Abstract
Purpose
The appearance of geographic atrophy (GA) on color photography (CP) is preceded by specific features on spectral domain optical coherence tomography (SDOCT). We aimed to build SDOCT-based risk assessment models for 5-year new onset of GA and central GA on CP.
Design
Prospective longitudinal study.
Participants
Age-related macular degeneration (AMD) patients with bilateral large drusen and/or non-central GA, and at least one eye without advanced disease (n=317) enrolled in the multicenter Age-Related Eye Disease Study 2 (AREDS2) Ancillary SDOCT study.
Methods
For one eye per participant, qualitative and quantitative SDOCT variables were derived, respectively, from standardized grading and semi-automated segmentation at baseline. Up to 7 years later, annual outcomes were extracted and analyzed to fit multivariate logistic regression models and build a risk calculator.
Main Outcome Measures
New onset of CP-visible GA and central GA.
Results
Over a follow-up median of 4.0 years and among 292 AMD eyes (without advanced disease at baseline) with complete outcome data, 46 (15.8%) developed central GA. Among a subset of 265 eyes without any GA on baseline CP, 70 (26.4%) developed CP-visible GA. Final multivariate models were adjusted for age. In the model for GA, the independent predicting SDOCT factors (p <0.001 to 0.03) were (1) hyperreflective foci (HF) and (2) retinal pigment epithelium layer atrophy or absence (RPEA), followed by (3) choroidal thickness in absence of subretinal drusenoid deposits, (4) photoreceptor outer segment loss, (5) RPE drusen complex (RPEDC) volume and (6) RPEDC abnormal thinning (RAT) volume. For central GA, the independent predicting SDOCT factors (p<0.001) were (1) RAT volume, (2) intraretinal fluid or cystoid spaces, (3) HF, and (4) RPEA. The models yielded a calculator that computes the probabilities of CP-visible new-onset GA and central GA after 1 through 5 years.
Conclusions
For AMD eyes with large drusen and no advanced disease, we built a novel risk assessment model – based on age and SDOCT segmentation, drusen characteristics, and retinal pathology – for progression to CP-visible GA over up to 5 years. This calculator may simplify SDOCT grading and, with future validation, have a promising role as a clinical prognostic tool.
Keywords: Retina, macular degeneration, age-related macular degeneration, geographic atrophy, optical coherence tomography, OCT, spectral domain optical coherence tomography, SDOCT, risk assessment model, prognostic factors, statistical models
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in developed countries. 1 The vision-threatening advanced stages of AMD may be predicted from the clinical phenotype; however, the currently available tools are not sufficient to monitor disease activity and detect early points of change. In the staging of AMD and the assessment of its progression, the imaging modality most commonly used in major epidemiological studies and severity scales is color photography (CP). 2–7 While CP shows a two-dimensional view of the retina, spectral domain optical coherence tomography (SDOCT) provides three-dimensional visualization, with high-resolution cross-sectional views, that allows in-depth examination of retinal tissue including delineation of retinal layers and characterization of substructures of AMD pathology. 8–12
The AREDS2 Ancillary SDOCT Study (A2A SDOCT Study) aimed to identify specific SDOCT patterns in AMD that can predict vision loss and disease progression from intermediate to advanced stages. 12 In the intermediate stage of AMD, various drusen-related patterns of reflectivity on SDOCT have been discovered, 8–14 and found to be early indicators of advancing disease. 14–21 It had been unknown if such SDOCT features implied a risk of progression to choroidal neovascularization or played a role in the sequence of degeneration in geographic atrophy (GA). Previous reports from the A2A SDOCT Study discovered features of intermediate AMD on SDOCT that can serve as biomarkers of disease progression, such as hyperreflective foci (HF). 14,18–21 Incidence of new-onset GA on CP followed after the qualitative SDOCT findings of atypical drusen (i.e. high or low internal reflectivity)12, OCT-reflective drusen substructures (ODS, i.e. cores within drusen)14, and HF18,19, as well as the quantitative SDOCT measurements of the retinal pigment epithelium drusen complex (RPEDC) volumes, which were accompanied by the SDOCT observation of RPE layer atrophy or absence (RPEA). 21 Also, as shown by Wu et al., atrophy of the RPE and overlying photoreceptors on SDOCT immediately preceded new onset of drusen-associated GA on CP. 22,23 Briefly, these findings suggested that SDOCT is useful to visualize early indicators of atrophy, rather than neovascularization. 14–21 In an attempt to further clarify the sequence of degenerative events in the atrophic pathway, we focused this report on the A2A SDOCT Study aims pertinent to atrophy and its possible precursors. Specifically, the aim of this paper was to identify the SDOCT features that, without inter-dependence, can collectively predict the probability of new progression from intermediate AMD to more advanced non-neovascular stages over 5 years. We sought to determine an algorithm, using SDOCT features, to predict GA, which is visible on CP.
Methods and Subjects
The A2A SDOCT Study (ClinicalTrials.gov identifier NCT00734487) was an ancillary observational prospective study of a subset of eyes from the Age-Related Eye Disease Study 2 (AREDS2), with a group of control eyes of aged adults. The AREDS2 (ClinicalTrials.gov identifier NCT00345176) was a multicenter prospective randomized trial conducted to test the effect of oral nutritional supplements on the progression of AMD on CP. 24 The A2A SDOCT Study recruited 349 participants with AMD from four AREDS2 clinical sites in the United States (National Eye Institute, Duke Eye Center, Emory Eye Center, and Devers Eye Institute). 12 All AMD participants had been consented and enrolled in the AREDS2 study. At each of the clinical sites, the A2A SDOCT Study was approved by the Institutional Review Board (IRB). Informed research consent was obtained prior to participation from each study participant. The protocol followed tenets of human research as presented in the Declaration of Helsinki. Data were collected, stored, and managed in compliance with Health Insurance Portability and Accountability Act guidelines.
Study Design
The A2A SDOCT Study has been described previously and is summarized here. 12 We studied eyes enrolled in the AREDS2 study. Participants were receiving the AREDS supplements as part of the standard of care, and were randomly assigned to take one of the following AREDS2 study supplements daily: (1) placebo, (2) lutein and zeaxanthin, (3) omega-3 long-chain polyunsaturated fatty acids, or (4) both. 24,25 The AREDS2 inclusion criteria included age between 50 and 85 years, and CP assessed by the reading center (University of Wisconsin Fundus Photograph Reading Center) to be of adequate quality. 24 AREDS2 enrollment was restricted to people determined to be at high risk of progression to advanced AMD with either (1) bilateral large drusen ≥125 μm or non-central GA (no advanced AMD) or (2) large drusen or non-central GA in one eye and advanced AMD (neovascularization or central GA) in the fellow eye. 24 These eyes could have an AREDS Simple Scale Score of 2, 3, or 4. 7 The study eye was required to lack Advanced AMD as defined in the AREDS and AREDS2: neovascularization or central GA. 7,24 It is important to note that non-central GA was not considered advanced AMD per AREDS2 criteria (see Table 1). 24 Accordingly, an AREDS2 study eye (without advanced AMD) may have definite GA not involving the center of the macula, with or without evidence of drusen.
Table 1.
AREDS2 Ancillary SDOCT Study Primary Annual Outcomes of Progression to Non-Neovascular Atrophic Stages on Color Fundus Photography.
| Study A | Study B | |
|---|---|---|
|
|
|
|
| Outcome a | New-onset central Geographic Atrophy | New-onset Geographic Atrophy |
| Measure | Geographic Atrophy (GA) at the foveal center | Any GA (non-central or central) |
| AREDS terminology b | Non-neovascular Advanced AMD | Intermediate AMD with GA or Non-neovascular Advanced AMD |
| Beckman classification c | Non-neovascular Late AMD | Non-neovascular Late AMD |
| Modality | Color Fundus Photography | Color Fundus Photography |
|
|
|
|
| Total eyes at risk d,e | 292 | 265 |
AREDS2 = Age-Related Eye Disease Study 2, SDOCT = spectral domain optical coherence tomography, AMD = age-related macular degeneration, GA = geographic atrophy.
Each outcome was defined as new onset of the respective measure on the designated imaging modality.
Per AREDS and AREDS2, Advanced AMD is defined as neovascularization or central GA on color fundus photography. 7,24,57
The Beckman classification defined Late AMD as neovascularization or any Geographic Atrophy. 26
One study eye per participant.
By definition, eyes at risk of developing the outcome are outcome-free at baseline.
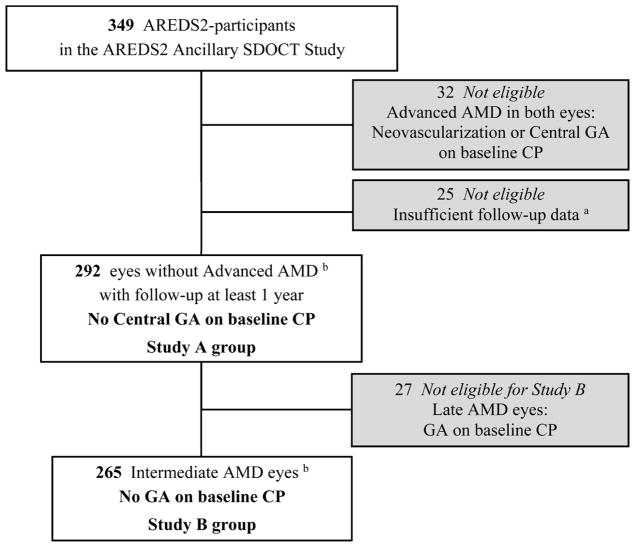
In the intervening years during the progress of the A2A SDOCT Study, we recognized new onset of any GA as a critical outcome, and SDOCT precursors to CP-defined GA as perhaps more meaningful indicators that are awaiting the progression to GA and central GA. In 2013, the Beckman classification put forward a clear clinical phenotyping definition of Intermediate AMD: large drusen ≥125 μm and/or any AMD pigmentary abnormalities, without neovascularization or any GA; the presence of any non-central GA was considered part of the definition of the more advanced class: Late AMD. 26 Per the original A2A SDOCT Study design, the outcomes were at 2 and 5 years of follow-up, and the primary outcome of atrophy was the CP-based central GA. In this report, the primary outcomes are progression (A) to non-neovascular Advanced AMD and (B) to non-neovascular Late AMD based on the first occurrence of the respective outcome measures on color fundus photography. The CP-based outcome measures are (A) new onset of central GA, in consistency with the traditional definition of non-neovascular Advanced AMD as described in the A2A SDOCT Study Protocol, and (B) new onset of any GA, including both central and non-central GA, in consistency with the more up-to-date classification of non-neovascular Late AMD (Table 1). For study A, the group at risk of developing the outcome of central GA was, by definition, free of central GA on CP at baseline. Similarly, for study B, the group at risk of developing the outcome of GA was, by definition, free of any GA on CP at baseline. Accordingly, all AREDS2-eligible eyes with SDOCT imaging were eligible for Study A, while Study B required the additional selection criterion of no baseline GA on CP (Figure 1).
