Abstract
Background: Sacubitril/Valsartan has been shown to improve mortality and reduce hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF). The effect of Sacubitril/Valsartan on ejection fraction (EF) and reverse remodeling parameters have not been previously described. Methods: We performed a single-center, retrospective, cohort study of HFrEF patients (n=48) who were treated with Sacubitril/Valsartan for a median duration of 3 months (Interquartile range 2-6 months). Clinical and echocardiographic parameters were reviewed at three time points (pre-baseline which was median of 18 months before starting Sacubitril/Valsartan, baseline before treatment started, and post-Sacubitril/Valsartan). Paired sample t-test and one-way repeated measures ANOVA were used for normally distributed data, while Wilcoxon Signed Rank test for non-normally distributed data. Results: Sacubitril/Valsartan use was associated with an average 5% (±1.2) increase in EF, from a mean baseline of 25.33% to 30.14% (p<0.001) with a median duration of treatment 3 months. There was no significant change in mean LVEF over a median duration of 11 months (IQR 5.5-15.5) between pre-baseline and baseline time points prior to treatment (p=1.0). The mean increase in ejection fraction tended to be marginally greater in the medium/high dose cohort as compared to the low dose cohort, with a mean increase of 5.09% (±1.36) and 4.03% (±3.17), respectively (p=0.184). There was a 3.36 mm reduction in left ventricular end-systolic diameter (p=0.04), a 2.64 mm reduction in left ventricular end-diastolic diameter (p=0.02), and a 14.4 g/m2 reduction in left ventricular mass index (p<0.01). Conclusion: Sacubitril/Valsartan was found to improve EF and multiple measures of reverse remodeling beyond the effects of concomitant optimal medical therapy. Though these results are encouraging, our small sample, observational study requires confirmation in larger cohorts with longer follow-up periods.
Keywords: Sacubitril/Valsartan, reverse remodeling, ejection fraction improvement
Introduction
Heart failure affects more than 23 million people worldwide. Patients with heart failure have an estimated 50% 5-year mortality [1-3]. The mainstay of medical treatment for patients with heart failure with reduced ejection fraction (HFrEF) are beta blockers (BB), angiotensin converting enzyme inhibitors (ACEi)/angiotensin receptor blockers (ARB), and mineralocorticoid receptor antagonists (MRA). More recently in 2014, the first in-human randomized controlled trial of angiotensin receptor neprilysin inhibitor Sacubitril/Valsartan (Entresto, Novartis) was demonstrated to improve survival and decrease hospitalizations compared to enalapril [4].
Although the physiological mechanisms of action of Sacubitril/Valsartan are well described, its effects on left ventricular remodeling and left ventricular ejection fraction (LVEF) have not been well studied. Left ventricular remodeling is a major mechanism underlying disease progression in patients with HFrEF [5]. The degree of improvement in left ventricular end-diastolic volume (LVEDV), left ventricular end-systolic volume (LVESV), LV dimensions, and LVEF with therapies are strongly correlated with clinical outcomes, including survival [6].
Beta blockers, ACEi/ARBs, and MRAs have demonstrated potent effects on reverse remodeling and improvement in LVEF in multiple studies [7-10]. Animal studies have shown that treatment with Sacubitril/Valsartan compared to Valsartan alone is associated with a statistically significant increase in LVEF and a trend towards improved reverse remodeling [11]. The goal of our study is to evaluate the effects of Sacubitril/Valsartan on LVEF and reverse remodeling parameters among patients with HFrEF.
Methods
The study population comprised of patients with a diagnosis of HFrEF treated with Sacubitril/Valsartan for more than 1 month in the Heart Function clinic at the University of Ottawa Heart Institute (UOHI) between Jan 1, 2015 and June 30, 2017. Patients with new diagnosis of heart failure within 1 year before starting Sacubitril/Valsartan were excluded. Clinical information collected included demographics, comorbidities, New York Heart Association fun-ctional class, duration of heart failure diagnosis, medications and laboratory parameters. No patient in our study discontinued Sacubitril/Valsartan due to an adverse event including acute kidney injury, hypotension or hyperkalaemia.
Cardiac imaging was performed by transthoracic echocardiography (80%), radionuclide angiography (14.6%) or cardiac MRI (6.1%). The tests were performed and interpreted in a standard fashion at a single center (UOHI) [12]. Imaging data were analyzed for LVEF and measures of reverse remodeling including left ventricular end-systolic diameter (LVESD), left ventricular end-diastolic diameter (LVEDD), LVESV, LVEDV, LV mass, and right ventricular systolic pressure (RVSP). These imaging and other laboratory parameters (serum potassium and creatinine levels) were collected at two time points; at baseline prior to initiating treatment, and the most recently available test after a period of treatment with Sacubitril/Valsartan. Data for LVEF were collected at an additional earlier time point at 18 months before starting Sacubitril/Valsartan.
