Summary
Gut epithelial organoids are routinely used to investigate intestinal biology; however, current culture methods are not amenable to genetic manipulation, and it is difficult to generate sufficient numbers for high-throughput studies. Here, we present an improved culture system of human induced pluripotent stem cell (iPSC)-derived intestinal organoids involving four methodological advances. (1) We adopted a lentiviral vector to readily establish and optimize conditioned medium for human intestinal organoid culture. (2) We obtained intestinal organoids from human iPSCs more efficiently by supplementing WNT3A and fibroblast growth factor 2 to induce differentiation into definitive endoderm. (3) Using 2D culture, followed by re-establishment of organoids, we achieved an efficient transduction of exogenous genes in organoids. (4) We investigated suspension organoid culture without scaffolds for easier harvesting and assays. These techniques enable us to develop, maintain, and expand intestinal organoids readily and quickly at low cost, facilitating high-throughput screening of pathogenic factors and candidate treatments for gastrointestinal diseases.
Keywords: intestinal organoids, human iPSCs, suspension culture, conditioned medium, epithelial organoids, IECs, IESCs, lentiviral infection, organoid differentiation
Highlights
-
•
We optimized conditioned medium preparation from L cells by lentiviral infection
-
•
We modified a protocol to differentiate iPSCs into small intestinal organoids
-
•
We succeeded in transducing a gene into organoids by 2D culture
-
•
We showed that intestinal human organoids self-proliferate in suspension culture
Takahashi et al. developed several user-friendly culture methods for human induced pluripotent stem cell (iPSC)-derived intestinal organoids. The methodological improvements include preparation of conditioned medium for organoid culture, efficient differentiation from iPSCs, gene transduction in organoids, and growth in suspension culture. This system will facilitate high-throughput screening of pathogenic factors and treatments for intestinal diseases using physiologically robust intestinal organoids.
Introduction
Cell-based high-throughput screening for drug discovery is usually conducted using established cell lines or primary cells in 2D culture. However, current culture systems cannot satisfactorily recapitulate the complexity of the microenvironment and generally do not fully reflect cell phenotype in vivo (Ranga et al., 2014). Furthermore, evaluation of test compounds in animal models is not only costly but also carries uncertainty due to fundamental differences in physiology between humans and experimental animals. Indeed, pre-clinical animal studies cannot predict the toxicity of drug candidates in humans due to species differences (Olson et al., 2000, Pritchard et al., 2003). Considering that species differences are also observed in the microtissues and cell clumps in vitro (Kostadinova et al., 2013), it is desirable to establish physiologically faithful 3D tissues from human cells as organ models.
Gut epithelial organoid culture is an emerging technique for investigating the molecular and cellular biology of the intestine in vitro (Sato et al., 2009, Sato et al., 2011a, Yui et al., 2012). Intestinal organoids are derived from intestinal epithelial stem cells (IESCs) and maintain self-propagation capacity because organoid crypt regions retain IESCs in addition to differentiated cells. WNT3A, R-spondin (RSPO) 1, and Noggin (NOG) are considered as key factors that enable self-proliferation of crypt IESCs. These organoids have been found to contain enterocytes, Paneth cells, goblet cells, and enteroendocrine cells derived from IESCs, as well as villus-like structures (Sato et al., 2009, Sato et al., 2011b). Thus, intestinal organoids possess many enteric characteristics found in vivo, and allow for greater use of human-derived culture systems in gut research, mitigating the uncertainty imposed by species differences.
Although epithelial organoids are powerful tools to investigate intestinal physiology and pathology, some cytokines essential for organoid growth are expensive if purchased commercially. Therefore, some attempts have been made to prepare conditioned medium (CM) for organoid culture from cells overexpressing specific cytokine genes (Miyoshi and Stappenbeck, 2013, Willert et al., 2003). However, stable cell lines usually express only one transgene, thereby requiring the production of multiple conditioned media from different cell lines, each overexpressing a single desired factor, or requiring several steps to transduce multiple genes in the same cell lines. Moreover, controlling their expression levels can usually be difficult. A retroviral or lentiviral expression system can overcome these disadvantages by enabling transduction of multiple genes simultaneously and efficiently in a single cell line, with control of expression levels by adjusting viral titers (Frimpong and Spector, 2000). Usually, high titers can transduce more than one gene copy into 100% of exposed cells, eliminating the need for selection and cloning, and enabling establishment of stable cell lines in several days.
Matrigel, a proprietary extracellular matrix (ECM) gel, is often used as a scaffold material for 3D organoid culture (Sato et al., 2009, Sato et al., 2011a). However, use of Matrigel is unlikely to be appropriate for large-scale assays, such as drug screening, due to lot-by-lot variation in trace components and trial-by-trial variation in gel structure (porosity) (Goldstein et al., 2011). For instance, Matrigel contains variable levels of growth factors such as fibroblast growth factor (FGF) 2, epidermal growth factor, and insulin-like growth factor 1 (Vukicevic et al., 1992). Furthermore, Matrigel is generated from mouse sarcoma and therefore may contain carcinogenic factors. It is thus not well suited for human studies, including clinical trials (Lee et al., 2010). Some investigators have used collagen type I gel instead of Matrigel for organoid culture to avoid such concerns (Jabaji et al., 2013, Yui et al., 2012); however, both collagen I gel and Matrigel require complicated procedures to recover cells for collection. Therefore, alternative methods for culture of human organoids are required.
Induced pluripotent stem cells (iPSCs), under appropriate conditions, can differentiate into multiple cell types, including cardiomyocytes, neurons, hepatocytes, and pancreatic cells (Karumbayaram et al., 2009, Si-Tayeb et al., 2010, Zhang et al., 2009a, Zhang et al., 2009b), making them invaluable for understanding embryonic development, drug toxicity testing, individualized research, and regenerative medicine (Inoue et al., 2014, Sterneckert et al., 2014). Furthermore, iPSCs can be more readily obtained than embryonic stem cells, and their isolation carries fewer ethical concerns (Nishikawa et al., 2008). More importantly, use of human iPSCs from patients could lead to the development of disease-specific human drug-screening models, even when the cause of the disease is unclear. It has been reported that intestinal organoids with specific properties (e.g., crypt-villus axis) can be generated from human iPSCs (McCracken et al., 2011). Although iPSC-derived organoids are useful for studies on intestinal development and in vivo transplantation (Watson et al., 2014), their application in high-throughput screening remains difficult due to limited culture scalability. In addition, stable gene transduction in human organoids can be precious, especially for regenerative medicine and drug screening. Although some researchers reported successful gene transduction in human intestinal organoids (Fujii et al., 2015, Spence et al., 2011), an easier and more rapid approach to modify the genes of interest is desired.
In this study, we developed or improved a number of methods to handle iPSC-derived intestinal organoids easily. First, we adopted lentiviral vector to readily establish and modify CM for human intestinal organoid culture. Second, we differentiated human iPSCs into intestinal organoids more efficiently by supplementing WNT3A and FGF2 during the differentiation into definitive endoderm (DE). Third, we were able to transduce an exogenous gene efficiently into these organoids through 2D culture. Fourth, we successfully cultured human iPSC-derived organoids in Happy Cell Advanced Suspension Medium (ASM), which does not require Matrigel and enables organoids to be easily collected. The combination of these techniques enables more efficient intestinal organoid culture and provides a scalable strategy to produce large numbers of organoids for therapeutic drug screening.
