Abstract
Background: Giant cell arteritis (GCA) is a chronic vasculitis of large and medium vessels in which no targetable biomarkers exist to allow selective treatment, predict disease activity and monitor therapeutic responses. The accessibility of the temporal artery (TA) for biopsy allows morphologic studies to characterize macrophages and T cells in the microenvironment of the arterial wall. We evaluated the expression of folate receptor beta (FRB), a candidate diagnostic/therapeutic biomarker, compared its expression with key macrophage markers and correlated it with GCA severity. Methods: Formalin-fixed paraffin-embedded tissue sections were examined from 6 patients with GCA and 2 controls. Immunohistochemistry was performed using FRB, ETB, CD68 and CD3 antibodies to evaluate for activated macrophages and T cells, assess FRB distribution along the intima, media and adventitial layers and composition of inflammatory infiltrates. We compared the expression of FRB, ETB and CD68 in GCA versus negative controls and in severe (with visual loss) versus mild (without visual loss) disease. Results: In GCA, moderate to severe inflammation was accompanied by >90% destruction of the internal elastic lamina. Macrophages comprised 36.3 ± 4.1% while CD3+ lymphocytes accounted for 61.7 ± 4.1% of total leukocytes. FRB was selectively expressed in macrophages and localized to the adventitia. GCA patients had marginally increased median FRB (9.8 cells/hpf vs. 0; p=0.095), ETB (20.5 vs. 0; p=0.095) and CD68 (38.8 vs. 5; p=0.071) expression versus controls. ETB was found in endothelial cells, smooth muscle cells and macrophages in intima/media. FRB positively correlated with ETB (r=0.90; p-0.037) and CD68 levels (r=0.90; p=0.037). ETB expression positively correlated with CD68 (r=1.0; p<0.0001). There was no difference in FRB between severe and mild GCA. Conclusion: FRB is a potential diagnostic and therapeutic biomarker with restricted expression in GCA macrophages. FRB+ macrophages localized to the adventitia and their expression correlated with ETB and CD68 macrophages, suggesting that they contribute to GCA pathogenesis.
Keywords: Giant cell arteritis, folate receptor beta, macrophages
Introduction
Giant cell arteritis (GCA) is a granulomatous vasculitis that targets the aorta and its branches [1,2]. Symptoms range from fevers, weight loss, musculoskeletal, temporal, jaw or head pains to severe complications of vision loss, stroke and tongue gangrene resulting from critical vessel insufficiency. GCA is the most common adult vasculitis with a prevalence of 15-25 cases per 100,000 and shows preference for affecting older adults, in whom genetics and immune vascular microenvironment render susceptibility to autoimmunity [2-6].
Compared to other forms of vasculopathy like aortic aneurysm and atherosclerosis, GCA allows translational studies more readily because of the accessible temporal artery (TA) which is obtained for biopsy. The TA is a prototypical medium/large artery and in GCA, demonstrates an extensive inflammatory infiltrate that spans all 3 layers of the tunica intima, media and adventitia with accompanying destruction of the internal elastic lamina [4,7]. The pathogenesis of GCA starts with an unknown antigen which is captured in the adventitia and presented by dendritic cells to subsets of T helper cells, Th1 and Th17, that produce interferon gamma (IFG) and interleukin 17 (IL17) cytokines respectively. IFG potentiates macrophages which differentiate into multinucleated giant cells and enable pathways that release metalloproteases and reactive oxygen species, interleukins (IL) 1 and 6, tumor necrosis factor (TNF) alpha, IL12 and growth factors which promote proteolysis, inflammation and vascular remodeling [8,9]. The influence of vasoactive peptides like endothelin 1 (ET-1), has also been studied and shown to mediate vascular remodeling. ET-1 promotes cellular hyperplasia and vasoconstriction thru endothelin A (ETA) and endothelin B (ETB) receptors which were found in the endothelial and smooth muscle cells of the intima and media respectively. Previous studies of GCA demonstrated that ETA and ETB receptors were also expressed in macrophages but the ETB appears more dominant in affecting the remodeling process [10-12].
