Abstract
Distant metastasis (DM) from head and neck cancers (HNC) portends a poor patient prognosis. Despite its important biological role, little is known about the cells which seed these DM. Circulating tumour cells (CTCs) represent a transient cancer cell population, which circulate in HNC patients’ peripheral blood and seed at distant sites. Capture and analysis of CTCs offers insights into tumour metastasis and can facilitate treatment strategies. Whilst the data on singular CTCs have shown clinical significance, the role of CTC clusters in metastasis remains limited. In this pilot study, we assessed 60 treatment naïve HNC patients for CTCs with disease ranging from early to advanced stages, for CTC clusters utilizing spiral CTC enrichment technology. Single CTCs were isolated in 18/60–30% (Ranging from Stage I-IV), CTC clusters in 15/60–25% (exclusively Stage IV) with 3/15–20% of CTC clusters also containing leukocytes. The presence of CTC clusters associated with the development of distant metastatic disease(P = 0.0313). This study demonstrates that CTC clusters are found in locally advanced patients, and this may be an important prognostic marker. In vivo and in vitro studies are warranted to determine the role of these CTC clusters, in particular, whether leukocyte involvement in CTC clusters has clinical relevance.
Introduction
Head and neck cancers (HNC) accounts for the fifth most common non-skin cancer globally1. Despite the fact that primary treatment is usually both intensive and highly morbid, up to 50% of HNC patients still fail locoregionally or systemically. The more advanced the locoregional disease, the greater the risk of presenting with established metastatic disease, or harbouring micrometastatic disease, resulting in systemic failure at a later point. Circulating tumour cells (CTCs), which are shed from primary or secondary tumours and circulate in patients’ blood represent an important window into the mechanisms and characteristics of tumour metastasis2–4. They may also help direct locoregional and systemic treatment, by stratifying patients’ risk of systemic failure, allowing better selection of treatment individually.
CTCs were first described by Thomas Ashworth in 1869, as ‘cells identical with those of the cancer itself’, and the field has rapidly advanced in the last decade5–8. CTCs can be measured non-invasively from the blood and have direct clinical application9. In 2004, the FDA approved the first CTC enumeration platform, CellSearch (Janssen Diagnostics)10,11. It was demonstrated on this platform that single CTCs, in a number of tumour types, had clinical utility10. The enumeration of CTCs and cut off values of 5 or more CTCs in 7.5 ml of blood for metastatic breast cancer and prostate cancer has been associated with a poor prognosis and predictive of shorter progression free survival (PFS) and overall survival (OS)2,12. However, due to its inherent pre-selection of epithelial tumour cells expressing EpCAM, this system has shown poor sensitivity in detecting CTCs isolated from HNC patients bloods13,14.
Of recent, there has been a shift, from marker-based CTC assays to marker-independent assays, to capture a greater population of CTCs from circulation, including CTC clusters2,15–17. In a number of studies, CTC clusters or circulating tumour microemboli (CTM), composed of platelets, stromal and hematopoietic cells, have been reported13,16,18. Critically, these tumour cell aggregates, are protected from the shear stressors in the blood, and are better suited to survive the journey through the circulation by cooperation of heterogeneous cell types within the cluster, which can include immune evading cells2,17,19. The prevalence and the number of CTC clusters can be underestimated due to their short detection window and lack of appropriate detection methods20. Studies have also documented that CTC clusters have a shorter circulation half-life, with faster entrapment within distant organs where metastatic growth may initiate10,19,21,22. CTC clusters may provide clues to their evolution during the course of cancer treatment and the mechanisms of cluster mediated treatment resistance23. CTC clusters have been associated with decreased metastasis-free survival and a greater metastatic capacity than single CTCs16,17,21. Notably, it has been shown that CTC clusters, held by plakoglobin-dependent adhesions, have arisen from oligoclonal expansion of tumour cell groupings, rather than aggregation or proliferation of single CTCs17. Recent studies suggest that CTC clusters have the ability to traverse narrow capillaries in a ‘single-file’ and retain the ability to re-form the intact cluster upon exiting, highlighting the metastatic seeding capacity of these large cellular aggregates that were previously thought to extravasate upon reaching narrow capillaries16,24.