Figure 1.
AREDS2 Ancillary SDOCT Study Participants and Selection of Groups At Risk of New-Onset Central Geographic Atrophy (N=292), and New-Onset Geographic Atrophy (GA) (N=265).
AREDS2 = Age-Related Eye Disease Study 2, SDOCT = spectral domain optical coherence tomography, GA = geographic atrophy, AMD = age-related macular degeneration, CP = color photography. a On color photography (CP), 25 eyes of 25 participants with no Advanced AMD (per AREDS2 definition) were excluded due to insufficient CP data on follow-up: 14 lost to follow-up before 1 year + 11 with CP ungradable for GA on follow-up visits. b One eye per participant.
Out of 349 AMD participants enrolled from AREDS2, and by the time the A2A SDOCT Study obtained baseline imaging, 32 participants had progressed to bilateral advanced AMD and were excluded, leaving a total of 317 study participants with at least one eligible eye with no Advanced AMD. We studied one eye per participant. In patients with both eyes eligible, the right eye was arbitrarily chosen as the study eye. In addition, subjects included in the analyses in this report had to have at least one follow-up visit with acceptable imaging for outcome assessment. Out of the 317 A2A SDOCT study eyes, Study A included the 292 that were at risk of new-onset central GA and had at least one adequate follow-up visit with valid color fundus photography grading (Figure 1). Out of the 292 eyes, 265 were free of any GA on color fundus photos at baseline and were thus eligible for Study B.
Procedures
In addition to the baseline visit at enrollment, participants were followed annually with a follow-up goal of 5 years. The A2A SDOCT Study started after the initiation of the AREDS2, thus to improve the long-term analysis of AMD features, subjects were recruited to return for an additional extension visit that allowed for data capture up to 7 years. At baseline we captured data on age, sex, and smoking status. 24 At all visits, we captured color fundus photography per AREDS2 protocol, in addition to SDOCT imaging. Color fundus photography images were graded by certified readers at the Wisconsin Fundus Photography Reading Center (University of Wisconsin, Madison, WI). 21,24 For the extension visits, color fundus photos were graded for the outcomes of interest by a retinal expert (CAT) masked to the SDOCT images, per AREDS2 protocol and Wisconsin grading system criteria. A2A SDOCT Study imaging was done using a research SDOCT imaging system (SDOIS; Bioptigen, Research Triangle Park, NC) using the imaging specifications previously described. 12 At every visit, each eye had 2 raster scans captured, oriented at 0° and 90°, each consisting of 100 B-scan lines, 1000 A-scans per line, and an axial resolution of 4.5 μm per pixel, covering an area of 6.7 mm × 6.7 mm centered on the fovea. Each de-identified SDOCT volume scan was then graded using the Duke Optical Coherence Tomography Retinal Analysis Program by Farsiu and Chiu, a viewing program developed in MATLAB (MathWorks, Natwick, MA) and previously described in detail that displays SDOCT cross-sectional B-scans and facilitates retinal layer segmentation. 20,27,28 Because Enhanced Depth Imaging had not been available at the time of the study, Bioptigen linear B-scans oriented at 0° repeated at the fovea were aligned and summed post-acquisition, in order to visualize and segment the posterior boundary of the choroid. Certified graders in the Duke Advanced Research in SS/SDOCT Imaging laboratory analyzed the SDOCT scan volumes for quality and ability to be graded, then for the presence of qualitative SDOCT characteristics of macular pathology described by Leuschen et al12. On average, for a grader to review and record all variables from an OCT volume, up to 20 minutes were required. In order to avoid peripapillary atrophy, qualitative grading and semi-automated segmentation of retinal layers were both focused on a 5 mm diameter macular region centered on the fovea. When graders could not agree, the grading was arbitrated by a senior investigator (CAT).
Outcome Measures
Measures of the primary outcomes were derived from color fundus photography features defined in the AREDS2 study (Table 1). GA was defined as any area of partial or complete depigmentation of the RPE within the macula, not adjacent to disciform scars, with at least 2 of the following 3 characteristics: visibility of underlying large choroidal vessels, roughly round or oval shape, and sharp margins on color fundus photography. 6,29 GA was considered definitely present if the lesion area was at least 0.146 mm2 (AREDS circle I-2, diameter = 433 μm) on color fundus photography. 29 Central GA was defined as GA involving the center of the macula. 24 Color fundus photography images were deemed ungradable for any GA either due to poor image quality or due to development of choroidal neovascularization as defined in the AREDS2 Study. 24 GA and central GA were assessed only in absence of neovascularization: The CP was considered ungradable for GA or central GA once it is flagged with neovascularization either (1) on CP per AREDS2 definition, (2) from clinical diagnosis, or (3) from history of treatment for neovascularization.
SDOCT-Derived Measures
The qualitative and quantitative SDOCT-derived variables utilized in this report are listed in Table 2. On scan volumes of at least acceptable quality, study eyes were graded for subretinal drusenoid deposits (SDD) based on a count of at least five independent SDD per volume, in consistency with previous definitions of SDD and reticular pseudodrusen. 30 A single SDD was called independent and was counted if it met the following four criteria. (1) Deposition was stage 2 or more as defined by Zweifel et al., 31 consisting of hyperreflective material accumulation above the RPE sufficient at least to alter the contour of the ellipsoid zone boundary. The deposit’s axial location on the adjacent B-scans did not contain (2) an extension of the same deposit or (3) the edge of a RPE elevation similar in appearance to or continuous with the deposit. (4) The eye had at least one SDD without underlying RPE elevation. The remaining qualitative SDOCT features graded in the A2A SDOCT Study eyes had previously been described (see Table 2). 12,14,19,32,33 Intraretinal fluid or cystoid spaces (IRF), previously reported under the term “intraretinal cysts”12, designate round or oval hyporeflective areas within the retinal layers usually located within the nuclear layers, without hyperreflective rim. As previously reported, the term IRF was retained because it was not possible to distinguish outer retinal tubulations without hyperreflective border from hyporeflective cystoid structures originating from fluid leakage. 34 Qualitative SDOCT variables that constituted rare findings – namely serous pigment epithelium detachment and foveal findings – were excluded from this analysis. Also, haze over RPE elevation was not included in this analysis because it occurred over all drusen as a result of the effect of beam entry position on the reflectivity of the Henle Fiber Layer. 35 RPE layer atrophy or absence (RPEA) – equivalent to the previously reported “RPE atrophy or absence” 12 – referred to either a clear degradation of the reflectivity and thickness of the RPE layer, a complete absence of RPE, or a contour break. It is critical to note that this feature is different from the presence of complete OCT-GA, which consists of a triad of (1) RPE layer atrophy or absence with (2) overlying loss in photoreceptor layer and (3) increased signal in underlying choroid.12,23 These features may or may not be considered as complete OCT-GA in case residual drusen is present, such as certain cases of nascent GA or in case the triad above does not meet a minimal diameter requirement.22,23
Table 2.a.
Baseline Distributions of Demographics and SDOCT Variables, with Contingency Proportions and Univariate Associations with 5-Year Progression to New Onset of Central GA on Color Fundus Photography in Study A.