To date, there is no known cure for GCA. The current management is limited to immunosuppression with glucocorticoids (GC) which are partially effective and normalize Th17 and IL17 levels but fail to suppress IFG/TH1 [8]. Recent approval of the IL-6 inhibitor tocilizumab has added to therapeutic choices, but long-term effects are not yet known [13,14]. The course of treatment is protracted and hampered by relapses and uncertainty because biomarkers that reflect ongoing disease activity and assist in targeted therapies are lacking. GCA fails to remit in 30-50% of patients and requires long term steroids which leads to complications of osteoporosis, skin fragility, diabetes, cataract and neurocognitive abnormalities [15,16].
The mechanism behind IFG persistence remains unclear and may account for the relapsing nature of GCA. This presented a novel question of whether the upregulation of IFG is due to specific pathogenic macrophages. When incubated in a local microenvironment rich in IFG, a macrophage is activated to a pro-inflammatory or classic M1 phenotype that releases nitric oxide [17] and IL-12 which can induce TH1 to produce more IFG thus creating a continuous cycle of cross activation between macrophage and T cells [7]. The folate receptor beta (FRB) is part of the folate receptor family and is made up of glycosyl-phosphatidylinositol-anchored glycoprotein with a high affinity for folic acid and 5-methyltetrahydrofolate. In normal hematopoiesis, FRB is expressed in the myelomonocytic lineage but in a non-functional form, i.e., the receptor is unable to bind its cognate ligand, folate. However, functional FRB is expressed in cells of myeloid leukemia, tumor associated macrophages in ovarian and colon cancer and pro-inflammatory monocytes and activated macrophages in rheumatoid arthritis (RA) [18]. FRB anchored on these pathogenic cell surfaces demonstrated increased uptake of folic acid and folate conjugates which are transported from cell membrane to the internal cell compartment [19-23]. This unique attribute and location of the FRB has made it a potential strategic target for selective FRB-targeted approaches and has seen numerous trials using folate-conjugated immunosuppressive and imaging agents, vaccines and multiple antifolates in cancer and rheumatoid arthritis but has not been explored in vasculitis where activated macrophages play a key role.
In this pilot study, we determined the expression and distribution of FRB in GCA macrophages and correlated them with other effector macrophage subsets and clinical severity (complication of vision loss). By demonstrating that FRB is expressed selectively in adventitial macrophages, we highlight a novel macrophage subtype that can contribute to disease pathogenesis and provide a potential target for mechanistic studies in GCA.
Material and methods
Subjects
After obtaining Institutional Review Board Approval, the electronic medical and surgical pathology records of the Penn State Medical Center dated 2001 to 2011 were reviewed and 6 subjects with GCA and 2 controls were selected. GCA was confirmed based on positive histopathologic findings and the American College of Rheumatology clinical criteria [24]. Under the GCA group, 4 subjects with severe disease as defined by vision loss from anterior ischemic optic neuropathy and 2 with mild disease and no vision loss were identified. Controls were defined as those who underwent a biopsy with initial suspicion of GCA which was ruled out after both negative pathology and clinical follow up were satisfied.
Histopathologic analysis
Formalin-fixed paraffin embedded hematoxylin-eosin stained tissue sections were used for confirmation of GCA. Immunohistochemical stains were performed using FRB, ETB, CD68 and CD3 antibodies to enhance recognition of activated macrophages, T lymphocytes, and assess their distribution along the intima media and adventitial layers and composition of inflammatory infiltrates.
