Summary
Adrenomedullary chromaffin cells are catecholamine (CA)-producing cells originating from trunk neural crest (NC) via sympathoadrenal progenitors (SAPs). We generated NC and SAPs from human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) in vitro via BMP2/FGF2 exposure, ascertained by qPCR and immunoexpression of SOX10, ASCL1, TFAP2α, and PHOX2B, and by fluorescence-activated cell sorting selection for p75NTR and GD2, and confirmed their trunk-like HOX gene expression. We showed that continuing BMP4 and curtailing FGF2 in vitro, augmented with corticosteroid mimetic, induced these cells to upregulate the chromaffin cell-specific marker PNMT and other CA synthesis and storage markers, and we demonstrated noradrenaline and adrenaline by Faglu and high-performance liquid chromatography. We showed these human cells' SAP-like property of migration and differentiation into cells expressing chromaffin cell markers by implanting them into avian embryos in vivo and in chorio-allantoic membrane grafts. These cells have the potential for investigating differentiation of human chromaffin cells and for modeling diseases involving this cell type.
Keywords: human embryonic stem cells, human induced pluripotent cells, sympathoadrenal progenitors, neural crest, chromaffin cells
Highlights
-
•
Human ESCs and iPSCs differentiated to chromaffin cells via sympathoadrenal stage
-
•
Chromaffin-like cells are identified by PNMT expression
-
•
Adrenaline and noradrenaline production is revealed by FAGLU and by HPLC
-
•
Selection of GD2+ sympathoadrenal cells improves chromaffin cell differentiation
In this article, Newgreen and colleagues show that human pluripotent cells can be induced to form chromaffin-like cells, which produce adrenaline and noradrenaline in vitro. The method uses the dual-inhibitor method to induce neural crest progenitor and sympathoadrenal-like cells then extended BMP4 and corticosteroid exposure for the chromaffin-like stage, with curtailed FGF2 exposure to limit sympathetic neuron induction.
Introduction
Adrenal chromaffin cells are neural crest (NC) derivatives (Le Douarin and Teillet, 1974) and, via sympathoadrenal progenitors (SAPs), are closely related to sympathetic neurons (Huber et al., 2009, Shtukmaster et al., 2013). Both produce catecholamine (CA) biosynthesis enzymes including tyrosine hydroxylase (TH) and dopamine-β-hydroxylase (DβH). The major CA for sympathetic neurons is noradrenaline since they lack the adrenaline-synthesizing enzyme phenylethanolamine-N-methyltransferase (PNMT), while chromaffin cells secrete both due to the presence of PNMT (Levitt et al., 1965). The CA vesicles of chromaffin cells and the synaptic vesicles of sympathetic neurons (Huber et al., 2009, Teichberg and Holtzman, 1973) possess chromogranin A, chromogranin B/secretogranin I (CgB/SgI; predominant in human adrenal chromaffin cells), and chromogranin C/secretogranin II (CgC/SgII) (Crivellato et al., 2008, Trifaro, 2002), which have diverse functions.
In addition to TH, DβH, PNMT, and Cg, a number of other molecules are markers of NC and SA development. P75NTR is indicative of early NC, i.e., NC stem/progenitor cells (NCPCs), as is, in some species, HNK1. There are several NC (SOX10, TFAP2) and SA transcription factors (ASCL1, PHOX2B, GATA3, and HAND2). The SA antibodies and the ganglioside GD2 indicate SAPs and chromaffin cells and the B2B1 antigen, MYCN, peripherin (PRPH), and neurofilament (NF) distinguish sympathetic neuron lineage progression (Anderson et al., 1991, Howard, 2005, Huber et al., 2009, Lumb and Schwarz, 2015, Mobley et al., 2015, Shtukmaster et al., 2013, Troy et al., 1990, Zhu et al., 2012).
Functionally, adrenal chromaffin cells are central to the “fight or flight” response. They also release enkephalin-related peptides to produce analgesic effects (Jozan et al., 2007, Livett et al., 1981) and also many growth factors (Krieglstein et al., 1998, Schober et al., 1998). Pathologically, chromaffin cells are affected in the pheochromocytoma associated with multiple endocrine neoplasia type 2A and 2B (MEN2A and MEN2B) (Wells et al., 2013) and are also related to neuroblastoma as they both originate from the SA lineage (Szabo et al., 2012). Clinically, autologous intra-brain transplantation of chromaffin cells ameliorated Parkinson's disease but the beneficial effects were transient (Drucker-Colin and Verdugo-Diaz, 2004). Chromaffin cell implants have also been suggested to alleviate cancer pain, and phase II clinical trials showed their feasibility (Lazorthes et al., 2000). However, the availability only of allogenic donor material limited the wider application of this approach (Jozan et al., 2007).
There are many studies on the production of NCPCs from pluripotent cells, and the differentiation of these cells into autonomic neurons (Lee et al., 2007, Oh et al., 2016). Production of SAPs from mouse embryonic stem cells (ESCs) and selection for GD2 has been demonstrated (Saxena et al., 2013). Here we describe modifications to these methods applied to human pluripotent ESCs (hESCs) and human induced pluripotent stem cells (iPSCs) to produce human adrenal chromaffin-like cells. A human cell resource will provide a platform to study development and physiology of these cells as well as allowing insight into the mechanisms of diseases that involve the SA and adrenal chromaffin cell lineage, such as neuroblastoma and MEN2A and 2B. In addition, the potential capacity to produce these cells in large numbers from human autologous sources may assist in the clinical practices that have been hampered by the previous unavailability of donor material and also provide an initiative in regenerative therapies (Soto-Gutierrez et al., 2010).
Results
NCPCs Differentiated from Human Pluripotent Cells Express SAP, Neuronal, and Chromaffin Markers
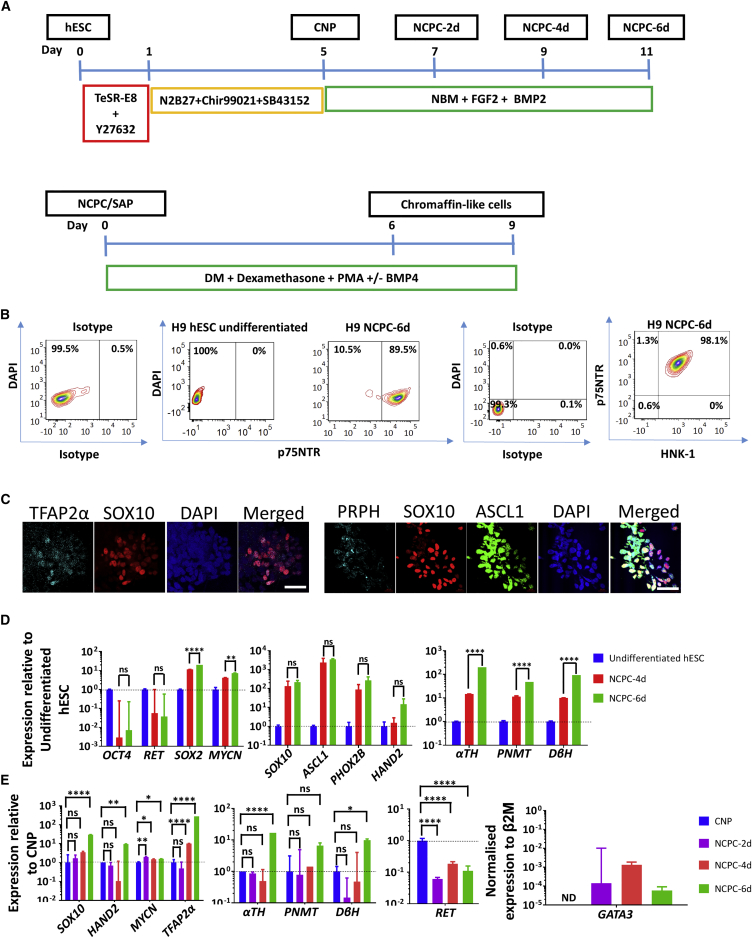
We differentiated H9 hESCs into neurectoderm-like caudal (i.e., caudal to forebrain) neural progenitors (CNPs) by TGF-β and GSK3β inhibition. This was followed by FGF2 and BMP2 treatment to generate NCPC neurospheres (we refer to these cells as NCPC-xd, where x is the duration of FGF2/BMP2 treatment in days) (Denham et al., 2015) (Figures 1A, S1A, and S1B). To quantitate the efficiency of generation of NCPCs, we fluorescence-activated cell sorting (FACS) analyzed dissociated neurospheres using p75NTR antibody. Most cells (89% ± 5%, N = 5) were p75NTR+ (Figure 1B), and almost all the p75NTR+ cells expressed another NC marker, HNK1 (Figure 1B). We analyzed the NCPCs with antibodies to SOX10 and TFAP2α, two NC transcription factors, and to pro-neuronal marker, ASCL1, which is expressed by pro-neuronal SAPs but later downregulated, and PRPH, which is expressed in sympathetic neuroblasts and neurons but not chromaffin cells (Lo et al., 1991, Troy et al., 1990). The majority of the SOX10+ cells expressed TFAP2α, and ASCL1 and PRPH markers were elevated especially in cells with lower SOX10 levels (Figure 1C).
Figure 1.
Characterization of Human NCPCs/SAPs
(A) Differentiation protocol of hESCs to NCPC/SAP-like cells and further to chromaffin-like cells. For details, refer to Figure S1.
(B) FACS analysis of NCPC-6d showing co-expression of the NCPC markers p75NTR and HNK1. Representative of ten independent inductions of H9 cells. Non-specific antibody binding is shown as antibody isotype control.
