villi. The volume criteria are expressed in millilitres and were calculated by dividing the assessed criterion by placental weight (grams). Volume estimates were performed according to the Cavalieri principle on single thin (4–6 micrometres) histological sections using a computerized stereology workstation, which comprised a modified light microscope (Axioskop;Zeiss) with motorized specimen stage for automatic sampling [MBF Bioscience, Williston, VT, United States of America (USA)] and stage controller [Type MAC 6000, Ludl Electronics, Hawthorne, New York, USA], focus encoder (Type MT 1271, Heidenhain, Germany), CCD colour video camera (1600H 3 1200 V pixels, MBF Bioscience, Williston, VT, USA) and stereology software (Stereo Investigator version 10;MBF Bioscience). This approach delivers volume fractions as raw data, which were stratified to the villous tree substructures (villous stroma, vessel lumen, endothelium, and syncytiotrophoblast), intervillous space and fibrinoid. Then, absolute volumes were calculated by multiplying volume fractions with total placental volume [placental volume is defined by placental weight divided by the density of placental tissue (1.03 g/millilitre)]. To determine villous barrier thickness, we used the Nearest Neighbor option in the stereology software (Stereo Investigator version 10, MBF Bioscience). The villous barrier thickness is expressed in micrometres. Figures 2 and 3 show illustrations of the placental morphology criteria measurements.
Figure 2.
Immunohistochemical double labeling of perivascular sheath and fetal villous endothelium. (A) Shows an example of a γ-smooth-muscle-actin(SMA)-positive villus (indigo-blue in perivascular position; white arrows). The endothelium of fetal vessels is labelled (CD34: brown; black arrows), the villous stroma marked by asterisks and the trophoblast (bluish nuclei; shaded arrows) visible. (B) Exemplifies a SMA-negative villus (no SMA reactivity in perivascular position). The endothelium of fetal vessels is labelled (CD34: brown; black arrows), villous stroma marked by asterisks and trophoblast (bluish nuclei; shaded arrows) visible. Red arrow heads label vasculo-syncytial-membrane spots. (A) Scale bar is 25 µm and valid for (B).
Figure 3.
An illustration of the villous membrane thickness measurement principle. (A,B) Show examples of SMA-negative villi with endothelium labelled by CD34-reactivity (brown reaction product). (C,D) Show examples of SMA-positive villi (indigo-blue reaction product in perivascular position) with endothelium labelled by CD34-reactivity (brown reaction product). (A–D) We measured membrane thickness with the Nearest Neighbor analysis and the shortest distance (red line) from the trophoblast to the fetal endothelium by increasing a circle (red) from the trophoblast surface until first intersection with the endothelium. Exemplary measurement positions are marked with black arrows. Scale bar is 25 µm.
Maternal Depressive Symptoms
The women assessed their depressive symptoms fortnightly up to 14 times during pregnancy from pregnancy weeks + days 12 + 0/13 + 6 until delivery or 38 + 0/39 + 6 pregnancy weeks + days with the Center for Epidemiological Studies Depression Scale (CES-D)53, which comprises 20 questions on the frequency of depressive symptoms during the preceding week. CES-D sum-scores range from 0 to 60. Higher scores indicate more depressive symptoms. The CES-D cutoff score of ≥16 indicates risk for clinical depression53. The CES-D has excellent psychometric properties52–54,56,57, and it has been validated also among pregnant women57,58. In our sample, the CES-D had high internal consistency (Cronbach’s α varying from =0.85 to =0.93).
Toddler Psychiatric Problems
When the toddlers were 1.9–3.1 years old, the mothers completed the Child Behavior Checklist for Ages 1½-5 (CBCL1½-5) which is a well-validated and very widely used scale on child psychiatric problems39,51. It comprises 99 items, rated from 0 to 2. Higher t-scores reflect more problems on the CBCL1½-5 main scales of internalizing, externalizing and total problems51, on the seven CBCL1½-5 Syndrome Scales and on the five CBCL1½-5 Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition-oriented scales51.