Consecutive 4 μm thick sections were cut from formalin-fixed, paraffin-embedded blocks and dried at 60°C for 1 hour. After dewaxing and rehydration, the sections were treated with 10 mmol/L citrate buffer pH 6.0 for 20 minutes in a steamer followed by 20 minutes to cool at room temperature (RT). Slides were incubated in methanol containing 0.3% H2O2 to inactivate endogenous peroxidases. Slides were pre-incubated with 1% normal Swine serum (Dako) for 10 minutes at RT. Slides were washed with PBS and then incubated with the following primary antibodies: (1) anti-FRB antibody (Manohar Ratnam) (diluted 1:800) with placental tissue used as positive control; (2) rabbit polyclonal anti-human ETB (GeneTex, Irvine CA) diluted 1:200; (3) anti-CD68 (M876 Dako, Glostrup, Denmark) diluted 1:200; (4) anti-CD3 (M701 Dako) diluted 1:600. The immunohistochemistry was carried out in an automated immunostainer, TechMate 500 (Ventana Biotek, Tucson, AZ) using the biotin-streptavidin-peroxidase method with diaminobenzidine as the chromogen (Dako REAL Detection System, peroxidase/diaminobenzidine+, rabbit/mouse). Samples were examined using an Olympus BX60 microscope (Tokyo, Japan) and photographed using a Pixera Pro 600ES digital camera (Los Gatos, CA). Measurements were done on 10 randomly selected high power field sections, and the mean numbers of cells were calculated. Numbers of FRB, ETB and CD68 macrophages were compared between GCA and controls, and between mild and severe GCA. Distribution of the immunostaining intensity of FRB and ETB macrophages was compared among the intima, media and adventitia. The expression and distribution of FRB was compared between GCA and controls. FRB expression was correlated with erythrocyte sedimentation rate and other macrophage markers including ETB and CD68.
Statistical analyses were performed using SAS Enterprise Guide (SAS Institute, Cary, NC). FRB, ETB and CD 68 expression were compared between GCA and controls as well as between severe GCA (defined as those with vision loss) and mild GCA (defined as those without vision loss) using the Wilcoxon rank sum test. The biomarkers were correlated using the Spearman’s rank correlation coefficient. Categorical variables were compared using the Fisher’s exact test. Significance was defined as P<0.05.
Results
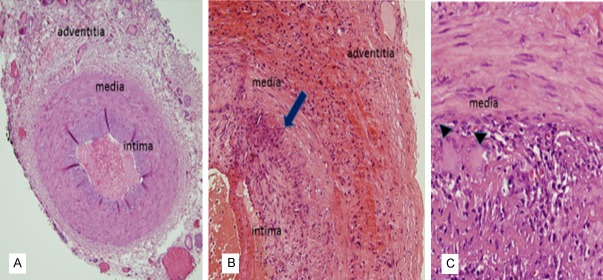
TA biopsies from 6 GCA+ subjects had a median age of 81.5 years (range 72-87), while the 2 negative controls were younger, with a median age of 55.5 years. Median erythrocyte sedimentation rate (ESR) for GCA subjects was 81 mm/hour (range 52-120). Four out of 6 GCA had severe disease (vision loss). The H and E stains demonstrated a moderate to severe lympho-histiocytic infiltrate with >90% destruction of the internal elastic lamina. Activated macrophages identified by positive CD68 staining comprised 36.3 ± 4.1% of total leukocytes while CD3 (+) lymphocytes accounted for 61.7 ± 4.1%. In contrast, the negative cases showed an intact internal elastic lamina and no inflammation (Figure 1).
Figure 1.
Comparison of normal versus GCA affected temporal arteries. Representative H and E images of a normal temporal artery with minimal immune cells (A) and GCA positive temporal arteries (B, C) with granulomatous infiltrates spanning the hyperplastic intima, media and adventitia. Arrow demonstrates fragments of internal elastic lamina. Arrowheads show 2 multinucleated giant cells. Magnification ×200 in (A); ×400 in (B); ×600 in (C).