(C) Immunofluorescence of NCPC-6d cells with the NCPC/SAP markers SOX10, TFAP2α, ASCL1, and PRPH. Note the co-expression of early NC markers TFAP2 and SOX10 and a trend for markers of lineage progression, ASCL1 and PRPH, to segregate from early NC marker SOX10. Scale bars, 50 μm.
(D) qPCR analysis of NCPC-4d and NCPC-6d normalized to undifferentiated hESC, showing upregulation of diverse NC/SAP markers.
(E) Time point qPCR analysis NCPC-2d, NCPC-4d, and NCPC-6d normalized to CNP showing NC/SAP markers appear progressively. β2M, β2-microglobullin (housekeeping gene). N ≥ 3 independent experiments.
Error bars represent mean ± SEM. ns, not significant, ∗p > 0.05, ∗∗p > 0.01, ∗∗∗∗p > 0.0001.
The duration and level of BMP exposure effects differentiation of SAPs (Huber et al., 2008, Schneider et al., 1999), and FGF2 induces neuronal differentiation (Carnahan and Patterson, 1991b). We investigated whether there would be an effect of prolonging FGF2/BMP2 exposure (that is NCPC-6d compared with NCPC-4d). qPCR analysis showed, compared with the starting H9 hESCs, an upregulation of SOX10, and also a modest increase in MYCN expression, a marker for NC cells, SAPs, and neurons (Mobley et al., 2015, Saxena et al., 2013). The expression of the pro-neuronal transcription factors, ASCL1, HAND2, and PHOX2B, and the CA synthesis enzymes was increased (Figure 1D). Also increased was SOX2, which is important in pluripotency and in the neuroectoderm, but is downregulated in early NC then later upregulated (Cimadamore et al., 2011), while the pluripotency gene OCT4 was suppressed (Figure 1D).
Using the CNP cells as a basis (see Figure 1A), the early NC genes SOX10 and MYCN commenced upregulation quickly (detectable in NCPC-2d cells). mRNA for the SA specification transcription factor GATA3 (Moriguchi et al., 2006) was also detected in NCPC-2d cells, increased about 9-fold in NCPC-4d, before decreasing slightly in NCPC-6d cells (Figure 1E). In contrast the increase in the pro-neuronal gene HAND2 and the CA synthesis enzyme genes, which reflect later SA differentiation, were only apparent after 6 days of FGF2/BMP2 treatment (Figure 1E). RET, coding for the receptor for GDNF, is highly expressed in human neuroectoderm (Attie-Bitach et al., 1998), is absent in early NC cells and is re-expressed in the sympathetic primordia but declines in adrenal chromaffin cells (Allmendinger et al., 2003, Huber et al., 2002). In the H9 hESC system, compared with neuroectoderm-like CNPs, RET expression followed a similar undulating trajectory (Figure 1E).
Increasing αTH, DβH, and PNMT expression by qPCR analysis is consistent with SA differentiation related to duration of FGF2/BMP2 exposure (Figures 1D and 1E). SA1 immunoreactivity marks SAPs, increasing in chromaffin cells and decreasing in sympathetic neurons (Carnahan and Patterson, 1991a, Lumb and Schwarz, 2015). FACS showed that almost 80% of p75NTR+ cells of NCPC-4d were SA1+, declining to 66% 2 days later (Figure 2B). In contrast, the proportion of NCPCs co-expressing the SA marker ganglioside GD2 and the pro-neuronal marker B2B1 increased from 4 to 6 days (see below). NF expression was also detected in NCPCs by FACS, using SK-N-BE(2)C human neuroblastoma cells and undifferentiated hESCs as positive and negative controls for SA and sympathetic marker expression (Figure S3). This is consistent with NCPCs progressing to an SAP state initially, but longer FGF2/BMP2 favoring neuronal lineages at the expense of chromaffin properties (Anderson et al., 1991, Carnahan and Patterson, 1991b, Stemple et al., 1988). Nevertheless, the NCPC-6d population was still heterogeneous (see Figures 1C and S2).
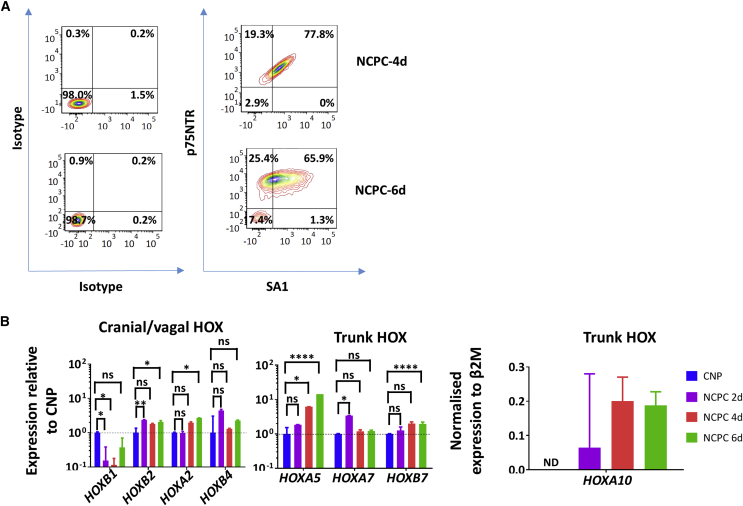
Figure 2.
Human NCPCs Express SA Markers and Possess the Positional Identity of Trunk NC Cells
(A) FACS analysis of differentiation of H9 NCPC-4d and NCPC-6d (both representative of ten separations) with heightened expression of NCPC marker p75NTR and SAP marker SA1.
(B) qPCR HOX gene analysis of CNP, NCPC-2d, NCPC-4d, and NCPC-6d. CNP (cranial positional identity, low-number HOX) was used to normalize the expression except for HOXA10, which was normalized to β2M. NCPC/SAP induction is accompanied by decreased expression of lowest-number and increased expression of higher-number HOX paralogs. ND, not detectable, pooled from N = 4 different inductions each, PCRs in triplicate.
Error bars represent mean ± SEM. ns, not significant, ∗p > 0.05, ∗∗p > 0.01, ∗∗∗∗p > 0.0001.
NCPCs Have a Trunk NC Identity
Antero-posterior positional information is important in NC development (Lee et al., 2005, Zhang et al., 2010), and a major mediator is the HOX gene code (Nelms and Labosky, 2010). For trunk positional identity consistent with SAPs, the hESC-derived NCPCs should express higher-number trunk HOX genes (Huber et al., 2012) rather than the low-number cranial and vagal HOX genes (Figures 2B and S4). We performed qPCR analysis for HOXB1, A2, B2, B4, A5, A7, B7, and A10 (Bhatt et al., 2013). Cranial gene HOXB1 was downregulated relative to CNP and HOXA2, B2 and B4 were not elevated (Figure 2B). HOXA5 expression, marking the vagal/trunk transition, was upregulated relative to CNP cells by the NCPC/SAP differentiation process but not to the degree seen in NCPCs with vagal properties (Figures 2B and S4). HOXB7 was upregulated over 6 days of FGF2/BMP2 treatment relative to CNPs (Figure 2B), and HOXA10 expression was also increased especially compared with vagal NCPCs (Figure S4).
Differentiating hESC-Derived NCPCs to Chromaffin Cells
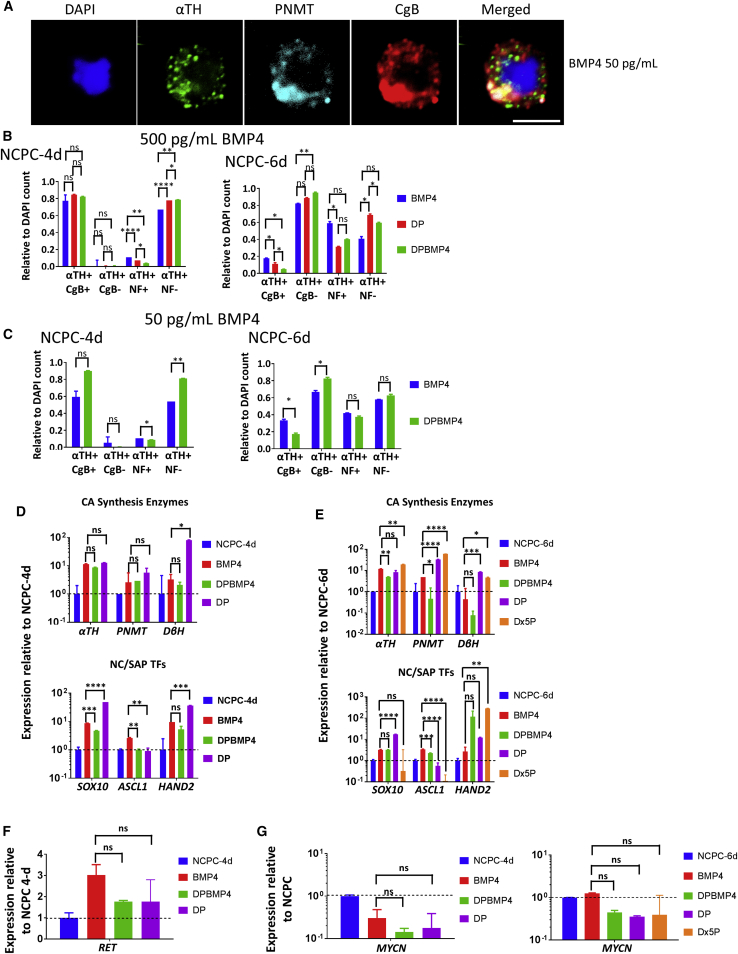
We developed a protocol (Figures 1A and S1B) based on that used for mouse cells (Saxena et al., 2013), and for human adrenal chromospheres (Santana et al., 2012), to obtain human chromaffin-like cells, using markers used by the latter. We subjected the H9 NCPCs (i.e., cells previously exposed to FGF2/BMP2) in vitro for 6–9 days with 500 pg/mL human recombinant BMP4 (without FGF2), or with dexamethasone plus phorbol 12-myristate 13-acetate (PMA) (Dex + PMA = DP), or both (DPBMP4). This resulted in upregulation of SAP/chromaffin markers αTH and CgB and, most importantly, chromaffin-restricted marker PNMT by immunofluorescence, thus demonstrating the chromaffin differentiation capacity of the hESC-derived NCPCs (Figure 3A).
