Abstract
Magnesium ions (Mg2+) are essential for various enzymatic reactions in the body associated with energy production and activation of the muscles and nerves. Mg2+ is also involved in blood pressure regulation, maintenance of body temperature, and glucose metabolism. Although various factors including foods and physical conditions have been reported to change serum Mg2+ status in humans, serum Mg2+ in dogs exposed to external stress has been unclear. In this study, we examined serum levels of Mg2+ in dogs at different conditions using the guide dog candidates for the blind. Serum Mg2+ was decreased in winter and increased in summer. Guide dog candidates in an elementary class of the training showed markedly lower levels of serum Mg2+, compared with that of dogs in an advanced class. When healthy adult dogs were subjected to forced exercise using a treadmill, a significant reduction in serum Mg2+ levels was observed, particularly in winter. These findings suggest that serum levels of Mg2+ may be influenced by weather fluctuation such as air temperature, nervousness in unaccustomed situations, age, and physical stress induced by exercise. The results indicate that Mg2+ supplementation should be considered for working dogs, dogs moving or traveling to a new environment, and dogs during winter.
Keywords: Exercise, Guide dogs, Seasonality, Serum magnesium ions, Training
Introduction
Magnesium ions (Mg2+) are one of the essential minerals necessary to maintain life. Most Mg2+ is stored in the cells of organs and tissues, particularly in the bones and teeth. Small amounts of Mg2+ are present in extracellular spaces, where Mg2+ binds with either proteins or anions. Mg2+ is needed to generate energy by assisting in the reaction of various enzymes with Mg2+ binding sites in their active region (Cowan, 2002). Mg2+ is necessary for the synthesis of proteins, energy metabolism (Pfeiffer and Barnes, 1981; He et al., 2006), contraction of the muscles (Altura and Altura, 1981), blood pressure regulation (Resnick et al., 2000; He et al., 2005), and modulating blood glucose levels (Dominguez et al., 1998; Singh et al., 1998), as well as a considerable number of enzymatic reactions within the body (Cowan, 2002). Lack of Mg2+ induces deterioration in energy production, leading to fatigue (Lukaski and Nielsen, 2002). Mg2+ deficiency also causes poor concentration, chronic fatigue, loss of appetite, and cardiovascular abnormalities in humans (Bohl and Volpe, 2002). Concentration of Mg2+ in blood is regulated by the interaction of several hormones, including, noradrenaline, parathyroid hormone, glucagon, and cortisol (Soria et al., 2014). Intravenous injection of catecholamine induced a marked increase of Mg2+ excretion in the urine (Rayssiguier, 1977; Joborn et al., 1985), suggesting catecholamine may reduce blood Mg2+ levels.
In humans, abnormalities in serum Mg2+ levels have been reported in various diseases (Elin, 1988; Rude and Gruber, 2004; Sinert et al., 2005; Baltaci et al., 2013), and, interestingly, the morbidity of these diseases has been reported to correlate with Mg2+ intake (Elin, 1988; Singh et al., 1997; Eby and Eby, 2006). However, the dietary intake of Mg2+ reduces with age (Bazzarre et al., 1993; Durlach et al., 1993; Tucker et al., 1999). The market for Mg2+ supplements has expanded for prophylactic amelioration of lifestyle related diseases (Seelig and Altura, 1997). In addition, Mg2+ supplementation in training athletes has also increased (Haymes, 1991). Moreover, reduction in Mg2+ intake has been reported to be associated with severity of depression and anxiety in community-dwelling adults, and administration of Mg2+ to patients improved their conditions (Jacka et al., 2009).
Changes in the serum Mg2+ levels of dogs have not been fully explored. The aim of this study was to analyze changes in serum Mg2+ levels of dogs before and after a training or exercise load.
Materials and Methods
Animals
All animal experiments complied with the standards specified in the guidelines of the University Animal Care and Use Committee of the Tokyo University of Agriculture and Technology as well as the guidelines for the use of laboratory animals provided by the Science Council of Japan. The procedures conducted were approved by the University Animal Care and Use Committee of the Tokyo University of Agriculture and Technology (No. 27-62; July, 27, 2015). For blood collection from guide dog candidates, all procedures were informed approved by The Eye Mate Inc. (Tokyo, Japan). Young Labrador retrievers (aged from 17 to 35 months, mean age was 23 ± 0.8 months old.) that had been selected as candidates for guide dogs for the blind were subjected to the measurement of serum Mg2+ in the experiment 1 (12 dogs) and 2 (24 dogs). They were housed in individual cages in a room illuminated daily from 6:00–21:00 with a temperature of 15–25 ± 3°C. The room temperature of the kennel was set according to that of the outside, because the training of the dog was carried out in city areas. They were fed with appropriate food once a day at 7:00 and were given water ad libitum. They were all neutered and belonged to The Eye Mate Inc. The Eye Mate Inc. has the longest history of dog training in Japan and provides the largest number of well-trained guide dogs to the blind. Healthy Labrador retrievers that worked as guide dogs for the blind (mean age was 6.9 ± 0.4 years old, 6 neutered males and 8 neutered females) were subjected to the measurement of serum Mg2+ in the experiment 2 (14 dogs). They were all neutered and belonged to their user. They were fed by their users. Three of them were fed in the morning and 11 of them were fed in the night. All the guide dog candidates and guide dogs were fed with the same food.