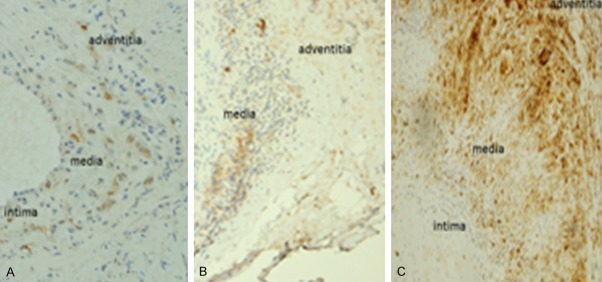
FRB expression was identified only in macrophages as opposed to ETB expression which localized in macrophages, endothelial cells and smooth muscle cells. GCA patients had marginally increased median expression of FRB (9.8 cells/high power field vs. 0; p=0.095), ETB (20.5 vs. 0; p=0.095) and CD68 (38.8 vs. 5; p=0.071) compared to controls (Figure 2). The distribution of the FRB macrophages was found highest in the adventitia followed by the media, with none seen in the intima. ETB was expressed in smooth muscle cells (SMCs), endothelial cells (ECs) and macrophages in the intima and media with none seen in the adventitia (Figure 3).
Figure 2.
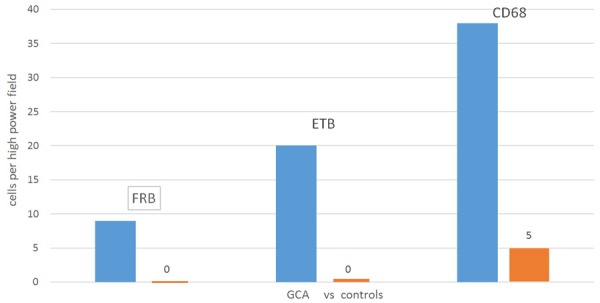
FRB+, ETB+ and CD68+ macrophage expression (median) in immunohistochemical stains of temporal artery biopsies in GCA (in blue) vs. controls (in orange).
Figure 3.
Differential distribution of FRB and ETB macrophage subtypes. Paraffin embedded tissue sections of temporal arteries were stained with the following antibodies: anti-FRB which localized primarily to adventita > media (A), anti-ETB to the intima and media (B) and anti-CD68 pan macrophage marker to all 3 layers (C). Magnification ×200.
GCA subjects with severe disease/vision loss did not have a significantly higher expression of FRB, ETB and CD68 compared with those without vision loss (data not shown). FRB expression positively correlated with ETB levels (r=0.90; p=0.037) and CD68 levels (r=0.90; p=0.037). ETB macrophage expression positively correlated with CD68 (r=1.0; p<0.0001) (Figure 4). FRB did not correlate with ESR levels (r=0.21; p=0.74).
Figure 4.
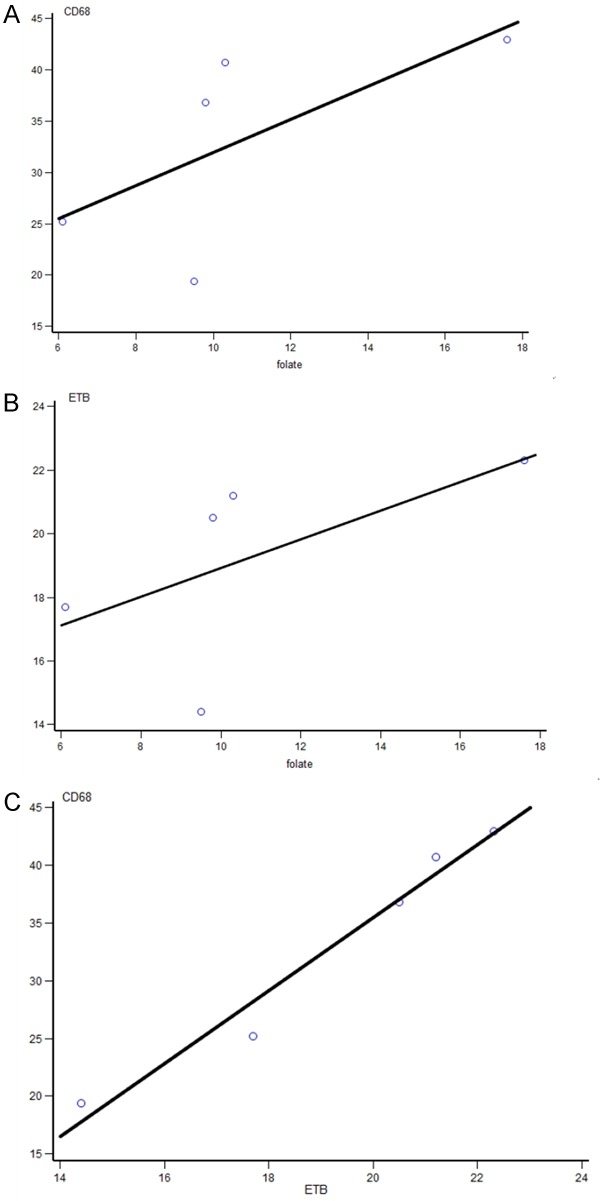
Correlation of macrophage subtype expression in GCA patients. (A) Folate vs. CD68 (r=0.90; p=0.037) (B) Folate vs. ETB (r=0.90; p=0.037) (C) ETB vs. CD68 (r=1.0; p<0.0001).