Results
Preparation of CM for Organoid Culture
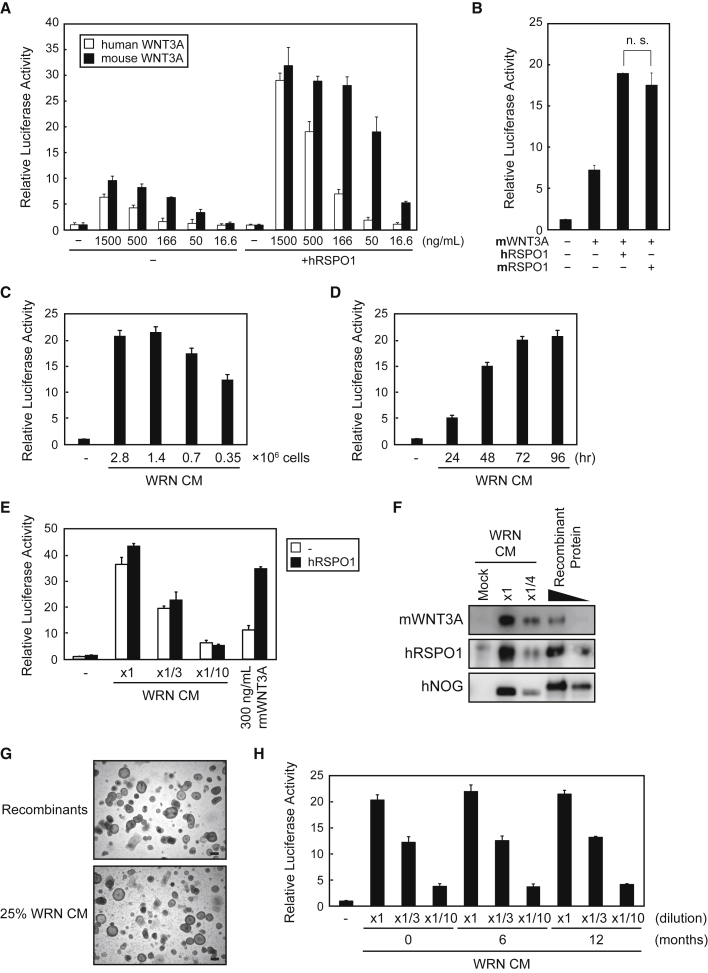
We first established a cell line that can stably express the cytokines WNT3A, RSPO1, and NOG, to reduce costs and labor for the development and maintenance of human organoids. Although a cell line simultaneously expressing mouse WNT3A (mWNT3A), mouse RSPO3, and mouse NOG has already been established and deposited to the American Type Culture Collection (Miyoshi and Stappenbeck, 2013), it was originally developed for using mouse organoid culture (Miyoshi et al., 2012). Therefore, we selected a lentiviral expression system for rapid establishment of our original cell line and characterized the CM produced by these cells. Prior to the establishment of such cells, we unexpectedly found that recombinant mWNT3A exhibited higher activity than recombinant human WNT3A (hWNT3A), as measured by a luciferase assay using a TOPflash reporter gene plasmid, which can detect Wnt signal activation (Korinek et al., 1997) (Figure 1A). In contrast to WNT3A, RSPO1 activities, which enhance Wnt signals, were comparable between mice and humans (Figure 1B). Therefore, we chose mWNT3A, human RSPO1 (hRSPO1), and human NOG (hNOG) (WRN) as the ingredients of CM for human organoid culture. Moreover, we compared Wnt activities of culture supernatants among three host cells, HEK293T cells, Chinese hamster ovary cells, and L cells, all of which were similarly infected with lentiviral vectors for WRN expression. Results indicated that L cells had the highest capacity to secrete WRN proteins among the three cell types (data not shown); therefore, we chose L cells as hosts to prepare WRN CM.
Figure 1.
Preparation of WRN CM by Lentiviral Infection
(A and B) Wnt activities of recombinant hWNT3A or mWNT3A (A) and recombinant hRSPO1 or mRSPO1 (B). Activities in the absence of the hRSPO1 expression plasmid and recombinant proteins were considered as “1.” The assays were performed in three independent biological replicates. Data expressed as mean ± SEM. n.s., not significant (Student’s t test).
(C–H) Mouse L cells stably expressing WRN were generated by lentiviral infection of each gene. (C–E) Wnt signaling activities of CM from L-WRN cells at densities of 2.8, 1.4, 0.7, and 0.35 × 106 cells/35-mm dish after 48 hr of culture (C); at a density of 1.4 × 106 cells/dish cultured between 24 and 96 hr (D); and at 1.4 × 106 cells/dish cultured for 72 hr in diluted CM or 300 ng/mL recombinant mWNT3A, with or without an hRSPO1 expression plasmid (E). Activities in the absence of WRN CM were considered as “1.” The assays were performed in three independent biological replicates. Data expressed as mean ± SEM. (F) Western blot analysis of the supernatants used in (C) undiluted (8 μL) and 4-fold diluted, and the recombinant proteins mWNT3A (1 and 0.3 ng), hRSPO1 (30 and 10 ng), and hNOG (10 and 3 ng) using anti-WNT3A, anti-RSPO1, and anti-NOG antibodies. (G) Images of human primary ileal organoids cultured in 25% WRN CM or the corresponding recombinant proteins for 7 days after a passage. Scale bars, 200 μm. (H) Wnt signaling activities of WRN CM (×1, ×1/3, ×1/10) stored in the deep freezer for 0, 6, or 12 months. The activities in the absence of CM were considered as “1.” The assay was performed in three independent biological replicates. Data expressed as mean ± SEM.
To optimize the conditions for preparing WRN CM, we seeded WRN-expressing L cells in 35-mm dishes at densities ranging from 0.35 × 106 to 2.8 × 106 cells in 2 mL medium, and harvested the medium 48 hr later. We found that a density of 1.4 × 106 cells/dish yielded CM the maximum Wnt signaling activity (Figure 1C). We then examined the appropriate culture period by seeding WRN-expressing L cells at 1.4 × 106 cells/35-mm dish and culturing them for 24–96 hr, which showed that the activity peaked at 72 hr (Figure 1D). Using these conditions, we found that WRN CM activity decreased with dilution and that undiluted WRN CM had higher activity than 300 ng/mL recombinant mWNT3A, even in hRSPO1 overexpressing cells (Figure 1E). We then examined the expression of each cytokine in WRN CM by western blot analysis (Figure 1F), and estimated that the concentrations of mWNT3A, hRSPO1, and hNOG were approximately 400 ng/mL, 3 μg/mL, and 800 ng/mL, respectively. Based on previous reports (Grabinger et al., 2014, Sato et al., 2011a), we assumed that 25% WRN CM would contain sufficient concentrations of these factors for human organoid culture. In fact, human primary small intestinal organoids could be cultured with medium containing 25% WRN CM as a source of required cytokines (Figure 1G). Importantly, the biological activities of WRN CM did not change even after 1 year of storage at −80°C (Figure 1H), which is an asset for the consistency of human organoid culture.