| Eyes at risk | Progression to Central GA b,f | Univariate Associations g | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| N=292 a | Yes (n=46 eyes) | No (n=246 eyes) | Crude OR (95% CI) | p-value | |
| Demographics b | |||||
|
| |||||
| Female % (n) | 60.6% (177) | 52.2% (24) | 62.2% (153) | 0.76 (0.39–1.48) | 0.43 |
| Smoking history % (n) | 55.5% (162) | 65.2% (30) | 53.7% (132) | 2.07 (1.05–4.06) | 0.04 |
| Age (years) mean ± SD (n) | 73.6 ± 7.5 (292) | 76.6 ± 6.4 (46) | 73.7 ± 7.6 (246) | 1.43 (1.11–1.84) | 0.006 |
| Qualitative SDOCT variables b,12 % (n) | |||||
|
| |||||
| RPE layer Atrophy or absence c | 22.6% (66) | 63.0% (29) | 15.0% (37) | 14.06 (7.08–27.92) | <0.001 |
| Hyperreflective Foci 19 | 58.6% (171) | 82.6% (38) | 54.1% (133) | 4.53 (1.97–10.43) | <0.001 |
| OCT-reflective Drusen Substructures 14 | 23.6% (69) | 41.3% (19) | 20.3% (50) | 2.64 (1.32–5.28) | 0.006 |
| Photoreceptor Layer thinning without underlying RPE changes 43 | 6.8% (20) | 6.5% (3) | 6.9% (17) | 1.13 (0.30–4.32) | 0.86 |
| Outer Segment Loss without underlying RPE changes | 6.2% (18) | 6.5% (3) | 6.1% (15) | 1.29 (0.33–5.03) | 0.71 |
| Atypical Drusen with Low Reflectivity | 41.8% (122) | 58.7% (27) | 38.6% (95) | 2.22 (1.13–4.35) | 0.02 |
| Atypical Drusen with High Reflectivity | 61.6% (180) | 80.4% (37) | 58.1% (143) | 3.20 (1.41–7.25) | 0.005 |
| Intraretinal Fluid or cystoid changes | 15.1% (44) | 45.7% (21) | 9.3% (23) | 6.74 (3.30–13.77) | <0.001 |
| Subretinal Drusenoid Deposits (SDD) | 50.7% (148) | 47.8% (22) | 51.2% (126) | 0.89 (0.46–1.72) | 0.72 |
| Photoreceptor Layer thinning over Drusen 43 | 96.2% (281) | 97.8% (45) | 95.9% (236) | 1.59 (0.18–14.04) | 0.68 |
| Subretinal Fluid | 5.8% (17) | 6.5% (3) | 5.7% (14) | 1.17 (0.30–4.55) | 0.82 |
| Subretinal Hyperreflective Material 32 | 7.2% (21) | 13.0% (6) | 6.1% (15) | 1.77 (0.66–4.73) | 0.25 |
| Vitreomacular Adhesion or Epiretinal Membrane | 54.5% (159) | 63.0% (29) | 52.8% (130) | 1.37 (0.69–2.69) | 0.37 |
| Quantitative SDOCT variables d,20,21,33 mean ± SD (n) | |||||
|
| |||||
| Choroidal Thickness (μm) e | 267 ± 63 (265) | 241 ± 65 (41) | 272 ± 61 (224) | 0.34 (0.16–0.72) | 0.005 |
| Choroidal Thickness by SDD | 0.41 | ||||
| Choroidal Thickness (μm) in absence of SDD | 273 ± 62 (131) | 232 ± 64 (23) | 281 ± 58 (108) | 0.35 (0.17–0.74) | 0.006 |
| Choroidal Thickness (μm) in presence of SDD | 262 ± 63 (134) | 253 ± 66 (18) | 263 ± 63 (116) | 0.31 (0.13–0.73) | 0.008 |
| RPEDC Abnormal Thinning (RAT) volume (10−3 mm3) | 0.82 ± 2.16 (242) | 2.88 ± 4.46 (40) | 0.41 ± 0.84 (202) | 1.43 (1.28–1.59) | <0.001 |
| RPE Drusen Complex (RPEDC) volume (10−1 mm3) | 6.7 ± 2.1 (242) | 6.5 ± 1.6 (40) | 6.8 ± 2.2 (202) | 0.91 (0.75–1.10) | 0.33 |
| Neurosensory Retina (NSR) volume (mm3) | 5.2 ± 0.4 (242) | 5.0 ± 0.5 (40) | 5.3 ± 0.4 (202) | 0.17 (0.06–0.50) | 0.001 |
| OCT Drusen volume (10−1 mm3) | 0.76 ± 1.58 (242) | 0.60 ± 0.98 (40) | 0.79 ± 1.68 (202) | 0.89 (0.69–1.15) | 0.38 |
SDOCT = spectral domain optical coherence tomography, GA = geographic atrophy, SD = standard deviation, RPE = retinal pigment epithelium, SDD = subretinal drusenoid deposits, OR = odds ratio.
One eye per participant.
Binary variables are reported as percentage (count); contingency proportions are reported within outcome (progression or no progression). Variables are listed in order consistent with table 2.b.
RPE layer Atrophy or absence is different from the presence of complete OCT-GA, which necessitates the additional findings of overlying loss in photoreceptor layer and increased signal in underlying choroid. 12,22
Continuous variables are summarized as mean ± SD (number of valid measurements).
Choroidal Thickness was adjusted for age, gender and estimated axial length.
Contingency proportions are reported for the outcome of progression on at least one follow-up visit, without the repeated measures from each eye.
From repeated measures over 5 years, univariate logistic regressions associations are reported. The odds ratio (OR) corresponds to the increase in odds after 5 years of follow-up associated with either (1) presence of the qualitative variable, or (2) every 1 unit increase in the continuous (quantitative) variable, except for Age (OR per 5 years of age) and Choroidal thickness (OR per 100 μm). Chi-square p-value of 0.05 constituted the significance level.
Choroidal thickness (CT) was extracted from the summed linear B-scans acquired in the naso-temporal direction at the fovea. The average count of scans aligned and summed post-acquisition was 15 scans (min = 2, max = 40). The choroid boundaries for stromal CT were segmented manually from the outer border of Bruch’s membrane to the outer border of the choroid stroma – or the inner border of the suprachoroidal layer when visible – as defined and proven reproducible by Yiu et al. 36–38 Disagreements were adjudicated by two graders (KS and KPW) or arbitrated by a senior choroid grader (GY). In order to minimize the variability in measurements taken at single point locations, we reported the average choroidal thickness from point measurements at every A-scan within the central 3 mm segment (from 1.5 mm nasal to 1.5 mm temporal to the fovea), based on the middle circle of the AREDS maculopathy grading grid. 3 The dependence of CT on age, gender and axial length, based on a normal control sample, 39 was adjusted for. To this end, we used the following linear regression formula: Adjusted CT (μm) = measured CT − [−2.5 × (age− 60 years)] − [−32.9 × gender (female=1)] − [−24.9 × (axial length − 23 mm)]. Axial length was estimated from the AREDS2-measured refractive error spherical equivalent based on the following linear regression formula: Axial length (mm) = 23.75 − [0.35 × spherical equivalent (D)], 40 as high myopes had been excluded at the eligibility criteria of the A2A SDOCT Study. Choroidal thickness measurements were adjusted for age, gender and axial length, but not the time of day at which the measures were taken, because the exact scan acquisition time was not available for all eyes. However, all scans were obtained between 9 am and 5 pm, and more than 2 hours after waking time. From normative data, CT measurements vary no more than 10μm during office hours (9 am to 6 pm). 41 Accordingly, for participants in clinical trials, the CT variations are minimal. For this population, the estimated median imaging time – reported previously from a subset as 12:54 pm 38– almost coincides with the mean nadir time (12:26 pm) and is farthest from the mean acrophase time (11:26 pm) of the diurnal cycle of choroidal thickness fluctuations. 42 In this paper, the adjusted CT described above was the only CT variable analyzed.
The quantitative SDOCT volumes were derived from semi-automated segmentation of SDOCT volume scans. 9,43 The neurosensory retinal (NSR) thickness was defined as the volume between the internal limiting membrane and the inner aspect of the RPE-drusen complex. The RPEDC volume was defined as the thickness from the apex of the drusen and RPE layer to the outer border of Bruch’s membrane. From outlier RPEDC thicknesses at each pixel, the following two measurements of abnormal RPEDC volume were calculated and previously described in detail: the OCT-derived Drusen volume and the RPEDC Abnormal Thinning (RAT) volume. 20 Briefly, OCT Drusen was derived from RPEDC thickness ≥3 SD from the mean of a normative dataset for non-AMD eyes. From RPEDC thickness ≤2 SD from the mean of the normative dataset, RAT was derived to represent the volume of difference lost from normal RPE thickness. 21 The normative database for the retinal SDOCT volumes had been derived in an earlier study by Farsiu et al using 118 age-appropriate non-AMD control participants recruited from the Duke and Emory sites using the same eligibility criteria as AREDS2, except lacking AMD. 20 Quantitative variables were continuous and qualitative variables were binary.
Statistical Modeling and Analyses
All the analyses reported herein, were performed in the Statistical Analysis Systems (SAS) software version 9.4 (SAS Institute Inc, Cary, NC). Univariate and multivariate logistic regression models were fitted using the GENMOD procedure. Repeated measures of the outcome over the follow-up visits were corrected for using the generalized estimating equations (GEE) methodology using a first order autoregressive model AR(1). Separately for each of the two studies (A and B), we explored the long-term relationships between each of the SDOCT variables and the outcome, then fitted a comprehensive risk assessment model and used it to build a risk calculator. SDOCT features that preceded the outcomes were found through examining the longitudinal associations using univariate models. Based on the significant univariate associations, the predecessors to the outcomes were suggested as potential candidate predictors of the outcomes. Candidate predictors were considered in the fitting of the multivariate model. Also, based on previous observations in the literature, when the effect of one variable on progression was expected to be modified by another variable, both variables were included in a multiplicative term and used to test for the interaction. The interaction between choroidal thickness (CT) and subretinal drusenoid deposits (SDD) was planned for inclusion because it had been studied previously: The known association between CT and GA is attenuated by the presence of SDD. 44 Apart from the single candidate variables, the combinations of the two interacting variables were attempted for inclusion in the multivariate models. On the other hand, when two candidate predictors were overlapping in distribution, they were considered collinear; although both were considered for inclusion in the multivariate model, only one was selected.
In order to determine the independent SDOCT predictors at highest risk of developing the outcome, a multivariate model was built from (1) covariates with univariate associations reaching significance (p<0.05), and using forward stepwise fitting to adjust for (2) potentially confounding systemic factors (age, gender and smoking) and (3) covariates with univariate associations approaching significance (p≤0.2). During the model fitting exercise, goodness-of-fit was monitored using the Quasilikelihood under the Independence model Criterion statistic (QICu). The choice between collinear variables was made based on univariate and multivariate p-values and the QIC. Also, variables that approached but did not reach significance in the model were dropped unless (1) dropping them caused a substantial increase in the QIC or (2) their univariate association had been highly significant (p<0.001). Associations were summarized by the Chi-square p-value and the odds ratio (OR) with 95% confidence interval. The OR was reported as crude for the univariate associations and as adjusted for the multivariate associations in the final model. Repeated measures over the years of follow-up necessitated the use of the years as a discrete variable, with follow-up at 5 years or beyond being assigned as a reference category. In the risk calculation formulas, the effects of years of follow-up on the risk of progression were reported as an estimate for each year, relative to the maximal risk at 5 years (reference category). The risk calculator was based on the logistic regression formula from the final multivariate model. The calculator computes the probability of developing the outcome at each of the annual time points.