In the experiment 3, we used 6 laboratory dogs. They were housed in individual cages in a room illuminated daily from 7:00–19:00 with a temperature of 21 ± 4°C. They were fed with appropriate food once a day at 19:00 and were given water ad libitum. They were allowed to take a walk or free exercise at the outside of their facilities with animal care staffs for 30–60 min in one day for their welfare, except the day of the experiment. They were all neutered, fed with the same food, and managed in the same circumstances.
Foods supplied to dogs used in the current study are shown in Table 1. Natural Harvest Maintenance (13–15 g/kg body weight/day) (Vanguard International Foods Co., Chiba, Japan) was given to guide dog candidates and guide dogs for the blind, and Acana Pacifica for dogs (15–20 g/kg body weight/day) (Champion Pet Foods Ltd., AB, Canada) was given to laboratory dogs.
Table 1.
Foods supplied to dogs in the current study
| Calorie and components | Natural Harvest Maintenance* | Acana Pacifica for dogs** |
|---|---|---|
| Metabolic calorie (kJ/g food) | 13.4 | 14.7 |
| Crude protein (% wt.) | 18 | 33 |
| Crude fat (% wt.) | 9.5 | 17 |
| Crude fiber (% wt.) | 4 | 5 |
| Moisture (% wt.) | 10 | 10 |
| Minerals | ||
| Magnesium (% wt.) | 0.1 | 0.1 |
| Calcium (% wt.) | 1.6 | 1.5 |
| Sodium (% wt.) | 0.36 | 0.6 |
| Chloride (% wt.) | 0.57 | 1.1 |
| Potassium (% wt.) | 1.0 | 1.0 |
| Iron (mg/kg food) | 265 | 170 |
| Zinc (mg/kg food) | 404 | 230 |
| Copper (mg/kg food) | 23.8 | 20 |
| Manganese (mg/kg food) | 51.2 | 23 |
| Iodine (mg/kg food) | 4.6 | 1.8 |
| Selenium (mg/kg food) | 0.34 | 1.5 |
Natural Harvest was given to guide dog candidates and guide dogs for the blind.
Acana Pacifica was given to laboratory dogs used in the treadmill experiment.
Dogs used in each experiment
In the experiment 1, 12 guide dog candidates in the advanced classes (aged from 21 to 35 months) were used to confirm seasonal changes of serum Mg2+ levels. The advanced class is a final stage of their training. Blood samples collected in January, May, and August from different dogs in the advanced class at each month, and serum Mg2+ levels were analyzed. Each group was consisted of 4 dogs (1 neutered male and 3 neutered females). In Eye Mate Inc., 4 dogs finish their trainings every month and start working as mature guide dogs for the blind.
In the experiment 2, the total of 24 guide dog candidates and 14 working guide dogs were used to measure serum Mg2+ levels. Training phases of those candidates are divided into three classes as described below. The elementary training class included dogs that could walk with their instructor on their leads for 10–15 min. The intermediate class included dogs that could wear harnesses to walk under the simple commands of their instructor on an empty street for 20–30 min. The advanced class included dogs that could wear harnesses to walk under the commands of their instructor on a busy street for 40–50 min. Six dogs (the mean age was 19.8 ± 0.8 months old, 1 neutered male and 5 neutered females) in the elementary class, 10 dogs (the mean age was 21.1 ± 0.9 months old, 4 neutered males and 6 neutered females) in the intermediate class, and 8 dogs (the mean age was 26.5 ± 1.5 months old, 2 neutered males and 6 neutered females) in the advanced class were subjected to the study. Fourteen healthy Labrador retrievers that worked as guide dogs for the blind (mean age was 6.9 ± 0.4 years old, 6 neutered males and 8 neutered females) were subjected to the measurement of serum Mg2+ in the experiment 2 under the informed consent of their owners as adult controls.
In the experiment 3, we used a treadmill to test the effects of forced exercise. In this experiment, three healthy mixed breed dogs (aged from 5 to 6 years), one Beagle dog (6 years old), one Jack Russell Terrier (6 years old) and one Miniature Dachshund (9 years old), belonging to the colony of our laboratory were used. They included 3 neutered males and 3 neutered females. Before and after the treadmill training performed in January and August, blood samples were collected. To assess the effects of forced exercise on serum Mg2+ levels, laboratory dogs undertook 20 min of physical exercise on a treadmill that was set at 3–6.5 km/h. The speed of the treadmill was set according to the physical ability of each dog.