The primary outcome was change in LVEF over the three studied time points; pre-baseline, baseline and post Sacubitril/Valsartan. The secondary outcomes were the changes in left ventricular reverse remodeling parameters, including LVESD, LVEDD, LVESV, LVEDV, LV mass and RVSP.
All data analysis was completed using SPSS software (V 24.0). Continuous variables are presented as mean and standard deviation (SD) for normally distributed variables and median and interquartile range (IQR) for non-normally distributed variables. Categorical variables are summarized as frequencies and percentages. Outcome variables were tested for normality using Shapiro-Wilk test. Paired sample t-test and one-way repeated measures ANOVA were used for normally distributed data, while Wilcoxon Signed Rank test for non-normally distributed data. Pearson chi-squared was used for categorical variables. The two-sided significance level for all final tests was set to 0.05.
Results
A total of 48 patients with HFrEF (median duration of HF diagnosis 25.7 months, IQR 15.5-43.2) were treated with Sacubitril/Valsartan. The baseline characteristics of this patient population are shown in Table 1. The vast majority of our patients were on all guideline-directed optimal heart failure therapy with 97.5% on BBs and 87.5% on MRAs. Over half (55%) of patients were on high dose (97/103 mg) Sacubitril/Valsartan. The median duration of treatment was 3 months, IQR 2-6 months (Table 1).
Table 1.
Baseline Characteristics and number of patients on low, medium, and high Sacubitril/Valsartan dose
| Sacubitril/Valsartan (n=48) (± SD or 25th-75th quartile IQR) | |
|---|---|
| Mean age (Years) | 70 (±11.1) |
| Median NYHA (IQR) | 2 (2.0-2.5) |
| Mean systolic BP mmHg | 114 (±16.7) |
| Mean baseline creatinine (umol/L) | 93.4 (±21.8) |
| Mean baseline potassium level (mmol/L) | 4.25 (±0.42) |
| Mean pre-baseline ejection fraction | 26.91% (±8.9) |
| Mean baseline ejection fraction | 26.41% (±7.7) |
| Female (%) | 10 (20.8%) |
| Non-ischemic etiology for HF (%) | 25 (53.2%) |
| Hypertension (%) | 23 (47%) |
| Diabetes (%) | 13 (27%) |
| Hyperlipidemia (%) | 22 (45.8%) |
| History of atrial fibrillation (%) | 7 (14.6%) |
| Previous CVA (%) | 3 (6.3%) |
| Medications at baseline | |
| On a Beta Blocker (%) | 47 (97.9%) |
| Sacubitril/Valsartan Dose | |
| Low dose (24.3/25.7 mg) | 10 (23.8%) |
| Medium dose (49/51 mg) | 9 (21.4%) |
| High dose (97.2/102.8 mg) | (54.8%) |
The primary outcome defined as a change in LVEF over the treatment period increased significantly following treatment with Sacubitril/Valsartan from 25.33% at baseline to 30.14% at follow-up (p<0.001), with a median duration of treatment 3 months. There was no significant change in mean LVEF over a median duration of 11 months (IQR 5.5-15.5) between pre-baseline and baseline time points prior to treatment (p=1.0) (Figure 1). When the change in ejection fraction was evaluated according to the Sacubitril/Valsartan dose received, there was an increase in the mean ejection fraction regardless of whether the patient was receiving the medium/high dose, or the low dose. However, the mean increase in ejection fraction tended to be marginally greater in the medium/high dose cohort as compared to the low dose cohort, with a mean increase of 5.09% (±1.36) and 4.03% (±3.17), respectively (p=0.184). (Figure 1) A total of 73% of study patients had a response to Sacubitril/Valsartan (defined as any improvement in EF). The rate of response was not statistically different between patients with ischemic (68.2%) and nonischemic (76.0%) (p=0.550) cardiomyopathy. Furthermore, response did not differ between patients with comorbidities such as diabetes, hypertension or atrial fibrillation.
Figure 1.