Figure 3.
Human NCPCs/SAPs Differentiate into Chromaffin-like Cells In Vitro
(A) Immunofluorescence of H9 NCPCs/SAPs differentiated with BMP4, showing co-expression of SAP marker, αTH, chromaffin marker, PNMT, and storage vesicle marker, CgB. Scale bar, 5 μm.
(B and C) Immunofluorescence count of differentiation of NCPC-4d and NCPC-6d cells with 6 days high or low BMP4 stained for αTH, NF-200kDa, and CgB (see Figures 1A and S1B). Longer initial FGF2/BMP2 exposure resulted in a higher proportion of neuronal (NF+) and a lower proportion of CgB+ SAP cells. DAPI stain was used to assess the total number of cells. N = 8 independent experiments.
(D and E) qPCR analysis of NCPC-4d and NCPC-6d cells differentiated with BMP4 and/or DP for 6 days. BMP-4 and corticosteroids increase chromaffin marker PNMT as well as SA markers. N = 4 independent experiments.
(F and G) qPCR analysis of neuronal markers RET and MYCN of NCPC-4d and NCPC-6d cells with BMP4 and/or DP for 6 days. Inclusion of corticosteroids associated with lower neuronal marker expression. N = 4 independent experiments.
Error bars represent mean ± SEM. ns, not significant, ∗p > 0.05, ∗∗p > 0.01, ∗∗∗p > 0.001, ∗∗∗∗p > 0.0001.
We investigated the efficiency of differentiating chromaffin cells from dissociated NCPC-4d and NCPC-6d cells, the latter having a longer initial treatment with FGF2, with both having continuous exposure to BMP2/4. αTH was highly efficiently induced in all conditions, but DP plus BMP4 treatment of NCPC-4d cells resulted in a high proportion of cells that were CgB+, but few cells that were NF+ (i.e., neuronal) (Figure 3B left). However, with NCPC-6d as the starting point, BMP4 induced more NF+ cells while CgB+ cells were reduced (Figure 3B right), which is consistent with elevated pro-neuronal B2B1+ cells at the NCPC-6d stage (see below). Genes for CA synthesis enzymes and NC and SA transcription factors increased expression (Figure 3D). MYCN favors the differentiation of sympathetic neurons and acts against differentiation of chromaffin cells (Mobley et al., 2015, Zhu et al., 2012). Cells differentiated from NCPC-4d cells under BMP4, DP, and DPBMP4 all showed less MYCN expression by qPCR than the starting NCPC-4d SAP cells (Figure 3G). On the other hand, cells differentiated from NCPC-6d cells under BMP4-only showed no decrease in MYCN expression, but addition of DP, or DP alone, did decrease MYCN (Figure 3F). This was also reflected in the immunofluorescence count in Figure 3B. These results suggest that, after 4 days of FGF2/BMP2, withdrawal of FGF2 but continuance of BMP allows SAPs to divert from neuronal differentiation and toward the chromaffin lineage, whereas after 6 days of FGF2/BMP2, glucocorticoids are required to achieve this. Thus, timely withdrawal of the neuronal differentiation factor FGF2 (Anderson, 1993) is important for chromaffin cell differentiation.
The concentration of BMP4 influences SAP development (Reissmann et al., 1996). We confirmed that BMP4 as low as 50 pg/mL activated its downstream signaling pathway in αTH+ cells by employing an antibody to phosphorylated SMAD1/5 (Figure S5A). Reducing BMP4 from 500 to 50 pg/mL decreased the proportion of CgB+ cells for both NCPC-4d and NCPC-6d cells, but NF+ cells were not greatly altered. The decreased CgB+ cell numbers at NCPC-4d with low BMP4 was prevented by simultaneous DP (Figure 3C), confirming the importance of both BMP4 and glucocorticoids in chromaffin differentiation (Hodel, 2001, Saxena et al., 2013). We also investigated glucocorticoid concentration by qPCR for αTH, PNMT, DβH, SOX10, ASCL1, and HAND2, comparing 10 and 50 mM dexamethasone. This increase in dexamethasone did not significantly alter αTH and PNMT expression, whereas HAND2 was strongly increased and ASCL1 and SOX10 were reduced (Figure 3E).
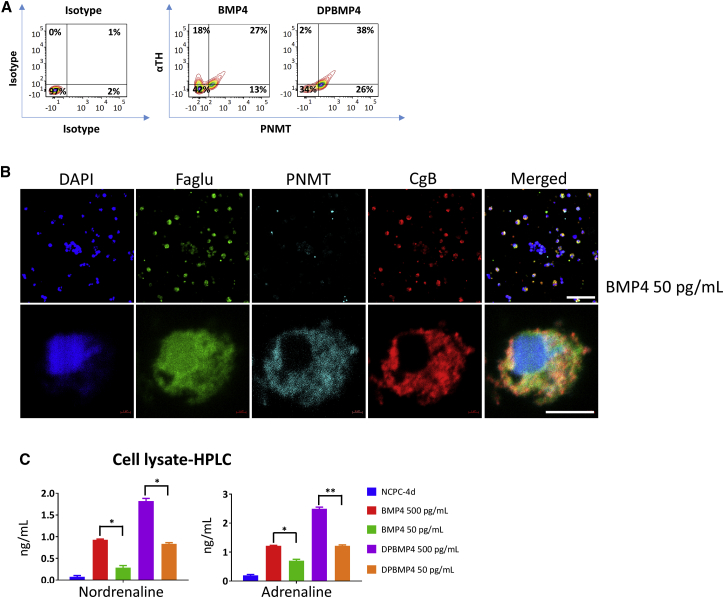
To investigate the efficiency of differentiation, we fixed and permeabilized cells after 6 days of chromaffin differentiation of NCPC-4d cells, and stained them with antibodies to αTH, CgB, and PNMT. FACS showed that BMP4 alone upregulated the percentage of cells expressing αTH, CgB, and PNMT, with no significant difference in DP and DPBMP4 as we have already shown with the immunofluorescent counts (Figures 4A, 3B, 3C, and S3). In vivo, αTH+ adrenal chromaffin cells are both PNMT+ and PNMT− (Huber, 2006). In our case, about 30%–40% of αTH+ cells were PNMT+ (Figure 4A).
Figure 4.
Chromaffin-like Cells Differentiated from NCPCs/SAPs Produce CAs
(A) Representative FACS plot of NCPCs differentiated to chromaffin-like cells as analyzed using αTH and PNMT antibodies. The plot suggests emergence of αTH+/PNMT− and αTH+/PNMT+ sub-populations in BMP4-only conditions, while addition of DP reduces the PNMT sub-population. N = 8 independent experiments; error bars represent mean ± SEM.
(B) Immunofluorescence of H9 NCPC-4d cells differentiated with BMP4 (50 pg/mL) for 9 days with the chromaffin markers, PNMT, CgB, and Faglu, markers of CA synthesis and storage. Scale bars, 200 and 5 μm.
(C) HPLC analysis of CA content in lysates of NCPC-4d cells differentiated for 9 days with BMP4 (500 pg/mL), BMP4 (50 pg/mL), DPBMP4 (500 pg/mL), and DPBMP4 (50 pg/mL). BMP4 and corticosteroid mimetic have a stimulatory effect on CA levels. N = 3 independent experiments. Error bars represent mean ± SEM. ∗p > 0.05, ∗∗p > 0.01.
Sub-populations from rodent and bovine adrenal chromaffin cells proliferate, unlike human chromaffin cells (Sicard et al., 2007, Tischler and Riseberg, 1993). To investigate proliferation, we immunostained the 9-day BMP4DP-treated human cells with Ki67 antibody. Ki67 staining was confined to weakly stained αTH+/CgB+ cells, so proliferation ability in the human chromaffin-like cells at this stage is likely to be minimal (Figure S5B).
We hypothesized that the NCPCs treated with BMP4 or DP will possess CAs including adrenaline. These cells were processed for Faglu (Furness et al., 1977), which converts CAs to a fluorescent product. These were co-immunostained for SAP marker CgB and chromaffin marker PNMT. Most of the cells staining with Faglu co-stained for PNMT and CgB, consistent with CA synthesis and storage (Figure 4B). To measure CA directly, we performed high-performance liquid chromatography (HPLC) on sonicated cell lysates of chromaffin-like cells differentiated from H9 NCPCs with BMP4 only and with DPBMP4. HPLC detected both noradrenaline and adrenaline, with DPBMP4-treated cells showing moderately higher levels than BMP4-only treated cells (Figure 4C). The results also showed that lowering the BMP4 concentration from 500 to 50 pg/mL reduced the concentration of CAs which corresponds with the data in Figures 3B and 3C.