Blood sampling
To investigate serum Mg2+ levels of dogs, guide dog candidates at different phases of training were examined. We collected blood samples from dogs in the elementary and intermediate classes on the first day of their training. We collected blood samples from dogs in the advanced class on the day that the training was completely over. Blood samples (1.5 mL/dog) of laboratory dogs were collected at each time point of their exercise. Samples were taken from the cephalic vein by experienced veterinarians and were collected into serum-separator tubes (SST II; Becton, Dickinson & Co.). The tubes were allowed to stand for 30 min at room temperature and were then centrifuged for 10 min at 425 g. Separated serum was collected and stored at -30°C until use.
Measurement of serum Mg2+ levels
Serum Mg2+ was measured by the quantitative colorimetric determination method using the QuantiChrom Magnesium Assay Kit (DIMG-250; BioAssay Systems, Hayward, CA), according to the manufacturer’s protocol. Using the assay kit, we can directly measure magnesium ions in serum samples without any pretreatment. All assays were performed in flat-bottom 96 well plates (Nunc PolySorp®; Thermo Fisher Scientific, Inc., Tokyo, Japan). A calmagite dye used in the assay kit forms a colored complex specifically with magnesium ions. The absorbance values were measured at 500 nm using a micro plate reader (ImmunoMini (NJ-2300); BioTec, Suffolk, UK). Serum Mg2+ values were expressed as the mean of triplicate measurements.
Measurement of plasma adrenaline and noradrenaline levels
Previous studies have described that increase in blood levels of adrenaline might associate with of hypomagnesemia in humans and ewes (Rayssiguier, 1977; Joborn et al., 1985). Since exercise stress induces increase in blood catecholamine levels, we measured adrenalin and noradrenalin after exercise loads and tried to delineate possible association of those markers in blood Mg2+ levels. Plasma of each dog was isolated using EDTA-2Na and stored at -30°C until use as described above. Plasma adrenaline and noradrenaline levels were measured by using the high-speed liquid chromatography systems (Shimadzu Corp., Kyoto, Japan) in SRL Inc. (Tokyo, Japan).
Statistical analysis
Data were analyzed using IBM SPSS Statistics ver. 22.0. In the experiment 1, the comparison between three groups was analyzed using multiple comparisons with Bonferroni correction (P < 0.05/3 was estimated as a level of significance). In the experiment 2, the comparison between four groups was analyzed using a one-way ANOVA and Dunnett test (P < 0.05). In the experiment 3, the influences of seasonal variation, and before and after the exercise load, were analyzed using a two-way ANOVA within-participant design (P < 0.05). Serum Mg2+ levels were quoted as median and interquartile ranges in all figures.
Results
Experiment 1; Seasonality of serum Mg2+ levels
To examine the serum Mg2+ levels of dogs with very few individual differences due to their environments and foods, we used guide dog candidates with the agreement of The Eye Mate Inc., a Japanese public incorporated foundation that raises guide dogs. Since serum Mg2+ levels are influenced by temperature and the seasons in humans (Owaki et al., 1996), we first assessed seasonal variations of serum Mg2+ levels in dogs. We collected blood samples from the guide dog candidates in the advanced class in January (winter, average temperature for the last five years in Tokyo, Japan is 5.7°C), May (spring, 19.4°C), and August (summer, 28.6°C). The serum Mg2+ levels of January, May, and August were 16.7 ± 0.2 μg/mL (the median is 16.9 μg/mL), 20.7 ± 0.3 μg/mL (the median is 21.0 μg/mL), and 21.2 ± 0.2 μg/mL (the median is 21.7 μg/mL), respectively (Fig. 1a). Serum Mg2+ levels of dogs in the advanced class were significantly lower in winter. On the other hand, serum Mg2+ levels increased in summer.
Fig. 1a.
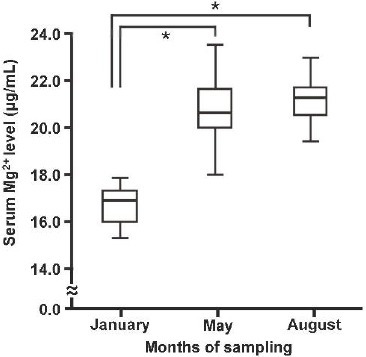
Seasonal variation in serum Mg2+ levels of guide dog candidates in the advanced class. The median values of serum Mg2+ levels are indicated by the bar within each box. The statistical significance of differences between the three groups was tested using Bonferroni correction. n = 4 in each group. (* P < 0.05).