To date, the role of CTC clusters, including CTM, has not been well established. Despite their biological importance, in comparison to single CTCs, the data on CTC clusters remains unclear. In this pilot study, we investigated whether CTC clusters and CTM were found in the circulation of HNC patients. This study evaluated 60 treatment-naive HNC patients, with disease ranging from early to advanced stages, for CTC clusters using spiral microfluidic CTC enrichment technology. This technology enriches for CTCs by a function of cell size and deformability and provides a robust methodology for CTC enrichment15,25,26.
Results
Patients’ HNC disease staging ranged from early to advanced stages of HNC (Stage I-IV). CTCs and CTC clusters were successfully isolated using the spiral microfluidic chip. All patients had no evidence of distant metastatic disease upon presentation, however, at later time points (3–6 months follow up rescan), 7 stage IV patients developed lung and/or liver lesions (Fig. 1). CTC clusters were present in the blood of 6/7 patients that progressed to have distant disease. The patient demographics and clinicopathological features are presented in Table 1 and Table 2 respectively.
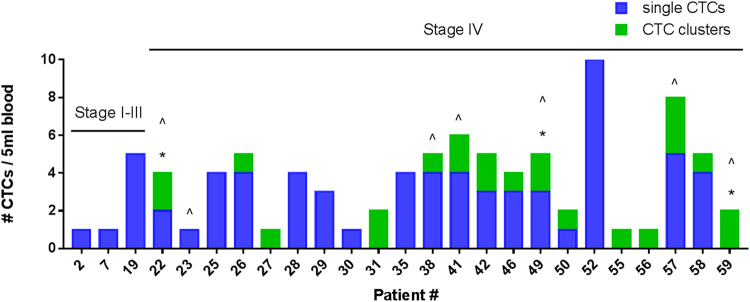
Figure 1.
Stacked bar graph showing single CTCs (blue), CTC clusters (green) for each of the 25 head and neck cancer patients positive for either/both cell types from 5 ml blood draw. CTC positive: 3/22 Stage I-III, 22/39 Stage IV patients). Asterix (*) represent the CTC clusters with white blood cell involvement. Caret (^) refers to patients that developed lung or liver lesions within 3–6 month period.
Table 1.
Patient demographics (n = 60).
| Variables | N |
|---|---|
| Total | 60 (100%) |
| Gender | |
| Male | 53 (88.3%) |
| Female | 7 (11.7%) |
| Age (years) | |
| ≤60 | 25 |
| >60 | 35 |
| Anatomic site of primary | |
| Oral Cavity | 24 |
| Oropharyngeal | 27 |
| Larynx | 5 |
| Hypophaynx | 3 |
| Salivary Glands | 1 |
| Tumour Staging | |
| I | 6 |
| II | 6 |
| III | 9 |
| IV | 39 |
| Distant metastases | |
| M0 | 60 |
| M1 | 0 |
| HPV status | |
| HPV-positive | 26 |
| HPV-negative | 29 |
| HPV status unknown | 5 |
| CTC status | |
| CTC-positive (single cells) | 20/60 (33.3%) (Range from 1–10CTCs/5 ml blood) |
| CTC-positive (clusters) | 15/60 (25%) (Range from 1–3/5 ml blood) |
| CTC clusters including WBCs | 3/15 (20%) |
*WBCs: White blood cells.
Table 2.
Clinicopathological findings (n = 60).