Discussion
This clinical and immunohistochemical study demonstrated 3 novel findings. Firstly, FRB was found only in GCA positive temporal arteries and FRB expression was restricted to activated macrophages only. Secondly, the FRB macrophages localized predominantly to the adventitial arterial wall. Thirdly, the FRB macrophages correlated with known pathogenic CD68+ and ETB+ macrophage subtypes.
Delivering drugs accurately and precisely to a disease target while sparing normal cells is the ultimate goal of selective drug therapy. Examples include anti-bacterials which target vulnerable areas of infectious pathogens, and blockade of angiotensin converting enzyme that exerts mechanistic effects that control chronic hypertension. Molecularly targeted therapeutics such as antagonists of tyrosine kinase, vascular endothelial growth factor and programmed cell death-1 receptors have improved the morbidity and mortality of solid organ and hematologic malignancies [25]. The FRB, with its unique position in the activated macrophages in GCA and properties that bind folate and anti-folates, can be exploited as a potential diagnostic and therapeutic target.
The consequences of targeting the FRB macrophage should be analyzed in the context of the use of the prototypical anti-folate methotrexate plus folic acid, a standard treatment regimen in rheumatoid arthritis and vasculitis. Folic acid is an essential vitamin that uses 3 transport pathways in humans which include the FR mechanism, the reduced folate carrier and the proton coupled folate transporter. After cellular uptake, folate induces cellular growth thru DNA synthesis, methylation and repair. In recent years, folate was shown to modulate endothelial function by providing tetrahydrobiopterin (BH4), which is an endothelial nitric oxide synthase co-factor used for nitric oxide (NO) production [26]. The NO produced through this folate assisted mechanism, can potentially mediate vascular remodeling by activating fibroblasts, smooth muscle cells and endothelial cells. On the other hand, methotrexate is a disease modifying anti-rheumatic drug that exerts its anti-inflammatory effects thru purine antagonism, DNA synthesis blockade and induction of adenosine release via multiple pathways including the FRB transport system [27]. In a study by van der Heijden and colleagues, the FRB displayed a stronger affinity for folic acid than methotrexate in-vitro, but the latter nonetheless was still able to reduce FRB synovial macrophages and RA clinical disease activity in vivo although outcomes failed to reach statistical significance [28].