In the previous studies, it mentioned that intravenous injection of adrenaline decreased blood levels of Mg2+ in humans (Joborn et al., 1985) and ewes (Rayssiguier, 1977), and that the consequent increase in adrenaline induced magnesium loss (Seelig, 1994). Therefore, we examined adrenaline and noradrenaline levels in winter and summer (Fig. 1b, Fig. 1c). Plasma adrenaline and noradrenaline levels were higher in winter; on the other hand, they became lower in summer, showing the inverse correlation with serum Mg2+ levels.
Fig. 1b.
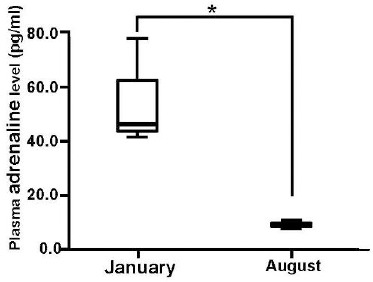
Seasonal variation in plasma adrenaline levels of guide dog candidates in the advanced class. The statistical significance of differences between January and August was tested using paired t-test. n = 4 in each group. (* P < 0.05).
Fig. 1c.
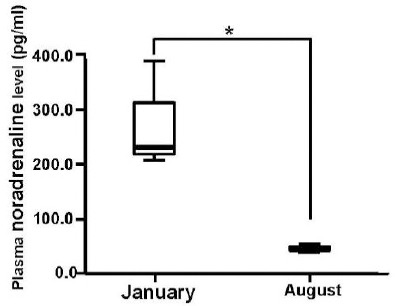
Seasonal variation in plasma noradrenaline levels of guide dog candidates in the advanced class. The statistical significance of differences between January and August was tested using paired t-test. n = 4 in each group. (* P < 0.05).
Experiment 2; Serum Mg2+ levels of guide dog candidates in different training phases
To investigate serum Mg2+ levels of dogs exposed to different amounts of external stress, we selected dogs in elementary, intermediate, and advanced training classes. These different training stages were classified according to the total of training hours and days. Serum samples were collected from May to August and Mg2+ levels were measured. The steady state levels of serum Mg2+ of dogs in elementary, intermediate, and advanced classes were 13.0 ± 0.3 μg/mL (the median is 13.0 μg/mL), 19.3 ± 0.9 μg/mL (the median is 19.9 μg/mL), 21.5 ± 0.5 μg/mL (the median is 21.7 μg/mL), respectively. Serum Mg2+ levels were lower in dogs in the elementary class than those of dogs in other classes (Fig. 2).
Fig. 2.
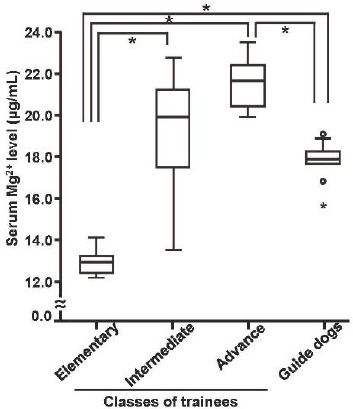
Serum Mg2+ levels of guide dog candidates in different stages of training. The medians of serum Mg2+ levels measured in samples collected from May to August are indicated by the bar within each box. The statistical significance of differences between classes was tested using a one way ANOVA Dunnet. Elementary class, n = 6; intermediate class, n = 10; advanced class, n = 8; healthy adult guide dogs for the blind, n = 14. (* P < 0.05).
On the other hand, serum Mg2+ levels were significantly higher in dogs in the advanced class comparing to those of dogs in the elementary class (Fig. 2). Interestingly, serum Mg2+ levels of dogs in the intermediate class showed intermediate levels with some variation (Fig. 2).
Serum Mg2+ levels of working guide dogs for the blind were markedly higher than those of guide dog candidates in the elementary class, but lower than those of dogs in the advanced class. The right column of Fig. 2 shows the serum Mg2+ levels of healthy guide dogs for the blind without any clinical abnormalities. The serum samples were collected in May. Serum Mg2+ levels of mature guide dogs for the blind were 17.8 ± 0.7 μg/mL (the median is 17.9 μg/mL), and no individual differences were observed.
Means of serum Mg2+ levels of each group with or without breakfast were 17.5 ± 0.4 μg/mL and 17.9 ± 0.3 μg/mL respectively, and there was no the statistical significant difference (t = -0.676, df = 12, p > 0.05). In the mature guide dogs, the Mg2+ value of female dogs was 17.7 ± 0.4 μg/mL and that of male dogs was 17.9 ± 0.3μg/mL, and no significant difference due to the distinction of sex was observed (t = 0.456, df = 12, p > 0.05). All dogs used were clinically healthy throughout the experiment 1 and 2.