Results
Baseline SDOCT Predecessors of Color Fundus Photography GA Outcomes
Parallel analyses were run for Studies A and B separately. Eyes with no central GA on baseline CP (n=292) were included as eyes at risk in Study A. Eyes without any GA on baseline CP (n=265) were included in Study B. For that reason, this report presents their baseline variables distributions separately without statistical comparison between the two studies (Table 2). The baseline characteristics of eyes analyzed in the two studies were generally similar with the following exceptions. RPE layer atrophy (RPEA) and IRF, which are qualitative SDOCT variables related to existing GA, appeared to be less common in Study B. These eyes also had a smaller volume of RPE-drusen complex Abnormal Thinning (RAT) relative to Study A eyes. Also, photoreceptor loss overlying RPE layer atrophy was strongly associated with RPE layer atrophy: Out of the 40 GA-free eyes with RPE layer atrophy at baseline (Table 2b), only 4 had no overlying photoreceptor loss at baseline, but developed it within 1 to 2 years. Rare findings – RPE changes without elevation or atrophy (2.9%), vitelliform lesion (2.2%) and outer retinal tubulation (0.9%) – were not included in this analysis.
Table 2.b.
Baseline Distributions of Demographics and SDOCT Variables, with Contingency Proportions and Univariate Associations with 5-Year Progression to New Onset of GA on Color Fundus Photography in Study B.
| Eyes at risk | Progression to any GA b,f | Univariate Associations g | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| N=265 a | Yes (n=70 eyes) | No (n=195 eyes) | Crude OR (95% CI) | p-value | |
| Demographics b | |||||
|
| |||||
| Female % (n) | 61.9% (164) | 58.6% (41) | 63.1% (123) | 0.81 (0.46–1.43) | 0.47 |
| Smoking history % (n) | 53.2% (141) | 54.3% (38) | 52.8% (103) | 1.30 (0.75–2.27) | 0.35 |
| Age (years) mean ± SD (n) | 73.4 ± 7.6 (265) | 76.2 ± 7.2 | 73.1 ± 7.6 | 1.41 (1.15–1.73) | 0.001 |
| Qualitative SDOCT variables b,12 % (n) | |||||
|
| |||||
| RPE layer Atrophy or absence c | 15.1% (40) | 37.1% (26) | 7.2% (14) | 12.07 (5.90–24.73) | <0.001 |
| Hyperreflective Foci 19 | 57.4% (152) | 85.7% (60) | 47.2% (92) | 5.72 (2.75–11.90) | <0.001 |
| OCT-reflective Drusen Substructures 14 | 21.5% (57) | 34.3% (24) | 16.9% (33) | 3.54 (1.91–6.57) | <0.001 |
| Photoreceptor Layer thinning without underlying RPE changes 43 | 7.2% (19) | 14.3% (10) | 4.6% (9) | 3.84 (1.65–8.98) | 0.002 |
| Outer Segment Loss without underlying RPE changes | 6.4% (17) | 12.9% (9) | 4.1% (8) | 4.47 (1.83–10.89) | 0.001 |
| Atypical Drusen with Low Reflectivity | 40.0% (106) | 54.3% (38) | 34.9% (68) | 2.12 (1.21–3.70) | 0.008 |
| Atypical Drusen with High Reflectivity | 60.4% (160) | 70.0% (49) | 56.9% (111) | 1.91 (1.05–3.46) | 0.03 |
| Intraretinal Fluid or cystoid changes | 11.7% (31) | 17.1% (12) | 9.7% (19) | 2.16 (0.99–4.67) | 0.05 |
| Subretinal Drusenoid Deposits (SDD) | 50.6% (134) | 57.1% (40) | 48.2% (94) | 1.52 (0.88–2.66) | 0.14 |
| Photoreceptor Layer thinning over Drusen 43 | 96.6% (256) | 98.6% (69) | 95.9% (187) | 3.57 (0.49–26.27) | 0.21 |
| Subretinal Fluid | 5.7% (15) | 4.3% (3) | 6.2% (12) | 0.63 (0.15–2.58) | 0.52 |
| Subretinal Hyperreflective Material 32 | 6.8% (18) | 7.1% (5) | 6.7% (13) | 0.84 (0.31–2.31) | 0.74 |
| Vitreomacular Adhesion or Epiretinal Membrane | 52.8% (140) | 58.6% (41) | 50.8% (99) | 1.03 (0.59–1.80) | 0.91 |
| Quantitative SDOCT variables d,20,21,33 mean ± SD (n) | |||||
|
| |||||
| Choroidal Thickness (μm) e | 271 ± 61 (241) | 255 ± 55 (65) | 277 ± 62 (176) | 0.47 (0.27–0.81) | 0.007 |
| Choroidal Thickness by SDD | 0.16 | ||||
| Choroidal Thickness (μm) in absence of SDD | 277 ± 57 (118) | 247 ± 34 (28) | 287 ± 60 (90) | 0.44 (0.26–0.74) | 0.002 |
| Choroidal Thickness (μm) in presence of SDD | 265 ± 64 (123) | 261 ± 66 (37) | 267 ± 63 (86) | 0.52 (0.29–0.93) | 0.03 |
| RPEDC Abnormal Thinning (RAT) volume (10−3 mm3) | 0.42 ± 0.88 (218) | 0.92 ± 1.46 (60) | 0.23 ± 0.36 (158) | 2.04 (1.14–3.67) | 0.02 |
| RPE Drusen Complex (RPEDC) volume (10−1 mm3) | 6.8 ± 2.1 (218) | 7.0 ± 2.1 (60) | 6.7 ± 2.1 (158) | 1.10 (0.99–1.22) | 0.09 |
| Neurosensory Retina (NSR) volume (mm3) | 5.3 ± 0.4 (218) | 5.2 ± 0.4 (60) | 5.3 ± 0.4 (158) | 0.60 (0.28–1.31) | 0.20 |
| OCT Drusen volume (10−1 mm3) | 0.79 ± 1.64 (218) | 0.96 ± 1.61 (60) | 0.73 ± 1.66 (158) | 1.08 (0.96–1.21) | 0.21 |
SDOCT = spectral domain optical coherence tomography, GA = geographic atrophy, SD = standard deviation, RPE = retinal pigment epithelium, SDD = subretinal drusenoid deposits, OR = odds ratio.
One eye per participant.
Binary variables are reported as percentage (count); contingency proportions are reported within outcome (progression or no progression). Variables are listed in order of decreasing significance.
RPE layer Atrophy or absence is different from the presence of complete OCT-GA, which necessitates the additional findings of overlying loss in photoreceptor layer and increased signal in underlying choroid. 12,22
Continuous variables are summarized as mean ± SD (number of valid measurements).
Choroidal Thickness was adjusted for age, gender and estimated axial length.
Contingency proportions are reported for the outcome of progression on at least one follow-up visit, without the repeated measures from each eye.
From repeated measures over 5 years, univariate logistic regressions associations are reported. The odds ratio (OR) corresponds to the increase in odds after 5 years of follow-up associated with either (1) presence of the qualitative variable, or (2) every 1 unit increase in the continuous (quantitative) variable, except for Age (OR per 5 years of age) and Choroidal thickness (OR per 100 μm). Chi-square p-value of 0.05 constituted the significance level.
The univariate associations between each covariate and the outcome (Table 2) represent the longitudinal relationship over 5 years of follow-up, adjusted for the repeated measures of outcomes at each of the four earlier years discretely. Contingency proportions were reported against an all-or-none outcome variable (progression at any time point or no progression over all points) summarizing the repeated measures for each eye over the interval of 5 years of follow-up. Results of the multivariate associations in the final risk assessment model for each of studies A and B are shown in Table 3. In the two final models respectively, a change in the risk of new-onset central GA or new-onset GA on color fundus photography can be conveyed by each SDOCT variable independently.
Table 3.a.
Central Geographic Atrophy Risk Assessment Model (Study A) – Multivariate Associations between Baseline Independent SDOCT Predictors and Progression to New Onset of Central GA on Color Fundus Photography over 5 years.
| Multivariate Associations (N=222) a | ||
|---|---|---|
|
| ||
| Adjusted OR (95% CI) b | p-value c | |
| Demographics | ||
| Age [per 5 years] | 1.32 (0.97–1.81) | 0.08 |
| Qualitative SDOCT variables 12 | ||
| Intraretinal Fluid or cystoid changes | 11.23 (4.96–25.46) | <0.001 |
| Hyperreflective Foci19 | 6.51 (2.87–14.74) | <0.001 |
| RPE layer Atrophy or absence | 5.82 (2.25–15.04) | <0.001 |
| Quantitative SDOCT variables c,20,21,33 | ||
| RPEDC Abnormal Thinning (RAT) volume [per 10−3 mm3] | 1.40 (1.24–1.57) | <0.001 |
| Choroidal Thickness [per 100 μm] d | 0.51 (0.22–1.16) | 0.11 |
| uantitative SDOCT variables 20,21,33 | ||
|---|---|---|
| Choroidal Thickness (CT) by SDD d,f | 0.11 | |
| CT in absence of SDD [per 100 μm] | 0.22 (0.09–0.56) | 0.002 |
| CT in presence of SDD [per 100 μm] | 0.68 (0.25–1.86) | 0.45 |
| RPE Drusen Complex (RPEDC) volume [per 10−1 mm3] | 1.18 (1.02–1.36) | 0.02 |
| RPEDC Abnormal Thinning (RAT) volume [per 10−3 mm3] | 1.65 (1.06–2.56) | 0.03 |
SDOCT = spectral domain optical coherence tomography, GA = geographic atrophy, RPE = retinal pigment epithelium, RPEDC = RPE drusen complex, OR = odds ratio.