Covariates
Information on maternal pre-pregnancy height and weight, of which pre-pregnancy BMI (kilograms/metres2) was calculated, maternal diabetes and/or hypertension in pregnancy (gestational and type 1 diabetes, preeclampsia, chronic and gestational hypertension; any vs. none), maternal age at delivery, toddler sex, birth date and gestation length was extracted from the Finnish Medical Birth Register and patient case records. Maternal history of physician-diagnosed mental disorders (depression, panic disorder, schizophrenia, other psychosis, other mental disorder) before pregnancy (no/yes; n = 80; 6 mothers with missing data were dummy-coded to an own category for regression analysis) and education level (primary/secondary vs. tertiary) was self-reported during pregnancy. Mothers assessed their depressive symptoms at toddler follow-up with the Beck Depression Inventory-II59. Toddler age at follow-up was calculated by subtracting birth date from CBCL1½-5 completion date.
Statistical Analyses
To account for skewness and improve linear model fitting, the maternal depressive symptom scores, volumes of SMA-positive villi per placenta- and capillaries per SMA-positive villi per placenta, the villous barrier thickness of SMA-negative villi and the SD of villous barrier thickness of SMA-positive villi were square-root transformed and maternal pre-pregnancy body mass index and the placental ratio of the distribution of SMA-positive villi to SMA-negative villi, the villous barrier thickness of SMA-positive villi and the SD of villous barrier thickness of SMA-negative villi were rank-normalized according to Blom’s formula. All continuous variables are expressed in SD units (mean = 0: SD = 1).
We examined the associations between maternal depressive symptoms and placental morphology with linear regression models. In these models, trimester-weighted mean maternal depressive symptoms across pregnancy (mean value calculated from the value in first trimester and mean values across second and third trimesters) and mean scores during each pregnancy trimester and depressive symptoms above the clinical cutoff (≥16 across pregnancy or 0, 1, or 2/3 times across pregnancy trimesters) predicted placental morphology criteria. We present unstandardized regression coefficients (B) and 95% CI in SD units from unadjusted regression models, regression models adjusted for maternal age, education, BMI, diabetes and hypertension in pregnancy and gestation length, and models adjusted further for maternal history of physician-diagnosed mental disorders before pregnancy.
We tested whether placental morphology criteria predicted toddler internalizing, externalizing and total problems using linear regression. We present B and 95% CI from unadjusted models, models adjusted for toddler age and sex, gestation length, maternal age, education, BMI, diabetes and hypertension in pregnancy, and models adjusted for maternal depressive symptoms concurrent to rating toddler’s psychiatric problems. To specify any possible effects of toddler psychiatric problems, we examined the syndrome- and DSM-IV-oriented CBCL as secondary outcomes for those placental criterion that showed significant associations with the main scales. The associations with these CBCL subscales were examined with tobit regressions, since these scales are left-censored51.
Data Availability
The datasets generated during and/or analysed during the current study are not publicly available due to prohibitions by national laws since the data include patient report data. However, the datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request in completely anonymized form. Due to the sensitive nature of the patient report data, data requests may require further approval by the PREDO Study Board, that also enables collaboration in PREDO data analysis through specific study proposals55.
Electronic supplementary material
Acknowledgements
The work was supported by the Academy of Finland, the Signe and Ane Gyllenberg Foundation, the Emil Aaltonen Foundation, EVO (a special state subsidy for health science research), the Finnish Medical Foundation, the Jane and Aatos Erkko Foundation, the Juho Vainio Foundation, MRC Career Development Award to MJC, DFG Fr1245/9–1 to HGF, the Novo Nordisk Foundation, the Päivikki and Sakari Sohlberg Foundation, the Sigrid Juselius Foundation, University of Helsinki Research Funds and the British Heart Foundation. The funders had no role in the conduct or design of the study. The authors acknowledge the skilful technical assistance and diligent work of the whole team of technicians of the Department of Anatomy II (LMU) Germany, namely B. Aschauer, A. Baltruschat and B. Mosler. We also acknowledge the support of the British Heart Foundation.