Differentiation of Human iPSCs into Intestinal Organoids
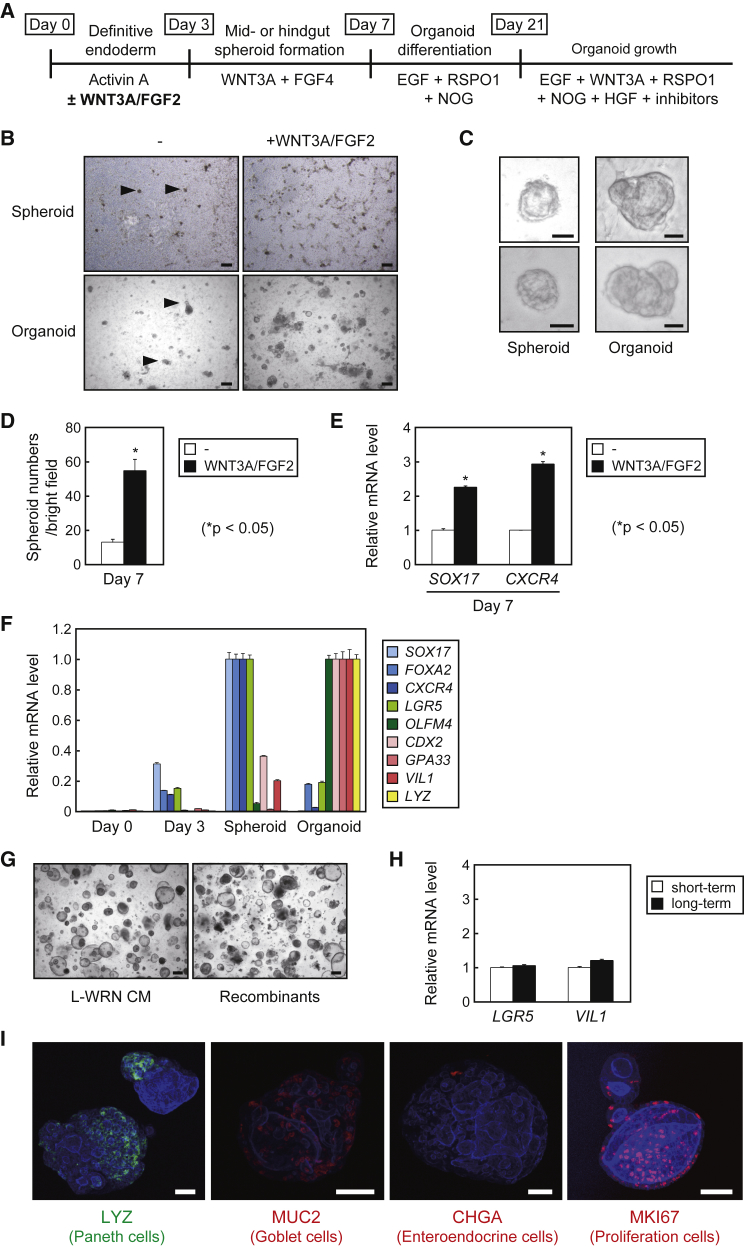
We attempted to establish human intestinal organoids from iPSCs as a first step toward developing healthy and disease models for future studies. We first differentiated the human iPSC line TkDN4-M (Takayama et al., 2010) into DE using activin A for 3 days, and then treated the cells with FGF4 and WNT3A for up to 4 days to obtain spheroids according to previous reports (McCracken et al., 2011, Spence et al., 2011) (Figure 2A). However, the generation efficiencies of the spheroid- and subsequent organoid-like cell clumps were not sufficient (Figure 2B, left panels), and we were unable to improve this even by use of another human iPSC line, TkDA3-4 (Takayama et al., 2010) (data not shown). We speculated that this limitation was caused by low differentiation efficiency into DE. Therefore, we also added WNT3A and FGF2 during the initial culture stage, as these factors have been reported to enhance endoderm differentiation (Massumi et al., 2014, Rezania et al., 2011, Shiraki et al., 2009) (Figure 2A). This modification greatly improved spheroid-like clump formation efficiencies (Figures 2B, right upper panel, and 2D), which was consistent with the upregulation of DE markers sex determining region Y-box 17 (SOX17) and C-X-C chemokine receptor type 4 (CXCR4) expression (Figure 2E), and further improved the following organoid-like clump formation efficiencies (Figure 2B, right lower panels).
Figure 2.
Differentiation of TkDN4-M into Human Intestinal Organoids
(A) Schematic procedures for intestinal organoid differentiation from human iPSC lines.
(B) Images of the human iPSC line TkDN4-M that had been differentiated into spheroids (upper panels) and organoids (lower panels) with (right panels) or without (left panels) recombinant hWNT3A and human FGF2 (hFGF2). Arrowheads indicate spheroid- and organoid-like cell clumps. Scale bars, 200 μm.
(C) Magnified images of the spheroids and organoids. Scale bars, 40 μm.
(D) The numbers of spheroids per microscopic bright field in the presence or absence of WNT3A/FGF2 supplementation. The assays were performed in three independent experiments. Data are presented as mean ± SEM. ∗p < 0.05, Student's t test.
(E) Relative mRNA levels of SOX17 and CXCR4 in TkDN4-M cells differentiated into spheroids in the presence or absence of WNT3A/FGF2 supplementation were determined by qPCR and normalized to HPRT. The assay was performed in three independent biological replicates. Expression levels presented as mean ± SEM. ∗p < 0.05, Student's t test.
(F) Relative mRNA levels of the indicated genes in TkDN4-M cells during the course of differentiation (days 0, 3, and 7 [spheroids], and organoids) were determined by qPCR and normalized to HPRT. The highest expression levels of each gene in each differentiation stage were considered as “1.” The assays were performed in three independent biological replicates. Expression levels presented as mean ± SEM.
(G) Images of TkDN4-M-derived organoids on day 7 after culture in 25% WRN CM, or the corresponding recombinant proteins. Scale bars, 200 μm.
(H) Relative mRNA levels of LGR5 and VIL1 in TkDN4-M organoids cultured for short-term (passage 19) and long-term (passage 53) were determined by qPCR and normalized to HPRT. The assays were performed in three independent biological replicates. Expression levels presented as mean ± SEM.
(I) Whole-mount images of TkDN4-M-derived organoids stained with phalloidin (blue), together with each indicated antibody. Scale bars, 50 μm.
The morphologies of these clumps were similar to those reported previously for spheroids and organoids (Figure 2C) (McCracken et al., 2011). Furthermore, the relative gene expression of the endoderm markers SOX17, CXCR4, and forkhead box protein A2 (FOXA2), peaked when the spheroid-like clumps were formed, whereas the intestinal epithelial markers caudal-related homeobox transcription factor 2 (CDX2), glycoprotein A33 (GPA33), and villin 1 (VIL1), as well as the Paneth cell marker lysozyme (LYZ), were induced during organoid-like clump formation (Figure 2F). Thus, these clumps clearly possessed the typical properties as spheroids and organoids, demonstrating that terminal differentiation into intestinal organoids proceeded successfully. We also confirmed that the organoids were able to proliferate in our 25% WRN CM, as well as in recombinant proteins such as the organoids prepared from ileum (Figures 2G and 1G). Furthermore, expression of LGR5 and VIL1 was comparable between short-term (100 days) and long-term (300 days) cultures, indicating that properties of human iPSC-derived organoids are stable over multiple passages (Figure 2H). Finally, whole-mount staining revealed the development of Paneth cells (LYZ+), goblet cells (MUC2+), enteroendocrine cells (Chromogranin A [CHGA]+), and proliferative cells (MKI67+), confirming successful organoid differentiation from TkDN4-M (Figure 2I).
We were also able to obtain organoid-like cell clumps from other human iPSC lines, TkDA3-4 (Figure S1A) and TkCBSev9 (Yagyu et al., 2015) (Figure S1B), by the addition of WNT3A and FGF2 to the differentiation medium, and similarly confirmed their authentic organoid properties through extensive gene expression analysis (Figures S1C and S1D). No organoids were obtained from TkCBSev9 without the addition of WNT3A and FGF2 during the initial stage of the differentiation (data not shown). These results indicate that our modified differentiation protocol, which enables higher differentiation capacities into intestinal organoids, can be generally applied.
Comparison between Human iPSC-Derived Organoids and Primary Organoids from the Small Intestine and Colon
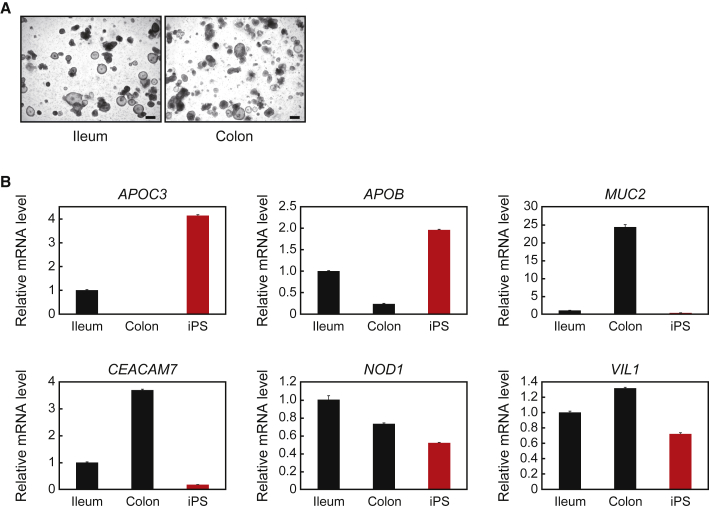
Spence et al. have reported, using transcriptome analysis, that intestinal organoids differentiated from human iPSCs show mid- and hindgut specification (McCracken et al., 2011) and possess small intestinal properties (Finkbeiner et al., 2015). To investigate whether our intestinal organoids, which were differentiated by the modified method, show small intestinal specifications, we prepared human primary organoids from healthy parts of the ileum and transverse colon of surgical specimens using human organoid culture medium containing 25% WRN CM, and found that both types of primary organoids showed an almost identical morphology to human iPSC-derived organoids (Figure 3A).