Experiment 3; Serum Mg2+ levels after exercise loads
To investigate the effect of physical exercise on serum Mg2+ levels, dogs were subjected to forced exercise using a treadmill for 20 min in the morning, and Mg2+ levels were measured in January (winter) and August (summer).
As shown in Fig. 3, serum Mg2+ levels of dogs after forced exercise were significantly lower than those of dogs before exercise in both January (before; 16.6 ± 0.4 μg/mL and after; 10.8 ± 0.4 μg/mL) and August (before; 18.2 ± 0.5 μg/mL and after; 12.5 ± 0.5 μg/mL). All dogs used were clinically healthy throughout the experiment 3.
Fig. 3.
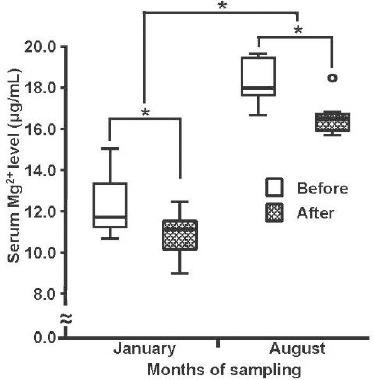
Serum Mg2+ levels before and after treadmill exercise in winter and summer. The medians of serum Mg2+ levels of each group of dogs (n = 6) measured before and after treadmill exercise are indicated by the bar within each box. The statistical significance of differences between the each groups were analyzed using a two-way ANOVA within-participant design (P < 0.05).
Discussion
Recently, decrease in blood Mg concentrations has been reported to associate with urinary Mg excretion (Disashi et al., 1996; Nielsen and Lukaski, 2006; Belluci et al., 2011). Moreover, abnormalities in Mg concentrations in both blood and urine have been identified in patients with neoplastic disorders (Wilhelm et al., 2002) and athletes (Nuviala et al., 1999; Nielsen and Lukashi, 2006), proposing the significance of Mg supplementation in some cases (Haymes, 1991; Bazzarre et al., 1993; Toba et al. 2000; Volpe, 2015). Although dogs have long history as good partners of humans, we found very few report on changes in Mg concentrations of dogs induced by external stress. In the current study, we focused on serum Mg2+ levels as one of biomarkers fluctuated by external stress. Since examination of urine during dogs’ training or working without contamination was quite difficult, we tried to evaluate serum Mg2+ levels before and after external stress.
First, by using young-adult guide dog candidates, we measured serum Mg2+ levels of dogs in January (winter), May (spring), and August (summer) to check seasonal changes. Serum Mg2+ levels were lower in winter; on the other hand, they became higher in summer. In previous research on humans, blood Mg2+ levels became lower in winter because the Mg2+ content of food was decreased during this season (Owaki et al., 1996). Dogs used in our study were the same breed, fed with the same dog food throughout the year in the quite similar environment. Therefore, the observed reduction in serum Mg2+ levels of dogs in winter is likely to have been caused by the effects of climatic changes, such as low temperature and the atmospheric pressure fluctuation, rather than the content of their diet. Since mammals need to maintain their body temperature during the winter, Mg2+ may be important for sustaining metabolic processes including enzymatic reactions.
Adrenaline decreased blood levels of Mg2+ in humans (Joborn et al., 1985) and exes (Rayssiguier, 1977), and induced magnesium loss (Seelig, 1994). Moreover, in humans, plasma free normetanephrine levels were higher in winter than summer (Pamporaki et al., 2014). Therefore, we checked levels of catecholamines and found that plasma adrenaline and noradrenaline levels were higher in winter than summer. Inversely, serum Mg2+ levels were higher in summer than winter, suggesting a possibility that the seasonal changes of serum Mg2+ levels were influenced by the increase in adrenaline and noradrenaline concentrations due to vasoconstriction and vasodilation in winter (Bolli et al., 1984).
Next, we measured serum Mg2+ levels of guide dog candidates at different stages in their training in order to evaluate the effects of mental and physical stress. Interestingly, serum Mg2+ levels were higher in dogs of the advanced class, compared to that of the elementary and intermediate classes. Since Mg2+ is necessary to protect individuals from environmental, physical, and mental stress (Golf et al., 1998; Soldatovic et al., 1998; Zieba et al., 2000), Mg2+ might have been applied to biologic reaction of cells and tissues in dogs in the elementary class, leading to the reduction of serum Mg2+ levels observed. In contrast, since dogs in the advanced class were accustomed to their situation, Mg2+ might have been maintained at a higher level. The average serum Mg2+ level of healthy adult guide dogs for the blind was 17.8 ± 0.7 µg/mL. In a previous study, the mean serum Mg2+ level in adult dogs was 18.8 ± 1 µg/mL (Bailie et al., 1988), which is similar to our finding. In addition, the normal reference range for canine serum Mg2+ described in Veterinary Drug Handbook (Plumb, 1999) and the list of a biochemical examination for dogs by LSI Medience Co. (Tokyo, Japan) is 17–27 µg/mL. However, sex, breeds, age, and seasons when Mg2+ levels were measured were not mentioned. Since the guide dogs in the present study were fed with the same food as the guide dog candidates (Table 1), the differences between the groups have not resulted from their diet, but may have been due to the higher age of the guide dogs when compared to the guide dog candidates.