Sorting for GD2 Expression Enriches for SAP-like Cells
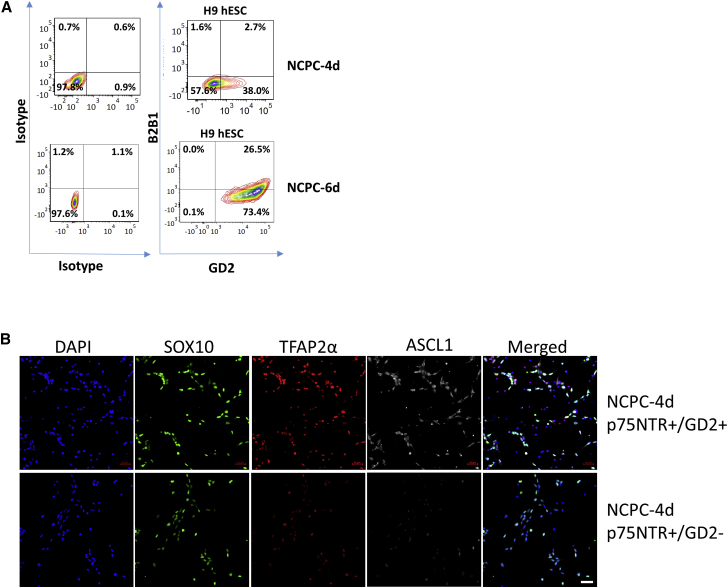
GD2 sorting enriched SAP-like cells in the mouse ESC model (Saxena et al., 2013). We asked firstly whether GD2 is expressed in human NCPCs. Then, because NCPC populations were heterogeneous (Figure 3B), we asked whether sorting NCPCs for GD2 would improve the selection for chromaffin-like cells.
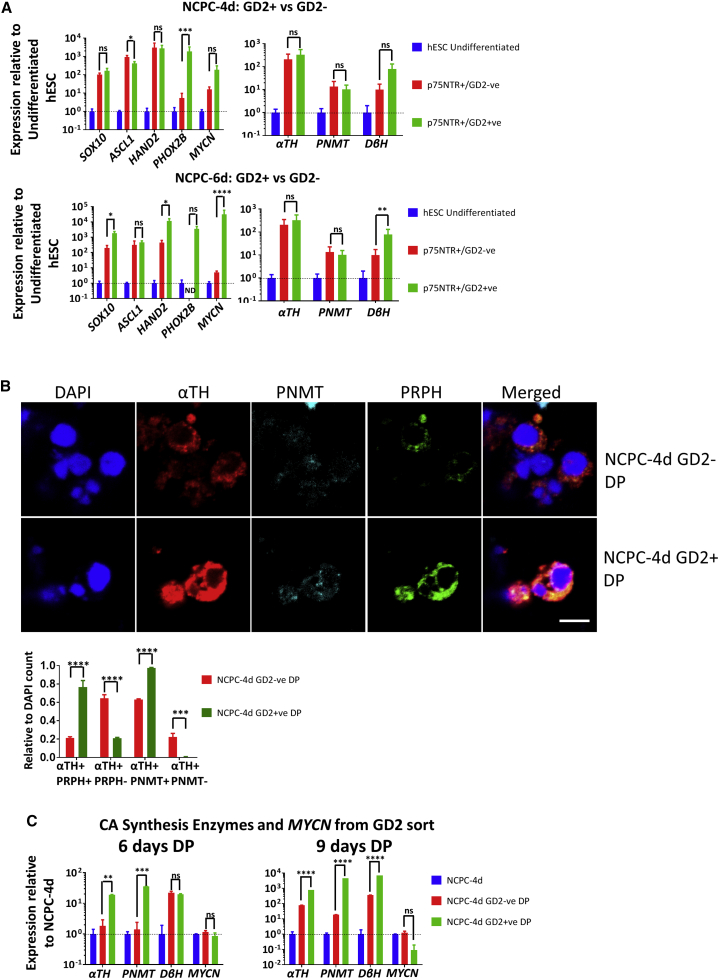
NCPCs show a maturation of SA (GD2) and pro-neuronal (B2B1) properties (Figure 5A). NCPC-4d cells showed GD2 expression in about 40% of p75NTR+ cells, but, by 6 days, GD2 was expressed by all p75NTR+ cells. The p75NTR+/GD2+ and p75NTR+/GD2− NCPC sub-populations expressed NC markers SOX10, TFAP2α, and ASCL1 by immunostaining, and, for the latter two, labeling was more intense in the p75NTR+/GD2+ sub-population (Figure 5C). Comparison by qPCR showed a trend for GD2-associated upregulation of all NC and SAP genes without attaining significance for all markers (Figure 6A).
Figure 5.
Expression of GD2 and B2B1 in NCPC/SAP Lineages and GD2 Selection
(A) FACS analysis of p75NTR+ H9 NCPC-4d and NCPC-6d cells with GD2 (SAP lineages) and B2B1 (neuroblast lineage) shows increase in SA and neuronal differentiation. Each representative of ten independent experiments.
(B) Immunofluorescence of NCPC-4d cells sorted as p75NTR+/GD2+ and p75NTR+/GD2−, and labeled with NCPC markers SOX10 and TFAP2α, and SAP marker ASCL1, the latter being enriched by GD2 selection. Scale bar: 50 μm.
Figure 6.
FACS for GD2 Enriches SAP-like Cells
(A) qPCR analysis of SA genes in p75NTR+/GD2+, p75NTR+/GD2− H9 NCPC-4d, and NCPC-6d cells. Expressions were normalized to that of hESCs. ND, not detectable at <35 cycles. N = 3 (4d) and N = 3 (6d) independent experiments.
(B) NCPC-4d p75NTR+/GD2+ and p75NTR+/GD2− cells after 6 days differentiation in DP express SA (αTH), chromaffin (PNMT), and neuronal (PRPH) markers. Scale bar, 5 μm. After DP differentiation, PNMT and PRPH are extensively co-expressed, and proportions of these cells are both increased in the GD2+ population, and all αTH+/GD2+ cells expressed PNMT. DAPI was used to count the total cells. N = 4 independent experiments.
(C) qPCR analysis of NCPC-4d p75NTR+/GD2+ cells differentiated with DP for 6 and 9 days. GD2 preselection augments PNMT expression as well as other SAP genes. ND, not detectable, N = 4 independent experiments.
Error bars represent mean ± SEM. ns, not significant, ∗p > 0.05, ∗∗p > 0.01, ∗∗∗p > 0.001, ∗∗∗∗p > 0.0001.
We differentiated the GD2– and GD2+ cells using DP treatment for 6 days and analyzed them by immunofluorescence with SA (αTH), chromaffin (PNMT), and neuronal markers (PRPH) (Saxena et al., 2013). Cells derived from GD2+ precursors showed relatively higher level and proportion of expression (Figure 6B), which paralleled results using mouse cells (Saxena et al., 2013). We also analyzed the differentiated cells by qPCR normalized to levels in NCPC-4d cells. GD2+ cells when differentiated with DP showed greater upregulation of αTH, PNMT, and DβH compared with GD2-cells, and this increased between 6 and 9 days differentiation (Figure 6C). We conclude that sorting for GD2 expression after NCPC induction is a useful means of increasing SA and chromaffin cell differentiation in the subsequent stage of differentiation.
Similarities and Differences with Different Pluripotent Cell Lines
Similar trends in NCPC generation were seen in H9 and HES3 hESC- and 007 iPSC-derived cells (Figures 1B, 5B, and S2). Upregulation of CA synthesis enzyme genes αTH and PNMT was shown with qPCR in HES3- and 007-derived NCPC-4d cells exposed to BMP4 and DPBMP4 although, compared with H9 cells, DβH was relatively more strongly expressed by HES3 cells and was not upregulated in 007 iPSCs (Figures 3D and S6A). There was a reduction in RET mRNA expression with BMP4 and DPBMP4 in 007 iPSC- and HES3 hESC-derived chromaffin-like cells, relative to NCPC-4d, whereas H9-derived cells showed a moderate upregulation, especially with BMP4 (Figures 3F and S6A). For mRNA expression of pro-neuronal MYCN from NCPC-4d cells treated with BMP4 or DPBMP4, 007 iPSC-derived cells showed a reduction similar to that in H9 cells, but HES3-derived cells displayed an upregulation of MYCN with BMP4 (Figures 3G and S6A). HES3-hESCs were differentiated to NCPCs, and the expression of GD2 (SA lineage marker) and B2B1 (pro-neuronal marker) were analyzed for p75NTR+ cells by FACS. Similar to H9s, a quarter of p75NTR+ HES3-derived NCPC-4d cells were GD2+, with very few co-expressing B2B1. However, after 6 days of FGF2/BMP2 exposure, a far higher proportion (over 90% compared with 26% in H9) of p75NTR+ HES3-derived cells co-expressed GD2 and B2B1 (Figures 5A and S6B).