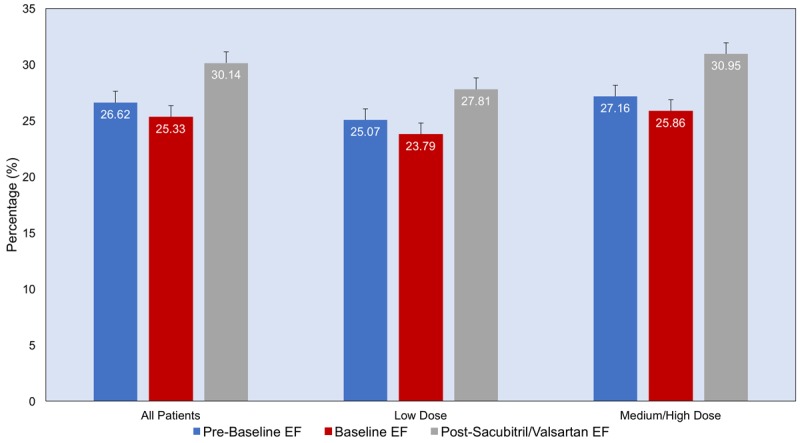
Mean ejection fraction at three time points: pre-baseline, baseline and post- Sacubitril/Valsartan treatment, in three groups: all patients, patients on low dose and patients on medium/High dose.
There were significant improvements in left ventricular remodeling parameters including reductions in LVESD (3.36±1.6 mm), LVEDD (2.64±1.1 mm), and LV mass index (14.4±3.9 g/m2 ), (all p values <.05). There were also non-statically significant reductions in LVESV and LVEDV (Table 2).
Table 2.
Patients left ventricular remodelling parameters immediately prior to initiating Sacubitril/Valsartan compared to most recent assessment post Sacubitril/Valsartan
| Initial | Final | Mean change (±SD) or median (IQR)* | p-value | |
|---|---|---|---|---|
| End Systolic Dimension mean (mm) n=33 | 56.3 | 52.9 | -3.36 (±1.6) | 0.038 |
| End Systolic Volume median (mL)) n=24 | 165.0 | 143.7 | -14.1 (IQR-45 to 21) | 0.424 |
| End Diastolic Dimension mean (mm) n=33 | 65.8 | 63.15 | -2.64 (±1.1) | 0.022 |
| End Diastolic Volume median (mL) n=25 | 221.4 | 207.5 | 4.9 (IQR-43 to 44) | 0.989 |
| Left Ventricular Mass Index mean (gm/m2 ) n=31 | 128.1 | 113.66 | -14.4 (±3.9) | 0.001 |
| Right Ventricular Systolic Pressure RVSP mean (mmHg) n=12 | 29.8 | 27.51 | -2.3 (±1.78) | 0.222 |
Mean and standard deviations were used for variables that were normally distributed.
Median and interquartile range were used for non-normally distributed variables.
Discussion
This is the first study to-date to describe improvements in LVEF and reverse remodeling parameters with Sacubitril/Valsartan. Akin to BB, ACEi, ARB and MRA therapies, our results demonstrate the ability of Sacubitril/Valsartan to significantly improve LVEF, and reduce LVESD, LVEDD and LV mass. This data proves a potent reverse remodeling effect of Sacubitril/Valsartan in a real-world setting outside of the context of clinical trials.
As per current heart failure management guidelines, Sacubitril/Valsartan is only prescribed in our centre in patients with HFrEF who have symptomatic heart failure despite optimal ACEi, BB, and MRA treatment [13]. We therefore believe that the observed benefits in this study are attributable to Sacubitril/Valsartan. This is also supported by our three-time-point LVEF analysis demonstrating unchanged LVEF over 6-12 months on stable ACEi/ARB, BB and MRA treatments preceding Sacubitril/Valsartan initiation, followed by a significant improvement in LVEF after its initiation. Moreover, when patients were stratified according to Sacubitril/Valsartan dose, there was a trend towards greater improvement in LVEF for patients treated with higher Sacubitril/Valsartan dose. Our findings support the previous animal work by Suematsu and colleagues, which showed that Sacubitril/Valsartan was associated with statistically significant improvement in LVEF [4,11]. A meta-analysis of over 69,000 patients by Kramer et al. demonstrated that improvement in LVEF and left ventricular remodeling parameters was associated with lower rates of mortality among patients with HFrEF [4,14]. Although an improvement in EF and reverse remodeling may be inferred from the improved mortality shown in the PARADIGM trial, our study represents the first human data documenting these effects [4].
Despite the promising results presented, there are several limitations to our study. The observational study design, small sample size and lack of a comparator group preclude a direct comparison of Sacubitril/Valsartan patients to those on optimal medical therapy (OMT). However, all Sacubitril/Valsartan patients were optimized on OMT prior to initiating Sacubitril/Valsartan, and patients with a recent diagnosis of HF (i.e. less than 1-year duration) were excluded from the analysis. Therefore, all patients in our study had received OMT for a minimum of one year prior to starting Sacubitril/Valsartan which suggests that improvements seen in EF are likely attributable to Sacubitril/Valsartan. As previous reports have demonstrated that patients are unlikely to attain further benefits from OMT if they failed to have significant reverse remodeling within the first 6-12 months of treatment, our cohort likely derived their maximum benefits from OMT prior to initiation of Sacubitril/Valsartan [15]. Furthermore, our analysis of EF at three-time points specifically addresses the above concerns and its definitive results suggest that the improvement seen in EF is very likely due to the introduction of Sacubitril/Valsartan above and beyond preceding OMT.