Finally, we found that the exercise load using a treadmill reduced serum Mg2+ levels of subjected dogs in both winter and summer. In experiment 3, seasonal variation between winter and summer of serum Mg2+ levels was remarkable, which is similar to results obtained in experiment 1. Since the laboratory dogs used were all fed with the same food, as indicated in Table 1, the influence of diet on serum Mg2+ levels could be excluded. Forced exercise might reduce serum Mg2+ levels because of activation of the muscles and nerves. These results suggest that serum Mg2+ levels are influenced by external physical stress.
In the current study, we demonstrated for the first time that serum Mg2+ levels of dogs might be influenced by air temperature, environment, age, and exercise load. Serum Mg2+ levels in dogs were reduced in winter, and they became higher in summer. Serum Mg2+ levels of guide dog candidates were lower in the elementary class.
As the training proceeds, serum Mg2+ levels were increased. Serum Mg2+ levels of dogs after the forced exercise were significantly lower than those before the exercise in both winter and summer. Further experiments with more dogs must take place; however, the variation of serum Mg2+ levels of dogs may become one of biomarkers that reflect physical status of dogs.
Previous research has shown that pigs became healthier after administration of the Mg2+ supplement (O’Driscoll et al., 2013). Supplementation of Mg2+ in healthy dogs with vigorous exercise or training regimes must be discussed for animal welfare. Mg2+ supplementation may also be necessary for aged dogs and beneficial for dogs that are subjected to new environments. Moreover, in winter, Mg2+ supplementation may be needed not only for working dogs but also for companion dogs, particularly those suffering from disease.
Acknowledgments
We would like to appreciate Assoc. Prof. Hideyuki Tanaka for his advice on the statistical analysis, and thank Dr. Akira Matsuda, Dr. Kumiko Oida, Dr. Yosuke Amagai, Dr. Hyosun Jang, Dr. Saori Ishizaka, and Ms Juri Toyama (Tokyo University of Agriculture and Technology) for their supports and animal care.
This work was partially supported by a joint research grant (No. 26-619) from Tateho Chemical Industries Co., Ltd. (Hyogo, Japan).
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Altura BM, Altura BT. Magnesium ions and contraction of vascular smooth muscles: relationship to some vascular diseases. Fed. ProcX. 1981;40(12):2672–2679. [PubMed] [Google Scholar]
- Bailie DS, Inoue H, Kaseda S, Ben-David J, Zipes DP. Magnesium suppression of early after depolarizations and ventricular tachyarrhythmias induced by cesium in dogs. Circulation. 1988;77(6):1395–1402. doi: 10.1161/01.cir.77.6.1395. [DOI] [PubMed] [Google Scholar]
- Baltaci AK, Mogulkoc R, Belviranli M. Serum levels of calcium, selenium, magnesium, phosphorus, chromium, copper and iron-their relation to zinc in rats with induced hypothyroidism. Acta Clin. Croat. 2013;52:151–156. [PubMed] [Google Scholar]
- Bazzarre TL, Scarpino A, Sigmon R, Marquart LF, Wu SM, Izurieta M. Vitamin-mineral supplement use and nutritional status of athletes. J. Am. Coll. Nutr. 1993;12(2):162–169. doi: 10.1080/07315724.1993.10718297. [DOI] [PubMed] [Google Scholar]
- Belluci MM, Gior G, del Barrio RA, Pereira RM, Marcantonio E, Jr, Orrico SR. Effects of magnesium intake deficiency on bone metabolism and bone tissue around osseointegrated implants. Clin. Oral Implants Res. 2011;22(7):716–721. doi: 10.1111/j.1600-0501.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- Bohl CH, Volpe SL. Magnesium and exercise. Crit. Rev. Food Sci. Nutr. 2002;42(6):533–563. doi: 10.1080/20024091054247. [DOI] [PubMed] [Google Scholar]
- Bolli P, Erne P, Ji BH, Block LH, Kiowski W, Buhler FR. Adrenaline induces vasoconstriction through post-junctional alpha 2 adrenoceptors and this response is enhanced in patients with essential hypertension. J. Hypertens. Suppl. 1984;2(3):S115–118. [PubMed] [Google Scholar]