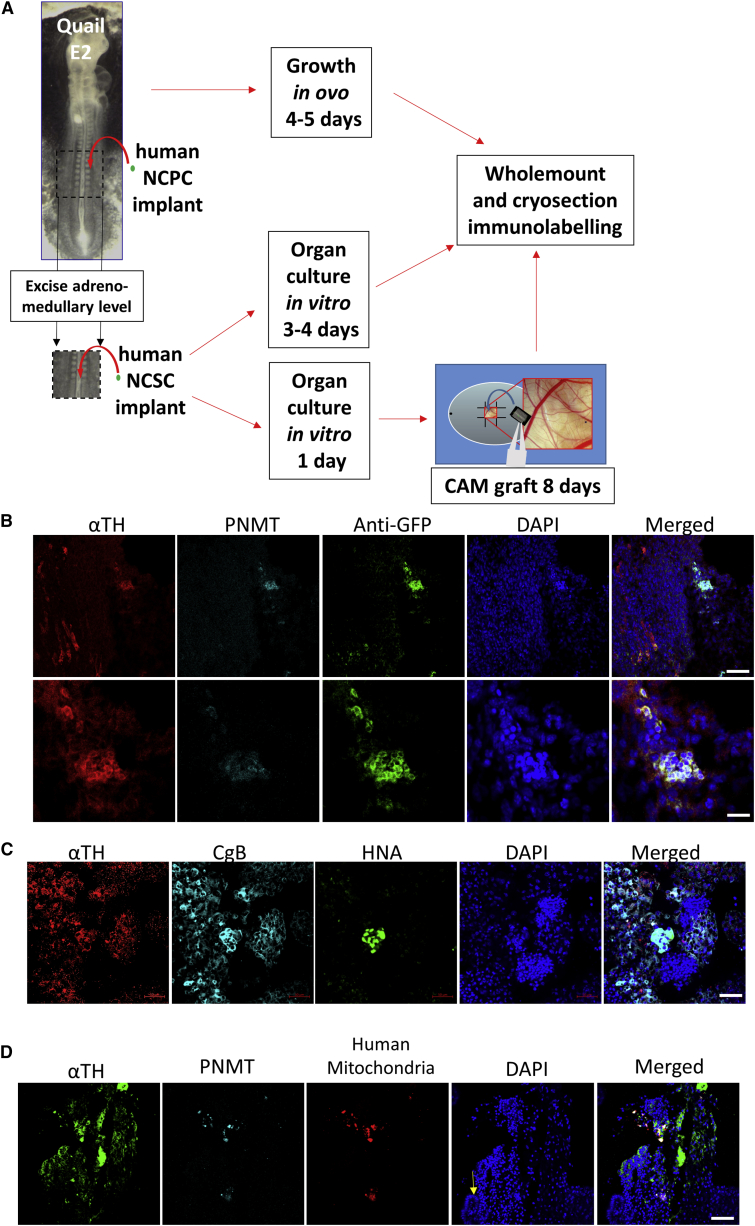
hESC-Derived NCPCs Migrate In Vivo and Differentiate into Cells Expressing Chromaffin Markers
To assess the behavior of hESC-derived NCPCs in vivo, we differentiated GFP+ ENVY-HES3 cells (Costa et al., 2005) along NCPC/SAP lines (Hotta et al., 2009) and transplanted them next to the “adrenomedullary” neural tube of quail embryos in ovo and incubated the embryos for 4–5 days (N = 9; Figure 7A). Confocal analysis of cryosections showed that human NCPC neurosphere cells migrated away from the implant and differentiated into αTH+ and PNMT+ cells. The human GFP+ cells had in general migrated ventral to the vertebral cartilage where sympathetic tissues occur. Frequently αTH+ human cells were associated with quail αTH+ cells. Other cells were GFP+ but TH−, or PNMT− (Figures 7B and S7). This is expected since the hESC-derived NCPCs include SAP and NC-like cells.
Figure 7.
NCPC/SAP-like Cells Migrate and Differentiate in Embryonic Tissues
(A) Scheme of the transplantation of hESC-derived NCPCs in quail E2 embryos and incubated in vivo or cultured in vitro or grown on CAM.
(B) Immunofluorescence with chromaffin markers, αTH and PNMT, of NCPCs derived from ENVY-HES3 hESCs transplanted for 4 days in QE2 embryo. This frontal-oblique section is further ventral to the section shown in Figure S7. The human αTH+ cells associate with similar lineage host cells. Scale bars, 50 μm (upper) and 10 μm (lower).
(C) Immunofluorescence with SA markers, αTH and CgB, and human cell-recognizing antibody, anti-human nuclear antigen, of NCPCs derived from H9 hESCs transplanted into QE2 tissue and cultured in vitro for 4 days. Scale bar, 50 μm.
(D) Immunofluorescence with chromaffin markers, αTH and PNMT, and anti-human mitochondria antibody of NCPCs derived from H9 hESCs transplanted into QE2 tissue and cultured on CAM for 8 days. Section is through the αTH-expressing tissue at the margin of mesonephric kidney tissue (arrow). Scale bar, 50 μm.
We also transplanted NCPCs derived from H9 hESCs to the excised “adrenomedullary” level of E2 quail embryos, and grew these as organ cultures for 3–4 days (N = 9; Figure 7A). Transplanted human cells in the quail tissue were identified in whole mounts with anti-human nuclear antigen (HNA); these co-expressed αTH and CgB and were surrounded by αTH- and CgB-expressing HNA-negative quail cells (Figure 7C).
To achieve extended time and greater growth, we used the same organ culture format as CAM grafts (N = 24; Figure 7A) (Zhang et al., 2010). CAM graft cryosections could not be defined by conventional anatomical planes, but sections which had structures resembling renal tubules were immunostained. Human cells identified with mouse anti-human mitochondria antibody were found adjacent to tubular tissue in the CAM sections (Figure 7D), showed immunoreactivity for αTH and PNMT, and were often associated with quail αTH+ cells.
Discussion
There are previous reports on differentiation of NCPCs and SAP-like cells from human pluripotent cells (Denham et al., 2015, Lee et al., 2010) and differentiation of sympathetic neurons (Oh et al., 2016), but the directed differentiation of human pluripotent cells to chromaffin-like cells has not yet been described. Previous reports have outlined the isolation, culturing, and characterization of human and bovine adrenal chromaffin cells (Santana et al., 2012). Here, starting from human pluripotent cells, we show the temporal increment in numerous SAP-like cell markers in NCPCs in relation to duration of exposure to BMP2/FGF2, as shown in mouse and human ESC-derived SAPs (Oh et al., 2016, Saxena et al., 2013). Our results suggest that the NCPC population at this stage is heterogeneous with both NC- and SAP-like cells. We also showed the utility of sorting for the ganglioside GD2 to concentrate on the SAP moiety. We also analyzed positional identity based on the HOX gene expression.
These NCPCs/SAPs could be differentiated in vitro such that SA and chromaffin markers (CgB, SA1, PNMT, and adrenaline) were enhanced and sympathetic neuronal markers (B2B1, MYCN, and RET) reduced. Culture conditions were modeled on those that have been shown to play an important role in development, in mouse ESCs differentiation in vitro, and in human chromaffin cell culture (Huber et al., 2008, Santana et al., 2012, Saxena et al., 2013). Our results emphasize the combined importance of continued BMP signaling to increase chromaffin differentiation and curtailment of FGF2 to reduce sympathetic neuron differentiation, the chromaffin lineage choice being augmented by corticosteroid mimetics.
The NCPC/SAP differentiation method was robust in that cells expressing p75NTR and HNK-1 were at high proportion in three pluripotent cell lines (H9, HES3 hESCs, and 007 iPSCs), with H9 hESCs and 007 iPSC at higher proportion than HES3 hESCs. More cells expressed chromaffin markers in H9- and 007 iPSC-derived cells than in HES3-derived cells, but the latter showed a bias toward expression of neuronal markers (MYCN, B2B1). There have been previous reports on differences in differentiation between hESC lines (Osafune et al., 2008).
Human NCPCs transplanted in vivo into the quail exhibited SAP properties by migrating and differentiating into cells expressing SA (αTH and CgB) and chromaffin cell markers (PNMT). Typically, these human cells were associated with quail cells of the same lineage. This confirms the post-transplantation survival, integration, and differentiation ability of the human NCPCs in situ.
In conclusion, these human chromaffin-like cells derived from pluripotent cells have the potential for investigating differentiation of human chromaffin cells, for modeling specific diseases such as pheochromocytoma, for cell replacement strategies in neurodegenerative diseases, and for pain management.
Experimental Procedures
Ethics Statement
All experiments were performed with the approval of the Murdoch Children's Research Institute Animal Ethics Committee AEC650 and AEC677 and Institutional Biosafety Committee 226–2015 PC2. Human Stem Cell studies were performed with approval from the University of Melbourne Human Ethics, ID 1545384 and 0605017.
NCPC Induction
hESCs (H9 [WiCell], HES3 and HES3-ENVY [ESI International]) and hiPSC (007 iPSC) were cultured to 80%–90% confluence and harvested using 0.5 μM EDTA in CMF-PBS for 4–6 min. The buffer was gently removed and 2 mL of medium per 35 cm2 tissue culture dish was gently added to harvest the cells, which were counted using a hemocytometer. Cells were seeded at 10,000 per cm2 into laminin-coated (10 μg/mL in PBS, 1 hr; Life Technologies) organoid culture dishes with TESR-E8 complete medium (STEMCELL Technologies) supplemented with 10 μM Y-27632 (Sigma-Aldrich) and cultured at 37°C and 5% CO2 in a humidified incubator overnight; this is recorded as day 0. On day 1, the medium was changed to N2B27 medium (Denham et al., 2015) with 3 μM CHIR 99021 and 10 μM SB431542 (both Tocris) and cultured for 5 days. Medium was replaced on the day 3 after CHIR-SB induction. On day 5, the cells were termed caudal neural progenitors (CNPs) as they had a positional identity caudal to the forebrain (Denham et al., 2015).
CNPs were harvested using EDTA-CMF-PBS for 4–6 min and centrifuged at 200 × g for 3 min. The pellet was resuspended in Neurobasal Medium (Denham et al., 2015) supplemented with 10 ng/mL BMP2 and 20 ng/mL FGF2 (both PeproTech), and plated in 96-well ultra-low attachment plates (In Vitro Technologies), in which the cells aggregated to form spheres. On day 7, 9, and 11 (i.e., 2, 4, and 6 days with BMP2/FGF2, termed NCPC-2d, NCPC-4d, and NCPC-6d), neurospheres were harvested and dissociated using EDTA-CMF-PBS buffer for 4–6 min and spun down. This is shown in Figure 1A. To assay for NCPC markers by immunofluorescence, cells were plated for 5–6 hr on an 8-well Nunc Lab-Tek II Chamber Slide System (Thermo Fisher Scientific) coated with laminin as above. Otherwise, cells were subjected to chromaffin cell differentiation (Figures 1A and S1B, see below).