| Pt # | Gender | Age | HPV status | Staging | Site | Single CTCs | CTC clusters | # cells per cluster | Follow up FDG Pet Scan |
|---|---|---|---|---|---|---|---|---|---|
| 1 | f | 58 | negative | I | Oral Cavity | 0 | 0 | 0 | |
| 2 | m | 57 | negative | I | Oral Cavity | 1 | 0 | 0 | |
| 3 | m | 69 | negative | I | Oropharynx | 0 | 0 | 0 | |
| 4 | m | 57 | negative | I | Oral Cavity | 0 | 0 | 0 | |
| 5 | m | 71 | negative | I | Oral Cavity | 0 | 0 | 0 | |
| 6 | f | 64 | unknown | I | Oral Cavity | 0 | 0 | 0 | |
| 7 | m | 78 | negative | II | Larynx | 1 | 0 | 0 | |
| 8 | m | 55 | negative | II | Oral Cavity | 0 | 0 | 0 | |
| 9 | m | 62 | negative | II | Oral Cavity | 0 | 0 | 0 | |
| 10 | m | 59 | positive | II | Oral Cavity | 0 | 0 | 0 | |
| 11 | m | 77 | negative | II | Oral Cavity | 0 | 0 | 0 | |
| 12 | m | 64 | negative | II | Oral Cavity | 0 | 0 | 0 | |
| 13 | m | 56 | negative | III | Oral Cavity | 0 | 0 | 0 | |
| 14 | m | 55 | negative | III | Oral Cavity | 0 | 0 | 0 | |
| 15 | m | 69 | negative | III | Larynx | 0 | 0 | 0 | |
| 16 | m | 63 | negative | III | Oropharynx | 0 | 0 | 0 | |
| 17 | m | 63 | negative | III | Oropharynx | 0 | 0 | 0 | |
| 18 | m | 78 | negative | III | Oral Cavity | 0 | 0 | 0 | |
| 19 | m | 66 | unknown | III | Larynx | 5 | 0 | 0 | |
| 20 | f | 62 | positive | III | Oral Cavity | 0 | 0 | 0 | |
| 21 | m | 63 | unknown | III | Larynx | 0 | 0 | 0 | |
| 22 | m | 74 | positive | IV | Oral Cavity | 2 | 2* | 13,8 | lung lesion |
| 23 | m | 88 | negative | IV | Oral Cavity | 1 | 0 | 0 | lung lesion |
| 24 | m | 81 | positive | IV | Oropharynx | 0 | 0 | 0 | |
| 25 | m | 60 | Positive | IV | Oropharynx | 4 | 0 | 0 | |
| 26 | m | 64 | negative | IV | Oral Cavity | 4 | 1 | 6 | |
| 27 | m | 50 | Positive | IV | Oropharynx | 0 | 1 | 3 | |
| 28 | f | 65 | negative | IV | Oral Cavity | 4 | 0 | 0 | |
| 29 | m | 74 | positive | IV | Oropharynx | 3 | 0 | 0 | |
| 30 | f | 45 | positive | IV | Oropharynx | 1 | 0 | 0 | |
| 31 | m | 58 | Positive | IV | Oropharynx | 0 | 2 | 3,3 | |
| 32 | m | 73 | negative | IV | Oropharynx | 0 | 0 | 0 | |
| 33 | m | 73 | negative | IV | Oral Cavity | 0 | 0 | 0 | |
| 34 | m | 55 | positive | IV | oropharynx | 0 | 0 | 0 | |
| 35 | m | 56 | negative | IV | Oropharynx | 4 | 0 | 0 | |
| 36 | m | 69 | positive | IV | Oropharynx | 0 | 0 | 0 | |
| 37 | m | 62 | positive | IV | Oropharynx | 0 | 0 | 0 | |
| 38 | m | 58 | positive | IV | Oropharynx | 4 | 1 | 5 | lung lesion |
| 39 | m | 66 | positive | IV | Oropharynx | 0 | 0 | 0 | |
| 40 | m | 79 | positive | IV | Oropharynx | 0 | 0 | 0 | |
| 41 | m | 66 | negative | IV | Larynx | 4 | 2 | 8,10 | lung and liver lesions |
| 42 | m | 50 | positive | IV | Oral Cavity | 3 | 2 | 3,5 | |
| 43 | m | 75 | positive | IV | Oral Cavity | 0 | 0 | 0 | |
| 44 | m | 54 | positive | IV | Oropharynx | 0 | 0 | 0 | |
| 45 | f | 70 | positive | IV | Oropharynx | 0 | 0 | 0 | |
| 46 | m | 53 | negative | IV | Oropharynx | 3 | 1 | 6 | |
| 47 | m | 77 | negative | IV | Hypophaynx | 0 | 0 | 0 | |
| 48 | m | 50 | unknown | IV | Oral Cavity | 0 | 0 | 0 | |
| 49 | m | 65 | negative | IV | Oropharynx | 3 | 2* | 5,7 | liver lesion |
| 50 | m | 58 | unknown | IV | Oropharynx | 1 | 1 | 3 | |
| 51 | m | 74 | positive | IV | Oropharynx | 0 | 0 | 0 | |
| 52 | m | 62 | positive | IV | Oropharynx | 10 | 0 | 0 | |
| 53 | m | 23 | negative | IV | Hypopharynx | 0 | 0 | 0 | |
| 54 | m | 61 | positive | IV | Hypopharynx | 0 | 0 | 0 | |
| 55 | m | 50 | positive | IV | Oral Cavity | 0 | 1 | 3 | |
| 56 | f | 59 | negative | IV | Oral Cavity | 0 | 1 | 4 | |
| 57 | m | 59 | positive | IV | Oropharynx | 5 | 3 | 5,5,7 | lung lesion |
| 58 | m | 50 | positive | IV | Oropharynx | 4 | 1 | 3 | |
| 59 | m | 64 | positive | IV | Oropharynx | 0 | 2* | 7,5 | lung lesion |
| 60 | m | 60 | negative | IV | Salivary Glands | 0 | 0 | 0 |
*CTC clusters with WBCs.