In the field of vasculitis, empirically administered glucocorticoids remain the cornerstone of management despite advances in understanding the crosstalk between macrophages and lymphocytes. The B cell depleting agent rituximab and cytokine directed therapies including the tumor necrosis alpha antagonists infliximab and etanercept, and the interleukin-6 blocker tocilizumab are variably effective for anti-neutrophil cytoplasmic antibody mediated vasculitis and giant cell arteritis but mechanistic studies to validate biomarkers that effectively indicate macrophage activity and response to these biologic interventions are lacking. We demonstrate that FRB is a possible indicator of macrophage mediated pathology and a potential target for antifolate therapy in GCA. FRB was selectively expressed in macrophages which stood in contrast with another novel target, the ETB, which was ubiquitously expressed in the smooth muscle and endothelial cells and which may not be amenable to more selective drug targeting. The long term efficacy and safety record of methotrexate compared to other anti-cytokine agents and our findings of FRB positive macrophages should fuel mechanistic studies to evaluate how methotrexate can best target GCA macrophages expressing the FRB. Concurrently, the practice of using folic acid in conjunction with methotrexate should be questioned. Since FRB binds folate/folic acid with more affinity than methotrexate, the use of folate can outcompete methotrexate and account for the reported drug resistance to methotrexate in the few clinical GCA trials [29,30]. In addition, folate conjugates of drugs or second generation antifolates which have higher affinities for FRB can be justified to target these GCA macrophages.
In comparison with other pathogenic macrophages, FRB expression was correlated with ETB and CD68 macrophage populations, both of which are implicated in proposed endothelin mediated mitogenesis, vasoconstriction and tissue destruction in GCA respectively [9,12].
The predominantly adventitial distribution of FRB macrophages was an unexpected finding and contrasted with ETB macrophages which localized to both the media and intima. This observation raises questions on how FRB macrophages affect the resident fibroblasts in the unique microenvironment of the adventitia. In a recent in-vitro study, Zhang and colleagues demonstrated that LPS activated macrophages mediated inducible NO synthase signaling in the adventitial fibroblasts, thereby promoting myofibroblast and collagen production [31]. This provides proof of concept that if FRB macrophages bind folic acid, the co-factor tetrahydrobiopterin can be recycled and used for NO production which can activate GCA adventitial fibroblasts to promote vascular remodeling. [32].
We demonstrated a trend towards higher FRB, ETB and CD68 expression in our GCA cohort versus controls but our results were limited by a small sample size and did not reach statistical significance. The retrospective design limited our FRB analysis to protein quantification in paraffin embedded blocks and will require further validation with mRNA analysis in larger prospective studies. We demonstrated no correlation with the severe complication of visual loss which is instructive in designing future studies to expand the spectrum of disease and include other ischemic complications. By establishing that FRB is present and restricted to GCA macrophages, we justify larger and prospective studies to characterize the FRB macrophage activation and assess its response to targeted approaches with folates, folate conjugates and antifolate drugs.
To date, this study is the first to demonstrate that FRB is present and limited to activated macrophages in GCA affected temporal arteries. FRB levels correlated with other effector macrophages and present a potential putative target in the pathogenesis of GCA.
Disclosure of conflict of interest
None.
References
- 1.Evans JM, Hunder GG. Polymyalgia rheumatica and giant cell arteritis. Rheum Dis Clin North Am. 2000;26:493–515. doi: 10.1016/s0889-857x(05)70153-8. [DOI] [PubMed] [Google Scholar]