For NCPC/vagal NC differentiation, CNP pellets were processed as above but with BMP2 50 ng/mL and 50 nM retinoic acid and plated for 6 days (Denham et al., 2015, Fattahi et al., 2016).
Chromaffin Cell Differentiation from NCPCs and GD2-Sorted Cells
For differentiation of NCPCs and GD2-sorted NC-derived SAP-like cells toward the chromaffin lineage (see Figure 1B), NCPC-4d and NCPC-6d neurospheres were harvested, spun down, and incubated in EDTA-CMF-PBS buffer for 4–6 min to achieve single cells. Then, 3 mL of NBM medium per 96-well plate of neurospheres was added and centrifuged, and cells were counted before seeding them for differentiation. For chromaffin cell differentiation, dissociated cells (8,000–10,000 cells per well) were differentiated for 6–9 days in 96-well ultra-low attachment plates in Differentiation Medium (DM) of DMEM/F12, 1× GlutaMAX, 2% B27, 0.5% BSA, 1× penicillin/streptomycin (Gibco) supplemented with 500 pg/mL (or 50 pg/mL) recombinant human BMP4 (R&D Systems) with or without 10 mM (or 50 mM) dexamethasone (Sigma-Aldrich) and 100 nM PMA (Millipore). Medium was half changed every 3 days. Cells were analyzed by qPCR (see below) and cells for immunofluorescence analysis (see below) were transferred directly from the 96-well plate into 12-well chamber slides with removable microscopy glass slides (ibidi, Germany) coated with human plasma fibronectin (EMD Millipore, 10 μg/mL in CMF-PBS for 1 hr at room temperature) and cultured for 4–5 hr. Cells from 2 wells of a 96-well plate were plated in one well of the 12-well slide.
For chromaffin cell differentiation of GD2 FACS-sorted cells, cells were dissociated as above and processed for FACS by antibody staining. FACS-sorted cells, p75+/GD2+ only, and p75+/GD2−, were differentiated for 6 days in 96-well ultra-low-attachment plates in DM. Cells were analyzed by qPCR (see below) and immunofluorescence as above.
RNA Extraction and SYBR Green qPCR
Using TRIzol (Invitrogen), total RNA was extracted from cultured cells and lysate were purified by acid-phenol chloroform and recovered by isopropanol/ethanol precipitation. Extracted RNA was digested with DNaseI (Promega), following the manufacturer's instructions, to remove residual DNA. cDNA was synthesized using the Bioline SensiFAST cDNA Synthesis Kit (BIO-65054). In brief, 30 ng total RNA was converted into cDNA following the manufacturer's directions. Reactions were performed using cDNA converted from 30 ng of RNA, 50 nM of each primer, and AccuPower 2X GreenStar Master Mix solution (Bioneer, South Korea), in a total volume of 10 μL. Primers for qPCR analysis are listed in Table S1. For TaqMan Assay analysis, reactions were performed using cDNA converted from 30 ng of RNA, 1× of TaqMan Probe, and GoTaq Probe qPCR Master Mix solution (Promega) in a total volume of 10 μL. All runs were in triplicates and more than 3 (N ≥ 3) independent experiments. β2-Microglobullin (β2M) housekeeping gene was used for data normalization when the relevant gene was undetectable in the control population. Cultured hESCs, CNP, NCPC-4d, and NCPC-6d were used as a calibration standard and relative gene expression changes were calculated using the 2–[Δ][Δ]Cq method.
Immunofluorescence
Cells were fixed with 1% paraformaldehyde (PFA) for 15 min, and permeabilized and blocked for 30 min with 0.2% Triton X-100 and 3% horse serum in CMF-PBS plus 0.02% (w/v) sodium azide. The cells were then washed once with CMF-PBS and stained with primary antibodies against αTH, PRPH, CgB, CgC, NF-200 kDa (all Abcam), ASCL1, SOX10 (both R&D Systems), PNMT (Thermo Scientific), and Phospho-Smad1/5 (Ser463/465) (41D10) (Cell Signaling Technology) diluted in blocking buffer at 4°C overnight (refer to Table S2 for full list of antibodies). For detection, cells were incubated with fluorochrome-conjugated secondary antibodies for 2–3 hr at room temperature. After counterstaining the nuclei with 10 ng/mL DAPI (Sigma-Aldrich) and mounting in 20 μg/mL 1,4-diazabicyclo[2.2.2]octane (DABCO) (Sigma-Aldrich) in glycerol, cells were viewed using a Zeiss LSM 780 confocal microscope (Oberkochen, Germany). Optical and electronic parameters were maintained for experiments comparing different conditions.
For whole-mount staining, tissues were stained as described (Abu-Bonsrah et al., 2016). In brief, tissues were fixed in 4% PFA in PBS at 4°C overnight then washed in PBS three times. Tissues were blocked and permeabilized with 3% horse serum and 0.2% Triton X-100 in PBS/azide for 1 hr. Primary antibodies were applied in 1% horse serum and 0.1% Triton X in PBS azide and incubated on a rocker at 4°C overnight. Washing with PBS was done for 3 hr, with changes every 30 min on a rocker at 4°C. Secondary antibodies and 500 ng/mL DAPI were applied and incubated for 3 hr on the rocker at 4°C. Tissues were washed with PBS three times and mounted using DABCO/glycerol mounting medium and then analyzed by confocal microscopy.
For cryostat sections, fixed tissues (4% PFA, 1 hr) were placed in 30% sucrose in CMF-PBS overnight, embedded in Tissue Tek OCT Medium in Tissue Tek Cryomoulds (from ProSciTech, Thuringowa, Australia) and frozen in dry ice-cooled isopentane. Twenty-μm sections were cut using a Leica CM 1900 cryostat microtome and collected on Superfrost microscope slides (Biolab Scientific, Auckland, NZ) coated with poly-L-lysine.
Faglu Fluorescence of CAs
NCPCs were fixed in 4% PFA and 0.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.0, for 1–2 hr at room temperature (Furness et al., 1977) to convert CAs to a fluorescent compound. Cells were washed thrice in CMF-PBS and permeabilized as described above for combination with immunostaining. The Faglu signal was detected using the 488 filter set of the Zeiss LSM 780 confocal microscope.
Cell Quantification
For immunofluorescence cell counts, Olympus IX70 and confocal images were selected and analyzed using the Zeiss Image Analyser and ImageJ (NIH). The total number of DAPI nuclei was used as the total number of cells in a field. With the DAPI channel and one other color channel, the cells in a field were counted. The ratio of the different colors to DAPI was calculated. The number of fields counted varied from three to ten, and images were blinded among counters.
Cell Culture
The human neuroblastoma line SK-N-BE(2)C (ECACC nos 95011817) was grown in DMEM supplemented with 10% fetal bovine serum (FBS) and 1× penicillin/streptomycin (both Life Technologies, Carlsbad, CA, USA), and maintained in a humidified incubator at 37°C with 5% CO2. Cells were then harvested for FACS.
HPLC for CA Detection
Differentiated cells for HPLC were prepared according to a previously published protocol (Saxena et al., 2013). In brief, 20,000 cells were homogenized in 250 μL sonication buffer of 0.1 M acetic acid (Sigma) and 2% EDTA (Gibco). QSonica sonicator (Newtown, CT, USA) was used to sonicate the cells on ice with 3 bursts (5 s each) at 50% amplitude, at 25-s intervals. Sonicated lysate was centrifuged for 5 min at 10,000 rpm and the supernatant was used to analyze CAs by HPLC with fluorescence detection. The manual derivatizations were performed as described previously (van der Hoorn et al., 1989). For the derivatization, 10 μL of 1 M KOH (to neutralize the acetic acid) was added to 40 μL of sample, which was followed by 68 μL of solution R1 (potassium ferricyanide:acetonitrile containing 10 μM isoprenaline as internal standard, 1,000:80) and 38 μL of solution R2 (1,2-diphenylethylediamine:bicine, 600:300). Incubation was for 45 min at 37°C. Injection volume was 100 μL.
For chromatography, the column was a 15 cm × 3.9 mm Waters Symmetry C18, and the mobile phase was 35% acetonitrile in 50 mM sodium acetate, pH 6.8, and run at 2 mL/min. The run-time was 60 min for each sample. Detection was by fluorescence at 350 nm excitation and 480 nm emission. Reference standards of CAs were obtained from the Department of Anaesthesia and Pain Management, Royal Melbourne Hospital.
FACS and Analysis
NCPC aggregates were harvested using the EDTA-CMF-PBS buffer for 4–6 min and centrifuged at 200 × g for 5 min. The pellet was then resuspended in 5 mL of NBM medium, and 10 μL was counted using a hemocytometer. Mouse IgM B2B1 antibody and rabbit p75NTR antibody (for antibodies, see Table S2) was used to indirectly label the cells for 30 min on ice. Cells were spun down and washed with CMF-PBS, and then followed by the respective secondary antibodies, donkey anti-rabbit (Alexa Fluor 594) and anti-mouse IgM (Alexa Fluor 488) for 30 min in the dark. The cells were washed with CMF-PBS and also directly labeled with mouse IgG GD2 antibody (Alexa Fluor 647) for 30 min on ice in the dark. For HNK1 and SA1 analysis, mouse HNK1 IgM antibody and mouse SA1 IgG antibody were used co-labeled with rabbit p75NTR antibody as above. Goat anti-mouse IgG-specific (Alexa Fluor 647) and goat anti-mouse IgM-specific (Alexa Fluor 488) were used to label the cells since these distinguish between the IgG and IgM primary antibodies. Mouse serum and rabbit IgG for isotype staining (1:500, Jackson ImmunoResearch) was used to gauge the degree of non-specific binding of antibody. The cells were washed with CMF-PBS, pelleted, and resuspended in CMF-PBS containing 2% FBS and strained (40 μm mesh; BD Falcon; Becton Dickinson) and FACS sorted using the BD FACSAria Fusion Cell Sorter (Becton Dickinson, Franklin Lakes, NJ), with separation based on secondary antibody, DAPI and live cell staining.