Figure 3.
Comparison of Human iPSC-Derived Organoids with Primary Intestinal Organoids
(A) Images of organoids from the human primary ileum or transverse colon cultured with human organoid culture medium for 6 days. Scale bars, 200 μm.
(B) Relative mRNA levels of the indicated genes in organoids derived from human primary ileum, colon, and iPSCs harvested at 6 days after passage. The relative mRNA levels were determined by qPCR and normalized to GAPDH. The assays were performed in three independent biological replicates. Data are presented as mean ± SEM.
Based on a recent DNA microarray that compared the gene expression profiles of different parts of the human intestinal epithelium (Wang et al., 2015), we chose to examine the expression of the apolipoprotein (APO) B and APOC3 genes, and found that their mRNA levels were much higher in organoids from the small intestine and iPSCs than in organoids from the colon (Figure 3B). We also examined the expressions of carcinoembryonic antigen-related cell adhesion molecule 7 (CEACAM7) and MUC2, which were higher in organoids from the colon than in those from the small intestine and iPSCs. As a control gene, we examined nucleotide-binding oligomerization domain containing 1 (NOD1), and confirmed that its expression, as well as that of VIL1, was comparable across the three types of organoids examined. These results indicate that characteristics of our human iPSC-derived organoids are much closer to those of small intestinal organoids than colonic organoids.
Gene Transduction into Human iPSC-Derived Organoids by Lentiviral Infection
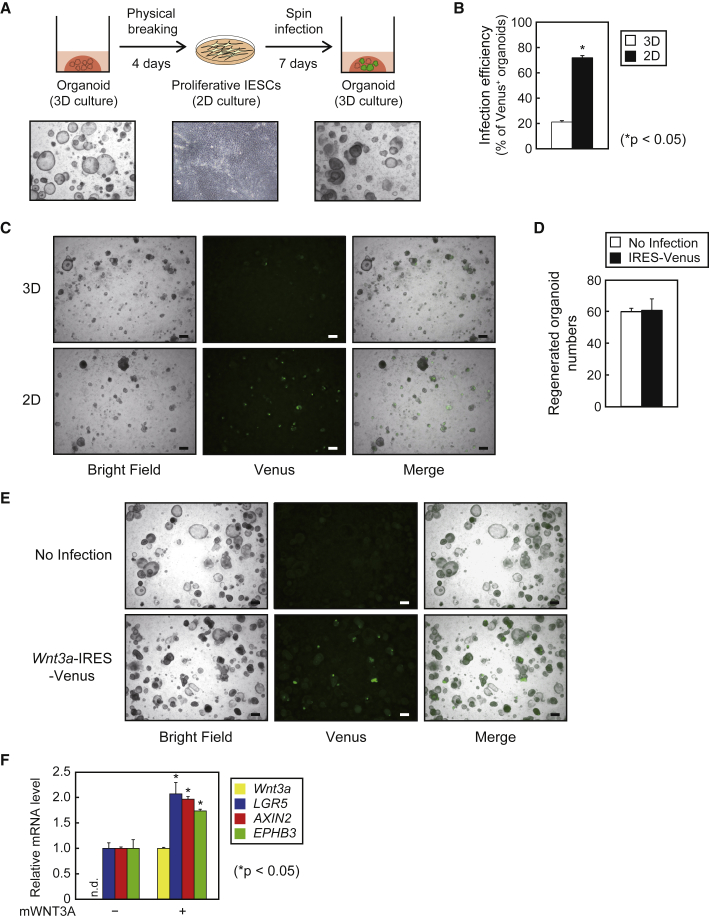
Stable gene transduction into human iPSC-derived organoids would be required to create organoids that mimic certain diseases or to screen for possible therapeutic genes. However, since organoids self-proliferate as cell clumps, and are usually seeded in solid ECM such as Matrigel, it is difficult to simply apply typical transfection and selection methods for stable gene expression. At first, in reference to a previous report to transduce exogenous genes into human iPSC-derived organoids using adenoviruses (Spence et al., 2011), we recovered organoids from Matrigel and incubated them with lentiviruses for Venus expression; however, the transduction efficiencies as well as Venus expression intensities were not sufficiently high (Figures 4B and 4C; 3D groups). Therefore, we devised a unique strategy to transduce a gene into human iPSC-derived organoids. We have found that when organoids were physically disrupted using needles and seeded on collagen I-coated dishes, 2D-proliferative cells emerged (Figure 4A). Intriguingly, when these cells were harvested and re-embedded in Matrigel, organoids were again generated, indicating that the cultures containing sufficient numbers of IESCs can reconstitute organoids in Matrigel (Figure 4A). Considering these results, we postulated that gene transduction into iPSC-derived organoids can be achieved by organoid disruption, followed by lentiviral spin infection of 2D-cultured IESCs and re-embedded them in Matrigel. Based on this notion, we examined lentiviral infection efficiency in organoids by monitoring Venus expression, and found that more than 70% of organoids were transduced (Figures 4B and 4C; 2D groups). We observed no significant difference in regenerated organoid yield at 1 week between infected and non-infected groups (Figure 4D), demonstrating that lentiviral infection had little effect on IESC viability. Last, to assess exogenous gene function in organoids, we introduced Wnt3a-internal ribosome entry site 2 (IRES2)-Venus plasmid into organoids using the above strategy, and found that they stably expressed mWNT3A and Venus even after several passages (Figures 4E and 4F). Furthermore, we found that the expression of Wnt target genes, namely LGR5, AXIN2, and EPHB3, was induced by exogenous mWNT3A (Figure 4F), suggesting that functional Wnt signaling was stably enhanced.
Figure 4.
Lentiviral Gene Transduction into Human iPSC-Derived Intestinal Organoids
(A) Schematic representation of gene transduction into organoids. TkDN4-M-derived organoids cultured in Matrigel were disrupted by a 29G needle, seeded in collagen I-coated plates, and cultured for 4 days. After 2D cultures from organoids were infected by lentiviruses, cells (including IESCs) were trypsinized, harvested, and re-embedded in Matrigel to reform organoids.
(B) The proportions of Venus+ organoids per microscopic bright field after 7 days of infection with IRES-Venus lentivirus. “3D” and “2D” indicate lentiviral infection into organoids harvested from Matrigel and 2D-cultured cells in collagen I-coated plates, respectively. The assay was performed in three independent biological replicates. Data are presented as mean ± SEM. ∗p < 0.05, Student's t test.
(C) Bright-field and fluorescent images of TkDN4-M-derived organoids as in (B). Scale bars, 200 μm.
(D) Regenerated organoid numbers per microscopic bright field followed by 2D cultures after 7 days of infection with or without IRES-Venus lentivirus. The assay was performed in three independent biological replicates. Data are presented as mean ± SEM.
(E) Bright-field and fluorescent images of TkDN4-M-derived organoids 3 weeks after infection with Wnt3a-IRES-Venus lentivirus in 2D cultures. Scale bars, 200 μm.
(F) Relative mRNA levels of exogenous Wnt3a and Wnt target genes (LGR5, AXIN2, and EPHB3) in TkDN4-M-derived organoids infected with lentiviruses as in (E). The relative mRNA levels were determined by qPCR and normalized to GAPDH. The assay was performed in three independent biological replicates. Expression levels presented as mean ± SEM. ∗p < 0.05, Student's t test. n.d., not detected.