- Cowan JA. Structural and catalytic chemistry of magnesium-dependent enzymes. Biometals. 2002;15(3):225–235. doi: 10.1023/a:1016022730880. [DOI] [PubMed] [Google Scholar]
- Disashi T, Iwaoka T, Inoue J, Naomi S, Fujimoto Y, Umeda T, Tomita K. Magnesium metabolism in hyperthyroidism. Endocr, J. 1996;43(4):397–402. doi: 10.1507/endocrj.43.397. [DOI] [PubMed] [Google Scholar]
- Dominguez LJ, Barbagallo M, Sowers JR, Resnick LM. Magnesium responsiveness to insulin and insulin-like growth factor I in erythrocytes from normotensive and hypertensive subjects. J. Clin. Endocrinol. Metab. 1998;83:4402–4407. doi: 10.1210/jcem.83.12.5327. [DOI] [PubMed] [Google Scholar]
- Durlach J, Durlach V, Bac P, Rayssiguier Y, Bara M, Guiet-Bara A. Magnesium and ageing II. Clinical data: aetiological mechanisms and pathophysiological consequences of magnesium deficit in the elderly. Magnes. Res. 1993;6(4):379–394. [PubMed] [Google Scholar]
- Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med. Hypotheses. 2006;67(2):362–370. doi: 10.1016/j.mehy.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Elin RJ. Magnesium metabolism in health and disease. Dis. Mon. 1988;34(4):161–218. doi: 10.1016/0011-5029(88)90013-2. [DOI] [PubMed] [Google Scholar]
- Golf SW, Bender S, Gruttner J. On the significance of Magnesium in extreme physical stress. Cardiovasc. Drugs Ther. 1998;12(2):197–202. doi: 10.1023/a:1007708918683. [DOI] [PubMed] [Google Scholar]
- Haymes EM. Vitamin and mineral supplementation to athletes. Int. J. Sport Nutr. 1991;1(2):146–169. doi: 10.1123/ijsn.1.2.146. [DOI] [PubMed] [Google Scholar]
- He K, Liu K, Daviglus ML, Morris SJ, Loria CM, Van Horn L, Jacobs D.R, Savage PJ. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113(13):1675–1682. doi: 10.1161/CIRCULATIONAHA.105.588327. [DOI] [PubMed] [Google Scholar]
- He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: Role of angiotensin II. Circ. Res. 2005;96(2):207–215. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- Jacka FN, Overland S, Stewart R, Tell GS, Bjelland I, Mykletun A. Association between magnesium intake and depression and anxiety in community-dwelling adults: the Hordaland Health Study. Aust. N. Z. J. Psychiatry. 2009;43(1):45–52. doi: 10.1080/00048670802534408. [DOI] [PubMed] [Google Scholar]
- Joborn H, Akerstrom G, Ljunghall S. Effects if exogenous catecholamines and exercise on plasma magnesium concentrations. Clin. Endocrinol. 1985;23(3):219–226. doi: 10.1111/j.1365-2265.1985.tb00217.x. [DOI] [PubMed] [Google Scholar]
- Lukaski HC, Nielsen FH. Dietary Magnesium Depletion Affects Metabolic Responses during Submaximal Exercise in Postmenopausal Women. J. Nutr. 2002;132(5):930–935. doi: 10.1093/jn/132.5.930. [DOI] [PubMed] [Google Scholar]
- Nielsen FH, Lukaski HC. Update on the relationship between magnesium and exercise. Magnes. Res. 2006;19(3):180–189. [PubMed] [Google Scholar]
- Nuviala RJ, Lapieza MG, Bernal E. Magnesium, Zinc, and copper status in women involved in different sports. Int. J. Sport Nutr. 1999;9(3):295–309. doi: 10.1123/ijsn.9.3.295. [DOI] [PubMed] [Google Scholar]
- O'Driscoll K, O'Gorman DM, Taylor S, Boyle LA. The influence of a magnesium-rich marine extract on behaviour, salivary cortisol levels, and skin lesions in growing pigs. Animal. 2013;7(6):1017–1027. doi: 10.1017/S1751731112002431. [DOI] [PubMed] [Google Scholar]
- Owaki A, Takatsuka N, Kawakami N, Shimizu H. Seasonal Variations of Nutrient Intake Assessed by 24-Hour Recall Method. J. Nutr. 1996;54(1):11–18. [Google Scholar]
- Pamporaki C, Bursztyn M, Reimann M, Ziemssen T, Bornstein SR, Sweep FC, Timmers H, Lenders JW, Eisenhofer G. Seasonal variation in plasma free normetanephrine concentrations: implications for biochemical diagnosis of pheochromocytoma. Eur. J. Endocrinol. 2014;170(3):349–357. doi: 10.1530/EJE-13-0673. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CC, Barnes B. Role of zinc, manganese, chromium, and vitamin deficiencies in birth defects. Int. J. Environ. Stud. 1981;17(1):43–56. [Google Scholar]