From 222 participants, 222 eyes had valid measurements for all continuous baseline variables.
Associations are reported from multivariate logistic regression with repeated measures over 5 years. The odds ratio (OR) corresponds to the increase in odds after 5 years of follow-up associated with either (1) the presence of the qualitative variable, or (2) every 1 unit increase in the continuous variable (for quantitative variables), as indicated [between brackets].
Chi-square p-value of 0.05 constituted the significance level.
Choroidal thickness was adjusted for age, gender and estimated axial length.
SDOCT = spectral domain optical coherence tomography, GA = geographic atrophy, RPE = retinal pigment epithelium, SDD = subretinal drusenoid deposits, Q1 = first quartile, Q2 = second quartile, Q3 = third quartile, RPEDC = RPE drusen complex, CT = choroidal thickness, OR = odds ratio.
From 201 participants, 201 eyes had valid measurements for all continuous baseline variables.
Associations are reported from multivariate logistic regression with repeated measures over 5 years. The odds ratio (OR) corresponds to the increase in odds after 5 years of follow-up associated with either (1) the presence of the qualitative variable, or (2) every 1 unit increase in the continuous variable (for quantitative variables), as indicated [between brackets].
Chi-square p-value of 0.05 constituted the significance level.
The presence of Subretinal Drusenoid Deposits (SDD) modifies the effect of Choroidal thickness with a p-value of 0.11 corresponding to the difference in effect.
OR for SDD is reported at different values of Choroidal thickness (around the quartiles of CT: Q1, Q2 and Q3) in order to illustrate the interaction.
Choroidal thickness was adjusted for age, gender and estimated axial length.
Study A: New-Onset Central GA
Over a median follow-up of 4.0 years (IQR=3.0–5.0, average=3.9±1.3), 15.8% of the eyes (46/292) progressed on color fundus photography to develop new-onset central GA. However, the analyses were based on repeated measures from several intervals for each eye. Accordingly, from the 292 eyes, the number of data points was 936 follow-up visits. Central GA was seen in 72 out of 936 follow-up visits. The univariate associations in Table 2a suggested the following SDOCT candidate predecessors to new-onset central GA: a greater volume of RPEDC Abnormal Thinning (RAT) (p<0.001), RPE layer atrophy or absence (p<0.001), intraretinal fluid or cystoid changes (p<0.001), hyperreflective foci (p<0.001), atypical drusen with low reflectivity (p=0.02) and with high reflectivity (p=0.005), a smaller neurosensory retina (NSR) volume (p=0.001), OCT Drusen Substructures (p=0.006), and a thinner Choroid (p=0.005). The univariate association between Choroidal Thickness and new-onset central GA was independent of the presence of SDD (p=0.41). Accordingly, the interaction term was not tested in the multivariate model. Of the above candidate variables, both atypical drusen types (low and high reflectivity), as well as OCT-reflective Drusen Substructures and NSR volume, lost significance in the multivariate model fitting exercise and thus were not independently associated with new-onset central GA. In the final multivariate model for central GA was based on the 222 eyes with complete data for all candidate predictors (Table 3a). Among the qualitative predictors, the one most strongly associated with an increased risk of color fundus photos new-onset central GA was IRF (OR=11.23, p<0.001) followed by hyperreflective foci (OR=6.51, p<0.001) and RPE layer atrophy (OR=5.82, p<0.001). Among the quantitative SDOCT measures, the only strong independent predictor of new-onset central GA was the RAT volume (OR=1.40 per 10−3 mm3, p<0.001). Choroidal Thickness did not significantly predict new-onset central GA (p=0.11), but was adjusted for in the model. Age (p=0.08) was also adjusted for in the final model.
Study B: New-Onset GA
In the cohort of 265 eyes followed-up on color fundus photography for a median of 4.1 years (IQR=3.0–5.0, average=3.9±1.3), new-onset GA occurred in 26.4% (70 eyes). From the 265 eyes at baseline, out of the repeated measures on 849 follow-up visits, GA event was present on 109 visits. As shown in Table 2b, candidate predecessors for new-onset GA on color fundus photography were as follows: RPE layer atrophy or absence (p<0.001), hyperreflective foci (p<0.001), OCT-reflective Drusen Substructures (p<0.001), photoreceptor Outer Segment Loss (OSL) without underlying RPE changes (p=0.001), the less specific photoreceptor layer thinning without underlying RPE changes (p=0.002), atypical drusen of low (p=0.008) and high reflectivity (p=0.03), larger RAT volume (p=0.02), and the marginally significant IRF (p=0.05), larger RPEDC volume (p=0.09), and smaller NSR volume (p=0.20). SDD approached significance (p=0.14), while a thinner Choroid preceded new-onset GA both in eyes with SDD (p=0.03) and without SDD (p=0.002). In other words, new-onset GA was preceded by a thinner Choroid overall regardless of SDD presence (p=0.007). The difference in effect that is due only to the presence of SDD approached significance (p=0.16). In the multivariate model fitting exercise, photoreceptor layer thinning without underlying RPE changes was dropped on account of using the subclass variable incorporating outer segment loss, a collinear and more significant predecessor to new-onset GA. IRF, atypical drusen with low reflectivity and the NSR volume were not independent predictors and were dropped from the model. The final multivariate model for GA was based on the 201 eyes with complete data for all candidate predictors (Table 3b). Among the qualitative predictors of new-onset GA on CP, the strongest was hyperreflective foci (OR=6.33, p<0.001), followed by RPE layer atrophy or absence (OR=5.32, p<0.001) and photoreceptor OSL without underlying RPE changes (OR=3.27, p=0.01). The volumes of RPEDC (OR=1.18 per 10−1 mm3, p=0.02) and RAT (OR=1.65 per 10−3 mm3, p=0.03) were also significant independent predictors of new-onset GA. In eyes without SDD, a thinner Choroid was a strong independent quantitative predictor of new-onset GA (OR=1/0.22=4.55 per μm of thinning, p=0.002). Eyes with SDD did not exhibit a significant association between Choroidal Thickness and new-onset GA (p=0.45). The effect of CT on the incidence of new-onset GA appeared different in SDD presence as compared to SDD absence, but such difference was not detected as significant (p=0.11). Features that only approached significance were included in the model for adjustment, namely atypical drusen with high reflectivity (OR=2.31, p=0.052), OCT Drusen Substructures (p=0.17), and the presence of SDD which approached significance only in eyes with Choroids thinner than the first quartile (at CT=220 μm, p=0.19). The final multivariate prediction model also included age as one of the strong predictors (OR=1.46, p=0.005).
Table 3.b.
Geographic Atrophy Risk Assessment Model (Study B) – Multivariate Associations between Baseline Independent SDOCT Predictors and Progression to New Onset of Any GA on Color Fundus Photography over 5 years.
| Multivariate Associations (N=201) a | ||
|---|---|---|
|
| ||
| Adjusted OR (95% CI) b | p-value c | |
| Demographics | ||
| Age [per 5 years] | 1.46 (1.12–1.90) | 0.005 |
| Qualitative SDOCT variables 12 | ||
| Hyperreflective Foci 19 | 6.33 (2.37–16.92) | <0.001 |
| RPE layer Atrophy or absence | 5.32 (2.28–12.40) | <0.001 |
| Photoreceptor Outer Segment loss without underlying RPE changes | 3.27 (1.32–8.08) | 0.01 |
| Atypical Drusen with High Reflectivity | 2.31 (0.99–5.39) | 0.052 |
| OCT-reflective Drusen Substructures 14 | 1.67 (0.80–3.47) | 0.17 |
| Subretinal Drusenoid Deposits (SDD) d,e,f | ||
| [for Choroidal Thickness = 220 μm < Q1] | 0.55 (0.22–1.35) | 0.19 |
| [for Choroidal Thickness = 270 μm ≈ Q2] | 0.96 (0.45–2.08) | 0.92 |
| [for Choroidal Thickness = 320 μm > Q3] | 1.70 (0.54–5.36) | 0.37 |
| Quantitative SDOCT variables 20,21,33 | ||
| Choroidal Thickness (CT) by SDD d,f | 0.11 | |
| CT in absence of SDD [per 100 μm] | 0.22 (0.09–0.56) | 0.002 |
| CT in presence of SDD [per 100 μm] | 0.68 (0.25–1.86) | 0.45 |
| RPE Drusen Complex (RPEDC) volume [per 10−1 mm3] | 1.18 (1.02–1.36) | 0.02 |
| RPEDC Abnormal Thinning (RAT) volume [per 10−3 mm3] | 1.65 (1.06–2.56) | 0.03 |
SDOCT = spectral domain optical coherence tomography, GA = geographic atrophy, RPE = retinal pigment epithelium, SDD = subretinal drusenoid deposits, Q1 = first quartile, Q2 = second quartile, Q3 = third quartile, RPEDC = RPE drusen complex, CT = choroidal thickness, OR = odds ratio.
From 201 participants, 201 eyes had valid measurements for all continuous baseline variables.
Associations are reported from multivariate logistic regression with repeated measures over 5 years. The odds ratio (OR) corresponds to the increase in odds after 5 years of follow-up associated with either (1) the presence of the qualitative variable, or (2) every 1 unit increase in the continuous variable (for quantitative variables), as indicated [between brackets].
Chi-square p-value of 0.05 constituted the significance level.
The presence of Subretinal Drusenoid Deposits (SDD) modifies the effect of Choroidal thickness with a p-value of 0.11 corresponding to the difference in effect.
OR for SDD is reported at different values of Choroidal thickness (around the quartiles of CT: Q1, Q2 and Q3) in order to illustrate the interaction.
Choroidal thickness was adjusted for age, gender and estimated axial length.