Characterization of Human iPSC-Derived Organoids Cultured in Suspension Medium
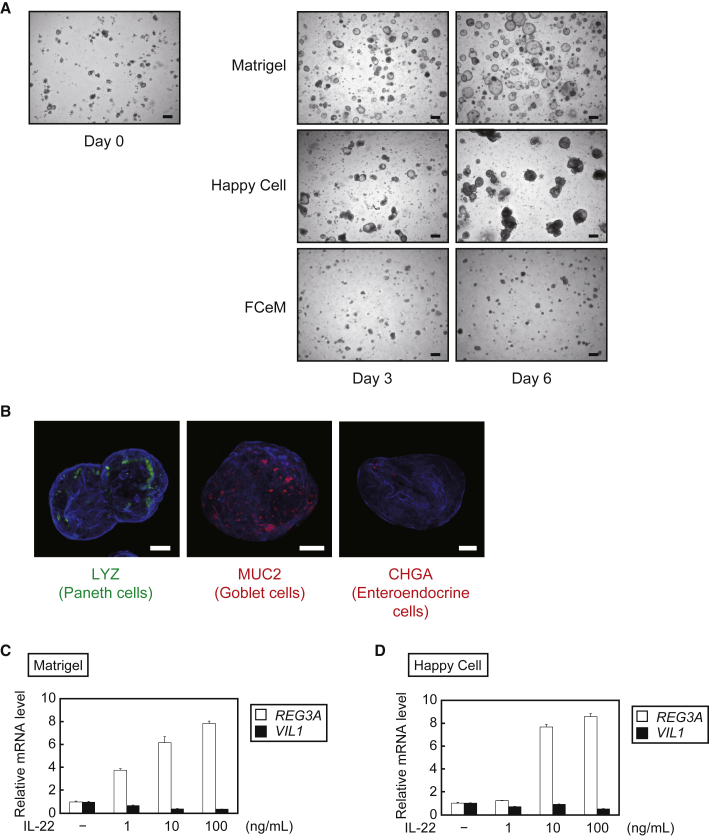
Although 3D culture in a solidified environment, such as Matrigel, is a well-established method for culturing intestinal organoids (Sato et al., 2011a), it is often laborious and inappropriate for large volume culture because the gel impedes cell recovery. To overcome this problem, we tried to maintain organoids in suspension culture without solid ECM. As suspension medium, we adopted Happy Cell ASM (Biocroi, Ireland) (Kulasinghe et al., 2016) and FCeM (Nissan Chemical Industries) (Otsuji et al., 2014), both of which are commercially available but mainly used for cancer spheroid culture. We then prepared a suspension medium supplemented with 25% WRN CM, and cultured TkDN4-M-derived organoids in low-attachment plates. Time-lapse microscopy revealed that the organoids were able to grow in Happy Cell ASM as well as in Matrigel; however, they failed to grow in FCeM (Figure 5A). We confirmed that the organoids grown in Happy Cell ASM contained Paneth cells, goblet cells, and enteroendocrine cells (Figure 5B), as in Matrigel (Figure 2I). Furthermore, we stimulated the organoids with interleukin-22 (IL-22), a potent inducer of epithelial cell cytokine expression and regeneration (Lindemans et al., 2015), to examine physiological responsiveness. Regenerating family member 3α (REG3A) expression was dose-dependently induced by IL-22 in organoids cultured in both Matrigel (Figure 5C) and Happy Cell ASM (Figure 5D), consistent with the IL-22 response in mouse intestinal organoids (Lindemans et al., 2015).
Figure 5.
Growth of Human iPSC-Derived Organoids by Suspension Culture Using Happy Cell ASM
(A) Time-lapse bright-field images of TkDN4-M-derived organoids cultured in Matrigel, Happy Cell ASM, or FCeM, each supplemented with 25% WRN CM and requisite ingredients for human organoid culture. Scale bars, 200 μm.
(B) Whole-mount images of TkDN4-M-derived organoids cultured in Happy Cell culture medium were stained with phalloidin (blue), together with each indicated antibody. Scale bars, 50 μm.
(C and D) Relative mRNA levels of the indicated genes in TkDN4-M-derived organoids cultured in Matrigel (C) or Happy Cell ASM (D) for 5 days and then exposed to each pre-stimulation medium containing the indicated concentrations of recombinant human IL-22 for 2 days. The relative mRNA levels were determined by qPCR and normalized to GAPDH. The assays were performed in three independent biological replicates. Data presented as mean ± SEM.
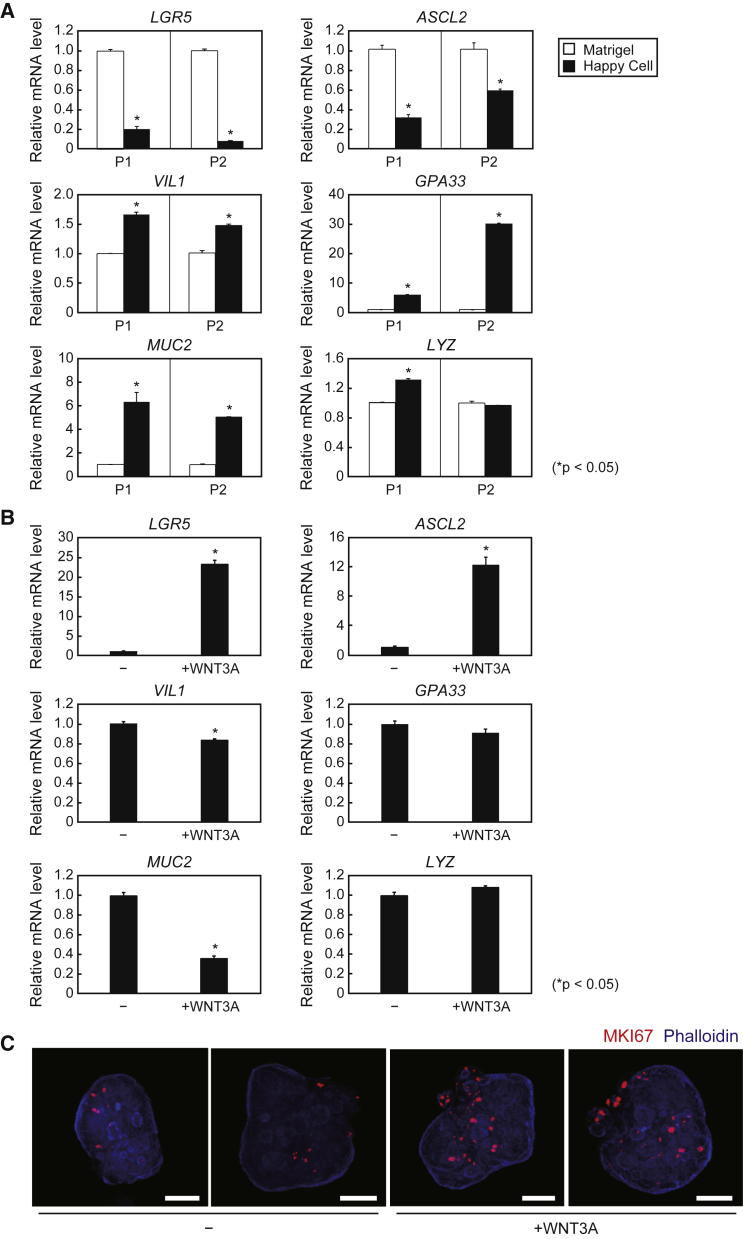
We next conducted serial PCR analyses and found that expression of the stem cell markers LGR5 and ASCL2 was lower in whole-mount organoids cultured in Happy Cell ASM than those in Matrigel, whereas expression levels of VIL1 and the goblet marker MUC2 were higher (Figure 6A), indicating that organoids cultured in Happy Cell ASM are more likely to be composed of differentiated IECs, rather than IESCs. Based on these results, we performed DNA microarray analysis to comprehensively compare gene expression changes caused by suspension culture after two passages. Representative genes showing up- and downregulation are presented in Tables S1 and S2. Among these genes, the expression of another intestinal epithelial marker, GPA33, was increased when organoids were cultured in Happy Cell ASM (Figure 6A), further indicating that suspension culture increased certain populations of differentiated IECs. In contrast, when recombinant mWNT3A was added to the medium, LGR5 and ASCL2 expression was induced and VIL1 and MUC2 expression was decreased (Figure 6B), which suggests that proportions of cell components can be modified by changing medium ingredients. This notion was also supported by whole-mount immunostaining against MKI67, which indicates that MKI67+ proliferative cells were increased by WNT3A supplementation in the organoids cultured in Happy Cell ASM (Figure 6C).