- Plumb DC. Veterinary drug handbook. Third Edition. Ames, Iowa, USA: Iowa State Press; 1999. p. 812. [Google Scholar]
- Rayssiguier Y. Hypomagnesemia resulting from adrenaline infusion in ewes: its relation to lipolysis. Horm. Metab. Res. 1977;9(4):309–314. doi: 10.1055/s-0028-1093519. [DOI] [PubMed] [Google Scholar]
- Resnick LM, Oparil S, Chait A, Haynes RB, Kris-Etherton P, Stern JS, Clark S, Holcomb S, Hatton DC, Metz JA, McMahon M, Pi-Sunyer FX, McCarron DA. Factors affecting blood pressure responses to diet: the Vanguard study. Am. J. Hypertens. 2000;13(9):956–965. doi: 10.1016/s0895-7061(00)01221-8. [DOI] [PubMed] [Google Scholar]
- Rude RK, Gruber HE. Magnesium deficiency and osteoporosis: Animal and human observations. J. Nutr. Biochem. 2004;15(12):710–716. doi: 10.1016/j.jnutbio.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Seelig M, Altura BM. How best to determine magnesium requirement: need to consider cardiotherapeutic drugs that affect its retention. J. Am. Coll. Nutr. 1997;16(1):4–6. doi: 10.1080/07315724.1997.10718643. [DOI] [PubMed] [Google Scholar]
- Seelig MS. Consequences of magnesium deficiency on the enhancement of stress reactions;preventive and therapeutic implications. J. Am. Coll. Nutr. 1994;13(5):429–446. doi: 10.1080/07315724.1994.10718432. [DOI] [PubMed] [Google Scholar]
- Sinert R, Spektor M, Gorlin A, Doty C, Rubin A, Altura BT, Altura BM. Ionized magnesium levels and the ratio of ionized calcium to magnesium in asthma patients before and after treatment with magnesium. Scand. J. Clin. Lab. Invest. 2005;65(8):659–670. doi: 10.1080/00365510500333825. [DOI] [PubMed] [Google Scholar]
- Singh RB, Beegom R, Rastogi SS, Gaoli Z, Shoumin Z. Association of low plasma concentrations of antioxidant vitamins, magnesium and zinc with high body fat percent measured by bioelectrical impedance analysis in Indian men. Magnes. Res. 1998;11(1):3–10. [PubMed] [Google Scholar]
- Singh RB, Niaz MA, Moshiri M, Gaoli Z, Shoumin Z. Magnesium status and risk of coronary artery disease in rural and urban populations with variable magnesium consumption. Magnes. Res. 1997;10(3):205–213. [PubMed] [Google Scholar]
- Soldatovic D, Matovic V, Vujanovic D, Stojanovic Z. Contribution to interaction between magnesium and toxic metals: the effect of prolonged cadmium intoxication on magnesium metabolism in rabbits. Magnes. Res. 1998;11:283–288. [PubMed] [Google Scholar]
- Soria M, Gonzalez-Haro C, Anson MA, Inigo C, Calvo ML, Escanero JF. Variations in serum magnesium and hormonal levels during incremental exercise. Magnes. Res. 2014;27(4):155–164. doi: 10.1684/mrh.2014.0372. [DOI] [PubMed] [Google Scholar]
- Toba Y, Kajita Y, Masuyama R, Takada Y, Suzuki K, Aoe S. Dietary magnesium supplementation affects bone metabolism and dynamic strength of bone in ovariectomized rats. J. Nutr. 2000;130(2):216–220. doi: 10.1093/jn/130.2.216. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Hannan MT, Chen H, Cupples LA, Wilson PW, Kiel DP. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr. 1999;69(4):727–736. doi: 10.1093/ajcn/69.4.727. [DOI] [PubMed] [Google Scholar]
- Volpe SL. Magnesium and the Athlete. Curr. Sports Med. Rep. 2015;14(4):279–283. doi: 10.1249/JSR.0000000000000178. [DOI] [PubMed] [Google Scholar]
- Wilhelm Z, Kleinova J, Kalábová R. Effect of magnesium administration on urinary ion excretion in healthy subjects and cancer patients. Scr. Med. (Brno) 2002;75(5):231–238. [Google Scholar]
- Zieba A, Tata R, Dudek D, Schlegel-zawadzka M, Nowak G. Serum trace elements in animal models and human depression: Part III. Magnesium. Relationship with copper. Hum. Psychopharmacol. 2000;15(8):631–635. doi: 10.1002/hup.231. [DOI] [PubMed] [Google Scholar]