- 2.Hunder GG. Giant cell arteritis and polymyalgia rheumatica. Med Clin North Am. 1997;81:195–219. doi: 10.1016/s0025-7125(05)70511-3. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Liao YJ, Goronzy JJ. The immunopathology of giant cell arteritis: diagnostic and therapeutic implications. J Neuroophthalmol. 2012;32:259–265. doi: 10.1097/WNO.0b013e318268aa9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol. 2013;9:731–740. doi: 10.1038/nrrheum.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobato-Berezo A, Alcalde-Villar M, Imbernon-Moya A, Martinez-Perez M, Aguilar-Martinez A, Collado-Ramos P. Tongue necrosis: an unusual clinical presentation of giant cell arteritis. Arthritis Rheumatol. 2014;66:2803. doi: 10.1002/art.38767. [DOI] [PubMed] [Google Scholar]
- 6.Zaragoza JR, Vernon N, Ghaffari G. Tongue necrosis as an initial manifestation of giant cell arteritis: case report and review of the literature. Case Rep Rheumatol. 2015;2015:901795. doi: 10.1155/2015/901795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hilhorst M, Shirai T, Berry G, Goronzy JJ, Weyand CM. T cell-macrophage interactions and granuloma formation in vasculitis. Front Immunol. 2014;5:432. doi: 10.3389/fimmu.2014.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121:906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittner HL, Kaiser M, Brack A, Szweda LI, Goronzy JJ, Weyand CM. Tissue-destructive macrophages in giant cell arteritis. Circ Res. 1999;84:1050–1058. doi: 10.1161/01.res.84.9.1050. [DOI] [PubMed] [Google Scholar]
- 10.Dimitrijevic I, Andersson C, Rissler P, Edvinsson L. Increased tissue endothelin-1 and endothelin-B receptor expression in temporal arteries from patients with giant cell arteritis. Ophthalmology. 2010;117:628–636. doi: 10.1016/j.ophtha.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Lozano E, Segarra M, Corbera-Bellalta M, Garcia-Martinez A, Espigol-Frigole G, Pla-Campo A, Hernandez-Rodriguez J, Cid MC. Increased expression of the endothelin system in arterial lesions from patients with giant-cell arteritis: association between elevated plasma endothelin levels and the development of ischaemic events. Ann Rheum Dis. 2010;69:434–442. doi: 10.1136/ard.2008.105692. [DOI] [PubMed] [Google Scholar]
- 12.Planas-Rigol E, Terrades-Garcia N, Corbera-Bellalta M, Lozano E, Alba MA, Segarra M, Espigol-Frigole G, Prieto-Gonzalez S, Hernandez-Rodriguez J, Preciado S, Lavilla R, Cid MC. Endothelin-1 promotes vascular smooth muscle cell migration across the artery wall: a mechanism contributing to vascular remodelling and intimal hyperplasia in giant-cell arteritis. Ann Rheum Dis. 2017;76:1624–1634. doi: 10.1136/annrheumdis-2016-210792. [DOI] [PubMed] [Google Scholar]
- 13.Villiger PM, Adler S, Kuchen S, Wermelinger F, Dan D, Fiege V, Butikofer L, Seitz M, Reichenbach S. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1921–1927. doi: 10.1016/S0140-6736(16)00560-2. [DOI] [PubMed] [Google Scholar]
- 14.Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, Brouwer E, Cid MC, Dasgupta B, Rech J, Salvarani C, Schett G, Schulze-Koops H, Spiera R, Unizony SH, Collinson N. Trial of tocilizumab in giant-cell arteritis. N Engl J Med. 2017;377:317–328. doi: 10.1056/NEJMoa1613849. [DOI] [PubMed] [Google Scholar]
- 15.Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, Tavera-Bahillo I, Corbera-Bellalta M, Planas-Rigol E, Espigol-Frigole G, Butjosa M, Hernandez-Rodriguez J, Cid MC. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore) 2014;93:194–201. doi: 10.1097/MD.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Lado L, Calvino-Diaz C, Pineiro A, Dierssen T, Vazquez-Rodriguez TR, Miranda-Filloy JA, Lopez-Diaz MJ, Blanco R, Llorca J, Gonzalez-Gay MA. Relapses and recurrences in giant cell arteritis: a population-based study of patients with biopsy-proven disease from northwestern Spain. Medicine (Baltimore) 2011;90:186–193. doi: 10.1097/MD.0b013e31821c4fad. [DOI] [PubMed] [Google Scholar]