Figure 6.
Characterization of Human iPSC-Derived Organoids Cultured in Happy Cell ASM
(A) Relative mRNA levels of the indicated genes in TkDN4-M-derived organoids cultured with Matrigel (open bars) or Happy Cell ASM (closed bars) plus 25% WRN CM and requisite ingredients for human organoid culture after one (P1) or two (P2) passages in each culture condition. The relative mRNA levels were determined by qPCR and normalized to GAPDH. The assays were performed in three independent biological replicates. Data presented as mean ± SEM. ∗p < 0.05, Student's t test.
(B) Relative mRNA levels of the indicated genes in TkDN4-M-derived organoids cultured in Happy Cell ASM with or without 300 ng/mL recombinant mWNT3A for 6 days. The relative mRNA levels were determined by qPCR and normalized to GAPDH. The assays were performed in three independent biological replicates. Data presented as mean ± SEM. ∗p < 0.05, Student's t test.
(C) Whole-mount images of TkDN4-M-derived organoids cultured in Happy Cell ASM with or without 300 ng/mL recombinant mWNT3A after staining with phalloidin (blue) and anti-MKI67 antibodies. Scale bars, 50 μm.
Discussion
Intestinal organoids derived from IESCs contain various IEC types observed in vivo, including enterocytes, Paneth cells, goblet cells, and enteroendocrine cells; they are, thus, regarded as “mini-organs” (Dedhia et al., 2016). Unlike normal cell lines or primary cultures, however, organoid culture is both expensive and labor intensive. Moreover, it is difficult to establish organoids stably expressing exogenous genes for research applications, particularly for studies requiring disease-mimicking organoids. In this study, we developed several original protocols and modified others to improve the efficiency, cost, and practicality of human iPSC-derived organoid culture. First, we produced a single line of L cells stably co-expressing mWNT3A, hRSPO1, and hNOG, factors required for IESC maintenance, by lentiviral infection. Importantly, unlike transfection methods (e.g., lipofection and electroporation), lentivirus infection enables not only transduction of many genes into the same cell genome promptly and simultaneously, but also control of expression levels by changing viral titers. Indeed, we were able to generate WRN CM for human organoid culture with desired and reproducible concentrations of mWNT3A, hRSPO1, and hNOG by adjusting the titers of each virus (Wnt3a:RSPO1:NOG = 15:3:1). Furthermore, it should also be possible to easily prepare other CM types by establishing L cells co-expressing different combinations of genes by lentiviral infection. As substantial examples, we further generated human hepatocyte growth factor (HGF)- and/or IL-22-producing L-WRN cells by lentiviral infection because these cytokines have the capacity to stimulate organoid growth (Lindemans et al., 2015, Yui et al., 2012). We found that L-WRN cells infected with both HGF- and IL22-expressing lentiviruses can simultaneously produce CM containing approximately 200 ng/mL of HGF and 80 ng/mL of IL-22 (Figure S2A). We used the CM for human iPSC-derived organoid culture at 25% concentration as normal, and confirmed that HGF- and IL-22-containing WRN CM cooperatively increased organoid growth, which was revealed by bright-field microscopic observation (Figure S2B) and quantification of viable cell numbers (Figure S2C). Therefore, our strategy can help researchers in various fields to readily develop original CM-producing cells stably expressing any gene of interest with a variable expression level. The customized CM could greatly reduce cost and labor for human organoid culture by obviating the need for commercial factors without sacrificing inter-trial reproducibilities.
It is widely known that iPSC differentiation capacity varies among iPSC lines (Nishizawa et al., 2016), and we found that several iPSC lines, including TkDN4-M and TkDA3-4, failed to generate substantial numbers of intestinal organoids using a previous culture method (McCracken et al., 2011). Therefore, we improved the conditions for differentiation of human iPSCs into intestinal organoids by the addition of WNT3A and FGF2 during the initial culture phase. Efficient yield of the organoids from human iPSCs mitigates not only the ethical concerns of using fetal tissue but also the low availability of surgical specimens. In addition, it is expected that intestinal organoids can be generated even from iPSC lines with relatively low differentiation capacities by adopting the modified conditions used in our study. In fact, we were able to obtain any organoids from TkCBSev9 only when supplemented with WNT3A and FGF2.
Furthermore, we developed a method to stably express exogenous genes in organoids involving disruption, seeding in 2D culture for lentiviral infection, and subsequent organoid regeneration. It is worth mentioning that many of IESCs can survive and grow in 2D conditions because the number of regenerated organoids after re-embedding in Matrigel was comparable with that of organoids that were not subjected to 2D cultures. The 2D-cultured cells can be treated like normal adherent cultured cells with a proliferation capacity, which makes it easier to perform preliminary experiments such as determination of the MOI of multiple viruses simultaneously. The regeneration can be completed in a week, which is sufficient time for subsequent assays, and the overall method is easy and simple, and usually requires less than 2 weeks to obtain genetically engineered organoids. These properties suggest that our culture method is useful for patient-specific studies and production of organoids with disease-specific genetic abnormalities, and also be applicable to modern gene-editing strategies, such as the CRISPR/Cas9 system (Ran et al., 2013). Moreover, we found that transient gene transduction by lipofection was also successfully achieved in 2D-cultured cells, and the expression of the exogenous gene was confirmed in regenerated organoids (Figure S3), suggesting that lipofection-mediated gene transduction into organoids is performable in our system.
Gene expression analysis indicated that our human iPSC-derived organoids resembled those derived from the small intestine rather than the colon. Considering that the expression of APOB, APOC3, and VIL1, exclusively expressed genes in the small intestine, was comparable between iPSC-derived and primary organoids, it is plausible that the iPSC-derived organoids would mainly comprise cells that terminally differentiated toward the intestinal lineage. Therefore, they could be used to improve our molecular and cellular understanding of physiological and inflammatory conditions of the small intestine. In turn, such studies may facilitate development of new treatment alternatives to surgical excision of the small intestine, which has a much more severe impact on quality of life than excision of the colon. On the other hand, since previous reports have proposed that intestinal regiospecificities depend on the intrinsic gene expression profiles of local stem cells rather than differences in microenvironment (Fukuda et al., 2014), it is desirable to also establish a method for generating human colon organoids from iPSCs. During the preparation of this manuscript, successful differentiation into colonic organoids from human iPSCs was reported by independent groups (Crespo et al., 2017, Munera et al., 2017). The differentiation protocols, particularly after CDX2+ spheroid formation, are different from the previous protocol that was used to obtain small intestinal organoids; therefore, there would be little chance of a contamination of colonic cells in the intestinal organoids differentiated using our method, which is a modified method of the original one to provide small intestinal properties.