Author Contributions
M.L.P. and M.J.C. contributed equally to the study and are the joint first authors. M.L.P., M.J.C., A.K.P., K.R., R.M.R., E.Hä., H.L., H.G.F., E.H.A., H.L., P.M.V., C.S. and E.K. designed the study; P.M.V., E.K., A.K.P., K.R., E.Hä., H.L. and S.M., collected the data; M.L.P., M.J.C., R.R., K.R., H.L., E.K., H.G.F., C.S. and E.H.A. analysed the data; all authors took part in interpreting the data, writing the manuscript and/or revising it for intellectual content.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Marius Lahti-Pulkkinen and Melissa Jane Cudmore contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-19133-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. The Lancet Psychiatry. 2016;3:973–982. doi: 10.1016/S2215-0366(16)30284-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gentile S. Untreated depression during pregnancy: Short- and long-term effects in offspring. A systematic review. Neuroscience. 2017;342:154–166. doi: 10.1016/j.neuroscience.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Lahti M, et al. Maternal Depressive Symptoms During and After Pregnancy and Psychiatric Problems in Children. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:30–39.e7. doi: 10.1016/j.jaac.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Molyneaux E, Poston L, Ashurst-williams S, Howard LM. Obesity and mental disorders during pregnancy and postpartum: a systematic review and meta-analysis. Obstet. Gynecol. 2014;123:857–867. doi: 10.1097/AOG.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017;219:86–92. doi: 10.1016/j.jad.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Beijers R, Buitelaar JK, de Weerth C. Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: beyond the HPA axis. European child & adolescent psychiatry. 2014;23:943–956. doi: 10.1007/s00787-014-0566-3. [DOI] [PubMed] [Google Scholar]
- 7.Korhonen M, Luoma I, Salmelin R, Tamminen T. A longitudinal study of maternal prenatal, postnatal and concurrent depressive symptoms and adolescent well-being. J. Affect. Disord. 2012;136:680–692. doi: 10.1016/j.jad.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell KJ, Glover V, Barker ED, O’Connor TG. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev. Psychopathol. 2014;26:393–403. doi: 10.1017/S0954579414000029. [DOI] [PubMed] [Google Scholar]
- 9.Van den Bergh BRH, et al. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev., 2017 doi: 10.1016/j.neubiorev.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Brunst KJ, et al. Maternal lifetime stress and prenatal psychological functioning are associated with decreased placental mitochondrial DNA copy number in the PRISM study. Am. J. Epidemiol., 2017 doi: 10.1093/aje/kwx183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Lee L, et al. Maternal choline status during pregnancy, but not that of betaine, is related to antenatal mental well-being: The growing up in Singapore toward healthy outcomes cohort. Depress. Anxiety. 2017;34:877–887. doi: 10.1002/da.22656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serati M, Redaelli M, Buoli M, Altamura AC. Perinatal Major Depression Biomarkers: A systematic review. J. Affect. Disord. 2016;193:391–404. doi: 10.1016/j.jad.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Räikkönen K, et al. Maternal depressive symptoms during pregnancy, placental expression of genes regulating glucocorticoid and serotonin function and infant regulatory behaviors. Psychol. Med. 2015;45:3217–26. doi: 10.1017/S003329171500121X. [DOI] [PubMed] [Google Scholar]
- 14.Chong MFF, et al. Relationships of maternal folate and vitamin B12 status during pregnancy with perinatal depression: The GUSTO study. J. Psychiatr. Res. 2014;55:110–116. doi: 10.1016/j.jpsychires.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Benirschke, K., Burton, G. J. & Baergen, R. N. Pathology of the human placenta, sixth edition. Pathology of the Human Placenta, 6th Edition, 10.1007/978-3-642-23941-0 (Springer Berlin Heidelberg, 2012).