To detect intracellular proteins, cells fixed with 1% PFA were permeabilized with 0.1% Triton X in 1% horse serum, blocked with 1% horse serum, and incubated with primary antibodies in blocking buffer at 4°C overnight. After washing with CMF-PBS and centrifuging, appropriate secondary antibodies were added for 1 hr and cells were washed and events were acquired with BD FACS X-20 Fortessa. Data were analyzed using CellQuest (both from BD Biosciences) and FCS Express 4 Flow (De Novo Software).
In Vivo Transplantation of hESC-Derived NCPCs
GFP+ ENVY-hESCs were differentiated to NCPCs and transplanted in ovo between the neural tube and somites at the “adrenomedullary” level of E2 quail embryos (Le Douarin and Teillet, 1974). Incubation was continued for 4–5 days in a 65% humidified incubator at 38°C. The adrenomedullary regions were excised and fixed, embedded in Tissue Tek OCT, and frontal cryosections were collected as above. Human cells were identified with antibody to GFP.
Organ Culture and CAM Grafts of hESC-Derived NCPCs
E2 quail embryos were harvested and the adrenomedullary region was excised. Aggregates of H9 NCPCs were incubated with Calcein AM cell-permeant dye (1/2000; Thermo Fisher Scientific) for 20–30 min to make the implant visible. After implantation as above, these were grown in Ham's F12 supplemented with 10% FCS, 1× pen-strep, and 5% E4 quail embryo extract in a 65% humidified incubator at 38°C. After 24 hr, tissues were checked to confirm that the implant cells had attached to the tissue. For organ culture, these were continued in vitro for a further 3–4 days. For extended time and greater growth the ensemble was transferred to embryonic day 8 chick embryo CAM and grown for 8 days (Zhang et al., 2010). Grafts were fixed, and either whole-mount immunolabeled for in vitro organ cultures or, for CAM grafts, embedded in Tissue Tek OCT and cryosectioned. Human cells were identified with mouse antibodies to HNA and to human mitochondria.
Statistical Analyses
Experiments were independently replicated (i.e., from time zero of Figure 1) three or more times (given as N), with technical replicates (e.g., qPCR) in triplicate. Data were analyzed by using one-way ANOVA (when analyzing only one variable: gene expression), two-way ANOVA (when analyzing two variables: time and gene expression), and unpaired t test with Welch's correction. Values were expressed as mean ± SEM. Changes were deemed significant if the p value was >0.05. Statistical significance is indicated as follows: ∗p > 0.05, ∗∗p > 0.01, and ∗∗∗p > 0.001. Graphs were drawn using GraphPad Prism.
Author Contributions
K.D.A.-B. designed and performed the in vitro experiments. D.Z. and K.D.A.-B. performed and harvested the in vivo transplantation experiments and the immunofluorescence evaluations. A.R.B. performed the HPLC experiments. K.D.A.-B. and D.F.N. wrote the manuscript. K.D.A.-B., D.Z., A.R.B., M.D., and D.F.N. approved the final draft of the manuscript. M.D. and D.F.N. provided the funding for this project.
Acknowledgments
We thank Matthew Burton and Paul Lau (Flow Cytometry-MCRI) for their technical advice and support. The αTH, B2B1, TFAP2α, and SA1 antibodies were developed and supplied by the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. We would like to also thank Dr. Marthe Howard (University of Toledo) for her advice on antibody selection, A/Prof Alice Pebay for providing the 007 iPSC line, Rachael Chatterton, Centre for Neural Engineering, The University of Melbourne, for her help in culturing the 007 iPSC cell line, Ana Antonic-Baker for her advice on the statistical methods, and Dr. David Elliot, Murdoch Children's Research Institute, for the pSMAD antibody. This research was supported by NHMRC grant 1069757, grant 1050692, Stem Cells Australia (SCA) 2013F-9, Australian Research Council Future Fellowship, The University of Melbourne, and MCRI. Vagal NC-related constructs were contributed to by Foundation for Children 2014-211. We acknowledge the Victorian Government's Operational Infrastructure Support Program to MCRI. K.D.A.-B. holds an IPRS and APA (Int) PhD Scholarship through the Department of Paediatrics, University of Melbourne.
Published: December 7, 2017
Footnotes
Supplemental Information includes seven figures and two tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.11.003.
Supplemental Information
References
- Abu-Bonsrah K.D., Zhang D., Newgreen D.F. CRISPR/Cas9 targets chicken embryonic somatic cells in vitro and in vivo and generates phenotypic abnormalities. Sci. Rep. 2016;6:34524. doi: 10.1038/srep34524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmendinger A., Stoeckel E., Saarma M., Unsicker K., Huber K. Development of adrenal chromaffin cells is largely normal in mice lacking the receptor tyrosine kinase c-Ret. Mech. Dev. 2003;120:299–304. doi: 10.1016/s0925-4773(02)00455-0. [DOI] [PubMed] [Google Scholar]
- Anderson D.J. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu. Rev. Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- Anderson D.J., Carnahan J.F., Michelsohn A., Patterson P.H. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J. Neurosci. 1991;11:3507–3519. doi: 10.1523/JNEUROSCI.11-11-03507.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attie-Bitach T., Abitbol M., Gerard M., Delezoide A.L., Auge J., Pelet A., Amiel J., Pachnis V., Munnich A., Lyonnet S. Expression of the RET proto-oncogene in human embryos. Am. J. Med. Genet. 1998;80:481–486. doi: 10.1002/(sici)1096-8628(19981228)80:5<481::aid-ajmg8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Diaz R., Trainor P.A. Signals and switches in Mammalian neural crest cell differentiation. Cold Spring Harb. Perspect. Biol. 2013;5 doi: 10.1101/cshperspect.a008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan J.F., Patterson P.H. The generation of monoclonal antibodies that bind preferentially to adrenal chromaffin cells and the cells of embryonic sympathetic ganglia. J. Neurosci. 1991;11:3493–3506. doi: 10.1523/JNEUROSCI.11-11-03493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan J.F., Patterson P.H. Isolation of the progenitor cells of the sympathoadrenal lineage from embryonic sympathetic ganglia with the SA monoclonal antibodies. J. Neurosci. 1991;11:3520–3530. doi: 10.1523/JNEUROSCI.11-11-03520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadamore F., Fishwick K., Giusto E., Gnedeva K., Cattarossi G., Miller A., Pluchino S., Brill L.M., Bronner-Fraser M., Terskikh A.V. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell Stem Cell. 2011;8:538–551. doi: 10.1016/j.stem.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M., Dottori M., Ng E., Hawes S.M., Sourris K., Jamshidi P., Pera M.F., Elefanty A.G., Stanley E.G. The hESC line Envy expresses high levels of GFP in all differentiated progeny. Nat. Methods. 2005;2:259–260. doi: 10.1038/nmeth748. [DOI] [PubMed] [Google Scholar]
- Crivellato E., Nico B., Ribatti D. The chromaffin vesicle: advances in understanding the composition of a versatile, multifunctional secretory organelle. Anat. Rec. (Hoboken) 2008;291:1587–1602. doi: 10.1002/ar.20763. [DOI] [PubMed] [Google Scholar]
- Denham M., Hasegawa K., Menheniott T., Rollo B., Zhang D., Hough S., Alshawaf A., Febbraro F., Ighaniyan S., Leung J. Multipotent caudal neural progenitors derived from human pluripotent stem cells that give rise to lineages of the central and peripheral nervous system. Stem Cells. 2015;33:1759–1770. doi: 10.1002/stem.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker-Colin R., Verdugo-Diaz L. Cell transplantation for Parkinson's disease: present status. Cell. Mol. Neurobiol. 2004;24:301–316. doi: 10.1023/B:CEMN.0000022764.94760.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattahi F., Steinbeck J.A., Kriks S., Tchieu J., Zimmer B., Kishinevsky S., Zeltner N., Mica Y., El-Nachef W., Zhao H. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531:105–109. doi: 10.1038/nature16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J.B., Costa M., Wilson A.J. Water-stable fluorophores, produced by reaction with aldehyde solutions, for the histochemical localization of catechol- and indolethylamines. Histochemistry. 1977;52:159–170. doi: 10.1007/BF00492292. [DOI] [PubMed] [Google Scholar]
- Hodel A. Effects of glucocorticoids on adrenal chromaffin cells. J. Neuroendocrinol. 2001;13:216–220. doi: 10.1046/j.1365-2826.2001.00628.x. [DOI] [PubMed] [Google Scholar]
- Hotta R., Pepdjonovic L., Anderson R.B., Zhang D., Bergner A.J., Leung J., Pebay A., Young H.M., Newgreen D.F., Dottori M. Small-molecule induction of neural crest-like cells derived from human neural progenitors. Stem Cells. 2009;27:2896–2905. doi: 10.1002/stem.208. [DOI] [PubMed] [Google Scholar]
- Howard M.J. Mechanisms and perspectives on differentiation of autonomic neurons. Dev. Biol. 2005;277:271–286. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Huber K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev. Biol. 2006;298:335–343. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Huber K., Bruhl B., Guillemot F., Olson E.N., Ernsberger U., Unsicker K. Development of chromaffin cells depends on MASH1 function. Development. 2002;129:4729–4738. doi: 10.1242/dev.129.20.4729. [DOI] [PubMed] [Google Scholar]