Single CTCs
Single CTCs were detected in 20/60 patients (33.3%) ranging from 1–10 CTCs/5 ml (Fig. 2) (CTC positive: 1/6 Stage I, 1/6 Stage II, 1/9 Stage III, 17/39 Stage IV).
Figure 2.
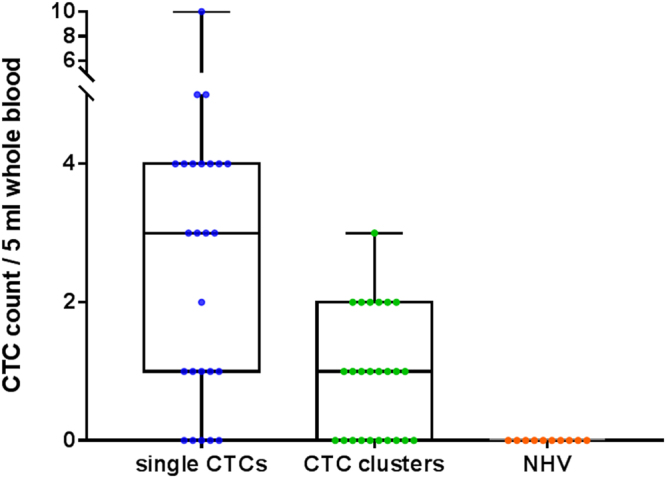
Box and whisker plot showing the number of single CTCs (blue) and CTC clusters (green) (pan-CK + EGFR+ DAPI +) per 5 ml blood for the 25 CTC positive head and neck patient samples and 10 normal healthy volunteers. The box and represent the minimum to maximum values with all individual data points.
CTC Clusters
CTC clusters were detected in 15/60 patients (25%) ranging from 1–3 clusters/5 ml (consisting of 3–13 cells) (Figs 2 and 3) (CTC positive: 0/6 Stage I, 0/6 Stage II, 0/9 Stage III, 15/39 Stage IV), with white blood cells present in 3/15 CTC clusters (20%) (Fig. 4).
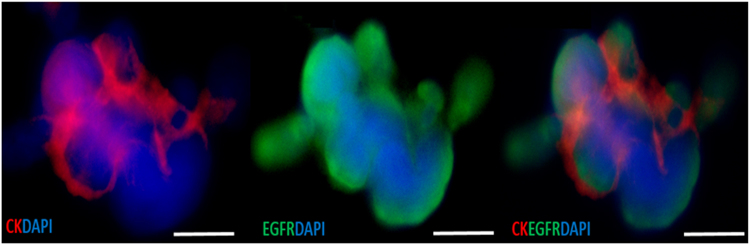
Figure 3.
Circulating tumour cell (CTC) clusters isolated from a head and neck cancer (HNC) patient stained positive for pan-cytokeratin −8, 18, 19 (Red), EGFR (Green), DAPI (Blue) and negative for CD45 (not shown). Scale bar represents 10 µm.