- 16.Barker DJP, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: A review. Placenta. 2013;34:841–845. doi: 10.1016/j.placenta.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 17.Longtine MS, Nelson DM. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Semin. Reprod. Med. 2011;29:187–96. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang, Y. & Zhao, S. Vascular Biology of the Placenta. Vascular Biology of the Placenta (Morgan & Claypool Life Sciences, 2010). [PubMed]
- 19.Demir R, Kosanke G, Kohnen G, Kertschanska S, Kaufmann P. Classification of human placental stem villi: Review of structural and functional aspects. Microsc. Res. Tech. 1997;38:29–41. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<29::AID-JEMT5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 20.Castellucci M, Scheper M, Scheffen I, Celona A, Kaufmann P. The development of the human placental villous tree. Anat Embryol. 1990;181:117–128. doi: 10.1007/BF00198951. [DOI] [PubMed] [Google Scholar]
- 21.Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2015;370:20140066. doi: 10.1098/rstb.2014.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treacy A, Higgins M, Kearney JM, McAuliffe F, Mooney EE. Delayed villous maturation of the placenta: quantitative assessment in different cohorts. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2013;16:63–66. doi: 10.2350/12-06-1218-OA.1. [DOI] [PubMed] [Google Scholar]
- 23.Kaufmann P, Bruns U, Leiser R, Luckhardt M, Winterhager E. The fetal vascularisation of term human placental villi. II. Intermediate and terminal villi. Anat Embryol. 1985;175:203–214. doi: 10.1007/BF00316301. [DOI] [PubMed] [Google Scholar]
- 24.Burton GJ, Tham SW. Formation of vasculo-syncytial membranes in the human placenta. J. Dev. Physiol. 1992;18:43–7. [PubMed] [Google Scholar]
- 25.Mayhew, T. M., Joy, C. F. & Haas, J. D. Structure-function correlation in the human placenta: the morphometric diffusing capacity for oxygen at full term. J. Anat., 691–708 (1984). [PMC free article] [PubMed]
- 26.Sankar KD, Bhanu PS, Kiran S, Ramakrishna BA, Shanthi V. Vasculosyncytial membrane in relation to syncytial knots complicates the placenta in preeclampsia: a histomorphometrical study. Anat. Cell Biol. 2012;45:86–91. doi: 10.5115/acb.2012.45.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meng Q, et al. Ultrastructure of Placenta of Gravidas with Gestational Diabetes Mellitus. Obstet. Gynecol. Int. 2015;2015:283124. doi: 10.1155/2015/283124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helbig, A., Kaasen, A., Malt, U. F. & Haugen, G. Does Antenatal Maternal Psychological Distress Affect Placental Circulation in the Third Trimester? PLoS One8 (2013). [DOI] [PMC free article] [PubMed]
- 29.Tegethoff M, Greene N, Olsen J, Meyer AH, Meinlschmidt G. Maternal psychosocial stress during pregnancy and placenta weight: Evidence from a national cohort study. PLoS One. 2010;5:1–7. doi: 10.1371/journal.pone.0014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalife N, et al. Placental size is associated with mental health in children and adolescents. PLoS One. 2012;7:e40534. doi: 10.1371/journal.pone.0040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahti M, et al. Prenatal origins of hospitalization for personality disorders: the Helsinki birth cohort study. Psychiatry Res. 2010;179:226–30. doi: 10.1016/j.psychres.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Lahti J, et al. Early-life origins of schizotypal traits in adulthood. Br. J. Psychiatry. 2009;195:132–137. doi: 10.1192/bjp.bp.108.054387. [DOI] [PubMed] [Google Scholar]
- 33.Burton GJ, Jones CJP. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan. J. Obstet. Gynecol. 2009;48:28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- 34.Jackson MR, Joy CF, Mayhew TM, Haas JD. Stereological studies on the true thickness of the villous membrane in human term placentae: a study of placentae from high-altitude pregnancies. Placenta. 1985;6:249–58. doi: 10.1016/S0143-4004(85)80054-0. [DOI] [PubMed] [Google Scholar]
- 35.Calvert SJ, et al. Studies of the dynamics of nuclear clustering in human syncytiotrophoblast. Reproduction. 2016;151:657–671. doi: 10.1530/REP-15-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayhew TM, Sørensen FB, Klebe JG, Jackson MR. Oxygen diffusive conductance in placentae from control and diabetic women. Diabetologia. 1993;36:955–60. doi: 10.1007/BF02374479. [DOI] [PubMed] [Google Scholar]
- 37.Mayhew TM, Manwani R, Ohadike C, Wijesekara J, Baker PN. The placenta in pre-eclampsia and intrauterine growth restriction: studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta. 2007;28:233–8. doi: 10.1016/j.placenta.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Achenbach, T. M. & Rescorla, L. A. Manual for the ASEBA Preschool Forms & Profiles. University of Vermont, Research Center for … (University of Vermont, Research Center for Children, Youth, & Families, 2000).