Risk Calculator for Progression to Atrophy on CP
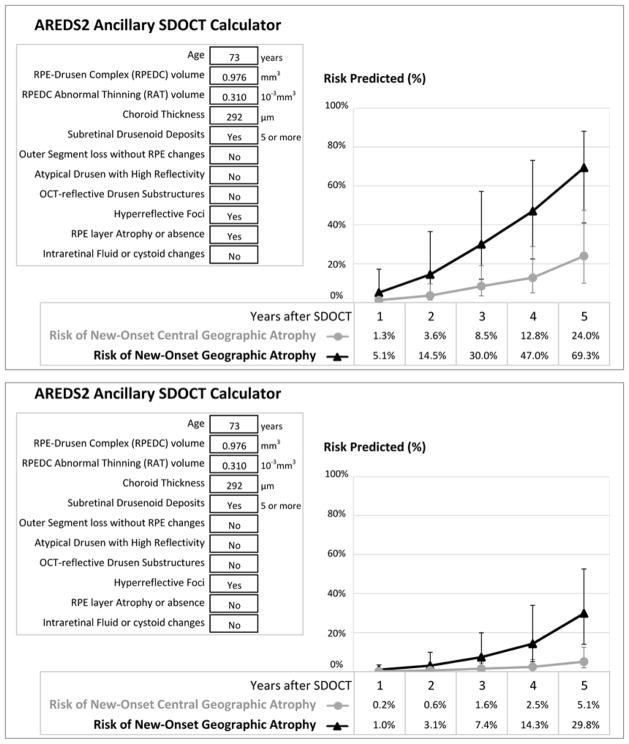
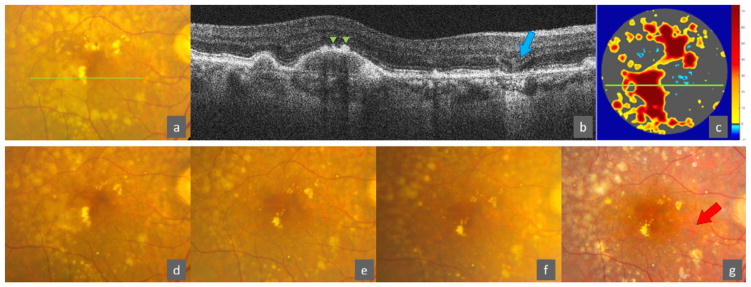
Based on the independent SDOCT predictors of color fundus photography outcomes of atrophy, the calculator in Equation 1 yields the predicted risk of progression to new-onset central GA and to new-onset GA after 1, 2, 3, 4 and 5 years. The probability of developing new-onset central GA or new-onset GA after a certain year is calculated using the logistic regression formula from the corresponding multivariate model (Equation 1). The predicted risk is maximal at the 5th year of follow-up. The year estimates included in the equations convey a reduction in risk for earlier years of follow-up, relative to the reference category of 5 years. As an example from one of the AREDS2 Ancillary SDOCT study participants, the predicted risk can be calculated for the 73 year old patient with a color fundus photo that shows no advanced AMD and a SDOCT volume scan of the central 5mm ring that shows a region of RPE layer atrophy or absence, one or more hyperreflective foci, with large drusen (RPEDC volume = 9.8×10−1 mm3 > 3rd quartile), some RPE thinning (RAT volume = 0.35×10−3 mm3 between the median and the 3rd quartile) and an age-adjusted choroidal thickness that is not thinner than the median (CT = 292 μm between the median and the 3rd quartile), with definite presence of SDD, but no IRF, OCT-reflective Drusen Substructures, atypical drusen with high reflectivity, or photoreceptor OSL without underlying RPE changes (Figure 2 – upper panel). The predicted risk of developing new-onset central GA is 3.6% in 2 years and 24% in 5 years, and for new-onset GA 14.5% in 2 years and 69.3% in 5 years. Had the SDOCT scan not shown any distinct atrophy or absence of the RPE layer, the predicted risk of developing new-onset central GA would have been 0.6% in 2 years and 5.1% in 5 years, and respectively 3.1% and 29.8% for new-onset GA (Figure 2 – lower panel). Study images from this subject are shown in Figure 3 as an example. New onset of GA on CP occurred at year 4 (Figure 3 – g).
Equation 1.
System of Equations for the Risk Calculator for New-Onset Central GA and New-Onset GA on Color Fundus Photography after x Years.
Figure 2.
Preview of the SDOCT-based Risk Calculator a for New-Onset b of Central GA and of GA. The upper panel corresponds to the risk calculation for a participant in the AREDS2 Ancillary SDOCT study with no GA on baseline color photography. The lower panel is for a hypothetical patient with similar findings, except without RPE layer Atrophy or absence on SDOCT.
SDOCT = spectral domain optical coherence tomography, GA = geographic atrophy, AREDS2 = Age-Related Eye Disease Study 2. a This calculator is a research tool, not validated, and not intended for clinical use. Actual outcome is shown in figure 3. b New-onset of atrophy on color fundus photography.
Figure 3.
AREDS2 Ancillary SDOCT Study imaging from the eye from example in Figure 2. Top row: baseline imaging. (a) Color fundus photography at baseline shows large drusen but no geographic atrophy. (b) Baseline SDOCT B-scan at location of green line in (a) showing hyperreflective foci (green arrowheads) and RPE layer Atrophy or absence on OCT (blue arrow). (c) Baseline map of abnormal thickness of RPE drusen complex (OCT Drusen in orange/red, RPE drusen complex Abnormal Thinning in blue). Green line shows location of B-scan in (b). Bottom row: follow-up imaging (chronologic from left to right). Color fundus photography from years 1, 2, 3, and 4 respectively in (d), (e), (f), and (g). In this eye, new onset of GA on color photography (red arrow) occurred in year 4 (g). The participant underwent cataract surgery between years 3 (f) and 4 (g), which explains the improvement in the focus and crispness of the photo (g).
AREDS2 = Age-Related Eye Disease Study 2, SDOCT = spectral domain optical coherence tomography, AMD = age-related macular degeneration, RPE = retinal pigment epithelium, GA = geographic atrophy.
Discussion
From demographics and SDOCT variables, we constructed risk assessment models for progression to new geographic atrophy on CP based on prospective data of a large clinical trial dataset. Consistent with the name of the disease, age was a strong predictor of progression independent of SDOCT findings. We found that hyperreflective foci independently predicted a higher risk of progression to both any GA and central GA, confirming that their long-term involvement in the pathologic process of atrophy is independent of other SDOCT findings. A greater RAT volume and a finding of RPE layer atrophy or absence on SDOCT share the hallmark concept of atrophy on OCT; they both strongly predicted higher risks for new-onset GA and new-onset central GA on CP.
The independent predictors of GA were found in different layers, suggesting different independent steps in the pathogenesis of atrophic AMD. Presence of intraretinal fluid or cystoid spaces, as the sole specific predictor for new-onset central GA, suggests that death of RPE at the center of the fovea may locally be heralded by more internal changes in the nuclear layers. Along the same lines, photoreceptor OSL without underlying RPE changes was an independent predictor of GA, suggesting that photoreceptors damage manifests prior to RPE death and regardless of SDD presence. In addition, although a weaker independent predictor of GA, atypical drusen with high reflectivity suggests that drusen composition constitutes an imperative part of the pathophysiology of atrophy. Likewise, in a separate study of regions containing OCT-reflective drusen substructures (ODS), we have shown that these cores within drusen herald GA in a location-specific manner. 14 In this analysis, both GA and central GA were preceded by ODS, but ODS was not found to be an independent predictor of GA in our multivariate models, possibly due to the presence of other stronger predictors evaluated in this study. Nevertheless, their highly significant and strong univariate longitudinal association with new-onset GA proves that they still constitute an important structural indicator. ODS, along with atypical drusen with high reflectivity, endorse the theory that a certain characteristic subtype of drusen and/or a dynamic change in drusen composition are involved in the local pathophysiology of atrophy. With more detail than previously described GA predictors such as internal reflectivity changes within drusen 13, ODS and high reflective drusen are independent precursors of GA, suggesting that these may reflect two different findings of atrophic pathology.
Furthermore, atrophy, choroidal thickness and SDD (or the corresponding CP finding of reticular pseudodrusen) are a triad of features that have been found, in smaller reports, 38,44–46 to have cross-sectional associations. Herein, they were found – in a large number of eyes – to have similar associations longitudinally. Although this study may have not been powered to detect an interaction between SDD and CT, such interaction term was tested only because suggested in previous literature. In other studies, SDD were considered a possible indicator of choroidal degenerative changes, were found to be associated with other systemic findings in the kidneys and heart, and were accordingly considered a subset of AMD and termed Reticular Macular Disease. 47–49 In absence of reticular pseudodrusen, Thorell et al. found no differences in choroidal thickness between eyes of normal controls, eyes with drusen but no GA, and eyes with GA, but they found thicker choroids in eyes with smaller GA areas. 44 In eyes with reticular pseudodrusen, choroid was thin regardless of the extent of GA. 44 Similarly, eyes with reticular pseudodrusen – whether they had drusen only, GA, or choroidal neovascularization – have been shown to have thinner choroids compared to eyes without reticular pseudodrusen. 50 In this study, we found that SDD were only slightly more prevalent in eyes that progressed to GA, and that this association might have been driven by eyes with thinner choroids. Overall, compared to eyes that progressed to GA, eyes that remained GA-free had thicker choroid (adjusted for age, gender and axial length). This difference in CT was widest among eyes without SDD; despite adjusting for the independent predictors of GA, this association remained solid. The same association was less pronounced in eyes with SDD; but in these SDD-eyes, it vanished upon adjusting for the other independent predictors of GA. While SDD stipulate thinner choroids that cannot prognosticate progression to GA, SDD-free eyes with choroidal thickness that is age-appropriate may be protected against new-onset GA. By asserting claims from other studies, 44,45 our findings showed that choroidal thickness and SDD will help distinguish at least two different pathophysiologic mechanisms. Briefly, the findings of this study pointed to structural SDOCT markers that act as independent contributors to the risk of GA, and which may help in deciphering the pathophysiologic mechanisms leading to GA.