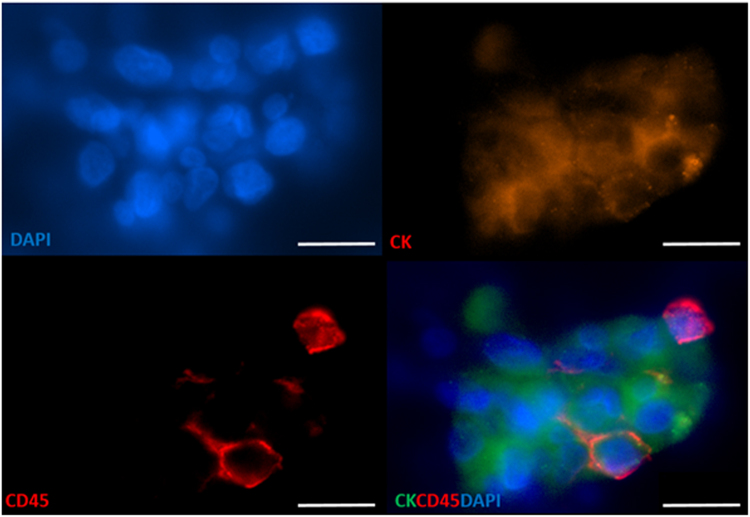
Figure 4.
Circulating tumour cluster (pan-cytokeratin + EGFR + DAPI+), containing white blood cells (CD45 + DAPI+ ) within the cellular aggregate. Scale bar represents 10 µm.
In 10/20 patients positive for single CTCs, CTC clusters were not present. Whereas, in 5/15 patients positive for CTC clusters, single CTCs were not found. Patients presented with both single and CTC clusters in 10/60 patient samples. No CTC-like (single/clusters) events were observed in the 10 normal healthy volunteer (NHV) cohort.
Discussion
Single CTCs have been previously reported in other solid tumour types including HNC5,6,25,27–29. CTC clusters are defined as ≥3 tumour cells, held in close proximity by strong cell-cell adhesions, detected in the blood of cancer patients2,30,31. Studies have reported that CTC clusters have a shorter half-life in blood, an increased metastatic capacity compared to single CTCs21,24,30, and that disrupting the interactions within clusters may provide a strategy to reduce CTC cluster mediated metastasis16. An example of this would be the knockdown of plakoglobin, a protein which is highly expressed in CTC clusters that may facilitate in the reduction of cluster generation21,32. The observation of CTC clusters or CTMs has been associated with adverse outcomes33.
To date, the clinical utility of CTCs in HNC remains limited, and few studies have reported on the presence of these subpopulations of CTC clusters2,13,14,34–37. In our cross sectional pilot study, single CTCs were found in 33.3% of patients (Stage I-IV) and CTC clusters in 25% of patients (Stage IV). Whilst the number of single CTCs is comparable to previous HNC studies2,14,26,34,38–41, the presence of CTC clusters in 25% of the cohort is of importance42,43. Furthermore, from 7 stage IV HNC patients in the study, that progressed to developing lung/liver metastasis (3–6 months later), 6 patients presented with CTC clusters (P = 0.0313). In patients with the absence of CTC clusters, the predicitive value of not developing distant disease within 6 months was found to be 95%. Chalmers et al., 2012 has shown that HNC CTC clusters can co-express Vimentin and CD44, epithelial-mesenchymal transition (EMT) and stemness traits which may represent an aggressive phenotype34. It is not fully understood whether the expression of mesenchymal traits on CTC clusters is due to single proliferating CTCs which had undergone EMT or an EMT transformed CTC cluster44. Single CTCs, CTC clusters, or both cell types were found in 25/60 of the sampled HNC patient bloods. Importantly, the presence of CTC clusters did not depend on single CTCs being present and could be potentially used as an independent prognostic marker44.
Notably, in 3/21 of the CTC clusters from stage IV HNC patients, white blood cells were found within the cluster. This feature in a number of CTC clusters is of importance as the incorporation of white blood cells (WBCs) in the CTC cluster may provide a mechanism by which these CTC clusters evade the immune system31,45–47. To this end, recent studies have highlighted that PD-L1 is frequently expressed on CTCs and may be involved in immune evasion45,46,48. Studies have shown that the presence of non tumour cells (e.g. platelets and leukocytes) within CTC clusters promotes metastasis, by protecting the clusters from the shear stressors and immune attacks44,49.
Conclusion
This pilot study challenges the notion of only reporting on individual CTCs in HNC studies. Whilst single CTCs were found in the screened population, a comparable population presented with CTC clusters. Whilst the role of CTC clusters, including clusters containing WBCs is not fully understood, studies into this area are warranted to understand cluster mediated immune escape and their role in metastasis.