- Huber K., Franke A., Bruhl B., Krispin S., Ernsberger U., Schober A., von Bohlen und Halbach O., Rohrer H., Kalcheim C., Unsicker K. Persistent expression of BMP-4 in embryonic chick adrenal cortical cells and its role in chromaffin cell development. Neural Dev. 2008;3:28. doi: 10.1186/1749-8104-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber K., Kalcheim C., Unsicker K. The development of the chromaffin cell lineage from the neural crest. Auton. Neurosci. 2009;151:10–16. doi: 10.1016/j.autneu.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Huber L., Ferdin M., Holzmann J., Stubbusch J., Rohrer H. HoxB8 in noradrenergic specification and differentiation of the autonomic nervous system. Dev. Biol. 2012;363:219–233. doi: 10.1016/j.ydbio.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Jozan S., Aziza J., Chatelin S., Evra C., Courtade-Saidi M., Parant O., Sol J.C., Zhou H., Lazorthes Y. Human fetal chromaffin cells: a potential tool for cell pain therapy. Exp. Neurol. 2007;205:525–535. doi: 10.1016/j.expneurol.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Krieglstein K., Henheik P., Farkas L., Jaszai J., Galter D., Krohn K., Unsicker K. Glial cell line-derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral and CNS neurons. J. Neurosci. 1998;18:9822–9834. doi: 10.1523/JNEUROSCI.18-23-09822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazorthes Y., Sagen J., Sallerin B., Tkaczuk J., Duplan H., Sol J.C., Tafani M., Bes J.C. Human chromaffin cell graft into the CSF for cancer pain management: a prospective phase II clinical study. Pain. 2000;87:19–32. doi: 10.1016/S0304-3959(00)00263-3. [DOI] [PubMed] [Google Scholar]
- Le Douarin N.M., Teillet M.A. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 1974;41:162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- Lee V.M., Bronner-Fraser M., Baker C.V. Restricted response of mesencephalic neural crest to sympathetic differentiation signals in the trunk. Dev. Biol. 2005;278:175–192. doi: 10.1016/j.ydbio.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Lee G., Kim H., Elkabetz Y., Al Shamy G., Panagiotakos G., Barberi T., Tabar V., Studer L. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat. Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- Lee G., Chambers S.M., Tomishima M.J., Studer L. Derivation of neural crest cells from human pluripotent stem cells. Nat. Protoc. 2010;5:688–701. doi: 10.1038/nprot.2010.35. [DOI] [PubMed] [Google Scholar]
- Levitt M., Spector S., Sjoerdsma A., Udenfriend S. Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J. Pharmacol. Exp. Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- Livett B.G., Dean D.M., Whelan L.G., Udenfriend S., Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981;289:317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- Lo L.C., Johnson J.E., Wuenschell C.W., Saito T., Anderson D.J. Mammalian achaete-scute homolog-1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5:1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- Lumb R., Schwarz Q. Sympathoadrenal neural crest cells: the known, unknown and forgotten? Dev. Growth Differ. 2015;57:146–157. doi: 10.1111/dgd.12189. [DOI] [PubMed] [Google Scholar]
- Mobley B.C., Kwon M., Kraemer B.R., Hickman F.E., Qiao J., Chung D.H., Carter B.D. Expression of MYCN in multipotent sympathoadrenal progenitors induces proliferation and neural differentiation, but is not sufficient for tumorigenesis. PLoS One. 2015;10:e0133897. doi: 10.1371/journal.pone.0133897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T., Takako N., Hamada M., Maeda A., Fujioka Y., Kuroha T., Huber R.E., Hasegawa S.L., Rao A., Yamamoto M. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;133:3871–3881. doi: 10.1242/dev.02553. [DOI] [PubMed] [Google Scholar]
- Nelms B.L., Labosky P.A. Morgan & Claypool Life Sciences; 2010. Transcriptional Control of Neural Crest Development. [PubMed] [Google Scholar]
- Oh Y., Cho G.S., Li Z., Hong I., Zhu R., Kim M.J., Kim Y.J., Tampakakis E., Tung L., Huganir R. Functional coupling with cardiac muscle promotes maturation of hPSC-derived sympathetic neurons. Cell Stem Cell. 2016;19:95–106. doi: 10.1016/j.stem.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osafune K., Caron L., Borowiak M., Martinez R.J., Fitz-Gerald C.S., Sato Y., Cowan C.A., Chien K.R., Melton D.A. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- Reissmann E., Ernsberger U., Francis-West P.H., Rueger D., Brickell P.M., Rohrer H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122:2079–2088. doi: 10.1242/dev.122.7.2079. [DOI] [PubMed] [Google Scholar]
- Santana M.M., Chung K.F., Vukicevic V., Rosmaninho-Salgado J., Kanczkowski W., Cortez V., Hackmann K., Bastos C.A., Mota A., Schrock E. Isolation, characterization, and differentiation of progenitor cells from human adult adrenal medulla. Stem Cells Transl. Med. 2012;1:783–791. doi: 10.5966/sctm.2012-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena S., Wahl J., Huber-Lang M.S., Stadel D., Braubach P., Debatin K.M., Beltinger C. Generation of murine sympathoadrenergic progenitor-like cells from embryonic stem cells and postnatal adrenal glands. PLoS One. 2013;8:e64454. doi: 10.1371/journal.pone.0064454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Wicht H., Enderich J., Wegner M., Rohrer H. Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron. 1999;24:861–870. doi: 10.1016/s0896-6273(00)81033-8. [DOI] [PubMed] [Google Scholar]
- Schober A., Wolf N., Huber K., Hertel R., Krieglstein K., Minichiello L., Kahane N., Widenfalk J., Kalcheim C., Olson L. TrkB and neurotrophin-4 are important for development and maintenance of sympathetic preganglionic neurons innervating the adrenal medulla. J. Neurosci. 1998;18:7272–7284. doi: 10.1523/JNEUROSCI.18-18-07272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtukmaster S., Schier M.C., Huber K., Krispin S., Kalcheim C., Unsicker K. Sympathetic neurons and chromaffin cells share a common progenitor in the neural crest in vivo. Neural Dev. 2013;8:12. doi: 10.1186/1749-8104-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard F., Ehrhart-Bornstein M., Corbeil D., Sperber S., Krug A.W., Ziegler C.G., Rettori V., McCann S.M., Bornstein S.R. Age-dependent regulation of chromaffin cell proliferation by growth factors, dehydroepiandrosterone (DHEA), and DHEA sulfate. Proc. Natl. Acad. Sci. USA. 2007;104:2007–2012. doi: 10.1073/pnas.0610898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Gutierrez A., Yagi H., Uygun B.E., Navarro-Alvarez N., Uygun K., Kobayashi N., Yang Y.G., Yarmush M.L. Cell delivery: from cell transplantation to organ engineering. Cell Transplant. 2010;19:655–665. doi: 10.3727/096368910X508753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemple D.L., Mahanthappa N.K., Anderson D.J. Basic FGF induces neuronal differentiation, cell division, and NGF dependence in chromaffin cells: a sequence of events in sympathetic development. Neuron. 1988;1:517–525. doi: 10.1016/0896-6273(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Szabo P.M., Pinter M., Szabo D.R., Zsippai A., Patocs A., Falus A., Racz K., Igaz P. Integrative analysis of neuroblastoma and pheochromocytoma genomics data. BMC Med. Genomics. 2012;5:48. doi: 10.1186/1755-8794-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichberg S., Holtzman E. Axonal agranular reticulum and synaptic vesicles in cultured embryonic chick sympathetic neurons. J. Cell Biol. 1973;57:88–108. doi: 10.1083/jcb.57.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler A.S., Riseberg J.C. Different responses to mitogenic agents by adult rat and human chromaffin cells in vitro. Endocr. Pathol. 1993;4:15–19. doi: 10.1007/BF02914484. [DOI] [PubMed] [Google Scholar]
- Trifaro J.M. Molecular biology of the chromaffin cell. Ann. N. Y. Acad. Sci. 2002;971:11–18. doi: 10.1111/j.1749-6632.2002.tb04427.x. [DOI] [PubMed] [Google Scholar]
- Troy C.M., Brown K., Greene L.A., Shelanski M.L. Ontogeny of the neuronal intermediate filament protein, peripherin, in the mouse embryo. Neuroscience. 1990;36:217–237. doi: 10.1016/0306-4522(90)90364-a. [DOI] [PubMed] [Google Scholar]
- van der Hoorn F.A., Boomsma F., Man in 't Veld A.J., Schalekamp M.A. Determination of catecholamines in human plasma by high-performance liquid chromatography: comparison between a new method with fluorescence detection and an established method with electrochemical detection. J. Chromatogr. 1989;487:17–28. doi: 10.1016/s0378-4347(00)83003-0. [DOI] [PubMed] [Google Scholar]
- Wells S.A., Jr., Pacini F., Robinson B.G., Santoro M. Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J. Clin. Endocrinol. Metab. 2013;98:3149–3164. doi: 10.1210/jc.2013-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Brinas I.M., Binder B.J., Landman K.A., Newgreen D.F. Neural crest regionalisation for enteric nervous system formation: implications for Hirschsprung's disease and stem cell therapy. Dev. Biol. 2010;339:280–294. doi: 10.1016/j.ydbio.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Zhu S., Lee J.S., Guo F., Shin J., Perez-Atayde A.R., Kutok J.L., Rodig S.J., Neuberg D.S., Helman D., Feng H. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21:362–373. doi: 10.1016/j.ccr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.