Whether the above features (1) are signs of existing atrophic changes awaiting detection on CP, (2) fit in a chronological sequence of events leading to atrophy, or (3) are markers of separate pathways to atrophy, SDOCT provides important information for estimating risk for progression to atrophy. Previously developed risk scores and prediction models for AMD progression have been based on patient demographics, genetics, and environmental risk factors and relied on color fundus photography. 51–53 Autofluorescence, although the most extensively studied imaging modality in AMD, has been most useful in measuring the extent of geographic atrophy, not specifically its onset. GA size and progression have best been monitored in clinical trials that focus on the progression of GA that already exists in eyes with Late AMD. In contrast, we focused on the SDOCT findings that preceded the first occurrence of GA, because there is evidence to support their significance in relation to visual function. In Wu et al’s study, unlike GA on CP, areas of complete OCT-GA were always the worst-performing point in retinal sensitivity on microperimetry. 54 Loss of retinal sensitivity also characterized SDOCT regions of photoreceptor loss preceding atrophy. 54 Compared to RPE layer atrophy on SDOCT, photoreceptor loss occurs earlier in time and exhibits a less severe loss of retinal sensitivity on microperimetry. 22,54 SDOCT risk factors associated with GA – which may be either features of atrophy on OCT that are not seen on CP, features of OCT-GA that is not complete (e.g. nascent GA), or features that precede OCT-visualized atrophy of the RPE layer – have been previously partially examined. 15,55,56 Ours is the first report to examine independent SDOCT predictors which collectively contribute to a higher risk of developing GA in non-neovascular AMD over up to 7 years. Strengths of this analysis include the regular nature of the imaging data, the length of follow-up, the use of a centralized reading center and the high quality of SDOCT imaging. However, this study is not without limitations. Results for the predictors of any GA differ from those for central GA predictors since central GA was an outdated outcome measure that was based on the location of atrophy and relevant to visual function, rather than biologic mechanisms of progression. In view of certain discrepancies between the results of univariate and those of multivariate analyses for new-onset GA, we recognize that the choroid thins with age and is extremely variable between individuals, and hence may have a weaker association in a multivariate model.
Although the validity of the models was not assessed in a different sample, this calculator displays potential applications of SDOCT imaging in non-neovascular AMD. Currently, and given the novel and unique nature of this dataset, no studies were available with matching samples and imaging needed for validation. A lack of validation of this model limits the ability of an average retinal specialist to make accurate predictions based on this calculator. The predicting features in the calculator are not necessarily specific surrogate outcomes, although they may be candidates for future consideration. There is hope in the future to develop a clinically useful tool, once more studies collect OCT data and extract similar measurements. Still, this is a unique and first of its kind dataset, which – using more sophisticated statistical tools – may be useful to make such models usable clinically. Unlike the current models that relied on the baseline visit, possible models may include longitudinal data to account for changes or patterns of change as predictors of progression, for example. Accordingly, this calculator is presented rather as a research tool and is mainly a proof of concept that highlights the importance of SDOCT imaging in non-neovascular AMD. A major benefit of these current models is to inform the experts on which measurements will be needed for future studies. Similar to a simplified score, this calculator points to the lack of utility in much of the grading performed and found to be not significant or relevant to disease progression. Thus, it shortens grading time from the 20 minutes needed to record all variables, reduces expenses in research and in the clinic, and accelerates future studies.
We suggest and recommend the transition to multimodal imaging that include SDOCT, until a single imaging modality is proven sufficient for the detection of late AMD. As demonstrated in this report, color photography is an outdated method for early detection of atrophy. In addition, by the time of study entry, the SDOCT signs of SDD might have disappeared; the changes that remain observable may possibly be photoreceptor loss without RPE atrophy. Accordingly, earlier occurrence of SDD may have been missed since they were graded using SDOCT only, without autofluorescence or infrared imaging. However, the possible post-SDD finding of photoreceptor loss without RPE atrophy is included in our models. Further, although the original AREDS2 study had shown that there was no effect of supplements on GA on CP, it is not known whether – in this cohort – supplements may have had any influence on the rate or amount of progression to SDOCT findings more subtle than GA. The sample size in this ancillary study may be too small to make appropriate the evaluation of the effect of supplements on rates of new onset of qualitative features, because of the small number of events. Alternative to studying the rates of progression, and rather than measuring events only in the participants in which they occur (i.e. binary outcomes), the effect of supplements may be studied on changes that can be measured on all participants in the cohort, such as change in Drusen volume or change in RAT volume. Still, this warrants further examination of such potential effects in upcoming reports from this study.
Our model can find use as future advancements in the understanding of AMD ensue. Once validated, the risk calculators can have direct applications in the clinic in determining risk of atrophy, especially in the setting of emerging treatments for atrophy, and consideration of pre-treatment with anti-neovascularization agents. In addition to identification of single hallmark features of pre-atrophy, SDOCT-based risk stratification will offer surrogate biomarkers for clinical trials investigating therapies that plan either to treat atrophy in its earliest stages or to prevent the onset of GA. Meanwhile, an online version of the calculator will be available and updated, if further SDOCT predictors or clinical risk factors were added to the models.
Abbreviations and Acronyms
- A2A SDOCT study
AREDS2 Ancillary SDOCT study
- AMD
age-related macular degeneration
- AREDS2
Age-Related Eye Disease Study 2
- CI
confidence interval
- CP
color photography
- CT
choroidal thickness
- GA
geographic atrophy
- GEE
generalized estimating equations
- HF
hyperreflective foci
- IRF
intraretinal fluid or cystoid changes
- NSR
neurosensory retina
- OCT
optical coherence tomography
- ODS
OCT-reflective drusen substructures
- OR
odds ratio
- OSL
photoreceptor Outer Segment Loss
- QICu
Quasilikelihood under the Independence model Criterion statistic
- RAT
RPEDC abnormal thinning
- RPE
retinal pigment epithelium
- RPEA
RPE layer Atrophy or absence
- RPEDC
RPE drusen complex
- SD
standard deviation
- SDD
subretinal drusenoid deposits
- SDOCT
spectral domain OCT
Footnotes
Meeting Presentation: Presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, May 1–5, 2016, Seattle, Washington, USA and at the 7th EURETINA Winter Meeting in Vienna, January 28, 2017, Vienna, Austria.
Financial Disclosure(s): Dr. Yiu reports grants from NIH (K08 EY026101), grants from E Matilda Ziegler Foundation for the Blind, grants from Alcon Research Institute, grants from Barr Foundation for Retinal Research, personal fees from Allergan, personal fees from Carl Zeiss Meditec, outside the submitted work.
Dr. Farsiu reports grants from NIH (EY022691), during the conduct of the study; In addition, Dr. Farsiu has a patent US Patent 8,811,745 and 9299155 issued.
Dr. Clemons reports other from Duke University, during the conduct of the study;.
Dr. Toth reports grants from EMMES and NIH, grants from Genentech, grants from Bioptigen, grants from Alcon, during the conduct of the study; personal fees from Thrombogenics, outside the submitted work; In addition, Dr. Toth has a patent SDOCT image processing pending, and a patent Alcon Laboratories with royalties paid.
Other authors have nothing to disclose.