Materials and Methods
Study design
This prospective study was conducted across three major academic hospitals in Brisbane, Austrlia. Ethics approval was obtained from the Metro South and Health Service District Human Research Ethics Committee (HREC/12/QPAH/381 and HREC/11/QPAH/331) in accordance with the National Health and Medical Research Council’s (NHMRC) guidelines to collect blood from the Royal Brisbane and Women’s Hospital (RBWH), Logan Hospital and Princess Alexandra Hospital (PAH). This study also has QUT ethics approval (1400000617 and 1100001420). All participants gave written informed consent and 10 ml blood samples were collected in BD Vacutainer K2E tubes (EDTA) from 60 HNC patients before treatment and 10 normal healthy volunteers (NHV), with CTC assessment made as described below.
Enrichment of CTCs using spiral technology
An initial red blood cell (RBC) lysis (Astral Scientifix) was performed to the 10 ml blood sample to reduce the cellular components passing through the spiral chip. Thereafter, cells were centrifuged and the pellet resuspended in 10 ml of sheath buffer (1xPBS, 2 mM EDTA, 0.5% BSA). The spiral device was setup as previously described15,26. In brief, after the spiral chip had an initial priming run, the sample was loaded onto a syringe and pumped through the spiral chip at 1.7 ml/min. The CTC output were collected and spun down at 300 × g for 5 mins.
CTC and CTC cluster characterization
CTC enriched cells were cytospun onto glass slides and CTCs/CTM identified using the CellSearch antibody cocktail (Cytokeratin-8,18,19, CD45, DAPI) (Janssen Diagnostics). Cells were further characterized for surface EGFR using anti-EGFR antibody (AY13, Biolegend, San Diego). Briefly, the glass slides were incubated with an antibody cocktail of CellSearch Reagents (20 µl staining reagent, 20 µl permeabilization buffer, 20 µl fixation buffer, 10 µl DAPI in 120 µl PBS) for 1 hour at room temperature, washed 3 times in PBS, coverslipped and imaged on the Olympus IX73 epifluorescence microscope.
CTC and CTM parameters
CTCs were visualized using immunofluorescence post enrichment. Cells were classified as CTCs after meeting the following criteria (i) high nucleus to cytoplasmic ratio (ii) morphologically larger than the background cells with intact nuclei (iii) cytokeratin-8,18,19 positive (iv) EGFR positive (v) CD45 negative. CTC clusters were reported as 3 or more CTCs in close proximity and CTM when CTC clusters included leukocytes (CD45 positive cells). The results were reported as the number of CTCs, CTC clusters, and CTM per 5 ml whole blood.
Statistical Analysis
The development of distant metastatic disease (confirmed by imaging and biopsy where possible) were compared to CTC groups using Fisher’s exact test. All statistical analysis were performed using Graphpad Prism 7.0 software, were two-sided, and P-values <0.05 considered statistically significant.
Data Availability
All data generated or analysed during this study are included in this published article.
Acknowledgements
This study is supported by the Queensland Centre for Head and Neck funded by Atlantic Philanthropies and the Queensland Government. The authors would like to thank Prof William B Coman for clinical guidance and Tony Blick for editorial assistance. The Clinical Trials coordinators: Ms Jenny Edmunds, Ms Charm Micklewright and Ms Trang Le for sample accruals. The study was supported by the QUT write up scholarship for AK and QUT VC Fellowship for CP.