Another limitation to our study is the variability between ventricular function assessment methods. While the majority of patients were evaluated before and after initiating Sacubitril/Valsartan with transthoracic echocardiogram, there were a minority of patients who were assessed with other modalities, such as MRI. However, we believe this is unlikely to have a major impact on our findings given demonstrated reproducibility for each cardiac imaging modality as well as the performance and interpretation of testing in a single centre with standardized pattern of performance and reporting.
In conclusion, Sacubitril/Valsartan was found to improve LVEF and multiple measures of reverse remodeling above and beyond the effect of pre-existing OMT. A trend towards greater improvement in LVEF was observed for patients treated with higher doses of Sacubitril/Valsartan. These encouraging results warrant confirmations in larger prospective cohorts with longer follow-up.
Disclosure of conflict of interest
Lisa Mielniczuk and Peter Liu had worked as consultants for Novartis.
References
- 1.Blair JE, Huffman M, Shah SJ. Heart failure in North America. Curr Cardiol Rev. 2013;9:128–146. doi: 10.2174/1573403X11309020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran DT, Ohinmaa A, Thanh NX, Howlett JG, Ezekowitz JA, McAlister FA, Kaul P. The current and future financial burden of hospital admissions for heart failure in Canada: a cost analysis. CMAJ Open. 2016;4:E365–E370. doi: 10.9778/cmajo.20150130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–1355. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 6.Wong M, Johnson G, Shabetai R, Hughes V, Bhat G, Lopez B, Cohn JN. Echocardiographic variables as prognostic indicators and therapeutic monitors in chronic congestive heart failure. Veterans Affairs cooperative studies V-HeFT I and II. V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI65–70. [PubMed] [Google Scholar]
- 7.Dubach P, Myers J, Bonetti P, Schertler T, Froelicher V, Wagner D, Scheidegger M, Stuber M, Luchinger R, Schwitter J, Hess O. Effects of bisoprolol fumarate on left ventricular size, function, and exercise capacity in patients with heart failure: analysis with magnetic resonance myocardial tagging. Am Heart J. 2002;143:676–683. doi: 10.1067/mhj.2002.121269. [DOI] [PubMed] [Google Scholar]
- 8.A placebo-controlled trial of captopril in refractory chronic congestive heart failure. Captopril Multicenter Research Group. J Am Coll Cardiol. 1983;2:755–763. doi: 10.1016/s0735-1097(83)80316-7. [DOI] [PubMed] [Google Scholar]
- 9.Matsumori A Assessment of Response to Candesartan in Heart Failure in Japan (ARCH-J) Study Investigators. Efficacy and safety of oral candesartan cilexetil in patients with congestive heart failure. Eur J Heart Fail. 2003;5:669–677. doi: 10.1016/s1388-9842(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 10.Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, Marino P, Zardini P. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol. 2002;40:304–310. doi: 10.1016/s0735-1097(02)01965-4. [DOI] [PubMed] [Google Scholar]
- 11.Suematsu Y, Miura S, Goto M, Matsuo Y, Arimura T, Kuwano T, Imaizumi S, Iwata A, Yahiro E, Saku K. LCZ696, an angiotensin receptor-neprilysin inhibitor, improves cardiac function with the attenuation of fibrosis in heart failure with reduced ejection fraction in streptozotocin-induced diabetic mice. Eur J Heart Fail. 2016;18:386–393. doi: 10.1002/ejhf.474. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 13.Biglane JB, Becnel MF, Ventura HO, Krim SR. Pharmacologic therapy for heart failure with reduced ejection fraction: closing the gap between clinical guidelines and practice. Prog Cardiovasc Dis. 2017;60:187–197. doi: 10.1016/j.pcad.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshikawa E, Matsumura Y, Kubo T, Okawa M, Yamasaki N, Kitaoka H, Furuno T, Takata J, Doi YL. Effect of left ventricular reverse remodeling on long-term prognosis after therapy with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and β blockers in patients with idiopathic dilated cardiomyopathy. Am J Cardiol. 2011;107:1065–1070. doi: 10.1016/j.amjcard.2010.11.033. [DOI] [PubMed] [Google Scholar]