Matrigel is a widely used ECM gel for organoid culture, but it is still cumbersome to handle because it must be dissolved before the recovery of organoids. In this study, we found that human iPSC-derived organoids self-propagated in suspension culture with Happy Cell ASM. Importantly, primary human ileal and colonic organoids could also proliferate in the suspension culture (Figure S4). Although we have not yet identified the factors in Happy Cell ASM contributing to growth (which are presumably absent in FCeM), suspension culture in Happy Cell ASM can save time and labor for seeding and recovery of organoids, especially when a large number of organoids are needed. However, organoids expanded in Happy Cell ASM showed differences in cell marker expression compared with organoids in Matrigel, presumably due to ECM and trace growth factors in Matrigel (Vukicevic et al., 1992), which may allow IESCs to grow more efficiently. Consistent with this, organoid growth rate in Happy Cell ASM was lower than that in Matrigel and gradually decreased as passage numbers increased (data not shown); therefore, the current suspension culture would be suitable for culture expansion immediately before performing large-scale assays. Notably, despite the probable higher proportion of differentiated IECs and lower proportion of IESCs, suspension-cultured organoids can respond to IL-22 and WNT3A, which indicates that drug screening, focusing on intestinal epithelial cell function and growth of IESCs, can be properly performed, and these cytokines can be used to monitor or control the assay quality. Moreover, DNA microarray analysis revealed that the expression of many other genes can fluctuate following the suspension culture (Tables S1 and S2). The significance of these changes is so far unclear; however, it may be a clue to resolve the issue for the improvement of organoid suspension culture. Meanwhile, we observed that WNT3A supplementation enhanced expression of stem cell markers (LGR5 and ASCL2), and concomitantly increased MKI67+ proliferative cells in whole-mount organoids. Nevertheless, the number of MKI67+ cells (Figure 6C) is still smaller than that cultured in Matrigel (Figure 2I); therefore, further modification of suspension culture medium components, such as supplementation of suitable ECM and small molecules/compounds, will be needed to improve IESC growth. In addition, it is of interest to investigate other 3D culture technologies, such as use of scaffold-based culture plates and bioreactors, to further improve conditions for organoid culture (Li and Cui, 2014).
In summary, we have developed a systematic methodology for human iPSC-derived intestinal organoid culture that is more convenient and cost-effective than currently available methods. The methods introduced in this study will aid in promoting organoid studies by permitting applications requiring large numbers of organoids, such as gene and therapeutic drug screening and translational research.
Experimental Procedures
Materials
All materials used in this study are listed in Table S3.
Mice
C57BL/6J mice were purchased from CLEA Japan. The institutional animal care and research advisory committee at the University of Tokyo approved all animal procedures.
Cell Culture and Differentiation Experiments
All cell culture and differentiation procedures of human iPSCs into intestinal organoids are described in the Supplemental Experimental Procedures.
Preparation of Human Intestinal Organoids
Human intestinal crypts were isolated as described previously (Sato et al., 2011a), with minor modifications. The healthy intestinal tissues (ileum and colon) of surgical specimens were chopped into approximately 5-mm pieces and washed with cold PBS. The fragmented tissues were then incubated in cold chelation buffer (Table S4) for 30 min on ice. Following removal of the chelation buffer, the tissue fragments were resuspended with cold PBS and vigorously mixed in a 50-mL tube for 40 s, and the supernatant was then centrifuged at 200 × g for 3 min. This resuspension/centrifugation procedure was usually performed eight times. The pellets were suspended with PBS, passed through 150-μm nylon filters, centrifuged at 300 × g for 3 min, and further passed through 100-μm nylon filters. Crypt culture largely followed the previously described protocol of the Tokyo Medical and Dental University (Yui et al., 2012), except that it was embedded in Matrigel rather than type I collagen gels. The experiments were approved by the human ethical committee of The University of Tokyo and Osaka University, and all tissues were sampled with informed consent.
Organoid Culture and Passage
Organoid culture and passage in Matrigel were performed as described previously (Takahashi et al., 2017). When using Happy Cell ASM (Biocroi, Ireland), disrupted organoids were suspended with Happy Cell human organoid culture medium (Table S4) and seeded on ultra-low-attachment cell culture plate (Corning, 3473) (0.5 mL/well). Equal amounts of medium were also supplemented every 3 days, and organoids were collected every 6 days by centrifugation at 440 × g for 5 min after adding 4 mL of Dulbecco's modified Eagle's medium (DMEM). For the IL-22 stimulation assays, the culture medium was replaced with pre-stimulation medium or Happy Cell pre-stimulation medium (Table S4) containing human IL-22 with various concentrations for 2 days.
Lentiviral Infection
Lentiviral expression plasmids for mWNT3A, hRSPO1, hNOG, human HGF, and human IL-22 were constructed by inserting the full-length PCR fragment into CSII-EF-MCS-IRES2-Venus, which is provided by Dr. Hiroyuki Miyoshi (BioResource Center, RIKEN, Japan). Following detail procedures are described in the Supplemental Experimental Procedures.
Organoid Regeneration from 2D-Cultured Cells
After harvesting organoids from Matrigel, they were disrupted using a 29G needle 10 times, seeded in collagen I-coated 12-well plates, and cultured with human organoid culture medium to obtain proliferative cells. Proliferative cells (including IESCs) were then infected with lentiviruses or transfected with Lipofectamine 2000 (Thermo Fisher Scientific), trypsinized for 5 min, and harvested. After centrifugation at 440 × g for 5 min, they were resuspended in Matrigel with 20% human organoid culture medium on ice, and suspensions were aliquoted into the wells of a 24-well plate. They were cultured as normal organoid passages.
Statistical Analysis
All results are presented as mean ± SEM. Data were analyzed using Student's t test for two groups and Dunnett's test for ≥3 groups to compare all groups with a control group. Differences were considered significant at p < 0.05.
Author Contributions
Y.T., S.S., T. Yamamoto, and S.K. performed the experiments. Y.K., Y.Y., T. Yamaguchi, and M.H. supported the data analysis and provided helpful discussions. N.T., S.U., C.-Y.L., M.O., H.M., H.O., T.M., and J.N. managed and provided human samples including iPSCs and surgical specimens. Y.T., S.S., and H.K. designed and directed the study and wrote the paper. All authors approved the final version of the manuscript.
Acknowledgments
We thank Dr. Atsushi Miyawaki (Brain Science Institute, RIKEN, Japan) for providing us Venus-containing plasmids and Drs. Haruna Takagi, Chizuru Tsuzuki, and Ayae Nishiyama for technical support. The project is supported by a joint grant from Japan Tobacco and grants from the Ministry of Education, Science, Sports and Technology of Japan (Grant-in Aid for Young Scientists B [25860353 to S.S.] and for Scientific Research S [23229004 to H.K.]) and the Japan Agency for Medical Research and Development (AMED) (to H.K.).
Published: December 7, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.stemcr.2017.11.004.
Contributor Information
Yu Takahashi, Email: yu.takahashi@jt.com.
Shintaro Sato, Email: shinta@biken.osaka-u.ac.jp.