Author Contributions
Study Design: A.K., H.S., C.P.1., B.W., L.K., C.N., M.E.W., C.P. Data Collection, experimental procedures: A.K., H.S., C.P.1., B.W., L.K., C.N., M.E.W. Data analysis: A.K., H.S., C.P., M.E.W. Manuscript preparation and review: A.K., H.S., L.K., C.N., M.E.W., C.P. C.P.: Chamindie Punyadeera C.P.1: Chris Perry.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vokes EE, Agrawal N, Seiwert TY. HPV-Associated Head and Neck Cancer. JNCI: Journal of the National Cancer Institute. 2015;107:djv344–djv344. doi: 10.1093/jnci/djv344. [DOI] [PubMed] [Google Scholar]
- 2.Kulasinghe A, Perry C, Jovanovic L, Nelson C, Punyadeera C. Circulating tumour cells in metastatic head and neck cancers. International journal of cancer. 2015;136:2515–2523. doi: 10.1002/ijc.29108. [DOI] [PubMed] [Google Scholar]
- 3.Alix-Panabieres C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clinical chemistry. 2013;59:110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 4.Hanssen A, et al. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci Rep. 2016;6:28010. doi: 10.1038/srep28010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pantel K, Alix-Panabieres C. Real-time liquid biopsy in cancer patients: fact or fiction? Cancer Res. 2013;73:6384–6388. doi: 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- 6.Bidard FC, et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer metastasis reviews. 2013;32:179–188. doi: 10.1007/s10555-012-9398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alix-Panabieres C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer discovery. 2016;6:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 8.Ashworth TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Australian Medical Journal. 1869;14:146–149. [Google Scholar]
- 9.Gorges TM, et al. Accession of Tumor Heterogeneity by Multiplex Transcriptome Profiling of Single Circulating Tumor Cells. Clinical chemistry. 2016;62:1504–1515. doi: 10.1373/clinchem.2016.260299. [DOI] [PubMed] [Google Scholar]
- 10.Allard WJ, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 11.Farace F, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. British journal of cancer. 2011;105:847–853. doi: 10.1038/bjc.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliano M, et al. Circulating tumor cells as early predictors of metastatic spread in breast cancer patients with limited metastatic dissemination. Breast Cancer Res. 2014;16:440. doi: 10.1186/s13058-014-0440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulasinghe A, et al. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget. 2016 doi: 10.18632/oncotarget.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols AC, et al. Detection of circulating tumor cells in advanced head and neck cancer using the CellSearch system. Head Neck. 2012;34:1440–1444. doi: 10.1002/hed.21941. [DOI] [PubMed] [Google Scholar]
- 15.Warkiani ME, et al. Ultra-fast, label-free isolation of circulating tumor cells from blood using spiral microfluidics. Nature protocols. 2016;11:134–148. doi: 10.1038/nprot.2016.003. [DOI] [PubMed] [Google Scholar]
- 16.Au SH, et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proceedings of the National Academy of Sciences. 2016;113:4947–4952. doi: 10.1073/pnas.1524448113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gkountela, S., Szczerba, B., Donato, C. & Aceto, N. Recent advances in the biology of human circulating tumour cells and metastasis. ESMO Open1, 10.1136/esmoopen-2016-000078 (2016). [DOI] [PMC free article] [PubMed]
- 18.Cho EH, et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Physical biology. 2012;9:016001. doi: 10.1088/1478-3975/9/1/016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dive C, Brady G. SnapShot: Circulating Tumor Cells. Cell. 2017;168:742–742.e741. doi: 10.1016/j.cell.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Au SH, et al. Microfluidic Isolation of Circulating Tumor Cell Clusters by Size and Asymmetry. Sci Rep. 2017;7:2433. doi: 10.1038/s41598-017-01150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aceto N, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng S, et al. Circulating tumor cells in patients with breast cancer dormancy. Clinical cancer research: an official journal of the American Association for Cancer Research. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 23.King MR, et al. A physical sciences network characterization of circulating tumor cell aggregate transport. American journal of physiology. Cell physiology. 2015;308:C792–802. doi: 10.1152/ajpcell.00346.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aceto, N., Toner, M., Maheswaran, S. & Haber, D. A. En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition. Trends in Cancer1, 44–52, 10.1016/j.trecan.2015.07.006. [DOI] [PubMed]
- 25.Warkiani ME, et al. Slanted spiral microfluidics for the ultra-fast, label-free isolation of circulating tumor cells. Lab Chip. 2014;14:128–137. doi: 10.1039/C3LC50617G. [DOI] [PubMed] [Google Scholar]
- 26.Kulasinghe A. T. T. et al. Enrichment of circulating head and neck tumour cells using spiral microfluidic technology Scientific Reports in press (2017). [DOI] [PMC free article] [PubMed]