The AREDS2 study was supported by the intramural program funds and contracts from the National Eye Institute (NEI) of the National Institutes of Health (NIH), Department of Health and Human Services, Bethesda, Maryland (Contract No. HHS-N-260-2005-00007-C. ADB Contract No. N01-EY-5-0007.). The following sponsors supported the AREDS2 Ancillary SDOCT Study and had no role in the design or conduct of this research: Genentech, San Francisco, California (research grant); Alcon, Fort Worth, Texas (research grant); and Bioptigen, Morrisville, North Carolina (research grant).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 3.The Age-Related Eye Disease Study Research G. The age-related eye disease study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the age-related eye disease study report number 6. American Journal of Ophthalmology. 2001;132(5):668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 4.Joachim N, Mitchell P, Burlutsky G, Kifley A, Wang JJ. The Incidence and Progression of Age-Related Macular Degeneration over 15 Years: The Blue Mountains Eye Study. Ophthalmology. 2015;122(12):2482–2489. doi: 10.1016/j.ophtha.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Meyers KJ, Liu Z, Millen AE, et al. Joint Associations of Diet, Lifestyle, and Genes with Age-Related Macular Degeneration. Ophthalmology. 2015;122(11):2286–2294. doi: 10.1016/j.ophtha.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis MD, Gangnon RE, Lee LY, et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman SR, Kozak I, Cheng L, et al. Optical coherence tomography-raster scanning and manual segmentation in determining drusen volume in age-related macular degeneration. Retina. 2010;30(3):431–435. doi: 10.1097/IAE.0b013e3181bd2f94. [DOI] [PubMed] [Google Scholar]
- 9.Jain N, Farsiu S, Khanifar AA, et al. Quantitative comparison of drusen segmented on SD-OCT versus drusen delineated on color fundus photographs. Invest Ophthalmol Vis Sci. 2010;51(10):4875–4883. doi: 10.1167/iovs.09-4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregori G, Wang F, Rosenfeld PJ, et al. Spectral domain optical coherence tomography imaging of drusen in nonexudative age-related macular degeneration. Ophthalmology. 2011;118(7):1373–1379. doi: 10.1016/j.ophtha.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yehoshua Z, Wang F, Rosenfeld PJ, Penha FM, Feuer WJ, Gregori G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology. 2011;118(12):2434–2441. doi: 10.1016/j.ophtha.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leuschen JN, Schuman SG, Winter KP, et al. Spectral-domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology. 2013;120(1):140–150. doi: 10.1016/j.ophtha.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouyang Y, Heussen FM, Hariri A, Keane PA, Sadda SR. Optical coherence tomography-based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology. 2013;120(12):2656–2665. doi: 10.1016/j.ophtha.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veerappan M, El-Hage-Sleiman AM, Tai V, et al. Optical Coherence Tomography Reflective Drusen Substructures Predict Progression to Geographic Atrophy in Age-related Macular Degeneration. Ophthalmology. 2016;123(12):2554–2570. doi: 10.1016/j.ophtha.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleckenstein M, Charbel Issa P, Helb HM, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(9):4137–4144. doi: 10.1167/iovs.08-1967. [DOI] [PubMed] [Google Scholar]
- 16.Brar M, Kozak I, Cheng L, et al. Correlation between spectral-domain optical coherence tomography and fundus autofluorescence at the margins of geographic atrophy. Am J Ophthalmol. 2009;148(3):439–444. doi: 10.1016/j.ajo.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bearelly S, Chau FY, Koreishi A, Stinnett SS, Izatt JA, Toth CA. Spectral domain optical coherence tomography imaging of geographic atrophy margins. Ophthalmology. 2009;116(9):1762–1769. doi: 10.1016/j.ophtha.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folgar FA, Chow JH, Farsiu S, et al. Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Sci. 2012;53(8):4626–4633. doi: 10.1167/iovs.12-9813. [DOI] [PubMed] [Google Scholar]
- 19.Christenbury JG, Folgar FA, O’Connell RV, et al. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013;120(5):1038–1045. doi: 10.1016/j.ophtha.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farsiu S, Chiu SJ, O’Connell RV, et al. Quantitative classification of eyes with and without intermediate age-related macular degeneration using optical coherence tomography. Ophthalmology. 2014;121(1):162–172. doi: 10.1016/j.ophtha.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folgar FA, Yuan EL, Sevilla MB, et al. Drusen Volume and Retinal Pigment Epithelium Abnormal Thinning Volume Predict 2-Year Progression of Age-Related Macular Degeneration. Ophthalmology. 2016;123(1):39–50. e31. doi: 10.1016/j.ophtha.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Wu Z, Luu CD, Ayton LN, et al. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121(12):2415–2422. doi: 10.1016/j.ophtha.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Luu CD, Ayton LN, et al. Fundus autofluorescence characteristics of nascent geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56(3):1546–1552. doi: 10.1167/iovs.14-16211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Group AR, Chew EY, Clemons T, et al. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1) Ophthalmology. 2012;119(11):2282–2289. doi: 10.1016/j.ophtha.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Age-Related Eye Disease Study 2 Research G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 26.Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu SJ, Li XT, Nicholas P, Toth CA, Izatt JA, Farsiu S. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express. 2010;18(18):19413–19428. doi: 10.1364/OE.18.019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu SJ, Izatt JA, O’Connell RV, Winter KP, Toth CA, Farsiu S. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Invest Ophthalmol Vis Sci. 2012;53(1):53–61. doi: 10.1167/iovs.11-7640. [DOI] [PubMed] [Google Scholar]
- 29.Danis RP, Domalpally A, Chew EY, et al. Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2) Invest Ophthalmol Vis Sci. 2013;54(7):4548–4554. doi: 10.1167/iovs.13-11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Z, Ayton LN, Luu CD, Baird PN, Guymer RH. Reticular Pseudodrusen in Intermediate Age-Related Macular Degeneration: Prevalence, Detection, Clinical, Environmental, and Genetic Associations. Invest Ophthalmol Vis Sci. 2016;57(3):1310–1316. doi: 10.1167/iovs.15-18682. [DOI] [PubMed] [Google Scholar]
- 31.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117(2):303–312. e301. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Daniel E, Toth CA, Grunwald JE, et al. Risk of scar in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(3):656–666. doi: 10.1016/j.ophtha.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan E, Folgar F, Chiu S, Farsiu S, Toth C. One-year macular volume change of the neurosensory retina in intermediate AMD by SDOCT semi-automated segmentation. Invest Ophthalmol Vis Sci. 2013;54:6293–6293. [Google Scholar]
- 34.Lee JY, Folgar FA, Maguire MG, et al. Outer retinal tubulation in the comparison of age-related macular degeneration treatments trials (CATT) Ophthalmology. 2014;121(12):2423–2431. doi: 10.1016/j.ophtha.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lujan BJ, Roorda A, Knighton RW, Carroll J. Revealing Henle’s fiber layer using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(3):1486–1492. doi: 10.1167/iovs.10-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yiu G, Pecen P, Sarin N, et al. Characterization of the choroid-scleral junction and suprachoroidal layer in healthy individuals on enhanced-depth imaging optical coherence tomography. JAMA Ophthalmol. 2014;132(2):174–181. doi: 10.1001/jamaophthalmol.2013.7288. [DOI] [PubMed] [Google Scholar]
- 37.Vuong VS, Moisseiev E, Cunefare D, Farsiu S, Moshiri A, Yiu G. Repeatability of Choroidal Thickness Measurements on Enhanced Depth Imaging Optical Coherence Tomography Using Different Posterior Boundaries. Am J Ophthalmol. 2016;169:104–112. doi: 10.1016/j.ajo.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yiu G, Chiu SJ, Petrou PA, et al. Relationship of central choroidal thickness with age-related macular degeneration status. Am J Ophthalmol. 2015;159(4):617–626. doi: 10.1016/j.ajo.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Abbey AM, Kuriyan AE, Modi YS, et al. Optical coherence tomography measurements of choroidal thickness in healthy eyes: correlation with age and axial length. Ophthalmic Surg Lasers Imaging Retina. 2015;46(1):18–24. doi: 10.3928/23258160-20150101-03. [DOI] [PubMed] [Google Scholar]
- 40.Lam AKC, Chan R, Pang PCK. The repeatability and accuracy of axial length and anterior chamber depth measurements from the IOLMaster((TM)) Ophthalmic and Physiological Optics. 2001;21(6):477–483. doi: 10.1046/j.1475-1313.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- 41.Kinoshita T, Mitamura Y, Shinomiya K, et al. Diurnal variations in luminal and stromal areas of choroid in normal eyes. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2016-308594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52(8):5121–5129. doi: 10.1167/iovs.11-7364. [DOI] [PubMed] [Google Scholar]
- 43.Schuman SG, Koreishi AF, Farsiu S, Jung SH, Izatt JA, Toth CA. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology. 2009;116(3):488–496. e482. doi: 10.1016/j.ophtha.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thorell MR, Goldhardt R, Nunes RP, et al. Association Between Subfoveal Choroidal Thickness, Reticular Pseudodrusen, and Geographic Atrophy in Age-Related Macular Degeneration. Ophthalmic Surg Lasers Imaging Retina. 2015;46(5):513–521. doi: 10.3928/23258160-20150521-02. [DOI] [PubMed] [Google Scholar]
- 45.Pilotto E, Guidolin F, Convento E, Stefanon FG, Parrozzani R, Midena E. Progressing geographic atrophy: choroidal thickness and retinal sensitivity identify two clinical phenotypes. Br J Ophthalmol. 2015;99(8):1082–1086. doi: 10.1136/bjophthalmol-2014-306338. [DOI] [PubMed] [Google Scholar]
- 46.Lindner M, Bezatis A, Czauderna J, et al. Choroidal thickness in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56(2):875–882. doi: 10.1167/iovs.14-14933. [DOI] [PubMed] [Google Scholar]
- 47.Leisy HB, Ahmad M, Marmor M, Smith RT. Association between Decreased Renal Function and Reticular Macular Disease in Age-Related Macular Degeneration. Ophthalmology Retina. 2017;1(1):42–48. doi: 10.1016/j.oret.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Martillo AM, Marsiglia M, Lee DM, Pumariega N, Bearelly S, Smith RT. Is Reticular Macular Disease a Choriocapillaris Perfusion Problem? Med Hypothesis Discov Innov Ophthalmol. 2012;1(2):37–41. [PMC free article] [PubMed] [Google Scholar]
- 49.Rastogi N, Smith RT. Association of age-related macular degeneration and reticular macular disease with cardiovascular disease. Surv Ophthalmol. 2016;61(4):422–433. doi: 10.1016/j.survophthal.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Ueda-Arakawa N, Ooto S, Ellabban AA, et al. Macular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomography. Am J Ophthalmol. 2014;157(5):994–1004. doi: 10.1016/j.ajo.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 51.Klein ML, Ferris FL, 3rd, Armstrong J, et al. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology. 2008;115(6):1026–1031. doi: 10.1016/j.ophtha.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 52.Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying GS, Maguire MG Complications of Age-related Macular Degeneration Prevention Trial Research G. Development of a risk score for geographic atrophy in complications of the age-related macular degeneration prevention trial. Ophthalmology. 2011;118(2):332–338. doi: 10.1016/j.ophtha.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Z, Ayton LN, Luu CD, Guymer RH. Microperimetry of nascent geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;56(1):115–121. doi: 10.1167/iovs.14-15614. [DOI] [PubMed] [Google Scholar]
- 55.Moussa K, Lee JY, Stinnett SS, Jaffe GJ. Spectral domain optical coherence tomography-determined morphologic predictors of age-related macular degeneration-associated geographic atrophy progression. Retina. 2013;33(8):1590–1599. doi: 10.1097/IAE.0b013e31828d6052. [DOI] [PubMed] [Google Scholar]
- 56.de Sisternes L, Simon N, Tibshirani R, Leng T, Rubin DL. Quantitative SD-OCT imaging biomarkers as indicators of age-related macular degeneration progression. Invest Ophthalmol Vis Sci. 2014;55(11):7093–7103. doi: 10.1167/iovs.14-14918. [DOI] [PubMed] [Google Scholar]
- 57.The Age-Related Eye Disease Study Research G. The Age-Related Eye Disease Study (AREDS) Controlled Clinical Trials. 1999;20(6):573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]