- 39.Rescorla LA, et al. International comparisons of behavioral and emotional problems in preschool children: parents’ reports from 24 societies. J. Clin. Child Adolesc. Psychol. 2011;40:456–67. doi: 10.1080/15374416.2011.563472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rescorla LA. Assessment of young children using the Achenbach System of Empirically Based Assessment (ASEBA) Ment. Retard. Dev. Disabil. Res. Rev. 2005;11:226–237. doi: 10.1002/mrdd.20071. [DOI] [PubMed] [Google Scholar]
- 41.Cuffe JSM, et al. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J. Physiol. 2014;592:3127–3141. doi: 10.1113/jphysiol.2014.272856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrone S, et al. Placental histological examination and the relationship with oxidative stress in preterm infants. Placenta. 2016;46:72–78. doi: 10.1016/j.placenta.2016.08.084. [DOI] [PubMed] [Google Scholar]
- 43.Cuffe JSM, O’Sullivan L, Simmons DG, Anderson ST, Moritz KM. Maternal corticosterone exposure in the mouse has sex-specific effects on placental growth and mRNA expression. Endocrinology. 2012;153:5500–5511. doi: 10.1210/en.2012-1479. [DOI] [PubMed] [Google Scholar]
- 44.Roberts CT, et al. Maternal food restriction reduces the exchange surface area and increases the barrier thickness of the placenta in the guinea-pig. Placenta. 2001;22:177–185. doi: 10.1053/plac.2000.0602. [DOI] [PubMed] [Google Scholar]
- 45.Schlabritz-Loutsevitch N, et al. Moderate Maternal Nutrient Restriction, but not Glucocorticoid Administration, Leads to Placental Morphological Changes in the Baboon (Papio sp.) Placenta. 2007;28:783–793. doi: 10.1016/j.placenta.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buss C, et al. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. USA. 2012;109:E1312–9. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown AS, et al. Elevated maternal C-reactive protein and autism in a national birth cohort. Mol. Psychiatry. 2014;19:259–264. doi: 10.1038/mp.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haeussner E, Buehlmeyer A, Schmitz C, von Koch FE, Frank H-G. Novel 3D microscopic analysis of human placental villous trees reveals unexpected significance of branching angles. Sci. Rep. 2014;4:6192. doi: 10.1038/srep06192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haeussner E, et al. Does 2D-Histologic identification of villous types of human placentas at birth enable sensitive and reliable interpretation of 3D structure? Placenta. 2015;36:1425–1432. doi: 10.1016/j.placenta.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Haeussner E, Schmitz C, Frank H-G, Edler von Koch F. Novel 3D light microscopic analysis of IUGR placentas points to a morphological correlate of compensated ischemic placental disease in humans. Sci. Rep. 2016;6:24004. doi: 10.1038/srep24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Achenbach TM, R. LA. Manual for the ASEBA Preschool Forms & Profiles (2000).
- 52.Cents RAM, et al. Trajectories of maternal depressive symptoms predict child problem behaviour: the Generation R study. Psychol. Med. 2013;43:13–25. doi: 10.1017/S0033291712000657. [DOI] [PubMed] [Google Scholar]
- 53.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl. Psychol. Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 54.Vilagut G, Forero CG, Barbaglia G, Alonso J. Screening for Depression in the General Population with the Center for Epidemiologic Studies Depression (CES-D): A Systematic Review with Meta-Analysis. PLoS One. 2016;11:e0155431. doi: 10.1371/journal.pone.0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girchenko, P. et al. Cohort Profile: Prediction and prevention of preeclampsia and intrauterine growth restriction (PREDO) study. Int. J. Epidemiol. dyw154, 10.1093/ije/dyw154 (2016). [DOI] [PubMed]
- 56.Maloni JA, Park S, Anthony MK, Musil CM. Measurement of antepartum depressive symptoms during high-risk pregnancy. Res. Nurs. Health. 2005;28:16–26. doi: 10.1002/nur.20051. [DOI] [PubMed] [Google Scholar]
- 57.Tsai AC. Reliability and validity of depression assessment among persons with HIV in sub-Saharan Africa: systematic review and meta-analysis. J. Acquir. Immune Defic. Syndr. 2014;66:503–11. doi: 10.1097/QAI.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Natamba BK, et al. Reliability and validity of the center for epidemiologic studies-depression scale in screening for depression among HIV-infected and -uninfected pregnant women attending antenatal services in northern Uganda: a cross-sectional study. BMC Psychiatry. 2014;14:303. doi: 10.1186/s12888-014-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beck, A. T., Steer, R. A. & Brown, G. Manual for the Beck Depression Inventory-II. (Psychological Corporation, 1996).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are not publicly available due to prohibitions by national laws since the data include patient report data. However, the datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request in completely anonymized form. Due to the sensitive nature of the patient report data, data requests may require further approval by the PREDO Study Board, that also enables collaboration in PREDO data analysis through specific study proposals55.