- 27.Pantel, K. & Alix-Panabieres, C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med16, 10.1016/j.molmed.2010.07.001 (2010). [DOI] [PubMed]
- 28.de Bono JS, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 29.Martin OA, Anderson RL, Narayan K, MacManus MP. Does the mobilization of circulating tumour cells during cancer therapy cause metastasis? Nat Rev Clin Oncol. 2017;14:32–44. doi: 10.1038/nrclinonc.2016.128. [DOI] [PubMed] [Google Scholar]
- 30.Fabisiewicz A, Grzybowska E. CTC clusters in cancer progression and metastasis. Medical Oncology. 2016;34:12. doi: 10.1007/s12032-016-0875-0. [DOI] [PubMed] [Google Scholar]
- 31.Jansson S, Bendahl P-O, Larsson A-M, Aaltonen KE, Rydén L. Prognostic impact of circulating tumor cell apoptosis and clusters in serial blood samples from patients with metastatic breast cancer in a prospective observational cohort. BMC Cancer. 2016;16:433. doi: 10.1186/s12885-016-2406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goto W, et al. Circulating tumor cell clusters-associated gene plakoglobin is a significant prognostic predictor in patients with breast cancer. Biomarker research. 2017;5:19. doi: 10.1186/s40364-017-0099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin OA, et al. Mobilization of Viable Tumor Cells Into the Circulation During Radiation Therapy. International Journal of Radiation Oncology*Biology*Physics. 2014;88:395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Balasubramanian P, et al. Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PloS one. 2012;7:e42048. doi: 10.1371/journal.pone.0042048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jatana KR, et al. Effect of surgical intervention on circulating tumor cells in patients with squamous cell carcinoma of the head and neck using a negative enrichment technology. Head & neck. 2016;38:1799–1803. doi: 10.1002/hed.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt H, Kulasinghe A, Kenny L, Punyadeera C. The development of a liquid biopsy for head and neck cancers. Oral Oncology. 2016;61:8–11. doi: 10.1016/j.oraloncology.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Strati. A. K. G. et al. PD-L1 expressing circulating tumor cells (CTCs) in patients with head and neck squamous cell carcinoma (HNSCC). AACR Cancer Research Clinical Research (2016).
- 38.McMullen KP, Chalmers JJ, Lang JC, Kumar P, Jatana KR. Circulating tumor cells in head and neck cancer: A review. World Journal of Otorhinolaryngology-Head and Neck Surgery. 2016;2:109–116. doi: 10.1016/j.wjorl.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tinhofer I, Hristozova T, Stromberger C, Keilhoiz U, Budach V. Monitoring of circulating tumor cells and their expression of EGFR/phospho-EGFR during combined radiotherapy regimens in locally advanced squamous cell carcinoma of the head and neck. International journal of radiation oncology, biology, physics. 2012;83:e685–690. doi: 10.1016/j.ijrobp.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Morosin T, et al. Circulating tumour cells in regionally metastatic cutaneous squamous cell carcinoma: A pilot study. Oncotarget. 2016;7:47111–47115. doi: 10.18632/oncotarget.9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulasinghe, A. et al. Short term ex-vivo expansion of circulating head and neck tumour cells. Oncotarget, 10.18632/oncotarget.11159 (2016). [DOI] [PMC free article] [PubMed]
- 42.Balasubramanian P, et al. Confocal images of circulating tumor cells obtained using a methodology and technology that removes normal cells. Molecular pharmaceutics. 2009;6:1402–1408. doi: 10.1021/mp9000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bozec A, et al. Significance of circulating tumor cell detection using the CellSearch system in patients with locally advanced head and neck squamous cell carcinoma. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2013;270:2745–2749. doi: 10.1007/s00405-013-2399-y. [DOI] [PubMed] [Google Scholar]
- 44.Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science (New York, N.Y.) 339, 10.1126/science.1228522 (2013). [DOI] [PMC free article] [PubMed]
- 45.Mazel M, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Molecular oncology. 2015;9:1773–1782. doi: 10.1016/j.molonc.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kulasinghe A, et al. PD-L1 expressing circulating tumour cells in head and neck cancers. BMC Cancer. 2017;17:333. doi: 10.1186/s12885-017-3316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong Y, Fang F, Zhang Q. Circulating tumor cell clusters: What we know and what we expect (Review) International journal of oncology. 2016;49:2206–2216. doi: 10.3892/ijo.2016.3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, et al. CTC immune escape mediated by PD-L1. Medical hypotheses. 2016;93:138–139. doi: 10.1016/j.mehy.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 49.Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. Journal of cellular physiology. 2014;229:1005–1015. doi: 10.1002/jcp.24539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.