Supplemental Information
References
- Crespo M., Vilar E., Tsai S.Y., Chang K., Amin S., Srinivasan T., Zhang T., Pipalia N.H., Chen H.J., Witherspoon M. Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat. Med. 2017;23:878–884. doi: 10.1038/nm.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhia P.H., Bertaux-Skeirik N., Zavros Y., Spence J.R. Organoid models of human gastrointestinal development and disease. Gastroenterology. 2016;150:1098–1112. doi: 10.1053/j.gastro.2015.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S.R., Hill D.R., Altheim C.H., Dedhia P.H., Taylor M.J., Tsai Y.H., Chin A.M., Mahe M.M., Watson C.L., Freeman J.J. Transcriptome-wide analysis reveals hallmarks of human intestine development and maturation in vitro and in vivo. Stem Cell Reports. 2015;4:1140–1155. doi: 10.1016/j.stemcr.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimpong K., Spector S.A. Cotransduction of nondividing cells using lentiviral vectors. Gene Ther. 2000;7:1562–1569. doi: 10.1038/sj.gt.3301283. [DOI] [PubMed] [Google Scholar]
- Fujii M., Matano M., Nanki K., Sato T. Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 2015;10:1474–1485. doi: 10.1038/nprot.2015.088. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Mizutani T., Mochizuki W., Matsumoto T., Nozaki K., Sakamaki Y., Ichinose S., Okada Y., Tanaka T., Watanabe M. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28:1752–1757. doi: 10.1101/gad.245233.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A.S., Drake J.M., Burnes D.L., Finley D.S., Zhang H., Reiter R.E., Huang J., Witte O.N. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nat. Protoc. 2011;6:656–667. doi: 10.1038/nprot.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabinger T., Luks L., Kostadinova F., Zimberlin C., Medema J.P., Leist M., Brunner T. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell Death Dis. 2014;5:e1228. doi: 10.1038/cddis.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Nagata N., Kurokawa H., Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 2014;33:409–417. doi: 10.1002/embj.201387098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaji Z., Sears C.M., Brinkley G.J., Lei N.Y., Joshi V.S., Wang J., Lewis M., Stelzner M., Martín M.G., Dunn J.C.Y. Use of collagen gel as an alternative extracellular matrix for the in vitro and in vivo growth of murine small intestinal epithelium. Tissue Eng. Part C Methods. 2013;19:961–969. doi: 10.1089/ten.tec.2012.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karumbayaram S., Novitch B.G., Patterson M., Umbach J.A., Richter L., Lindgren A., Conway A.E., Clark A.T., Goldman S.A., Plath K. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P.J., van Wichen D., de Weger R., Kinzler K.W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC–/– colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Kostadinova R., Boess F., Applegate D., Suter L., Weiser T., Singer T., Naughton B., Roth A. A long-term three dimensional liver co-culture system for improved prediction of clinically relevant drug-induced hepatotoxicity. Toxicol. Appl. Pharmacol. 2013;268:1–16. doi: 10.1016/j.taap.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Kulasinghe A., Perry C., Warkiani M.E., Blick T., Davies A., O'Byrne K., Thompson E.W., Nelson C.C., Vela I., Punyadeera C. Short term ex-vivo expansion of circulating head and neck tumour cells. Oncotarget. 2016;7:60101–60109. doi: 10.18632/oncotarget.11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.H., Arcidiacono J.A., Bilek A.M., Wille J.J., Hamill C.A., Wonnacott K.M., Wells M.A., Oh S.S. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng. Part B Rev. 2010;16:41–54. doi: 10.1089/ten.TEB.2009.0449. [DOI] [PubMed] [Google Scholar]
- Li Z., Cui Z. Three-dimensional perfused cell culture. Biotechnol. Adv. 2014;32:243–254. doi: 10.1016/j.biotechadv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Lindemans C.A., Calafiore M., Mertelsmann A.M., O'Connor M.H., Dudakov J.A., Jenq R.R., Velardi E., Young L.F., Smith O.M., Lawrence G. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massumi M., Hoveizi E., Baktash P., Hooti A., Ghazizadeh L., Nadri S., Pourasgari F., Hajarizadeh A., Soleimani M., Nabiuni M. Efficient programming of human eye conjunctiva-derived induced pluripotent stem (ECiPS) cells into definitive endoderm-like cells. Exp. Cell Res. 2014;322:51–61. doi: 10.1016/j.yexcr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- McCracken K.W., Howell J.C., Wells J.M., Spence J.R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 2011;6:1920–1928. doi: 10.1038/nprot.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Ajima R., Luo C.T., Yamaguchi T.P., Stappenbeck T.S. Wnt5a potentiates TGF-beta signaling to promote colonic crypt regeneration after tissue injury. Science. 2012;338:108–113. doi: 10.1126/science.1223821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H., Stappenbeck T.S. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 2013;8:2471–2482. doi: 10.1038/nprot.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munera J.O., Sundaram N., Rankin S.A., Hill D., Watson C., Mahe M., Vallance J.E., Shroyer N.F., Sinagoga K.L., Zarzoso-Lacoste A. Differentiation of human pluripotent stem cells into colonic organoids via transient activation of BMP signaling. Cell Stem Cell. 2017;21:51–64. doi: 10.1016/j.stem.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S., Goldstein R.A., Nierras C.R. The promise of human induced pluripotent stem cells for research and therapy. Nat. Rev. Mol. Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Chonabayashi K., Nomura M., Tanaka A., Nakamura M., Inagaki A., Nishikawa M., Takei I., Oishi A., Tanabe K. Epigenetic variation between human induced pluripotent stem cell lines is an indicator of differentiation capacity. Cell Stem Cell. 2016;19:341–354. doi: 10.1016/j.stem.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32:56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- Otsuji T.G., Bin J., Yoshimura A., Tomura M., Tateyama D., Minami I., Yoshikawa Y., Aiba K., Heuser J.E., Nishino T. A 3D sphere culture system containing functional polymers for large-scale human pluripotent stem cell production. Stem Cell Reports. 2014;2:734–745. doi: 10.1016/j.stemcr.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J.F., Jurima-Romet M., Reimer M.L., Mortimer E., Rolfe B., Cayen M.N. Making better drugs: decision gates in non-clinical drug development. Nat. Rev. Drug Discov. 2003;2:542–553. doi: 10.1038/nrd1131. [DOI] [PubMed] [Google Scholar]
- Ran F.A., Hsu P.D., Lin C.Y., Gootenberg J.S., Konermann S., Trevino A.E., Scott D.A., Inoue A., Matoba S., Zhang Y. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–1389. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranga A., Gjorevski N., Lutolf M.P. Drug discovery through stem cell-based organoid models. Adv. Drug Deliv. Rev. 2014;69-70:19–28. doi: 10.1016/j.addr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Rezania A., Riedel M.J., Wideman R.D., Karanu F., Ao Z., Warnock G.L., Kieffer T.J. Production of functional glucagon-secreting alpha-cells from human embryonic stem cells. Diabetes. 2011;60:239–247. doi: 10.2337/db10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki N., Higuchi Y., Harada S., Umeda K., Isagawa T., Aburatani H., Kume K., Kume S. Differentiation and characterization of embryonic stem cells into three germ layers. Biochem. Biophys. Res. Commun. 2009;381:694–699. doi: 10.1016/j.bbrc.2009.02.120. [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K., Noto F.K., Nagaoka M., Li J., Battle M.A., Duris C., North P.E., Dalton S., Duncan S.A. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J.R., Mayhew C.N., Rankin S.A., Kuhar M.F., Vallance J.E., Tolle K., Hoskins E.E., Kalinichenko V.V., Wells S.I., Zorn A.M. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterneckert J.L., Reinhardt P., Scholer H.R. Investigating human disease using stem cell models. Nat. Rev. Genet. 2014;15:625–639. doi: 10.1038/nrg3764. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Sato S., Kurashima Y., Lai C.Y., Otsu M., Hayashi M., Yamaguchi T., Kiyono H. Reciprocal inflammatory signaling between intestinal epithelial cells and adipocytes in the absence of immune cells. EBioMedicine. 2017;23:34–45. doi: 10.1016/j.ebiom.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama N., Nishimura S., Nakamura S., Shimizu T., Ohnishi R., Endo H., Yamaguchi T., Otsu M., Nishimura K., Nakanishi M. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J. Exp. Med. 2010;207:2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S., Kleinman H.K., Luyten F.P., Roberts A.B., Roche N.S., Reddi A.H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- Wang X., Yamamoto Y., Wilson L.H., Zhang T., Howitt B.E., Farrow M.A., Kern F., Ning G., Hong Y., Khor C.C. Cloning and variation of ground state intestinal stem cells. Nature. 2015;522:173–178. doi: 10.1038/nature14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C.L., Mahe M.M., Munera J., Howell J.C., Sundaram N., Poling H.M., Schweitzer J.I., Vallance J.E., Mayhew C.N., Sun Y. An in vivo model of human small intestine using pluripotent stem cells. Nat. Med. 2014;20:1310–1314. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K., Brown J.D., Danenberg E., Duncan A.W., Weissman I.L., Reya T., Yates J.R., 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Yagyu S., Hoyos V., Del Bufalo F., Brenner M.K. An inducible caspase-9 suicide gene to improve the safety of therapy using human induced pluripotent stem cells. Mol. Ther. 2015;23:1475–1485. doi: 10.1038/mt.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui S., Nakamura T., Sato T., Nemoto Y., Mizutani T., Zheng X., Ichinose S., Nagaishi T., Okamoto R., Tsuchiya K. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. 2012;18:618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- Zhang D., Jiang W., Liu M., Sui X., Yin X., Chen S., Shi Y., Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wilson G.F., Soerens A.G., Koonce C.H., Yu J., Palecek S.P., Thomson J.A., Kamp T.J. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.