- 17.Salim T, Sershen CL, May EE. Investigating the role of TNF-alpha and IFN-gamma activation on the dynamics of iNOS gene expression in LPS stimulated macrophages. PLoS One. 2016;11:e0153289. doi: 10.1371/journal.pone.0153289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar MD, Ratnam M. The folate receptor: what does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007;26:141–152. doi: 10.1007/s10555-007-9048-0. [DOI] [PubMed] [Google Scholar]
- 19.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113:438–446. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 20.Shen J, Hilgenbrink AR, Xia W, Feng Y, Dimitrov DS, Lockwood MB, Amato RJ, Low PS. Folate receptor-beta constitutes a marker for human proinflammatory monocytes. J Leukoc Biol. 2014;96:563–570. doi: 10.1189/jlb.2AB0713-372R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakashima-Matsushita N, Homma T, Yu S, Matsuda T, Sunahara N, Nakamura T, Tsukano M, Ratnam M, Matsuyama T. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1609–1616. doi: 10.1002/1529-0131(199908)42:8<1609::AID-ANR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41:120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 23.Puig-Kroger A, Sierra-Filardi E, Dominguez-Soto A, Samaniego R, Corcuera MT, Gomez-Aguado F, Ratnam M, Sanchez-Mateos P, Corbi AL. Folate receptor beta is expressed by tumorassociated macrophages and constitutes a marker for M2 anti-inflammatory/regulatory macrophages. Cancer Res. 2009;69:9395–9403. doi: 10.1158/0008-5472.CAN-09-2050. [DOI] [PubMed] [Google Scholar]
- 24.Hunder GG. Classification/diagnostic criteria for GCA/PMR. Clin Exp Rheumatol. 2000;18:S4–5. [PubMed] [Google Scholar]
- 25.Garcia-Bennett A, Nees M, Fadeel B. In search of the holy grail: folate-targeted nanoparticles for cancer therapy. Biochem Pharmacol. 2011;81:976–984. doi: 10.1016/j.bcp.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Chalupsky K, Kracun D, Kanchev I, Bertram K, Gorlach A. Folic acid promotes recycling of tetrahydrobiopterin and protects against hypoxia-induced pulmonary hypertension by recoupling endothelial nitric oxide synthase. Antioxid Redox Signal. 2015;23:1076–1091. doi: 10.1089/ars.2015.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan ES, Cronstein BN. Methotrexate--how does it really work? Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 28.van der Heijden JW, Oerlemans R, Dijkmans BA, Qi H, van der Laken CJ, Lems WF, Jackman AL, Kraan MC, Tak PP, Ratnam M, Jansen G. Folate receptor beta as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Rheum. 2009;60:12–21. doi: 10.1002/art.24219. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman GS, Cid MC, Hellmann DB, Guillevin L, Stone JH, Schousboe J, Cohen P, Calabrese LH, Dickler H, Merkel PA, Fortin P, Flynn JA, Locker GA, Easley KA, Schned E, Hunder GG, Sneller MC, Tuggle C, Swanson H, Hernandez-Rodriguez J, Lopez-Soto A, Bork D, Hoffman DB, Kalunian K, Klashman D, Wilke WS, Scheetz RJ, Mandell BF, Fessler BJ, Kosmorsky G, Prayson R, Luqmani RA, Nuki G, McRorie E, Sherrer Y, Baca S, Walsh B, Ferland D, Soubrier M, Choi HK, Gross W, Segal AM, Ludivico C, Puechal X International Network for the Study of Systemic Vasculitides. A multicenter, randomized, doubleblind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum. 2002;46:1309–1318. doi: 10.1002/art.10262. [DOI] [PubMed] [Google Scholar]
- 30.Mahr AD, Jover JA, Spiera RF, Hernandez-Garcia C, Fernandez-Gutierrez B, Lavalley MP, Merkel PA. Adjunctive methotrexate for treatment of giant cell arteritis: an individual patient data meta-analysis. Arthritis Rheum. 2007;56:2789–2797. doi: 10.1002/art.22754. [DOI] [PubMed] [Google Scholar]
- 31.Zhang G, Li X, Sheng C, Chen X, Chen Y, Zhu D, Gao P. Macrophages activate iNOS signaling in adventitial fibroblasts and contribute to adventitia fibrosis. Nitric Oxide. 2016;61:20–28. doi: 10.1016/j.niox.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25:81–88. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]